Abstract
Deep brain stimulation is effective for a wide range of neurological disorders; however, its mechanisms of action remain unclear. With respect to Parkinson’s disease, the existence of multiple effective targets suggests that putamen stimulation also may be effective and raises questions as to the mechanisms of action. Are there as many mechanisms of action as there are effective targets or some single or small set of mechanisms common to all effective targets? During the course of routine surgery of the globus pallidus interna in patients with Parkinson’s disease, the deep brain stimulation lead was placed in the putamen en route to the globus pallidus interna. Recordings of hand opening and closing during high-frequency and no stimulation were made. Speed of the movements, based on the amplitude and frequency of the repetitive hand movements as well as the decay in amplitude, were studied. Hand speed in 6 subjects was statistically significantly faster during active deep brain stimulation than the no-stimulation condition. There were no statistically significant differences in decay in the amplitude of hand movements. High-frequency deep brain stimulation of the putamen improves bradykinesia in a hand-opening and -closing task in patients with Parkinson’s disease. Consequently, high-frequency deep brain stimulation of virtually every structure in the basal ganglia-thalamic-cortical system improves bradykinesia. These observations, together with microelectrode recordings reported in the literature, argue that deep brain stimulation effects may be system specific and not structure specific.
Keywords: putamen, deep brain stimulation, bradykinesia, physiology, pathophysiology
Though a remarkably effective therapy, the precise mechanisms of action of deep brain stimulation (DBS) are unknown. A better understanding of these mechanisms could lead to improved therapies for a wider range of indications. Further, DBS must alter underlying neuronal pathophysiology, which must, in some way, reflect the physiology of the basal ganglia-thalamic-cortical network.
We report and examine the effects of DBS of the putamen on hand opening and closing in patients with PD undergoing DBS surgery to place DBS leads in the globus palladus internus (GPi) as part of routine medical care. Whether putamen DBS improving bradykinesia would have been expected or unexpected relates to previous understandings of the mechanism(s) of action of DBS and the neuronal pathophysiology of PD.
Patients and Methods
Following Institutional Review Board (IRB) approval, subjects previously approved for surgery by the institutional DBS Case Conference consented to participate in the research. The DBS Case Conference convenes physicians, nurses, and psychologists, not directly involved in the research, that review candidacy criteria for DBS surgery described elsewhere.1
Subjects withheld anti-Parkinson’s medications from the evening before surgery. Subjects underwent routine GPi DBS, as previously described.2 During surgery, microelectrode recordings were made to identify various nuclei traversed by the microelectrode. Recordings in the putamen are characterized by sparse encounters with neurons having low frequency or transient extracellular action potentials.3 Entry into the globus pallidus para externa (GPe) from the striatum demonstrates high densities of neurons, with most demonstrating high-frequency discharges interrupted by pauses and fewer demonstrating low frequency of bursting, thus defining the inferior boarder of the putamen.3 Surgery proceeded to identify appropriate targets in the GPi, defined by the presence of neurons responding to passive limb movement or active jaw or tongue movement. Once the GPi target was localized, a DBS lead consisting of four cylindrical electrical contacts, each 1.27 mm in diameter, 1.5 mm in length, and separated by 1.5 mm (Model 3387; Medtronic Neuromodulation, Inc., Minneapolis, MN), with the distal edge of the distal contact placed at the inferior boarder of the GPi. Fluoroscopic guidance was used throughout to verify the depth of the DBS lead with respect to the final position of the microelectrode. En route to the GPi, the lead paused, such that the distal end of the distal contact was at the neurophysiologically identified bottom of the putamen (Fig. 1). Test stimulation of the putamen was conducted, and then the DBS lead was further lowered to the final target in the GPi, consistent with standard care.
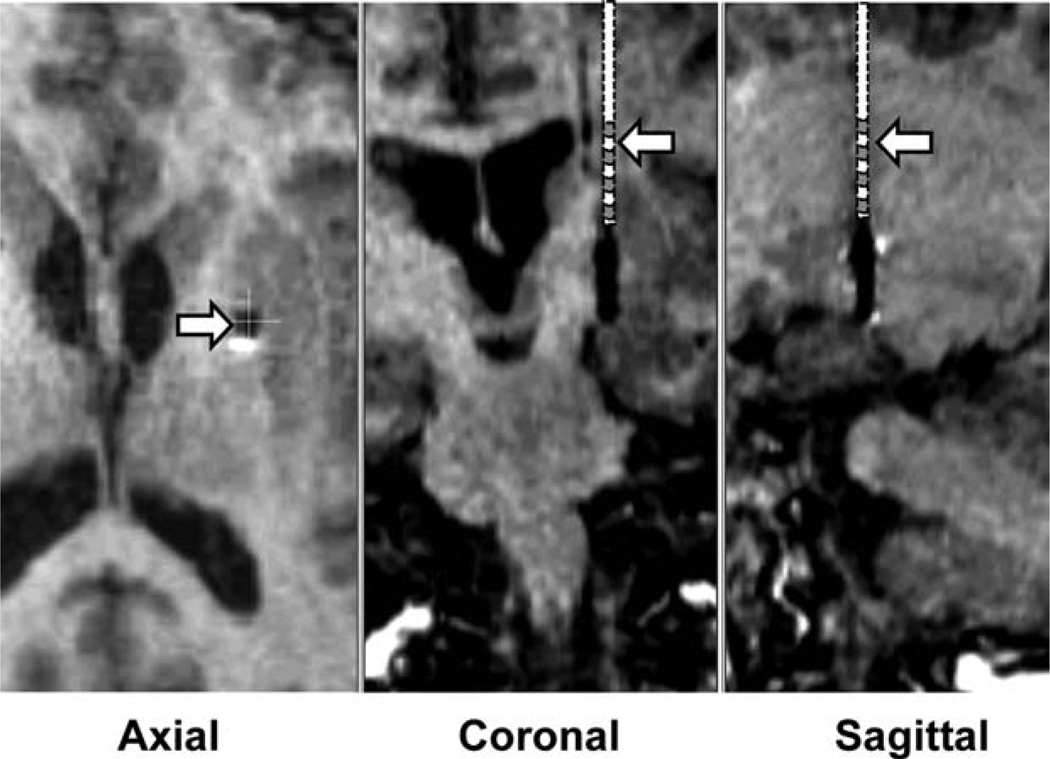
FIG. 1.
Postoperative MRI scans showing the projected location of the DBS lead during putamen DBS. The reconstruction was based on an MRI scan, with the DBS lead in its final location in the GPi. MRI shows the location of the DBS contacts in the GPi. The putamen DBS was in the same trajectory with the distal edge of the distal contact 10 to 13 mm above the location of the distal edge of the distal contact with the DBS lead in the GPi. Estimated positions of the DBS lead, shown schematically, in the coronal and sagittal plane are indicated by the arrows. Location of the putamen DBS lead in the axial section is indicated by the arrow. As can be seen, during putamen DBS, the lead was in the posterior medial region of the putamen.
The depth of the distal contact of the DBS lead was registered on the microelectrode drive, both in the putamen and the final GPi location. Thus, the distance of the distal edge of the distal contact during DBS of the putamen to the distal edge of the distal contact with the DBS lead in the GPi was determined. Postoperative MRI scans were performed in all subjects as a matter of routine clinical care, allowing verification that the distal contact of the DBS lead was in the GPi. The location of the distal edge of the distal contact of the DBS lead during putamen DBS could be estimated from the position of the distal edge of the distal contact in the GPi in the same trajectory.
Stimulation of the putamen used the most distal or ventral contact (contact 0) as the cathode and the most proximal or dorsal contact (contact 3) as the anode, which restricted the region of stimulation within the putamen. First, the initial high-voltage cathodal phase of the stimulation pulse, most responsible for activating neural elements, was restricted to contact 0. Further narrowing the volume of activation was accomplished by the use of bipolar stimulation, where the current density about contact 0 would fall off as the square of the distance from the contact. In monopolar configuration, the current density would fall off just as the distance from the contact. Finally, the initial anode phase in contact 3 provided a current sink, drawing the electrical lines of force toward contact 3 in the putamen and away from the GPe or GPi.
Stimulation parameters were rates of 160 pulses per second, 3.5 volts, and 90-microsecond pulse widths, typical of published studies, as time limitations prevented an optimization of stimulation configurations or parameters. Test stimulations were carried out in blocks in the order of 0, 3.5, 0, and then 3.5 V or the reverse to account for possible carryover effects. The patients were not informed of the stimulation voltage.
Subjects were repeatedly asked to open and close their hand as “big and as fast” as possible. Hand opening and closing were conducted for 15-second blocks interrupted with rest to avoid fatigue. The hand opposite the DBS stimulation was instrumented with a data glove (5DT Data glove series; Fifth Dimension Technologies, Irvine, CA) with position information of each finger and the thumb acquired at 200 samples per second (Fig. 2). The hand-opening and -closing excursion was previously normalized, such that a value of 0 represents a tightly closed fist, whereas a value of 1 represents full extension of the fingers. The output was the position relative to the fully open and closed hand positions. A value of 0.5 indicates a position midway between. The distance between the maximum and minimum position would be some fraction of the full range of motion. When the duration of the excursion was accounted for, the velocity would be in terms of fractions of full excursion per second.
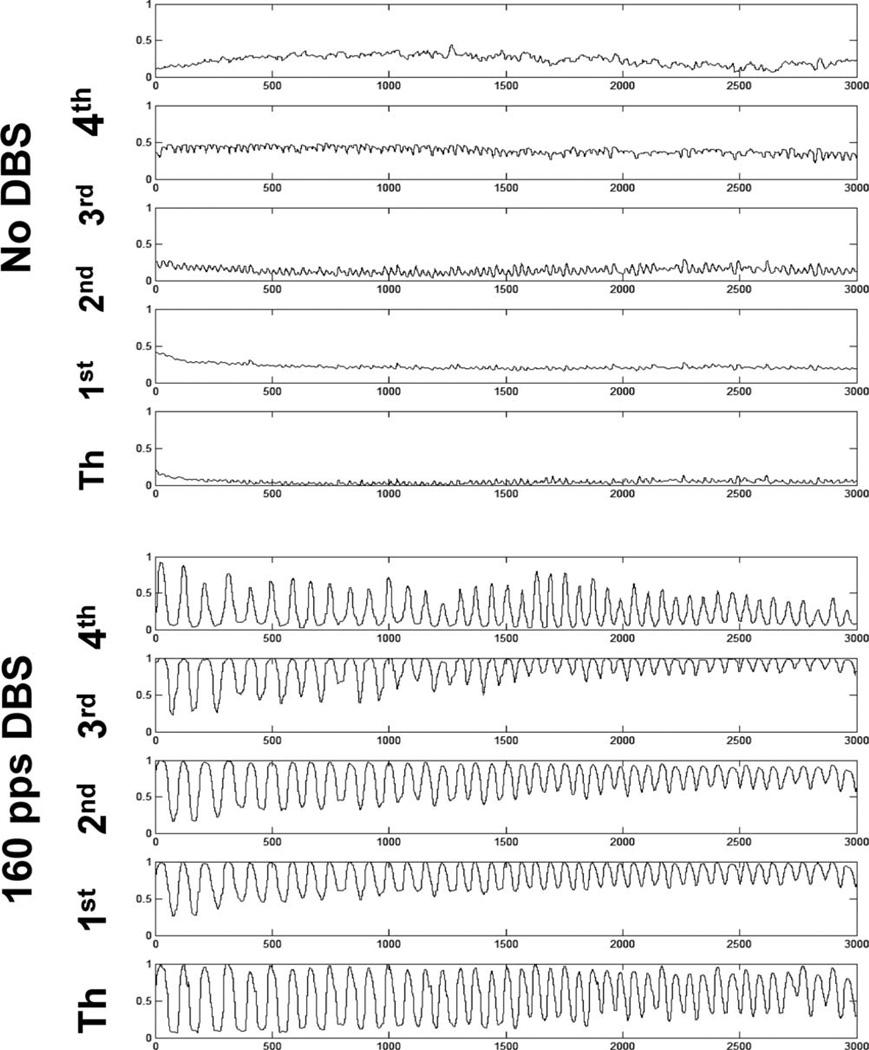
FIG. 2.
Representative tracing of finger (1st through 4th) and thumb positions during rapid and large hand opening and closing under no-stimulation and high-frequency stimulation.
Data from the first three fingers were analyzed in each subject under both conditions because of statistical concerns of having a reasonable representations of DBS effects versus the risks of alpha inflation resulting from multiple comparisons, if each and all fingers were analyzed, which would have reduced statistical power. The thumb and fourth fingers give the smallest excursions. In retrospect, there was considerable covariation of movements in all fingers (Fig. 2), and, consequently, the subset of fingers chosen a priori were reasonably representative of all the fingers.
Speed was determined as the product of frequency and magnitude of hand opening and closing multiplied by two to account for both hand opening and closing. Magnitude was defined as the difference between a “peak” and the subsequent “trough” (see Figs. 2 and 3). In addition, many patients demonstrate decay in the magnitudes of hand opening and closing over time, making determination of amplitude problematic (Figs. 2 and 3). Thus, decay from the maximum to minimum magnitudes was determined by fitting the curve of the difference between the peak and trough magnitudes, thus the excursion, over the time course, t, of hand opening and closing. This would account for both variability and decay. An exponential decay function characterized by a time constant, k, and amplitude, a (Fig. 3), was constructed. The equation for the curve fitting is given below as:
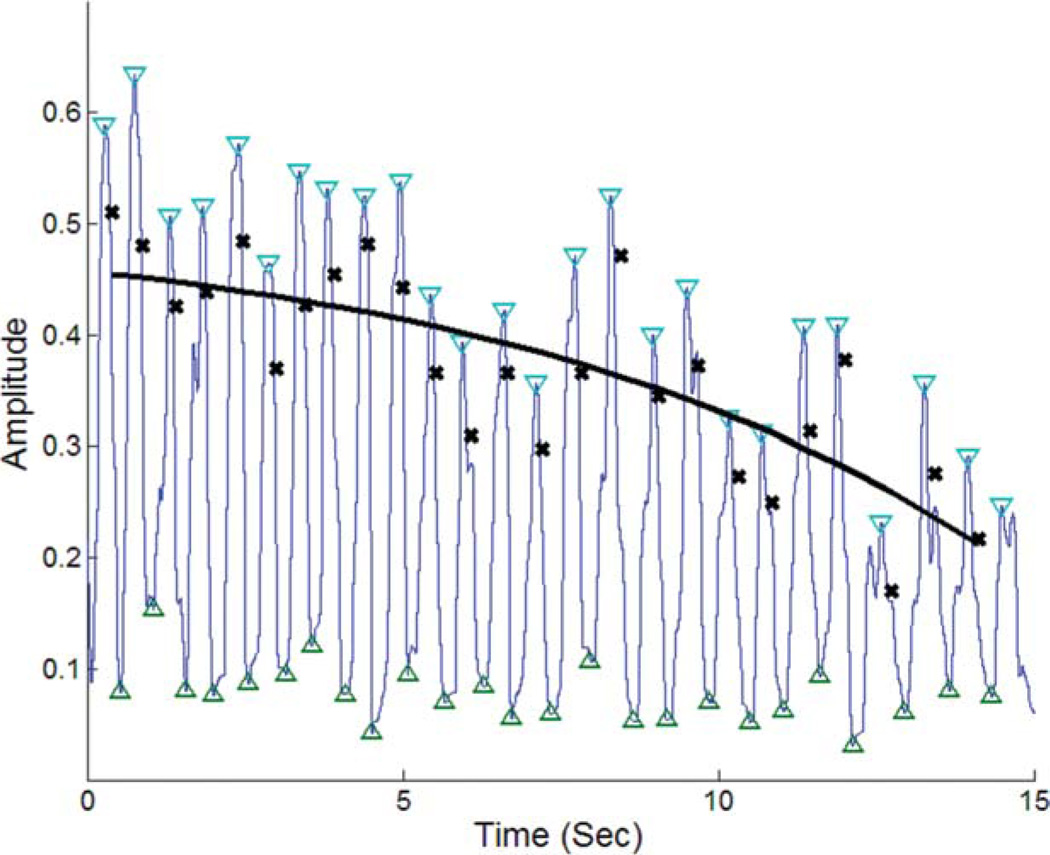
FIG. 3.
Tracings of the raw data for 1 finger in 1 subject and the fitted equation demonstrating the analysis methods to quantify the amplitude and decay of rapid hand opening and closing. Blue tracing shows the raw movement of a finger or thumb. Inverted blue triangles identify the “peaks,” whereas the green triangles identify the “trough.” Black crosses represent the magnitude of the difference between the peaks and troughs. Black solid line is the curve fitted to the differences between peaks and troughs.
Thus, amplitude a represents the y-intercept of the curve and can be considered the initial magnitude of hand opening and closing. The constant, r, is a proportionality constant for curve fitting. The constant, k, describes the decay in the peaks. Movement speed was taken as twice the product of the amplitude, given by a, and the frequency.
Observations were organized as pairs of “off” and “on” samples within each subject. Two samples were obtained in each patient under each condition. Pairing for analyses was randomized, such that each epoch of on condition for comparison was randomized to one of the two off condition epochs for each subject. For example, in a single subject, the first on condition may be paired with the second off condition and the second on paired with the first off condition. Thus, there were a total of two pairs of DBS conditions in each subject and a total of six pairs accounting for the three fingers tested.
As these were planned comparisons with the anticipation that speed would be increased with putamen DBS and that the decay would be less, one-tailed comparisons were made, initially using a statistical cut off of P < 0.05. It is understood that treating each of the two pairs of on and off conditions per subject as independent observations could be considered as inappropriately increasing the sample size and thus statistical power. However, taking the mean of only two on or off epochs would not be an accurate measure of the central tendency and could falsely reduce variance. To balance these concerns, the final P value for statistical significance was taken as P < 0.025.
Results
Six subjects, all males, participated, with an average age of 65.8 years (standard deviation [SD], 7.6) and an average duration of illness of 9.2 years (SD, 3.4). The left GPi was targeted in 4 subjects and the right in 2 subjects. Postoperative MRI scans were performed to confirm the location of stimulation in the putamen (a representative example is shown in Fig. 1). The estimated positions of the ventral contact of the DBS lead during putamen stimulation and the final position of the GPi lead relative to the midpoint of the line connecting the anterior to posterior commissure are given in Table 1. For 2 subjects only, single on and off epochs were obtained, resulting in 10 pairs of on and off for comparison for a single finger; thus, 30 pairs for all three fingers were studied.
TABLE 1.
Coordinates relative to AC-PC midpoint
| Coordinates relative to AC-PC midpoint of contact 0 in putamen |
Coordinates relative to AC-PC midpoint of contact 0 in GPi |
||||||
|---|---|---|---|---|---|---|---|
| Lateral | Anterior | Depth | Lateral | Anterior | Depth | Side | Distance of putamen contact 0 above GPi contact 0 |
| 23.79 | 6.470 | 5.27 | 21.19 | 5.01 | −5.65 | Left | 11.0 |
| 23.68 | 7.470 | 5.00 | 21.69 | 2.58 | −3.49 | Left | 10.0 |
| 18.17 | 7.270 | 6.55 | 16.52 | 3.94 | −5.84 | Left | 13.0 |
| 22.85 | 11.47 | 5.62 | 21.86 | 6.99 | −5.14 | Right | 12.0 |
| 17.51 | 13.89 | 5.14 | 17.22 | 4.03 | −2.70 | Right | 12.5 |
| 21.95 | 3.640 | 6.40 | 23.00 | 7.97 | −2.73 | Left | 10.0 |
Locations of the stimulation contact 0 supplying the initial high-voltage cathodal pulse relative to the midpoint of the line connecting the anterior commissure (AC) and posterior commissure (PC), the side of stimulation, and the distance between the putamen contact 0 and the final position of contact 0 in the globus pallidus interna (GPi). Note that the depths of the contacts are orthogonal to the AC-PC line, whereas the distance between the contacts is not the result of the angles of entry; consequently, the difference in the depths would not equal the distance between the contacts.
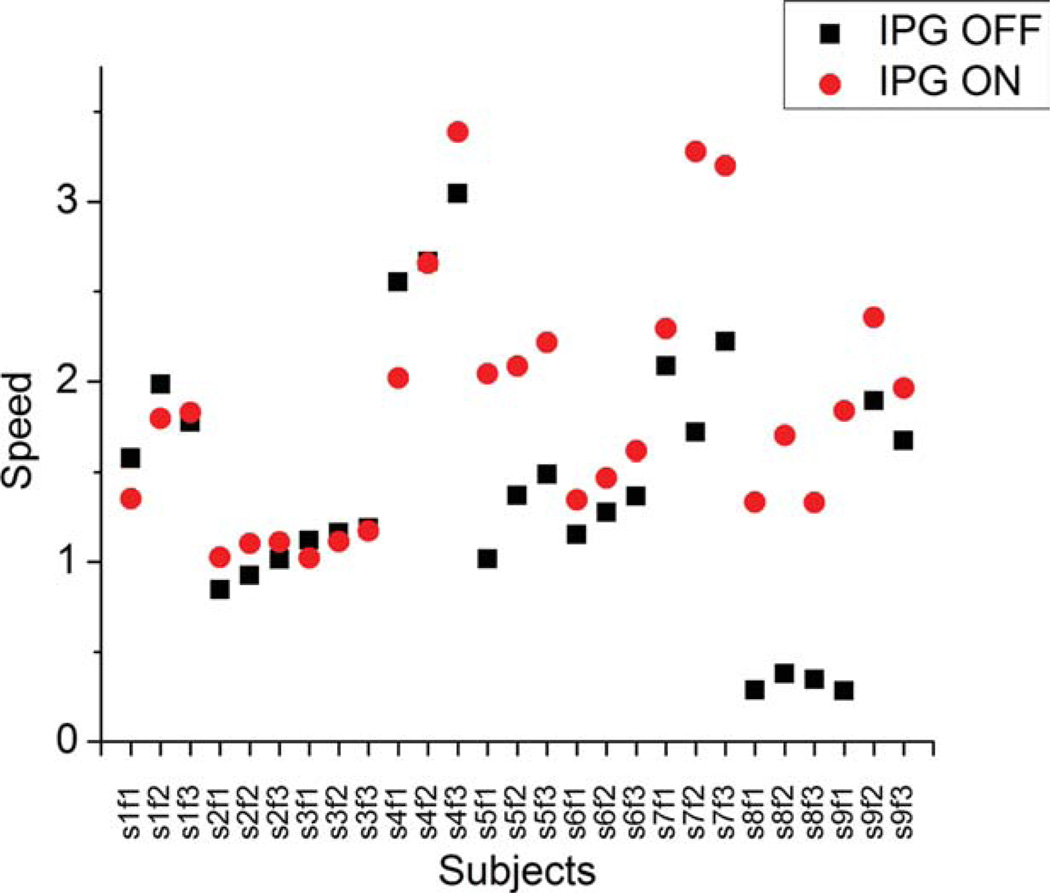
Figure 2 shows raw finger and thumb tracings for a representative subject in the no-stimulation and stimulation conditions. As can be seen by visual inspection, the subject opened and closed the hand with greater magnitude and frequency under the stimulation condition. Figure 3 shows a representative sample of curve fitting to the raw tracings. The curves fit the data well, and for all samples, the mean square error ranged from 0.002 to 0.03, indicating reasonable fit. Figure 4 shows the dataset of the speed for all subjects under the on and off DBS conditions. The speeds in the on condition ranged from 0.6 to 6.0 fractions of full excursion per second, whereas the speeds in the off condition ranged from 2 to 6.8 fractions of full excursion per second and were statistically significantly different (one-tailed, paired t-test; P < 0.0032). The differences in the pairs (an on epoch minus an off epoch) ranged from 0.4 to 3.0.
FIG. 4.
Speeds for all subjects under the DBS “on” and “off” conditions. Notation is sXfY, where X is the subject number and Y is the finger. For example, s2f3 means the 2nd subject and 3rd finger.
As secondary measures, effects on frequency and amplitude were analyzed. The mean (and SD) frequency in the off condition was 2.3 Hz (1.2 Hz) and in the on condition was 2.9 Hz (1.5 Hz). This was statistically significantly different at P < 0.0004, using a paired t-test, as described above. The mean (and SD) amplitude in the off condition was 1.2 fractions of full excursion per second (0.4 fractions of full excursion per second) and in the on condition was 1.4 fractions of full excursion per second (0.2 fractions of full excursion per second). This was not statistically significantly different at P < 0.048, based on the previous established criteria using a paired t-test, as described, above though a trend is suggested.
Similar analysis was applied to the decay constant, k, representing the decay of amplitudes over time. The decay constant, k, in the on condition ranged from −0.23 to 0.2 relative units, with a mean of 0.097 and in the off condition, from −0.21 to 0.16 relative units, with a mean of 0.090, and was not statistically significantly different (one-tailed, paired t-test; P <.7). Pairwise differences (an on epoch minus an off epoch) ranged from −0.26 to 0.24 relative units, with a 7% difference between the means. It is likely that the variance of the decay constant, k, was the substantial factor, in that assuming an effect size of 20%, 216 subjects would have needed to have had an 80% probability of detecting a difference at the P < 0.05 level.
Discussion
High-frequency putamen DBS improves bradykinesia, as measured by increased speed during hand opening and closing. There was no meaningful difference in the dampening of the hand opening and closing, as measured by the decay constant, k.
The location of the putamen DBS was consistent with the spatial distribution of the motor regions of the putamen demonstrated in labeling, neuronal recording, and functional magnetic resonance imaging (fMRI) in nonhuman primates and humans. In nonhuman primates, putamen neurons receiving projections from the motor cortex and the supplementary motor area (SMA) extend from the anterior commissure to the full extent of the putamen caudally.4, 5 The putamen DBS locations were well caudal to the anterior commissure. Further, the motor regions extended medially to the lateral border of the caudate nucleus and were just above the GPi. These spatial distributions were consistent with fMRI imaging during motor tasks in humans.6, 7
These findings are novel, but whether they were expected or unexpected would depend on what is understood to be the mechanism(s) of action of DBS and the neuronal pathophysiology of PD. One notion of DBS mechanism(s) is that the effects are local and specific to the stimulated target, termed the local mechanisms hypothesis. Note that these local effects can also include structures that receive efferents from the stimulated target. An alternative is that DBS mechanisms involve propagation of activations throughout the basal ganglia-thalamic-cortical system, such that stimulation anywhere within the reentrant network should have a similar effect on bradykinesia. If the effects are local, then the effects should be explainable in terms of the putative role of the stimulated structure in the pathophysiology of PD. With the results described above, DBS of nearly every component of the basal ganglia-thalamic-cortical system improves bradykinesia, though to varying degrees, including ventral intermediate nucleus of the thalamus (Vim), GPi, GPe, subthalamic nucleus, motor cortex, and, now, the putamen.
The situation with respect to Vim is problematic because of a selection bias for tremor with minimal bradykinesia. Thus, there is likely to be a “floor” effect mitigating against observing an effect on bradykineisa. As Limousin et al. wrote, “Symptoms other than tremor were very mild before surgery. Stimulation significantly reduced contralateral akinesia and rigidity … at 3 and 12 months follow up.”8 Most of the published studies have focused on tremor, and the few that have looked at bradykinesia have been mixed; however, at least one article carefully studied bradykinesia in PD subjects with Vim DBS and demonstrated improvement in bradykinesia.8
Evidence that STN and GPi DBS improve bradykinesia is based primarily on improvements in subscales of clinical measures, which, some may consider, are problematic. However, those few studies directly assessing bradykinesia in reaction time/movement velocity studies have consistently demonstrated improvements in bradykinesia.9–14 Reports of motor cortex15, 16 and GPe stimulation17–20 also describe improvement in clinical measures of bradykinesia. Again, the evidence is limited, but what evidence is available has been positive. Certainly, these observations do not exclude the possibility of unreported negative results.
The multiplicity of effective targets poses a conundrum. If different structures have different pathophysiological roles, then what is the nature of DBS such that stimulation in each and all produces the same effect on bradykinesia? The multiplicity of effective targets suggests that either there are multiple unique mechanisms or there is some single or small number of mechanisms in common.
There are at least three senses of what a common mechanism might involve. The first stimulation-induced inhibition of each target independently is sufficient to improve bradykinesia. An analogy would be an assembly line where the entire production stops if workers at any one station stop working. Alternatively, the same mechanism for each structure could be excitation. Second, there is a single structure that is activated, for example, the motor cortex, but that structure can be activated from anywhere within the basal ganglia-thalamic-cortical system through direct connections with the stimulated target.
A third sense of a common mechanism is that DBS of one target affects all structures, producing a stereotypic response throughout, and the effect in each target depends on specific interactions among the components of the basal ganglia-thalamic-cortical system (i.e., the systems mechanism hypothesis). This would be possible in a reentrant network consisting of multiple nodes formed by individual structures. For example, one reentrant system could be the motor cortex projecting to the striatum that projects to the GPi, which projects to the ventrolateral thalamus (VL) which projects back to the motor cortex. Each of these structures forms a node in the reentrant system. DBS excitation of any single node then generates activations that reenter and generate oscillations within the entire network. Such theories have been described elsewhere.21, 22 Although the sequential or assembly-line–type common mechanism described above could be considered a “system” in the sense of an interconnected anatomical system, the dynamics implied are not those implicit in the systems mechanism hypothesis.
Supporting evidence of the systems mechanism hypothesis comes from neuronal recordings in nonhuman primates and humans during DBS, demonstrating widespread activations over time scales of milliseconds. For example, DBS in the STN in nonhuman primates results in antidromic activation of the motor cortex and orthodromic activation of the putamen.23 In humans, STN DBS causes antidromic activation of the contralateral STN.24 GPi DBS produces antidromic activation of VL neurons in humans and orthodromic activation of GPi projection neurons in humans and nonhuman primates.2
Putamen DBS could cause the following: (1) antidromic activation of afferents to the putamen from MC and the centromedian and parafasciculus thalamic nuclei and/or (2) monosynaptic orthodromic efferent activations to target structures, such as the GPe, GPi, and substantia nigra pars reticulata. Finally, a local effect on putamen interneurons or the medium spiny neurons cannot be discounted.
Conclusion
Putamen DBS improves bradykinesia as does DBS of many structures within the basal ganglia-thalamic-cortical system. These findings suggest a single systems effect rather than multiple local effects due to individual targets. It is not claimed, from the findings presented here, that putamen DBS is an effective clinical treatment. Extrapolations for the acute intraoperative settings may not predict chronic clinical effects.
Acknowledgments
Funding agencies: The research was supported by the Dr. Sigmund Rosen Scholarship fund of the University of Alabama at Birmingham (to E.B.M.), the National Institutes of Health (K23 NS067053-01; to H.C.W.), the Lanier Family Foundation, and the Charles Ackerman Parkinson Research Fund.
Footnotes
Relevant conflicts of interest/financial disclosures: Nothing to report. Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Baker K, Montgomery EB, Jr, Rezai AR, Burgess R, Lüders HO. Subthalamic nucleus deep brain stimulus evoked potentials: physiology and therapeutic implications. Mov Disord. 2002;17:969–983. doi: 10.1002/mds.10206. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery EB., Jr Effects of GPi stimulation on human thalamic neuronal activity. Clin Neurophysiol. 2006;117:2691–2702. doi: 10.1016/j.clinph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Vitek JL, Bakay RAE, Hashimoto T, et al. Microelectrode-guided pallidotomy: technical approach and application for treatment of medically intractable Parkinson’s disease. J Neurosurg. 1998;188:1027–1043. doi: 10.3171/jns.1998.88.6.1027. [DOI] [PubMed] [Google Scholar]
- 4.Inase M, Sakal ST, Tanji J. Overlapping corticostriatal projections from the supplementary motor area and the primary motor cortex in the macaque monkey: an anterograde double labeling study. J Comp Neurol. 1996;373:283–296. doi: 10.1002/(SICI)1096-9861(19960916)373:2<283::AID-CNE10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Nambu A, Kaneda K, Tokuno H, Takada M. Organization of corticostriatal motor inputs in monkey putamen. J Neurophysiol. 2002;88:1830–1842. doi: 10.1152/jn.2002.88.4.1830. [DOI] [PubMed] [Google Scholar]
- 6.Maillard L, Ishii K, Bushara K, Waldvogel D, Schulman AE, Hallet M. Mapping the basal ganglia: fMRI evidence for somatotopic representation of face, hand, and foot. Neurology. 2000;55:377–383. doi: 10.1212/wnl.55.3.377. [DOI] [PubMed] [Google Scholar]
- 7.Gerardin E, Lehericy S, Pochon J-B, et al. Foot, hand, face and eye representation in the human striatum. Cereb Cortex. 2003;13:162–169. doi: 10.1093/cercor/13.2.162. [DOI] [PubMed] [Google Scholar]
- 8.Limousin P, Speelman JD, Gielen F, Janssens M study collaborators. Multicentre European study of thalamicstimulation in parkinsonian and essential tremor. J Neurol Neurosurg Psychiatry. 1999;66:289–296. doi: 10.1136/jnnp.66.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schubert T, Volkmann J, Müller U, et al. Effects of pallidal deep brain stimulation and levodopa treatment on reaction-time performance in Parkinson’s disease. Exp Brain Res. 2002;144:8–16. doi: 10.1007/s00221-002-1020-1. [DOI] [PubMed] [Google Scholar]
- 10.Vaillancourt DE, Prodoehl J, Metman LV, Bakay RA, Corcos DM. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease. Brain. 2004;127:1–14. doi: 10.1093/brain/awh057. [DOI] [PubMed] [Google Scholar]
- 11.Kumru H, Summerfield C, Valldeoriola F, Valls-Sole J. Effects of subthalamic nucleus stimulation on characteristics of EMG activity underlying reaction time in Parkinson’s disease. Mov Disord. 2004;19:94–100. doi: 10.1002/mds.10638. [DOI] [PubMed] [Google Scholar]
- 12.Temel Y, Blokland A, Ackermans L, et al. Differential effects of subthalamic nucleus stimulation in advanced Parkinson disease on reaction time performance. Exp Brain Res. 2006;169:389–399. doi: 10.1007/s00221-005-0151-6. [DOI] [PubMed] [Google Scholar]
- 13.Dorval AD, Kuncel AM, Birdno MJ, Turner DA, Grill WM. Deep brain stimulation alleviates parkinsonian bradykinesia by regularizing pallidal activity. J Neurophysiol. 2010;104:911–921. doi: 10.1152/jn.00103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldau B, Clayton DA, Gasperson LB, Turner DA. Analysis of the time course of the effect of subthalamic nucleus stimulation upon hand function in Parkinson’s patients. Stereotact Funct Neurosurg. 2011;89:48–55. doi: 10.1159/000323340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cilia R, Landi A, Vergani F, Sganzerla E, Pezzoli G, Antonini A. Extradural motor cortex stimulation in Parkinson’s disease. Mov Disord. 2007;22:111–114. doi: 10.1002/mds.21207. [DOI] [PubMed] [Google Scholar]
- 16.Pagni CA, Altibrandi MG, Bentivoglio A, et al. Extradural motor cortex stimulation (EMCS) for Parkinson’s disease. History and first results by the study group of the Italian neurosurgical society. Acta Neurochir Suppl. 2005;93:113–119. doi: 10.1007/3-211-27577-0_19. [DOI] [PubMed] [Google Scholar]
- 17.Krack P, Pollak P, Limousin P, et al. Opposite motor effects of pallidal stimulation in Parkinson’s disease. Ann Neurol. 1998;43:180–192. doi: 10.1002/ana.410430208. [DOI] [PubMed] [Google Scholar]
- 18.Vitek JL, Hashimoto T, Peoples J, DeLong MR, Bakay AE. Acute stimulation in the external segment of the globus pallidus improves parkinsonian motor signs. Mov Disord. 2004;19:907–915. doi: 10.1002/mds.20137. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K, Christine CW, Starr PA, Marks WJ., Jr Effects of unilateral subthalamic and pallidal deep brain stimulation on fine motor functions in Parkinson’s disease. Mov Disord. 2007;22:619–626. doi: 10.1002/mds.21300. [DOI] [PubMed] [Google Scholar]
- 20.Klostermann F, Wahl M, Marzinzik F, Vesper J, Sommer W, Curio G. Speed effects of deep brain stimulation for Parkinson’s disease. Mov Disord. 2010;25:2762–2768. doi: 10.1002/mds.23381. [DOI] [PubMed] [Google Scholar]
- 21.Montgomery EB., Jr Dynamically coupled, high-frequency reentrant, non-linear oscillators embedded in scale-free basal gangliathalamic- cortical networks mediating function and deep brain stimulation effects. Nonlinear Studies. 2004;11:385–421. [Google Scholar]
- 22.Montgomery EB., Jr Basal ganglia physiology and pathophysiology: a reappraisal. Parkinsonism Relat Disord. 2007;13:455–465. doi: 10.1016/j.parkreldis.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 23.MontgomeryEB EB, Jr, Gale JT. Mechanisms of action of deep brain stimulation (DBS) Neurosci Biobehav Rev. 2008;32:388–407. doi: 10.1016/j.neubiorev.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Walker HC, Watts RL, Schrandt CJ, et al. Activation of subthalamic neurons by contralateral subthalamic deep brain stimulation in Parkinson disease. J Neurophysiol. 2011;105:1112–1121. doi: 10.1152/jn.00266.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]