Abstract
The PI3K/Akt survival pathway is often dysregulated in cancer. Our previous studies have demonstrated that coexpression of activated Akt1 with activated ErbB2 or polyoma virus middle T antigen uncoupled from the PI3K pathway (PyVmT Y315/322F) accelerates mammary tumor development but cannot rescue the metastatic phenotype associated with these models. Here we report the generation of transgenic mice expressing activated Akt2 in the mammary epithelium. Like the MMTV-Akt1 strain, mammary-specific expression of Akt2 delayed mammary gland involution. However, in contrast to Akt1, coexpression of Akt2 with activated ErbB2 or PyVmT Y315/322F in the mammary glands of transgenic mice did not impact the latency of tumor development. Strikingly Akt2 coexpresssion markedly increased the incidence of pulmonary metastases in both tumor models demonstrating a unique role in tumor progression. Together these observations argue that these highly conserved kinases have distinct biological and biochemical outputs that play opposing roles in mammary tumor induction and metastasis.
Keywords: Akt, ErbB2, PyVmT, mammary tumorigenesis, metastasis
Introduction
The Akt family of serine/threonine kinases consists of three members; Akt1, Akt2 and Akt3. Akt has been implicated in a number of cellular processes including proliferation, cell survival and metabolism (reviewed in (1)). Several studies have demonstrated that expression of Akt1 in the mammary epithelium, while incapable of inducing mammary tumors, results in a profound involution defect (2–5). Coexpression of an activated Akt1 (Akt1-DD) with either Polyoma Virus middle T antigen (PyVmT) uncoupled from the PI3K pathway (PyVmT Y315/322F) or activated ErbB2 (NDL) results in decreased tumor latency in both tumor models (3, 6). Akt1 coexpression did not affect the incidence of lung metastases in PyVmT Y315/322F transgenic mice and decreased lung metastases in the NDL model (3, 6). Conversely, germline deletion of Akt1 in NDL and PyVmT mouse models resulted in a profound mammary tumor induction defect (7, 8). Interestingly, germline deletion of Akt2 resulted in slightly accelerated mammary tumor induction in both models (8). Together these observations suggest that Akt1 and Akt2 may have distinct roles in mammary tumor induction in both ErbB2 and PyVmT mammary tumor models.
Whereas these observations suggest that each Akt isoform may have distinct effects, the in vivo consequence of ectopic expression of activated Akt2 has not yet been examined. To directly explore this, we generated transgenic mice expressing a constitutively active Akt2 (Akt2-DD) from the mouse mammary tumor virus (MMTV) promoter. Like MMTV-Akt1 mice, we demonstrate that mammary-specific expression of activated Akt2 impairs mammary gland involution as a result of attenuated apoptotic cell death. To address the role of Akt2 in mammary tumorigenesis, activated Akt2 transgenic mice were interbred with separate strains of MMTV-PyVmT Y315/322F and MMTV-NDL transgenic mice. Unlike Akt1, the coexpression of Akt2 did not alter the latency of mammary tumor formation; however activated Akt2 coexpression resulted in a marked increase in the incidence of lung metastases in both models. Furthermore, we have isolated clones of an ErbB2-driven mammary tumor cell line differing in their metastatic capacity and the highly metastatic clones expressed elevated Akt2 protein. In addition, ectopic expression of activated Akt2 increased invasion in all clones whereas siRNA knockdown of Akt2 in the highly metastatic clones impaired invasion. Collectively the results of this study demonstrate that Akt1 and Akt2 perform distinct and non-redundant functions in mammary tumorigenesis and metastasis.
Materials and Methods
Transgenic mice
MMTV-Akt1-DD (3), MMTV-PyVmT Y315/322F (9) and MMTV-NDL (10) transgenic mice have been described previously. HA-tagged human Akt2-DD (T309D/S474D) cDNA was cloned downstream of the MMTV promoter/enhancer and followed by the SV40 PolyA sequence in the p206 vector. The fragment was linearized by SalI/SphI digestion and purified using the Qiaquick Gel extraction kit (Qiagen) as per the manufacturer’s protocol. DNA fragments were injected into one cell zygotes of FVB/n mice at the McGill Transgenic Core Facility and implanted into pseudopregnant females. Potential founder animals were screened by PCR and validated by Southern blot. MMTV-Akt1-DD and MMTV-Akt2-DD mice were interbred with MMTV-PyVmT Y315/322F and MMTV-NDL mice and routine genotyping performed by PCR. Experimental and control mice were monitored for tumor formation by physical palpation. All animals were maintained in accordance with the guidelines of the Royal Victoria Hospital Animal Care Committee.
Plasmid construction
HA-Akt1-DD and HA-Akt2-DD were subcloned from p206 into pMSCVpuro (Clontech) as an XhoI/EcoRI fragment. All constructs were verified by sequencing.
Tissue harvesting, immunoblotting and immunoprecipitations
Mammary gland and mammary tumor tissues were flash frozen in nitrogen and lysates prepared as described previously (9). For cell lines, extracts were prepared in lysis buffer (50 mM Hepes (pH 7.5), 150 mM NaCl, 10% Glycerol, 1% Triton X-100, 1 mM EGTA (pH 8.0), 1.5 mM MgCl2, 10 mM sodium fluoride, 10 mM sodium pyrophosphate) supplemented with 1 µg/ml aprotinin and leupeptin and 1 mM sodium orthovanadate. Antibodies for immunoblots include HA (HA.11, Covance), Neu (Ab3, Oncogene Research Products), ERα (clone AER311, Upstate), PyVmT (11), Akt1 (2H10, Cell Signaling), Akt2 (catalog # 2962, Cell Signaling), Grb2 (C-23, Santa Cruz) and β-actin (Clone AC-15, Sigma). For immunoprecipitations (IPs), cell lysate was incubated overnight with anti-pAkt (catalog # 9271 from Cell Signaling for IPs immunoblotted for Akt1 and catalog # 4051 from Cell Signaling for IPs immunoblotted for Akt2). Protein G beads (GE Healthcare) were added and rotated for an additional 3 h. The IPs were washed 5 times and analyzed by SDS-PAGE. All membranes were incubated with horseradish peroxidase conjugated secondary antibodies (Jackson Laboratories) and visualized using enhanced chemiluminescence (Amersham).
Histology
Tissues were fixed in 10% buffered formalin and blocked in paraffin. Embedded tissues were sectioned at 4 µm and hematoxylin and eosin stained. For lung examinations, 5 step sections were performed at 50 µm intervals and the slides were scanned using a ScanScope XT Digital Slide Scanner (Aperio). Mammary gland wholemounts were prepared as previously described (9).
Immunohistochemistry and in situ apoptosis assays
For immunohistochemistry, tissue sections were deparaffinized in xylenes and antigen retrieval performed in 10 mM sodium citrate (pH 6) using a pressure cooker. Blocking was performed using Power Block Universal Blocking Agent (Biogenex), primary antibody incubations performed in 2% BSA and processed using the Elite IgG VectaStain ABC kit (Vector Laboratories) according to the manufacturer’s instructions. ERα antibody was purchased from Novocastra (6F11). Stained sections were scanned using a ScanScope XT Digital Slide Scanner and a nuclear algorithm performed on 10 independent fields. TUNEL assays were conducted using the Apoptag Peroxidase In Situ Apoptosis Detection Kit (Chemicon) as per the manufacturer’s protocol.
Real time RT-PCR
Total RNA was isolated from 8 week virgin mammary glands and mammary tumor tissue using the Lipid tissue RNeasy Midi and RNeasy Midi Kits, respectively, from Qiagen as per the manufacturer’s instructions. For quantitative real-time RT-PCR, 50 ng of total RNA was used with the QuantiTect SYBR Green RT-PCR kit (Qiagen) and the LightCycler (Roche). Each amplification reaction was performed in triplicate and transcript levels normalized to GAPDH. Primer sequences are available upon request.
Cell culture, invasion assays, and orthotopic transplants
TM15 cells were maintained in DMEM supplemented with 10% FBS and SingleQuots (Clonetics). TM15 cell lines expressing activated Akt1 or Akt2 were generated by retroviral infection followed by puromycin selection. For in vitro invasion assays, cells were seeded in serum free medium in transwell chambers (Falcon) coated with 5% Matrigel (B.D. Biosciences) with complete medium as a chemoattractant. Cells were incubated for 24 h, formalin fixed and stained with crystal violet. Cells that passed through the membrane were visualized by microscopy and pixel counts determined using ImageJ software (NIH). For siRNA knockdown experiments, cells were transfected using HiPerfect (Qiagen) and the fast-forward protocol as per the manufacturers’ instructions. The siRNAs were purchased from Qiagen and included Akt1 (Mm_Akt1_5 HP siRNA), Akt2 (Mm_Akt2_2 HP siRNA) and AllStars Negative Control siRNA (Alexa Fluor 555). Cells were seeded for invasion assays as described above 24 h following transfection. For orthotopic transplants, 1.5×105 cells were injected into the inguinal mammary fat pad and tumor measurements performed twice weekly. Mice were sacrificed and tissues harvested once the primary tumor volume reached 1500 mm3.
Reverse Phase Protein Arrays
Frozen tumor tissue (≤10 mg) was homogenized in lysis buffer (1% Triton X-100, 50 mM HEPES, pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 100 mM NaF, 10 mM Na Pyrophosphate, 1 mM Na3VO4, 10% glycerol, supplemented with proteinase inhibitors (Roche Applied Science)) at 40 mg/ml by polytron (Power Gene). The concentration of the resulting protein lysates was corrected to 1 mg/mL using BCA reaction and additional lysis buffer. After centrifugation, post-nuclear detergent lysates (3 parts) were boiled with a solution (1 part) of 4XSDS (90%)/β-mercaptoethanol (10%). Five serial twofold dilutions were performed in lysis buffer containing 1% SDS (dilution buffer). The diluted lysates were spotted on nitrocellulose-coated glass slides (FAST Slides, Whatman Schleicher & Schuell BioScience, Inc. USA) by a robotic GeneTAC (Genomic Solutions, Inc.) G3 arrayer or an Aushon Biosystems 2470 arrayer.
The DAKO-catalyzed signal amplification system (Dakocytomation) was used for antibody blotting. Briefly, the non-specific binding sites on nitrocellulose were blocked by Re-Blot (mild, Chemicon International) and I-Block (Applied Biosystems). The endogenous peroxidase, avidin and biotin were blocked by hydrogen peroxidase and a Biotin Blocking system (Dakocytomation). Each slide was then incubated with a primary antibody (Table S1) in the appropriate dilution. The signal was captured by biotin-conjugated secondary antibody and amplified by tyramide deposition. The analyte was detected by avidin-conjugated peroxidase reactive to its substrate chromogen diaminobenzidine (DAB). Subsequently, the slides were individually scanned, analyzed, and quantitated using MicroVigene software (VigeneTech Inc.). This software provides automated spot identification, background correction, individual spot intensity determination (expressed in logarithmic units) and curve fitting (using a logistic fit model such as ln(y)=a+(b a)/(1+exp(c*(d-ln(x)).
The raw spot signal intensity data in .txt files from MicroVigene were processed by the R package SuperCurve (version 0.997- (12)) developed by the Department of Bioinformatics and Computational Biology at M. D. Anderson Cancer Center, currently available as version 1.01 at the repository “http://bioinformatics.mdanderson.org/OOMPA”. This program fits a single curve using all samples (i.e. all dilution series’) on each slide by utilizing signal intensity as the response variable and the dilution series as an independent variable. The assumption is that that the dilution series’ of all samples on any given slide stained with a specific antibody fall on the same curve. A fitted curve (called “supercurve”) is thus plotted with the signal intensities – both observed and fitted – on the Y-axis and the log2 concentration of each protein on the X-axis using the non-parametric, monotone increasing B-spline model. The protein concentrations are derived from supercurve for each sample lysate on the slide and then normalized by median polish. Each total and phosphoprotein measurement is subsequently corrected for loading using the average expression of all measured proteins in each sample.
Results
Generation of MMTV-activated Akt2 transgenic mice
To further assess the importance of the PI3K/Akt pathway in mammary gland development and tumorigenesis, we derived transgenic mice expressing an activated Akt2 (Akt2-DD) in the mammary epithelium. Akt2-DD cDNA was placed downstream of the MMTV promoter to direct transgene expression primarily to the epithelial cells of the mammary gland (Supplementary Fig. S1A). Akt2 transgene expression in the 7 founder lines was examined at the RNA level by quantitative RT-PCR and protein expression validated by immunoblotting for HA-tagged Akt2-DD (Supplementary Fig. S1B–C). Two founder lines expressed significant amounts of the transgene (lines 2-1 and 2–3) and the Akt2-1 line was used in subsequent studies.
Activated Akt2 expression delays mammary gland involution through an attenuation of apoptotic cell death
Given the importance of Akt signaling as a negative regulator of apoptosis, we first examined the impact of activated Akt2 expression on mammary gland involution, a process characterized by extensive apoptotic cell death. Mammary glands of age-matched wildtype and Akt2 transgenic mice were analyzed at days 1, 3, 7 and 10 post-parturition. In contrast to the rapid involution observed in the wildtype controls, Akt2 transgenic mice showed delayed mammary gland involution, which could be observed by both histological and wholemount analyses (Supplementary Fig. S2A). However, by day 10 post-parturition, Akt2 mammary glands had essentially completed the involution process and were indistinguishable from controls (data not shown). To explore whether this involution defect was due to differences in the induction of apoptosis, the mammary glands were analyzed by TUNEL staining. In accordance with the involution delay, Akt2 transgenics showed decreased apoptosis at day 3 compared to wildtype controls. However, at day 7 of involution, where the control glands showed very little apoptosis, Akt2 mammary glands still displayed significant levels (Supplementary Fig. S2B). These results argue that expression of an activated Akt2 interferes with the involution process by inhibiting apoptotic cell death.
Akt1 promotes mammary tumor induction, while Akt2 promotes metastasis in transgenic mice expressing a Polyoma Virus Middle T Antigen uncoupled from the PI3K pathway
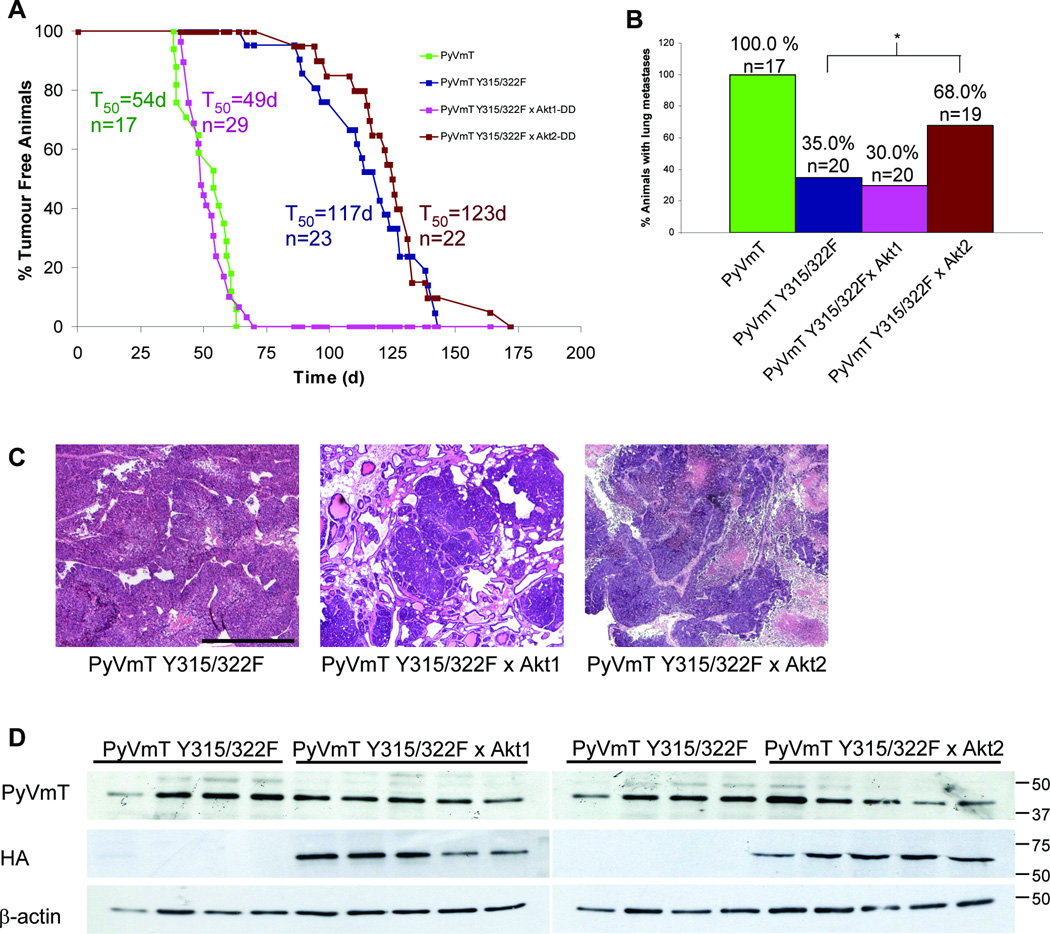
Although Akt2 attenuated apoptotic cell death in the mammary epithelium, the in vivo effect of expressing activated Akt2 in mammary tumorigenesis is unknown. Like MMTV-Akt1 mice (3), mammary epithelial expression of activated Akt2 was not sufficient for mammary tumor formation. To address the role of Akt2 in mammary tumorigenesis, we assessed whether activated Akt2 expression could accelerate mammary tumorigenesis in a strain of mice expressing Polyoma Virus Middle T Antigen uncoupled from the PI3K pathway (PyVmT Y315/322F) in the mammary epithelium. Cohorts of bigenic mice expressing the PyVmT Y315/322F and Akt2 transgenes and single transgene controls were monitored for tumor formation. PyVmT Y315/322F/Akt2 bigenic mice developed mammary tumors at 100% penetrance at a latency of 123 days (Fig. 1A). This tumor onset is similar to the latency observed for the PyVmT Y315/322F strain, indicating that unlike Akt1, Akt2 does not affect mammary tumor induction in the PyVmT Y315/322F mouse model. To directly compare the effect of Akt1 and Akt2, a separate cohort of PyVmT Y315/322F/Akt1 transgenic mice were generated. In accordance with our previous observations (3), Akt1 coexpression dramatically decreased tumor latency in the PyVmT Y315/322F model (Fig. 1A). We then examined whether Akt2 coexpression had any effect on the ability of the PyVmT Y315/322F tumor cells to metastasize. To ensure equal tumor bearing time, all mice were sacrificed 8 weeks following initial tumor detection. At this endpoint no significant difference in tumor burden in mice of different genetic combinations was noted (data not shown). Akt1 coexpression did not affect the incidence of lung metastases; however Akt2 coexpression resulted in approximately a 2-fold increase in the proportion of mice with lung metastases (Fig. 1B). Unlike PyVmT Y315/322F/Akt1 mammary tumors which display a more differentiated histology, PyVmT Y315/322F and PyVmT Y315/322F/Akt2 tumors displayed a similar pathology (Fig. 1C). Immunoblots demonstrated no significant difference in expression of the PyVmT Y315/322F oncogene was and all bigenic tumors expressed the activated Akt isoform (Fig. 1D). These results argue that in this mouse model where oncogene-dependent activation of PI3K is impaired, ectopic expression of activated Akt1 and Akt2 have opposing effects on either tumor onset or metastatic spread.
Figure 1.
Activated Akt2 does not affect mammary tumor onset in PyVmT Y315/322F mice but partially restores the metastatic defect associated with uncoupling PyVmT from PI3K. A, Cohorts of virgin female mice were monitored for tumor formation by physical palpation. T50 represents the time at which 50% of the mice had palpable mammary tumors and n the number of animals analyzed for each strain. B, Hematoxylin and eosin stained lung sections were scored for metastatic lesions. *, P = 0.038 (Fisher exact test). C, Representative hematoxylin and eosin stained mammary tumors. Bar, 0.5 mm. D, Lysates of mammary tumors from PyVmT Y315/322F, PyVmT Y315/322F/Akt1 and PyVmT Y315/322F/Akt2 mice were immunoblotted for PyVmT and HA-tagged activated Akt1 or Akt2. β-actin was detected as a control for loading. The PyVmT Y315/322F lysates were identical for the left and right panels allowing for direct comparison.
Akt1 promotes mammary tumor induction but impairs metastasis, while Akt2 promotes metastasis in activated ErbB2 transgenic mice
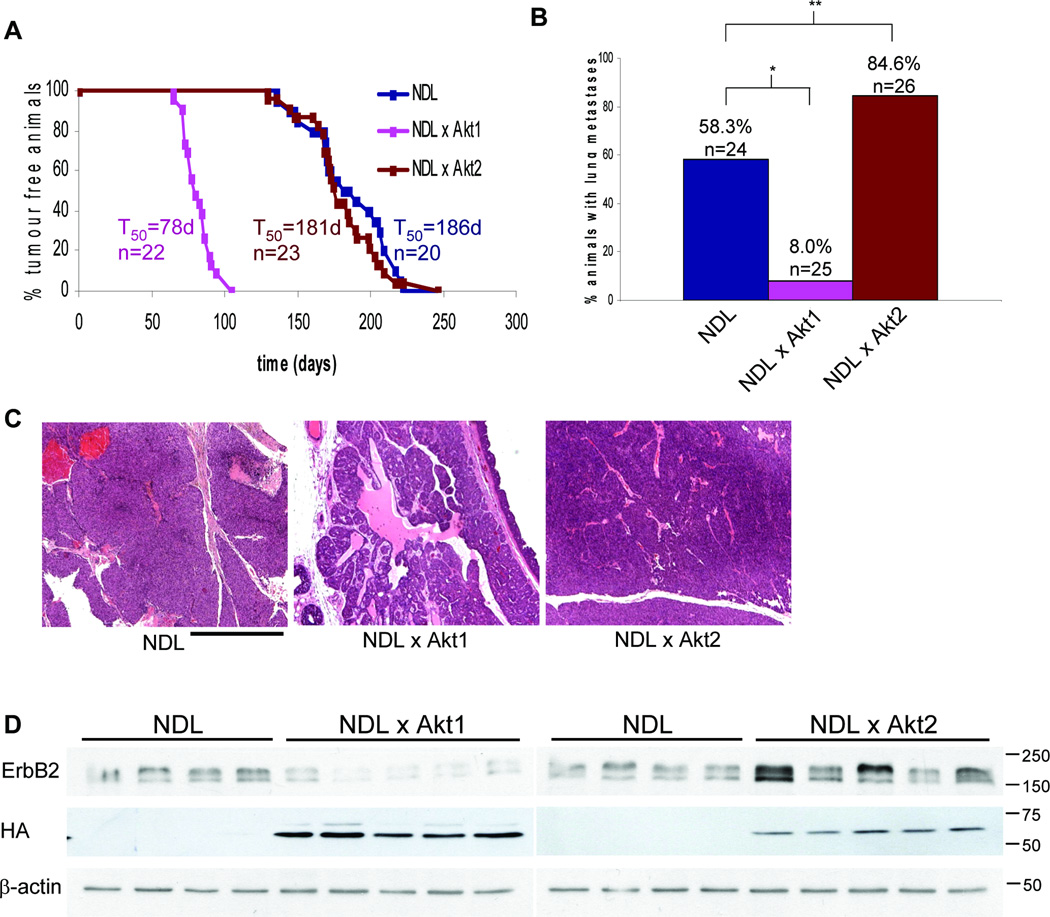
To further substantiate the distinct roles of Akt1 and Akt2 in mammary tumorigenesis, MMTV-Akt2 mice were interbred with MMTV-activated ErbB2 (NDL) mice. NDL/Akt2 bitransgenic mice developed mammary tumors with 100% penetrance at a median latency of 181 days, an onset similar to that of NDL controls (Fig. 2A). In accordance with our previous observations (6), Akt1 coexpression dramatically decreased tumor latency in NDL transgenic mice (Fig. 2A). We then examined whether Akt2 expression affected the ability of NDL-driven tumor cells to metastasize. Mice were sacrificed 8 weeks following initial tumor palpation, at which point no significant difference in tumor burden was noted (data not shown). Consistent with our previous findings (6), Akt1 coexpression impaired metastasis in NDL mice (from 58% to 8%). Akt2 coexpression however, increased the proportion of mice with metastatic lesions from 58% to 85% (Fig. 2B). Again in contrast to the poorly metastatic NDL/Akt1 mammary tumors which display a more differentiated pathology, no difference was noted between NDL and NDL/Akt2 tumors, both being of solid histology (Fig. 2C). To determine whether changes in tumor behavior were due to differences in transgene expression, immunoblots were performed for ErbB2 and the HA-tagged Akt1 and Akt2. ErbB2 levels were unchanged or slightly lower in the case of NDL/Akt1 mammary tumors, while Akt1 and Akt2 levels were not detectably different (Fig. 2D). The slight difference in ErbB2 protein was not due to erbB2 transcript differences (Supplementary Fig. S3), likely reflecting alternative post-transcriptional regulation. Together these observations suggest that similar to the PyVmT model, Akt1 plays a critical role in the induction of NDL mammary tumors whereas Akt2 selectively modulates the metastatic phase of mammary tumorigenesis.
Figure 2.
Activated Akt2 does not affect mammary tumor onset in NDL mice but increases lung metastases. A, Cohorts of virgin female mice were monitored for tumor formation by physical palpation. T50 represents the time at which 50% of the mice had palpable mammary tumors and n the number of animals analyzed for each strain. B, Hematoxylin and eosin stained lung sections were scored for metastatic lesions. *, P < 0.001; **, P = 0.039 (Fisher exact test). C, Representative hematoxylin and eosin stained mammary tumors. Bar, 0.5 mm. D, Lysates from NDL, NDL/Akt1 and NDL/Akt2 mammary tumors were immunoblotted for ErbB2 and HA-tagged activated Akt1 or Akt2. β-actin was detected as a control for loading. The NDL lysates were identical for the left and right panels allowing for direct comparison.
Endogenous Akt2 promotes invasion in mammary tumor-derived cell lines
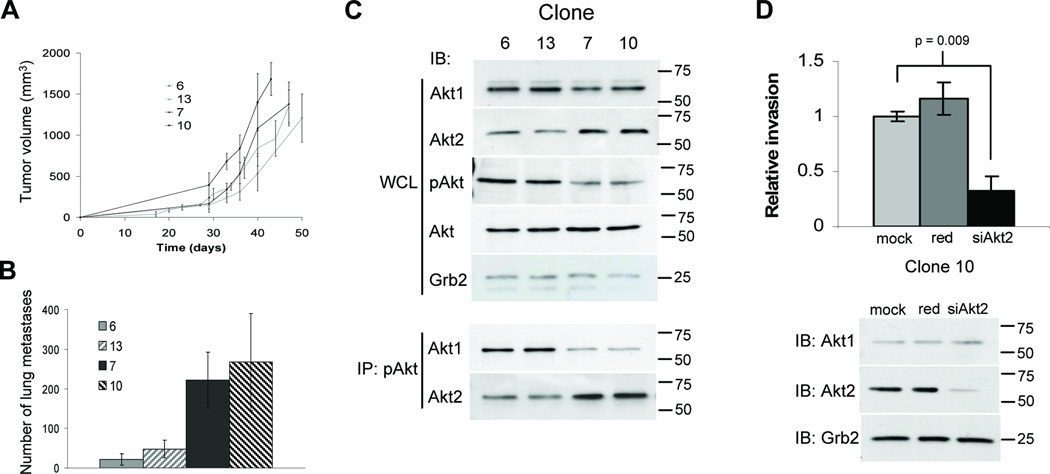
One important issue raised by the above studies is whether the effects of the Akt1 and Akt2 isoforms and the ErbB2 and PyVmT oncogenes are due to their constitutive activation state or high expression levels driven by a strong viral promoter. To further establish the physiological importance of the Akt isoforms, we took advantage of a mammary tumor cell line derived from mice expressing activated ErbB2 under its endogenous promoter (13). Like human ErbB2-induced mammary tumor progression, tumorigenesis in this strain of mice is associated with selective amplification of a core erbB2 amplicon comprising 10 genes (reviewed in (14)). One characteristic feature of mammary tumors and their derived cell lines in this strain is very low rates of spontaneous metastasis (13–15). Given the heterogeneity of the derived cell line (TM15), clonally expanded cells were injected into the mammary fat pad of athymic mice and lungs scored for metastatic lesions at tumor endpoint. Although the primary tumors grew at comparable rates, striking differences in the number of lung metastases were observed for the different clonal cell lines (Fig. 3A & B). Clones 6 and 13 consistently formed fewer metastatic lesions, compared to clones 7 and 10. We then examined Akt1 and Akt2 expression levels and found the highly metastatic clones displayed upregulated Akt2 expression compared to the low metastatic clones (Fig. 3C). Interestingly, the low metastatic clones 6 and 13 had the highest pAkt levels (Fig. 3C). However, from pAkt immunoprecipitates, the elevated pAkt in the low metastatic clones was shown to be due to elevated Akt1 phosphorylation, whereas highly metastatic clones showed markedly higher Akt2 phosphorylation (Fig. 3C). To establish the effect of ectopic activated Akt expression, stable cell lines expressing Akt1-DD or Akt2-DD were generated for in vitro invasion assays. All clones expressing Akt2-DD displayed increased invasion through Matrigel in transwell assays compared to empty vector controls, whereas Akt1-DD expression had little impact on invasion (Supplementary Fig. S4). Furthermore, siRNA knockdown of elevated endogenous Akt2 in the highly metastatic clones impaired invasion (Fig. 3D & Supplementary Fig. S5C). In contrast, siRNA downregulation of endogenous Akt1, the predominantly phosphorylated isoform in clones 6 and 13, did not affect their invasive ability (Supplementary Fig. 5A–B). Together with the transgenic mouse data above, these results strongly suggest that elevated Akt2 positively mediates breast cancer cell invasion and metastasis.
Figure 3.
Elevated Akt2 levels increase invasion and metastasis. A, Cells were injected into the mammary fat pad of athymic mice and primary tumors measured twice weekly. Tumor volumes represent the mean for 5 independent mice (+/− SD). B, Hematoxylin and eosin stained lungs were scored for the number of metastases. The values represent the mean number for 5 individual mice (+/− SD). C, Immunoblot analyses of Akt1, Akt2, pAkt and total Akt of TM15 clone whole cell lysates (WCL). To examine Akt isoform activation, pAkt (Ser473) was immunoprecipitated from lysates and immunoblots performed for Akt1 and Akt2. D, Invasion through Matrigel was assayed for mock, red negative control and Akt2-specific siRNA transfected TM15 clone 10. Pixel count analyses of Crystal Violet stained membranes were performed and relative invasion determined with mock transfection (mock) control set to 1. Values represent the mean of assays performed in triplicate (+/− SD) and statistical analyses performed using a student’s t-test. Immunoblots for Akt1 and Akt2 were performed on cell lysates, with Grb2 detected as a control for loading.
Akt1 and Akt2 expression in mammary tumors result in distinct signaling perturbations
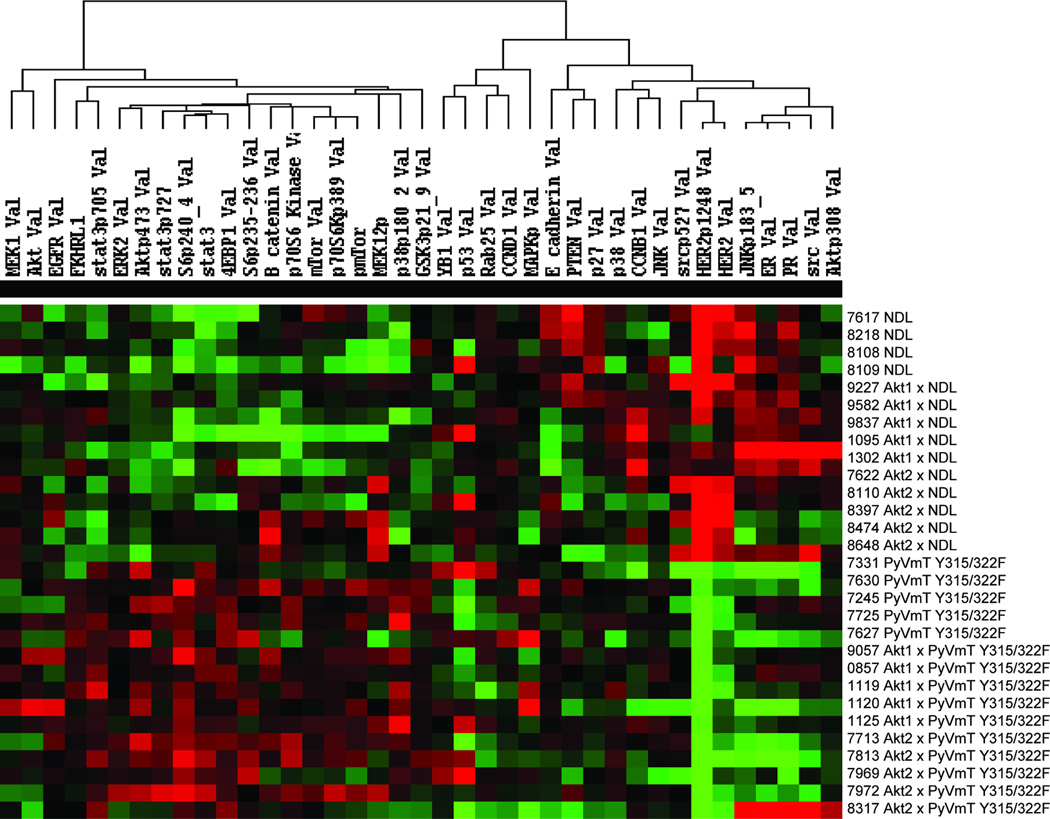
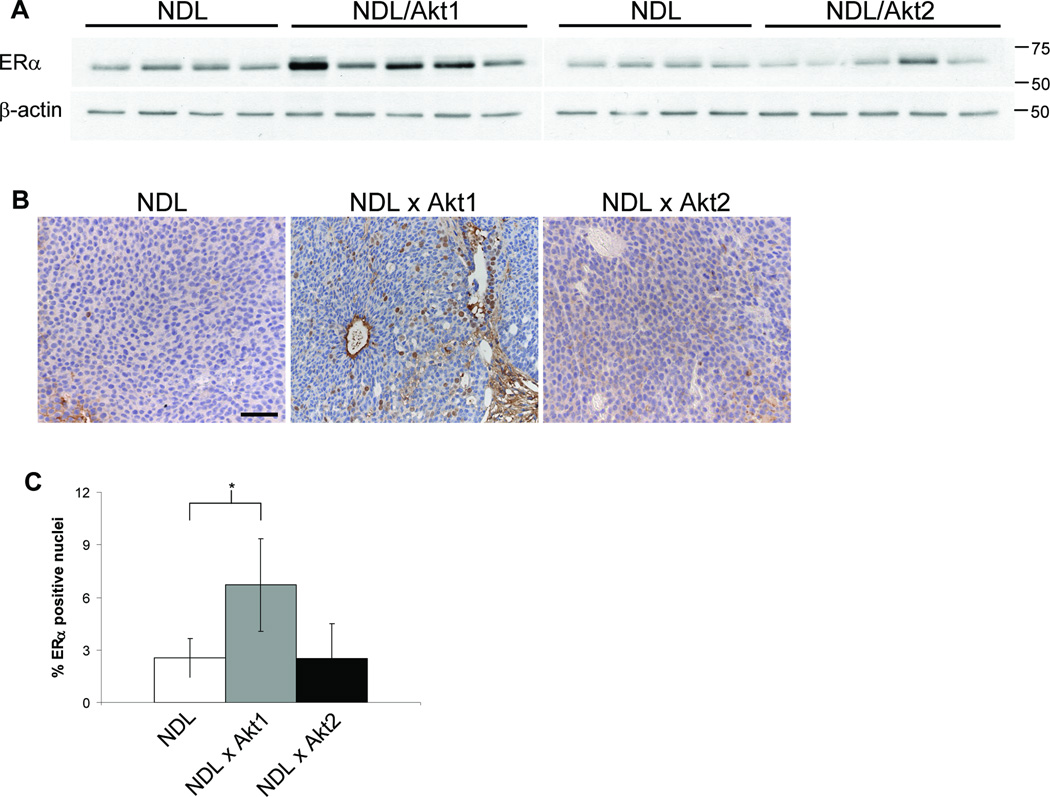
Given the distinct roles of Akt1 and Akt2 in mammary tumor induction and metastasis, we explored differences in signaling pathway activation in tumors of the different genotypes. A protein array approach (16–18) was employed using lysates of mammary tumors derived from five independent animals of each genotype and a number of proteins were found to be present and/or phosphorylated at different levels (Fig. 4). Not surprisingly, all NDL-based tumors displayed elevated Her2 and pHer2 levels compared to those of the PyVmT Y315/322F model. Unexpectedly, the protein arrays suggested that estrogen receptor α (ERα) was differentially expressed in the tumor models. In contrast to low ERα protein in NDL and NDL/Akt2 mammary tumors, NDL/Akt1 tumors displayed elevated ERα levels (Fig. 5A). We also examined ERα subcellular localization by immunohistochemistry and found a 3-fold increase in the proportion of ERα positive nuclei in NDL/Akt1 tumors compared to NDL and NDL/Akt2 tumors (Fig. 5B & C) suggesting functional ER signaling. Whether the observed increase in ERα is responsible for the differentiated and non-metastatic phenotype of NDL/Akt1 mammary tumors will require further investigation.
Figure 4.
Mammary tumors from mice of different genotypes display distinct protein expression and phosphorylation levels. Lysates of mammary tumors derived from mice expressing either the NDL or PyVmT Y315/322F oncogenes alone or in conjunction with Akt1 or Akt2 were analyzed by reverse phase protein arrays. The intensity levels are displayed as a heat map using supervised clustering.
Figure 5.
NDL/Akt1 mammary tumors express elevated ERα. A, Lysates of NDL, NDL/Akt1 and NDL/Akt2 mammary tumors were immunoblotted for ERα. β-actin was detected as a control for loading. The NDL lysates were identical for the left and right panels allowing direct comparison. B, Representative images of tumor sections stained with ERα-specific antibody. Bar, 50µm. C, The percentage of ERα positive nuclei was determined for 10 fields for each tumor section and represents the mean of 4 independent tumors for each genotype (+/− SD). *, P = 0.045 (student t-test).
Discussion
In this study we generated transgenic mice expressing an activated Atk2 (Akt2-DD) in the mammary epithelium. Activated Akt2 expression did not impact pubertal mammary gland development, nor did it affect the ability of transgene carrying females to lactate and nurse their pups. Activated Atk2 expression did however affect mammary gland involution due to delayed apoptotic cell death (Supplementary Fig. S2). The involution delay in Akt2 transgenic mice is similar to previous studies examining overexpression of either wildtype, activated, or constitutively membrane targeted Akt1 in the mammary epithelium (2–4), suggesting that Akt1 and Akt2 play similar roles in promoting cell survival in the context of the normal mammary gland. However, a recent study with Akt2 germline knockout mice reported delayed mammary gland involution (19). A couple of possibilities may account for this discrepancy. Firstly, Akt2 null mammary glands also displayed accelerated differentiation during pregnancy. However, transplantation experiments demonstrated this effect to be non-epithelial cell autonomous (19). Alternatively, a precise level of Akt2 activation may be required for proper mammary gland involution and increasing or decreasing Akt2 levels may affect this delicate balance.
In agreement with the non-oncogenic nature of Akt1-DD in earlier observations using chicken embryo fibroblasts (20) and our studies with MMTV-Akt1-DD mice (3), Akt2-DD was also non-transforming, with no transgenic mice developing mammary tumors. We then examined the impact of expressing activated Akt2 in two independent mouse models of breast cancer. In contrast to the dramatic acceleration of tumor induction by Akt1 coexpression in both MMTV-PyVmT Y315/322F and MMTV-NDL transgenic mouse models (3, 6), Akt2 expression had no effect on tumor latency (Fig. 1A & 2A). While Akt1 did not affect metastasis in PyVmT Y315/322F mice and actually impaired metastasis in the NDL background, Akt2 expression increased the incidence of lung metastases in both tumor models (Fig. 1B & 2B). Furthermore, highly metastatic clones isolated from a mammary tumor cell line expressing activated ErbB2 under its endogenous promoter had elevated Akt2 compared to low metastatic clones. Significantly, this was further correlated with a dramatic increase in pAkt2 (Fig. 3C). In addition, overexpression of activated Akt2 increased the invasive capacity of all clones, whereas siRNA knockdown of endogenous Akt2 in the highly metastatic clones impaired invasion (Fig. 3D & Supplementary Fig. S5C). Collectively, the results indicate that Akt1 promotes mammary tumor induction while Akt2 promotes progression through enhancing metastasis.
The distinct roles of Akt1 and Akt2 in promoting tumor induction and metastasis, respectively, are consistent with previous in vitro studies. The Brugge laboratory has demonstrated that Akt1 downregulation in IGF1-R expressing MCF10A cells increases motility and induces an epithelial to mesenchymal (EMT)-like phenotype (21). It has also been shown that Akt1 inhibits breast cancer cell invasion through nuclear factor of activated T cells (NFAT) downregulation (22).
Mice bearing germline deletions of Akt1 or Akt2 have been interbred with MMTV-ErbB2 and MMTV-PyVmT transgenic mice. Akt1 deletion delayed tumor development in both models (7, 8), consistent with the dramatic acceleration we observed upon activated Akt1 expression (3, 6). In agreement with increased metastasis in Akt2 expressing MMTV-PyVmT Y315/322F and MMTV-NDL tumors in the present study, Akt2 deletion in MMTV-PyVmT mice resulted in decreased metastasis (8). Interestingly, Akt1 ablation also decreased metastasis in PyVmT mice (8). The discrepancies between our studies with activated Akt1 expression (3, 6) and the study with Akt1 germline knockout mice (8) may be attributable to the fact that Akt1 is deleted in all tissues in the knockout mice, whereas our transgenic studies only express the transgene in the mammary epithelium. It is likely that a crosstalk between tumor cells and the surrounding stromal compartment is intimately associated with the metastatic process. In this regard mammary epithelial-specific ablation of Akt isoforms in mammary tumor models may provide important insights into the epithelial role. An alternative explanation for the decreased metastasis in Akt1-deficient MMTV-ErbB2 mice is that disseminated Akt1 null cells are incapable of surviving or fail to proliferate in the lung environment.
Tumor onset and metastatic differences between Akt1 and Akt2 were further correlated with modulated expression and activation levels of numerous proteins (Fig. 4). Interestingly, NDL/Atk1 mammary tumors displayed upregulated ERα compared to NDL or NDL/Akt2 tumors (Fig. 5). ERα expression in human breast cancer has been associated with more differentiated, less invasive tumors and more favorable prognosis. In cell culture, ERα expression in human breast cancer cell lines correlated with low motility and invasion (23, 24) and ERα positive breast cancer cells injected into nude mice required estrogen for tumor formation and were poorly metastatic in comparison to ERα negative cell lines (25). Interestingly, signal transduction pathway and ER signaling cross-talk has been associated with resistance to endocrine therapy (26–30). In particular, pAkt positive breast cancer patients have poorer response to various endocrine agents compared to pAkt negative patients (31). In this regard, it would be interesting to explore the involvement of the individual Akt isoforms in therapeutic resistance.
Collectively, the results of this study indicate that Akt1 promotes mammary tumor induction, whereas Akt2 promotes metastasis. The contrasting roles of Akt1 and Akt2 highlight the necessity for not only determining the expression/activation status of the individual isoforms in breast cancer patients, but also emphasize the importance of isoform-specific inhibitors. A more comprehensive understanding of Akt isoform-specific functions will enable the development of more efficacious therapeutic treatments.
Supplementary Material
Acknowledgements
We are grateful to Dr. Stephen Dilworth for the PyVmT antibody, Vasilios Papavasiliou and Cynthia Lavoie for assistance with injections and Marcin Bakinowski, Sonya Lam and Jo-Ann Bader for histological services.
Grant Support: NIH PO1 grant 5P01GA 099031-05 (GB Mills & WJ Muller); CIHR/CBCRA grant MOP-89751 (WJ Muller); Terry Fox Foundation grant 017003 (WJ Muller); Cancer Research Society/Quebec Breast Cancer Foundation (WJ Muller); Mouse Models of Human Cancer Consortium grant U01 CA105490 (WJ Muller); Canadian Research Chair in Molecular Oncology (WJ Muller).
References
- 1.Dillon RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene. 2007;26:1338–1345. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- 2.Ackler S, Ahmad S, Tobias C, Johnson MD, Glazer RI. Delayed mammary gland involution in MMTV-AKT1 transgenic mice. Oncogene. 2002;21:198–206. doi: 10.1038/sj.onc.1205052. [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson J, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt (protein kinase B) in mammary epithelium provides a critical cell survival signal required for tumor progression. Mol Cell Biol. 2001;21:2203–2212. doi: 10.1128/MCB.21.6.2203-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwertfeger KL, Richert MM, Anderson SM. Mammary gland involution is delayed by activated Akt in transgenic mice. Mol Endocrinol. 2001;15:867–881. doi: 10.1210/mend.15.6.0663. [DOI] [PubMed] [Google Scholar]
- 5.Schwertfeger KL, McManaman JL, Palmer CA, Neville MC, Anderson SM. Expression of constitutively activated Akt in the mammary gland leads to excess lipid synthesis during pregnancy and lactation. J Lipid Res. 2003;44:1100–1112. doi: 10.1194/jlr.M300045-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson JN, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt-1 (PKB±) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res. 2004;64:3171–3178. doi: 10.1158/0008-5472.can-03-3465. [DOI] [PubMed] [Google Scholar]
- 7.Ju X, Katiyar S, Wang C, et al. Akt1 governs breast cancer progression in vivo. Proc Natl Acad Sci U S A. 2007;104:7438–7443. doi: 10.1073/pnas.0605874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/Neu and MMTV-Polyoma middle T transgenic mice. Cancer Res. 2007;67:167–177. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- 9.Webster MA, Hutchinson JN, Rauh MJ, et al. Requirement for both Shc and phosphatidylinositol 3' kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel PM, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J. 1999;18:2149–2164. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dilworth SM, Griffin BE. Monoclonal antibodies against polyoma virus tumor antigens. Proc Natl Acad Sci U S A. 1982;79:1059–1063. doi: 10.1073/pnas.79.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu J, He X, Baggerly KA, Coombes KR, Hennessy BT, Mills GB. Non-parametric quantification of protein array lysates. Bioinformatics. 2007;23:1986–1994. doi: 10.1093/bioinformatics/btm283. [DOI] [PubMed] [Google Scholar]
- 13.Andrechek ER, Hardy WR, Siegel PM, Rudnicki MA, Cardiff RD, Muller WJ. Amplification of the neu/erbB-2 oncogene in a novel mouse model of mammary tumorigenesis. Proc. Natl Acad. Sci. USA. 2000;97:3444–3449. doi: 10.1073/pnas.050408497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ursini-Siegel J, Schade B, Cardiff RD, Muller WJ. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nat Rev Cancer. 2007;7:389–397. doi: 10.1038/nrc2127. [DOI] [PubMed] [Google Scholar]
- 15.Andrechek ER, Laing MA, Girgis-Gabardo AA, Siegel PM, Cardiff RD, Muller WJ. Gene expression profiling of Neu-induced mammary tumors from transgenic mice reveals genetic and morphological similarities to ErbB-2 expressing human breast cancers. Cancer Res. 2003;63:4920–4926. [PubMed] [Google Scholar]
- 16.Hennessy BT, Lu Y, Poradosu E, et al. Pharmacodynamic markers of perifosine efficacy. Clin Cancer Res. 2007;13:7421–7431. doi: 10.1158/1078-0432.CCR-07-0760. [DOI] [PubMed] [Google Scholar]
- 17.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PI3KCA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tibes R, Qui Y, Lu Y, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 19.Maroulakou IG, Oemler W, Naber SP, Klebba I, Kuperwasser C, Tsichlis PN. Distinct roles of the three Akt isoforms in lactogenic differentiation and involution. J Cell Physiol. 2008;217:468–477. doi: 10.1002/jcp.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK. The akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci U S A. 1998;95:14950–14955. doi: 10.1073/pnas.95.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irie HY, Pearline RV, Grueneberg D, et al. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial-mesenchymal transition. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoeli-Lerner M, Yiu GK, Rabinovitz I, Erhardt P, Jauliac S, Toker A. Akt blocks breast cancer cell motility and invasion through the transcription factor NFAT. Mol Cell. 2005;20:539–550. doi: 10.1016/j.molcel.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 23.Platet N, Prevostel C, Derocq D, Joubert D, Rochefort H, Garcia M. Breast cancer cell invasiveness: correlation with protein kinase C activity and differential regulation by phorbol ester in estrogen receptor-positive and -negative cells. Int J Cancer. 1998;75:750–756. doi: 10.1002/(sici)1097-0215(19980302)75:5<750::aid-ijc14>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Thompson EW, Paik S, Brunner N, et al. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in breast cancer cell lines. J Cell Physiol. 1992;150:534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- 25.Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50:717–721. [PubMed] [Google Scholar]
- 26.Johnston SR. Combinations of endocrine and biological agents: present status of therapeutic and presurgical investigations. Clin Cancer Res. 2005;11:889s–899s. [PubMed] [Google Scholar]
- 27.Johnston SR, Head J, Pancholi S, et al. Integration of signal transduction inhibitors with endocrine therapy: an approach to overcoming hormone resistance in breast cancer. Clin Cancer Res. 2003;9:524s–532s. [PubMed] [Google Scholar]
- 28.Kurokawa H, Arteaga CL. Inhibition of erbB (HER) tyrosine kinases as a strategy to abrogate antiestrogen resistance in human breast cancer. Clin Cancer Res. 2001;7:4436s–4442s. [PubMed] [Google Scholar]
- 29.Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res. 2004;10:331s–336s. doi: 10.1158/1078-0432.ccr-031212. [DOI] [PubMed] [Google Scholar]
- 30.Kurokawa H and Arteaga CL. ErbB (HER) receptors can abrogate antiestrogen action in human breast cancer by multiple signaling mechanisms. Clin Cancer Res. 2003;9:511s–515s. [PubMed] [Google Scholar]
- 31.Tokunaga E, Kataoka A, Kimura Y, et al. The association between Akt activation and resistance to hormone therapy in metastatic breast cancer. Eur J Cancer. 2006;42:629–635. doi: 10.1016/j.ejca.2005.11.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.