Abstract
Although physiologic jaundice of neonates is common, persistent neonatal cholestasis is life-threatening and has multiple etiologies. Among these etiologies, biliary atresia (BA) requires rapid diagnosis and treatment. In diagnosing BA, the surgical pathologist must recognize subtle histologic changes, often with only a small core liver biopsy. To aid in the differential diagnosis of neonatal cholestasis, we investigated Yes-associated protein (YAP), a regulator of organ size and bile duct development. We examined whether a YAP immunostain can highlight emerging hepatobiliary epithelium in BA [n=28] versus other causes of persistent cholestasis (non-BA) [n=15] and thus serve as a useful diagnostic marker in persistent neonatal jaundice. We show significantly (p≤0.01) more high-grade (≤2) fibrosis and ductular proliferation among BA versus non-BA cases. Likewise, there was significantly more high-grade (2–3/3) cytoplasmic and nuclear YAP staining in BA (97% and 89%) versus non-BA (20% and 13%). High-grade nuclear YAP staining was both sensitive (88%) and specific (87%) for the diagnosis of BA. In contrast to neonatal cholestasis, the differences in YAP localization in cholestatic/obstructed vs. non-obstructed adult livers were not significant. Lastly, we found that pharmacological inhibition of the YAP complex in both cholangiocyte and cholangiocarcinoma cell lines blocked compensatory bile duct proliferation, an early marker of BA that requires nuclear YAP expression, in a time- and dose-dependent manner. In summary, we show that YAP expression modulates both bile duct proliferation and liver damage/fibrosis while acting as a sensitive and specific marker in the differential diagnosis of persistent neonatal cholestasis.
Keywords: Biliary Atresia, Neonatal Cholestasis, Bile Duct Obstruction, Hippo, Neurofibromatosis 2 (NF2), verteporfin (VP), Yes-associated protein (YAP)
Introduction
Biliary atresia (BA) is a common cause of persistent neonatal cholestasis and liver transplantation in the pediatric population1. There are three forms of the disease. The first form, also called “isolated BA”, is characterized by biliary inflammation and thought to be either infectious or autoimmune related. The two congenital forms are associated with either laterality defects such as situs inversus, intestinal malrotation, polysplenia and dextrocardia2, or major congenital anomalies not related to laterality3. In all forms of BA, there is urgent need for early diagnosis and surgical intervention via a Kasai procedure (hepatoportoenterostomy). The goal of surgery is to ameliorate clinical symptoms, slow liver disease and delay or obviate the need for a liver transplant1.
The diagnosis can be suspected with a combination of clinical findings and noninvasive diagnostic approaches, such as: hepatobiliary iminodiacetic acid (HIDA) scan, magnetic resonance cholangiopancreatography (MRCP) and serum chemistries (transaminases, alkaline phosphatase (AlkP), gamma-glutamyl transpeptidase (GTT) and bilirubin). These tests, however, are often insufficient for a definitive diagnosis of BA4,5. As a result, a liver needle biopsy may guide the need for further invasive intervention and together with an intraoperative cholangiogram serves to diagnose BA6,7.
The liver biopsy in BA is characterized by periportal and interlobular bile ductular proliferation (also referred to as bile ductular reaction), bile plugs and portal fibrosis8. Using a liver needle biopsy to assess these criteria can pose a significant challenge. Surgical pathologists must often rely on only a small core biopsy and a few portal triads available for evaluation. The histologic findings vary with disease progression and potentially the age of the patient; moreover, any single histologic feature is non-specific, showing significant overlap with other disease entities in the differential diagnosis of persistent neonatal cholestasis9,10.
Several immunohistochemical (IHC) stains have been examined as ancillary tools in diagnosing neonatal cholestasis, but none have been widely adopted. Thus far, the efforts have been focused on epithelial markers and adhesion molecules with varied success10,11. General epithelial markers such as CK7 and CK19 stain biliary epithelium but not the newly emergent, proliferating bile ductules10. Alternatively, neural cell adhesion molecule (NCAM/CD56)9 and intercellular adhesion molecule 1 (ICAM-1)12 can stain both the existing bile ducts and the newly-formed bile ductules, but mark these bile ductules in a somewhat weak and non-specific fashion. The cytoplasmic/membranous staining pattern of both markers can be difficult to optimize and interpret. Lastly, there is no established relationship between CD56 or ICAM-1 and either bile duct development or the initiation and progression of BA. Therefore, the use of both markers as ancillary tools in the differential diagnosis of neonatal cholestasis has been controversial and debated13,14.
The ongoing need for ancillary histopathologic markers for the differential diagnosis of neonatal cholestasis has led us to investigate Yes-associated protein (YAP). YAP is the transcriptional effector of the Merlin (NF-2)-Hippo signaling cascade and together with its binding partner TEAD regulates expression of pro-proliferative target genes15. YAP is known to be dysregulated in carcinogenesis16 and is consistently up-regulated in a number of cancers, most pertinently hepatocellular carcinoma17,18. More recently, YAP has also been shown to be necessary for development of bile ducts17 and adaptive responses within the GI-tract, including intestinal response to toxic injury19 as well as perinatal growth and differentiation in the liver20. Importantly, verteporfin (VP), a readily available FDA-approved drug, specifically disrupts the pro-proliferative effects of the NF2-Hippo pathway and could be a therapeutic option in biliary diseases such as BA15 The objective of this study is to assess YAP expression in the setting of neonatal and adult cholestasis as well as its diagnostic potential in the differential diagnosis of BA.
Methods
Study Design
This study (NA_0006726) was approved by the Johns Hopkins School of Medicine Institutional Review Board. Search of Johns Hopkins Hospital archives over the past five years (2007–2012) identified 55 neonates with a liver biopsy performed to evaluate persistent cholestasis. Twenty-eight (28/33, 85%) BA and 15 (15/22, 73%) cholestatic control (non-BA) cases were available for further study. Non-BA cases included: 7 cases of giant cell hepatitis, 5 non-specific/descriptive diagnoses, 3 Allagille’s Syndrome, 2 Ductopenic syndrome and 5 other causes of cholestasis (single case each). As a control cohort, 19 adult patients with pancreatic adenocarcinoma and a non-mass directed liver biopsy performed at the time of pancreatoduodenectomy were also identified. These biopsy samples were separated into two groups: obstructed adult livers (due to adenocarcinoma in the pancreatic head; 12/19 or 63%) or non-obstructive (adenocarcinoma in the pancreatic body/tail; 7/19 or 37%). For all patients, we collected the following clinical information: general patient data (age, sex, date of surgery), retrospectively established diagnosis (BA vs. non-BA, location of pancreatic adenocarcinoma) and serum laboratory values (total bilirubin (tBili), alkaline phosphatase (AlkP)). To document pre-operative obstruction in adult patients, the percentage change from 24 hours pre-surgery (before) to 48 hours post-surgery for total bilirubin (%ΔtBili) and for alkaline phosphatase (%ΔAlkP) were calculated using: (Before − After)/(Before) × 100%.
Immunohistochemistry
All tissue was fixed in 10% neutral buffered formalin. Five-micron sections from paraffin-embedded tissue were stained with anti-YAP (Epitomics, Burlingame, CA) (diluted 1:200) followed by secondary anti-rabbit antibody (Envision, DAKO, Denmark) application and detected using the DAB substrate kit (Invitrogen, Carlsbad, CA) per manufacturer’s instructions. YAP staining distribution within the interlobular biliary epithelium and proliferating ductules was evaluated in at least 3 portal areas in 20× fields regardless of tissue sample size, according to the following grading scheme: 0 = none; 1 = 0–5%; 2 = 5–90%; 3= >90% of biliary epithelium staining for YAP. The intensity of YAP staining was graded (0–3), as follows: 0 = no staining; 1 = minimal/difficult to discern; 2 = moderate staining; 3 = strong staining. Although the extent of staining was also considered, focal/patchy staining was not observed; in short, the intensity of YAP immunopositivity varied but the extent was either diffuse or non-existent. Similarly, the histological assessment of bile duct proliferation was performed on a 0–3 scale, ranging from none (similar to control/healthy liver tissue) to 3 (marked/robust, i.e. ductular proliferation that nearly bridges adjacent portal tracts). The degree of liver damage/fibrosis was evaluated using archived H&E stain and if available MAS/trichrome histochemical stain (if available), using a modified Scheuer grading, as previously described21. The samples were retrieved and evaluated by a resident pathologist [GTG] and then re-evaluated by second pathologist [RAA], who was blinded to the diagnosis.
Cell Culture and Cell Proliferation Assay
Cell culture was performed using the human SV-40 immortalized, non-malignant intrahepatic bile duct cell line, H69 (a gift from Dr. Nicholas LaRusso, Mayo Clinic, Rochester, MN and Dr. Douglas Jefferson, Tufts University, Boston, MA) originally isolated from a normal liver prior to transplantation and cultured as previously described22. HuCCT1 cholangiocarcinoma cell line was a kind gift of Dr. Anthony J Demetris (University of Pittsburgh). Cells were plated in 96 well plates at a density of 2,500 cells/well and assessed for viability prior to experimentation. Verteporfin (VWR Radnor PA, USA) or vehicle control was added at day 0, for 24, 48 or 72 hours, at concentrations of 0μM, 1.25 μM, 2.5 μM and 5 μM. The number and cell viability were assessed by Vybrant® MTT Cell Proliferation Assay Kit (Invitrogen, Carlsbad, CA), per manufacturer’s instructions and assessed using SPECTRAMAX 340PC spectrophotometer (Molecular Devices, Sunnyvale, CA USA).
Statistics
Statistical analyses were performed using the GraphPad Prism software, unless otherwise specified (GraphPad Software, ver5, La Jolla, CA). Immunohistochemical (IHC) staining was evaluated using a non-parametric analysis of variance (Kruskall-Wallis), followed by Dunnet’s post-test. Categorical study cohort data and serum serologies were represented as percentages and evaluated by chi-squared test. Correlation between nuclear YAP staining and other histologic features/serological markers were examined by Spearman’s rank correlation coefficient and denoted as ρ. Error bars represent standard deviation (SD). For all analyses, differences were considered significant at p≤0.01.
Results
YAP expression in the liver of cholestatic neonates
Forty-three available neonatal liver needle and wedge biopsies were classified into 28 BA and 15 non-BA cases of neonatal cholestasis. Both neonatal cohorts had similar patient characteristics, including age and gender (Table 1). As further discussed below, patients with BA also had a statistically higher mean histological index of fibrosis (2.8 vs. 1.5, p≤0.01) and bile ductular proliferation (2.4 vs. 0.87, p≤0.01) compared to non-BA patients (Table 1).
TABLE 1.
Clinical patient characteristics – Pediatric Cohort Non-BA vs. Biliary Atresia (BA)
| Age Range | Male/Female (%) |
Fibrosis (0–4) |
Duct Prolif (0–3) |
YAP cyto (0–3) |
YAP nuc (0–3) |
|
|---|---|---|---|---|---|---|
|
| ||||||
| Non-BA | 1wk-12MO | 73/27 | 1.0 | 1.5 | 0.87 | 0.53 |
|
| ||||||
| BA | 2wks-13MO | 46/54 | 2.8 | 2.9 | 2.4 | 2.5 |
|
| ||||||
| Significance | ns | ns | ** | ** | ** | ** |
Legend: Clinical and histopathological characteristics; n=15 (non-BA); n=28 (BA)
ns (not significant) or
p≤0.01 by Student’s t-test or Chi-squared test
At baseline and similar to what we reported in the murine liver23, YAP IHC showed a weak cytoplasmic staining pattern of the bile duct epithelium. Likewise, in liver biopsy samples from non-BA neonatal cholestasis, YAP IHC demonstrated rare, weak positivity in bile duct epithelium (Figure 1A–B). In contrast, neonates with BA showed significantly greater YAP staining of bile duct epithelium, including newly formed bile ductules (Figure 1C–D). Because YAP functions as a transcriptional co-activator, we specifically examined whether YAP is localized to the nucleus or cytoplasm. In BA tissue, YAP IHC staining intensity was significantly (p≤0.01) higher (2–3 out of 3) compared to non-BA tissue (0–1 out of 3). This was true for both cytoplasmic (97% vs. 20%, Figure 2A) and nuclear YAP intensity (89% vs 13%, Figure 2B). In sum, we found significantly (p≤0.01) more YAP, both in the cytoplasm (2.4 vs 0.87) and perhaps more functionally important in the nucleus(2.5 vs 0.53) in BA compared to non-BA patients (Table 1).
Figure 1. YAP staining in neonatal cholestasis.
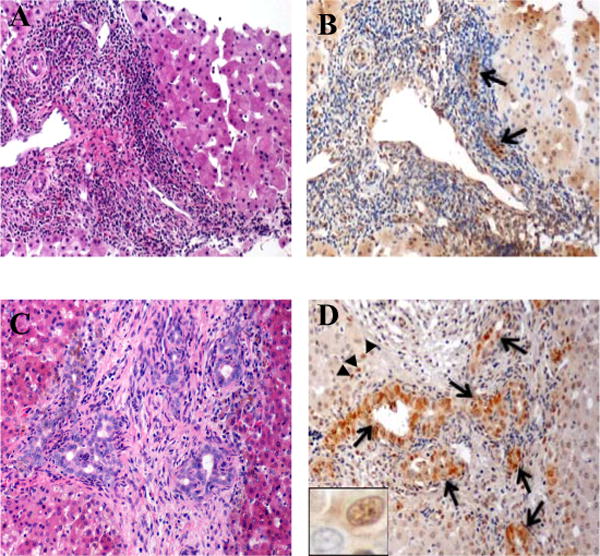
H&E (left) and corresponding YAP immunostain (right) – representative areas with bile duct proliferation at 40×. [a, b] Non-BA (Giant Cell Hepatitis); arrows highlight weak, diffuse cytoplasmic and focal nuclear YAP in remaining bile ducts. [c, d] Biliary atresia (BA); arrows highlight strong nuclear YAP staining in both mature bile ducts and proliferating bile ductules; arrowheads point to nuclear YAP staining in periportal hepatocytes. Nuclear YAP positivity in bile ductule nucleus and none in the adjacent hepatocyte (inset).
Figure 2. Semi-quantitative assessment of YAP in non-BA neonatal cholestasis versus neonatal biliary atresia (BA).
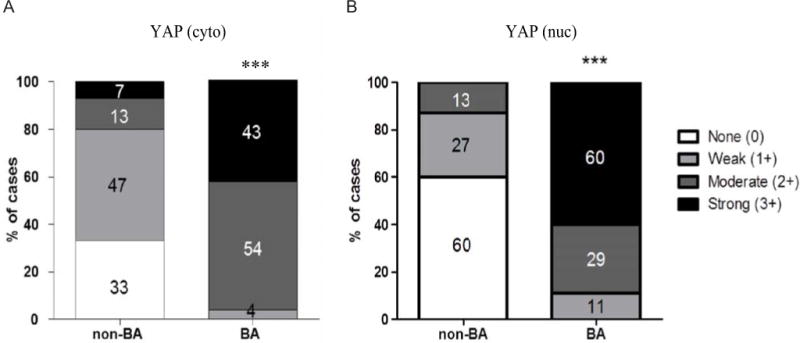
[a] Cytoplasmic YAP (scale 0–3) [b] Nuclear YAP (scale 0–3). Percentage of cases in each color-coded category are indicated within the bar graph; ** p≤0.01 by Chi-squared test.
We also examined the correlation between YAP expression and several other known quantifiable markers of cholestasis. We noted significant (p≤0.01) correlation between increased nuclear YAP staining intensity versus both increased rates of fibrosis and ductular proliferation (Supplementary Figure 1A&B). Conversely, pre-operative serum laboratory values (alkaline phosphatase and total bilirubin) did not show significant correlation with nuclear YAP (Supplementary Figure 1C&D)., High grade nuclear YAP staining was also highly sensitive (88%) and specific (87%) for the diagnosis of BA. Importantly, staining of the liver parenchyma was minimal, with the exception of weak, focalnuclear staining of periportal hepatocytes near the proliferating bile ductules (Figure 1D, arrowheads).
YAP expression in adult intrahepatic biliary epithelium
We collected liver biopsies from 19 adults with pancreatic adenocarcinoma undergoing pancreaticoduodenectomy. Control patients without bile duct obstruction were represented by cases of adenocarcinoma located in the pancreatic body or tail (non-obstructed, n=7), whereas cases with bile duct obstruction were represented by adenocarcinoma located in the pancreatic head (obstructed, n=13). Both patient cohorts had similar gender and age distribution (Table 2). To verify obstruction, we compared the change in serum biochemical markers associated with cholestasis before and after pancreaticoduodenectomy. As expected, obstructed patients showed a significantly (p≤0.01) greater reduction in total bilirubin (% ΔtBili: 65% vs. 5.9%) and alkaline phosphatase (% ΔAlkP: 65% vs. 20%) than non-obstructed patients. Adult patients with obstruction also showed significantly (p≤0.01) higher mean histological index of fibrosis (2.4 vs. 0.92, p≤0.01) and bile ductular proliferation (1.9 vs. 1.1, p≤0.01). For adults, however, there was no significant increase in nuclear (1.0 vs. 1.4) and cytoplasmic (1.9 vs. 2.1) YAP staining in the bile duct epithelium of obstructed versus non-obstructed patients (p=0.21) (Figure 3 and Table 2).
TABLE 2.
Clinical patient characteristics – Adult Cohort Non-obstructed vs. Obstructed
| Age Range (yo) |
Male/Female (%) |
% ΔtBili | %ΔAlkP | Fibrosis (0–4) |
Duct Prolif (0–3) |
YAP cyto (0–3) |
YAP nuc (0–3) |
|
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Non Obstructed | 41–79 | 43/57 | 5.9 | 20 | 0.92 | 1.1 | 1.9 | 1.0 |
|
| ||||||||
| Obstructed | 61–88 | 46/54 | 65 | 65 | 2.4 | 1.9 | 2.1 | 1.4 |
|
| ||||||||
| Significance | ns | ns | ** | ** | ** | ** | ns | ns |
Legend: ΔtBili and ΔAlkP laboratory values prior to surgery and 2 days (48 h) post-OP. n=7 (non-obstructed); n=12 (obstructed).
ns (not significant) or
p<0.01 by Student’s t-test or Chi-squared test
Figure 3. YAP staining in adult livers.
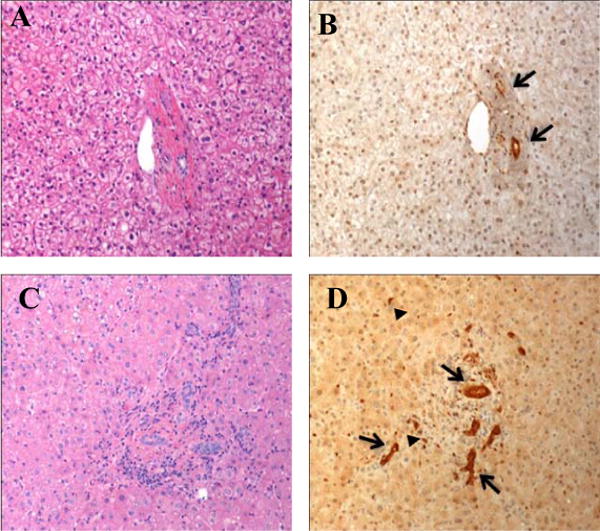
H&E (left) and corresponding YAP immunostain (right) – representative areas with bile duct proliferation at 100×. [a, b] Non-obstructed liver biopsy; arrows highlight baseline staining in mature bile ducts. [c, d] Obstructed liver biopsy; arrows highlight robust YAP staining in both mature bile ducts; arrowheads point to slightly weaker-staining bile ductules.
Functional significance of blocking YAP activity in proliferating bile duct epithelium
YAP is a transcriptional co-activator and acts as part of the TEA domain (TEAD) DNA binding complex. Verteporfin (VP), a recently uncovered cell-permeable small molecule, inhibits the YAP-TEAD association and has been shown to block the effect of YAP overexpression on the murine liver15,24. After optimizing VP-mediated inhibition of YAP in H69 cholangiocyte cell line, we found both a time- (24 to 72hr) and a dose-dependent (2.5 to 5 μM) VP inhibition of cell growth (Supplementary Figure 2). We then examined the effect of VP at the 72hr end time point in more detail, comparing H69 cholangiocyte s to the HuCCT1 cholangiocarcinoma cell line (Figure 4). H69 cholangiocytes showed minimal reduction of proliferation at 1.25 μM but significant (p≤0.01) reductions at higher concentrations, reducing proliferation by as much as 70–60% at 5 μM. A similar pattern of progressive inhibition of proliferation was seen for HuCCT1 cholangiocarcinoma cells; however, the effect was significant (p≤0.01) at even lower concentrations (1.25 μM) and more pronounced than in H69 cholangiocytes, decreasing proliferation by 80–90% at 5 μM (Figure 4).
Figure 4. Inhibition of YAP blocks growth in a dose-dependent fashion.
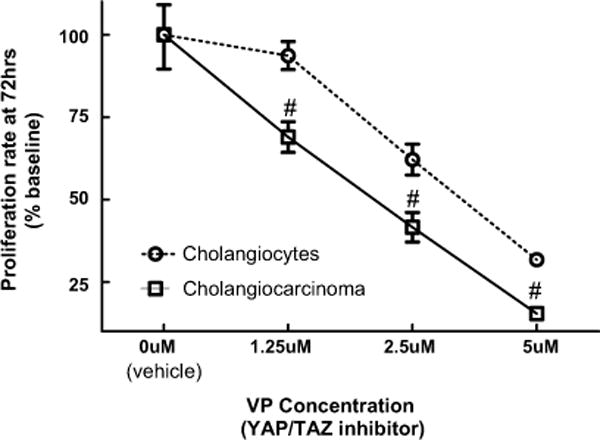
H69 human cholangiocyte cell line and HuCCT1 cholangiocarcinoma cell line treated with YAP/TAZ inhibitor VP for 72 hours. n=4 at each time point. Significant inhibition (#, p≤0.05) versus H69 at the same inhibitor concentration.
Discussion
Pathologic infant jaundice is a frequent cause of perinatal hospitalization, and among the many etiologies, biliary atresia (BA) remains the most common indication for liver transplantation in children25. Diagnostic workup of persistent neonatal cholestasis often requires a liver biopsy26; with current methods, biopsy interpretation is still a significant diagnostic challenge27,28. As a result, more accurate histopathological markers are needed to help diagnose BA – and to this end YAP may prove highly useful.
The YAP protein is critical in regulating liver response to cholestasis in animal models23. Here, we are the first to show YAP as a useful diagnostic marker in non-neoplastic, human hepatobiliary disease; we show that YAP highlights proliferating bile ducts, a hallmark of BA29. Importantly, the staining pattern of YAP is both strong and diffuse. YAP staining also appears to be the most sensitive and specific IHC markers tested as ancillary tools in the differential diagnosis of BA versus non-BA neonatal cholestasis9,11,12. The extent and intensity of YAP expression appears to be highly dependent on the clinical situation. Here, for instance, we did not see a significant difference in YAP expression between adults with or without biliary obstruction and persistent cholestasis. In addition, YAP appears to be more important in the developing neonatal liver. Deletion of YAP in the developing murine liver results in ductopenia, while deletion in the adult liver yields no phenotype unless the liver is challenged with bile duct ligation23. In addition, we note a robust correlation (ρ>0.6, p≤0.01) between nuclear YAP staining and both ductal proliferation and pre-operative fibrosis, two histologic hallmarks of early-mid course BA8. Conversely, pre-operative serum chemistry tests, including total bilirubin and alkaline phosphatase, do not correlate with YAP; hence, as previously noted30, serum chemistries are generally of limited diagnostic utility in this setting. In sum, these findings emphasize the importantrole of liver biopsy in the diagnosis of BA and the possible ancillary function for YAP.
Our study also suggests that at least in neonates, YAP may be mechanistically involved in reactive bile duct proliferation due to impaired bile outflow. YAP has been previously shown to regulate expression of Cyclin D1, an important mediator of G0 → S phase transition31. Here, we show that the inhibition of YAP significantly blocks the proliferation of the non-neoplastic H69 cholangiocyte cell line in a time- and dose-dependent manner. We also saw increased nuclear YAP expression in periportal hepatocytes. Periportal hepatocytes are candidates for a putative reserve pool of pleuripotent precursors for biliary epithelium/proliferating bile ductules32. This pool is likely to be activated in context of adaptive/reparative change such as BA, and manipulation of the process, perhaps via YAP, may hold future therapeutic potential. Lastly, it has been noted that ductal plate malformation (DPM) around the portal tract mesenchyme is more frequent in BA than non-BA etiologies of neonatal cholestasis and may be a post-operative prognostic indicator33. A study to examine YAP expression in DPM and its relationship to outcome and other clinical features is currently underway in our laboratory. Our H69 cell culture experiments and inferences based on YAP staining patterns by IHC should prompt additional work to firmly establish a mechanistic link between YAP expression and bile duct proliferation in vivo.
The mechanism of regulation between YAP expression and activity within the biliary epithelium remains unclear. Although NF2/Merlin has been shown to be an important regulator of YAP in hepatocellular carcinoma17,34, the effect of NF2 on the biliary tract is less clear. For example, thus far, there is no evidence of increased incidence of cholangiocarcinoma or primary biliary liver disease in neurofibromatosis. Here, we also show that blocking YAP by FDA-approved drug Vereteporfin markedly inhibits the proliferation of HuCCT1 cholangiocarcinoma cell line and to a lesser extent,H69 cholangiocytes. The significance of this observation is beyond the scope of this work, but may be due to increased dependence of cancer cells on trophic signals35. Though well-tolerated for current treatment of macular degeneration and now in clinical trials for pancreatic adenocarcinoma, Vereteporfin has yet to be approved for treatment of hepatobiliary disease. Lastly, there may also be a stromal contribution to YAP activation. The differences in stromal cell populations, expression of surface markers, and the composition or density of acellular matrix may influence bile duct proliferation, YAP expression and, in part, account for the differential expression/activation of YAP in BA versus non-BA neonatal cholestasis.
In summary, this study demonstrates that YAP protein is expressed at low levels in both adult and neonatal biliary epithelium, but its expression is significantly increased in proliferating ductules – a reactive process that is a hallmark of BA. Furthermore, elevated YAP expression correlates with bile duct proliferation and fibrosis, two important histological features of BA. As a parallel finding, inhibition of YAP in H69 cholangiocytes and HuCCT1, a cholangiocarcinoma cell line, significantly reduces proliferation. Taken together, these results show that a nuclear YAP immunoreactivity can be useful diagnostic marker and YAP may potentially be a mechanistically-important player in BA. The link between YAP expression and compensatory bile duct proliferation in BA should promote further translational work in this field.
Supplementary Material
Supplementary Figure 1: Correlation between nuclear YAP staining and clinical/histological measures of cholestasis. (a) YAP vs fibrosis (b) YAP vs ductular proliferation (c) YAP versus AlkPhos (d) YAP versus tBili. ρ = Spearman’s Rank correlation coefficient; **p≤0.01, otherwise not significant.
Supplementary Figure 2: Optimization of time- and dose-dependent YAP inhibition in H69 cholangiocytes. H69 human cholangiocytes were treated with YAP/TAZ inhibitor VP for 0, 24, 48 and 72 hours. n=4–5 at each time point; #p≤0.05 and ##p≤0.01 indicates significant inhibition versus vehicle at the same inhibitor concentration.
Acknowledgments
This work was presented, in part, at the Keystone Symposium (Hippo Tumor Suppressor Network, Monterey, CA in May 2013). We would like to thank Molly Van Appledorn for help with statistical analyses, Suresh K. Nayar for helpful discussions and review of the manuscript. The research was supported by the Joseph Eggleston Grant (GTG), NIH R01 grants (DK080736 and DK081417, to RAA) and Children’s Liver Disease Research and Education Network (UO1DK62503, to RAA/KS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cleghorn G. Biliary atresia and its micromanagement: does it really matter? J Pediatr. 2005 Aug;147(2):142–143. doi: 10.1016/j.jpeds.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 2.Chu AS, Russo PA, Wells RG. Cholangiocyte cilia are abnormal in syndromic and non-syndromic biliary atresia. Mod Pathol. 2012 May;25(5):751–757. doi: 10.1038/modpathol.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarz KB, Haber BH, Philip R, et al. Extra-hepatic anomalies in infants with biliary atresia: results of a large prospective North American multi-center study. Hepatology. 2013 May 23; doi: 10.1002/hep.26512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005 Jan-Feb;21(1):31–38. doi: 10.1002/dmrr.532. [DOI] [PubMed] [Google Scholar]
- 5.Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr. 2003 Jul;37(1):4–21. doi: 10.1097/00005176-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Shneider BL, Brown MB, Haber B, et al. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. 2006 Apr;148(4):467–474. doi: 10.1016/j.jpeds.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 7.Haber BA, Russo P. Biliary atresia. Gastroenterol Clin North Am. 2003 Sep;32(3):891–911. doi: 10.1016/s0889-8553(03)00049-9. [DOI] [PubMed] [Google Scholar]
- 8.Russo P, Magee JC, Boitnott J, et al. Design and validation of the biliary atresia research consortium histologic assessment system for cholestasis in infancy. Clin Gastroenterol Hepatol. 2011 Apr;9(4):357–362. e352. doi: 10.1016/j.cgh.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torbenson M, Wang J, Abraham S, Maitra A, Boitnott J. Bile ducts and ductules are positive for CD56 (N-CAM) in most cases of extrahepatic biliary atresia. Am J Surg Pathol. 2003 Nov;27(11):1454–1457. doi: 10.1097/00000478-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguti DC, Patricio FR. Morphometrical and immunohistochemical study of intrahepatic bile ducts in biliary atresia. Eur J Gastroenterol Hepatol. 2011 Sep;23(9):759–765. doi: 10.1097/MEG.0b013e32832e9df0. [DOI] [PubMed] [Google Scholar]
- 11.Davenport M, Gonde C, Redkar R, et al. Immunohistochemistry of the liver and biliary tree in extrahepatic biliary atresia. J Pediatr Surg. 2001 Jul;36(7):1017–1025. doi: 10.1053/jpsu.2001.24730. [DOI] [PubMed] [Google Scholar]
- 12.Ghoneim EM, Sira MM, Abd Elaziz AM, Khalil FO, Sultan MM, Mahmoud AB. Diagnostic value of hepatic intercellular adhesion molecule-1 expression in Egyptian infants with biliary atresia and other forms of neonatal cholestasis. Hepatol Res. 2011 Aug;41(8):763–775. doi: 10.1111/j.1872-034X.2011.00832.x. [DOI] [PubMed] [Google Scholar]
- 13.Mahjoub FE, Khairkhah RH, Sani MN, Irvanloo G, Monajemzadeh M. CD 56 staining in liver biopsies does not help in differentiating extrahepatic biliary atresia from other causes of neonatal cholestasis. Diagn Pathol. 2008;3:10. doi: 10.1186/1746-1596-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broome U, Nemeth A, Hultcrantz R, Scheynius A. Different expression of HLA-DR and ICAM-1 in livers from patients with biliary atresia and Byler’s disease. J Hepatol. 1997 Apr;26(4):857–862. doi: 10.1016/s0168-8278(97)80253-x. [DOI] [PubMed] [Google Scholar]
- 15.Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012 Jun 15;26(12):1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008 Nov;39(11):1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang N, Bai H, David KK, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010 Jul 20;19(1):27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu AM, Xu MZ, Chen J, Poon RT, Luk JM. Targeting YAP and Hippo signaling pathway in liver cancer. Expert Opin Ther Targets. 2010 Aug;14(8):855–868. doi: 10.1517/14728222.2010.499361. [DOI] [PubMed] [Google Scholar]
- 19.Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010 Nov 1;24(21):2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Septer S, Edwards G, Gunewardena S, et al. Yes-associated protein is involved in Proliferation and Differentiation During Postnatal Liver Development. Am J Physiol Gastrointest Liver Physiol. 2011 Dec 22; doi: 10.1152/ajpgi.00056.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheuer PJ, Standish RA, Dhillon AP. Scoring of chronic hepatitis. Clin Liver Dis. 2002 May;6(2):335–347. doi: 10.1016/s1089-3261(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 22.Bandyopadhyay AK, Das T, Sa G, Mukherjea M. Effect of fatty acid binding proteins on developing human placental malate dehydrogenase activity. Indian J Exp Biol. 1994 Nov;32(11):800–803. [PubMed] [Google Scholar]
- 23.Bai H, Zhang N, Xu Y, et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012 Sep;56(3):1097–1107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007 Sep 21;130(6):1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn E. Biliary atresia revisited. Pediatr Dev Pathol. 2004 Mar-Apr;7(2):109–124. doi: 10.1007/s10024-003-0307-y. [DOI] [PubMed] [Google Scholar]
- 26.Moyer V, Freese DK, Whitington PF, et al. Guideline for the evaluation of cholestatic jaundice in infants: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2004 Aug;39(2):115–128. doi: 10.1097/00005176-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Azar G, Beneck D, Lane B, Markowitz J, Daum F, Kahn E. Atypical morphologic presentation of biliary atresia and value of serial liver biopsies. J Pediatr Gastroenterol Nutr. 2002 Feb;34(2):212–215. doi: 10.1097/00005176-200202000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Zerbini MC, Gallucci SD, Maezono R, et al. Liver biopsy in neonatal cholestasis: a review on statistical grounds. Mod Pathol. 1997 Aug;10(8):793–799. [PubMed] [Google Scholar]
- 29.Moreira RK, Cabral R, Cowles RA, Lobritto SJ. Biliary atresia: a multidisciplinary approach to diagnosis and management. Arch Pathol Lab Med. 2012 Jul;136(7):746–760. doi: 10.5858/arpa.2011-0623-RA. [DOI] [PubMed] [Google Scholar]
- 30.Manolaki AG, Larcher VF, Mowat AP, Barrett JJ, Portmann B, Howard ER. The prelaparotomy diagnosis of extrahepatic biliary atresia. Arch Dis Child. 1983 Aug;58(8):591–594. doi: 10.1136/adc.58.8.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007 Dec 4;17(23):2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 32.Ernst LM, Spinner NB, Piccoli DA, Mauger J, Russo P. Interlobular bile duct loss in pediatric cholestatic disease is associated with aberrant cytokeratin 7 expression by hepatocytes. Pediatr Dev Pathol. 2007 Sep-Oct;10(5):383–390. doi: 10.2350/06-09-0171.1. [DOI] [PubMed] [Google Scholar]
- 33.Low Y, Vijayan V, Tan CE. The prognostic value of ductal plate malformation and other histologic parameters in biliary atresia: an immunohistochemical study. J Pediatr. 2001 Aug;139(2):320–322. doi: 10.1067/mpd.2001.117003. [DOI] [PubMed] [Google Scholar]
- 34.Benhamouche S, Curto M, Saotome I, et al. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010 Aug 15;24(16):1718–1730. doi: 10.1101/gad.1938710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehlen P. Dependence receptors: the trophic theory revisited. Sci Signal. 2010;3(151):47. doi: 10.1126/scisignal.3151pe47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Correlation between nuclear YAP staining and clinical/histological measures of cholestasis. (a) YAP vs fibrosis (b) YAP vs ductular proliferation (c) YAP versus AlkPhos (d) YAP versus tBili. ρ = Spearman’s Rank correlation coefficient; **p≤0.01, otherwise not significant.
Supplementary Figure 2: Optimization of time- and dose-dependent YAP inhibition in H69 cholangiocytes. H69 human cholangiocytes were treated with YAP/TAZ inhibitor VP for 0, 24, 48 and 72 hours. n=4–5 at each time point; #p≤0.05 and ##p≤0.01 indicates significant inhibition versus vehicle at the same inhibitor concentration.


