Abstract
Understanding cerebral oxygen metabolism is of great importance in both clinical diagnosis and animal experiments because oxygen is a fundamental source of brain energy and supports brain functional activities. Since small animals such as rats are widely used to study various diseases including cerebral ischemia, cerebrovascular diseases, and neurodegenerative diseases, the development of a noninvasive in vivo measurement method of cerebral oxygen metabolic parameters such as oxygen extraction fraction (OEF) and cerebral metabolic rate of oxygen (CMRO2) as well as cerebral blood flow (CBF) and cerebral blood volume (CBV) has been a priority. Although positron emission tomography (PET) with 15O labeled gas tracers has been recognized as a powerful way to evaluate cerebral oxygen metabolism in humans, this method could not be applied to rats due to technical problems and there were no reports of PET measurement of cerebral oxygen metabolism in rats until an 15O-O2 injection method was developed a decade ago. Herein, we introduce an intravenous administration method using two types of injectable 15O-O2 and an 15O-O2 gas inhalation method through an airway placed in the trachea, which enables oxygen metabolism measurements in rats.
1. Introduction
Since cerebral blood flow (CBF) and oxygen metabolism are fundamental for brain activity, the in vivo measurement of CBF, oxygen extraction fraction (OEF), and cerebral metabolic rate of oxygen (CMRO2) is of great importance in clinical diagnosis and for animal experiments. In particular, small animals such as mice and rats are widely used for research in a variety of diseases such as cerebral ischemia [1], dementia [2], Alzheimer's disease [3], and neurodegenerative diseases [4]. Small animals are also useful for the elucidation of glial function in pathological conditions [5] and for understanding the functional relationship between the brain and peripheral organs [6]. Therefore, the development of a noninvasive in vivo measurement method of such cerebral metabolic parameters in small animals has been eagerly sought.
Positron emitters, such as 18F, 15O, 11C, and 13N, emit positrons (β +) from which pairs of photons are detected by positron emission tomography (PET) to generate reconstructed images. This involves several corrections for random coincidence events, dead time count losses, detector inhomogeneity, photon attenuation, and scatter, among others. The annihilation radiation can noninvasively transmit through biological tissues. Thus positron-labeled compounds are used in combination with PET imaging to obtain biological information of living systems in research and clinical settings. For instance, 15O labeled O2 gas PET has been used to estimate cerebral oxygen metabolism in patients for diagnostic purposes since the 1970s [7–12]. Although the 15O-O2 gas PET technique also attracted researchers for the evaluation of cerebral oxygen metabolism in small animals, it was applied unsuccessfully due to technical challenges until the 1990s. To overcome these challenges, several methodological inventions have been tried, which have facilitated the evaluation of CMRO2 and OEF in small animals in the current research setting.
Herein, we introduce an intravenous administration method using injectable 15O-O2 and an inhalation method of 15O-O2 gas, both of which can measure CMRO2 and OEF with PET in living rats under anesthesia.
2. Intravenous Administration Method
Although the importance of evaluating cerebral oxygen metabolism in small animals has been recognized, application of the inhalation method using 15O-O2 gas in small animals could not be performed due to technical issues such as the potential influence of high radioactivity in the inhalation tube on the rat brain data acquisition. To overcome this situation, Magata et al. first developed an 15O-O2 injection method, which made rat OEF measurement possible using PET [13]. They collected blood from several rats and labeled the blood with 15O-O2 gas using an artificial lung (Figure 1). After 10 minutes of 15O-O2 uptake into the red blood cells, they had 15O labeled blood (72 MBq/mL) to use as an injectable for intravenous administration into normal rats for PET imaging. In fact, they performed two serial PET scans with 15O-water and injectable 15O-O2 and obtained 44 ± 4.5 mL/min/100 g of CBF and 0.54 ± 0.11 of OEF in normal rats under pentobarbital anesthesia. Subsequently, the same group evaluated the utility of the injectable 15O-O2 PET system using brain infarction rats [14], hypertensive rats [15], and normal monkeys [16]. The results indicated that the injectable 15O-O2 PET system could provide information on cerebral oxygen metabolism under normal and pathological conditions in rats as well as in larger animals. In particular, using the injectable 15O-O2 PET technique in spontaneously hypertensive rats (SHR), this research group clearly demonstrated that hypertension could intensify cerebral metabolic disturbances during the acute phase after the onset of stroke (Figure 2 [15]). This same group also applied the 15O-O2 injection technique to miniature pigs to evaluate myocardial oxygen metabolism, which was also considered to be a difficult target for evaluation by 15O-O2 gas inhalation because of the existence of radioactivity spillover from the gas volume in the lung to the myocardium due to limited spatial resolution [17]. Although the blood-based injectable 15O-O2 system provided a strong option that enabled oxygen metabolism measurement in small animals under normal and pathological conditions, some drawbacks were addressed for further applications. Namely, the blood-based injectable 15O-O2 system required that additional rats be sacrificed for blood collection and there was a possibility that the biological characteristics of the blood components might be damaged during the preparation process.
Figure 1.
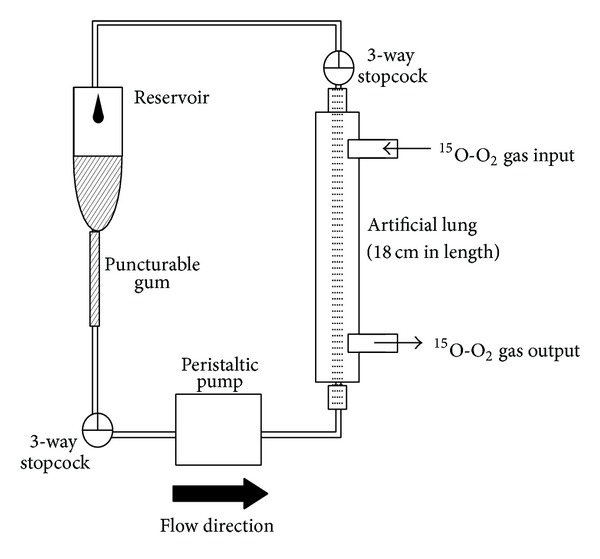
Injectable 15O-O2 preparation system using an 18 cm long artificial lung. The length of the artificial lung was 6 cm in the original report [13] and was changed to 18 cm in the latter studies for improvement of labeling efficiency [14, 15, 17].
Figure 2.
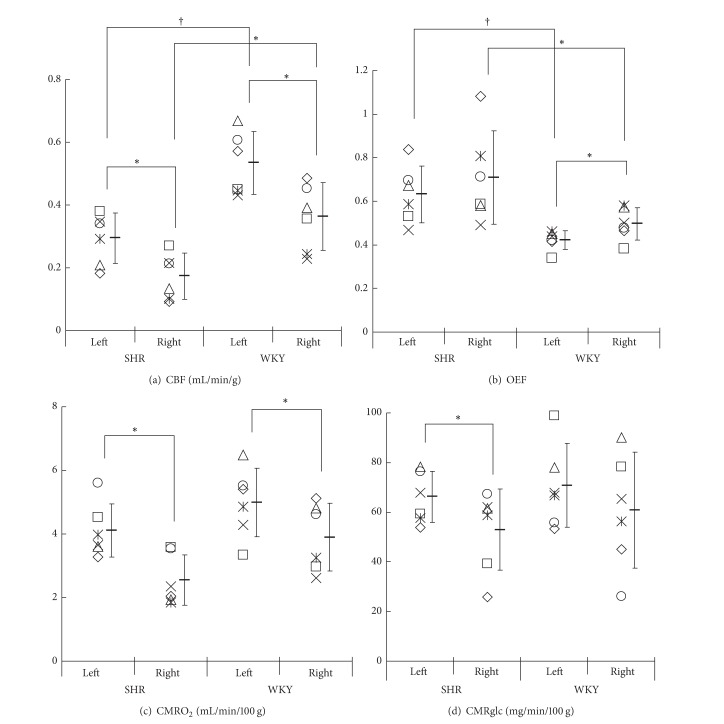
(Figure 1 in [15]) Quantitative values of CBF (a), OEF (b), CMRO2 (c), and cerebral metabolic rate of glucose (CMRglc) (d). PET with 15O-water and injectable 15O-O2 and an ex vivo autoradiography with 18F-FDG were performed one hour after the onset of a right middle cerebral artery occlusion using spontaneously hypertensive rats (SHR) and Wistar Kyoto rats (WKY). CBF, OEF, and CMRO2 were obtained from PET and CMRglc was obtained from ARG. Each of the six marks indicates the hemispheric average of 4 slices in an individual. Bar-shaped marks show the average and the error bars represent SD. Significant differences between hemispheres and between SHR and WKY were determined using the Wilcoxon signed rank test, *P < 0.05, and the Mann-Whitney U test, *P < 0.05, † P < 0.01.
Tiwari et al. then reported on a different injectable 15O-O2 system using hemoglobin-containing vesicles (HbV) to overcome these problems (Figure 3) [18]. The HbV, originally developed as an alternative oxygen carrier [19], was a liposome (about 300 nm in diameter) consisting of 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC), cholesterol, and 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol (DPPG) (5/5/1 at a molar ratio) and containing 10.8 g/dL hemoglobin molecules. The authors tested the feasibility of the HbV as an 15O-oxygen carrier, optimized a preparation system to obtain 15O-O2-HbV with a high labeling yield, and performed a PET study in normal rats after intravenous administration of 15O-O2-HbV. As a result, they achieved optimization of the labeling procedure using a direct bubbling method of 15O-O2 gas into the HbV solution containing L-cysteine using a vortex. They obtained 214 ± 7.8 MBq/mL 15O-O2-HbV, which is about 3-fold higher than the previous blood-based injectable 15O-O2 [13]. They also measured CBF, OEF, and CMRO2 values using the 15O-O2-HbV with PET imaging in normal rats. The same research group from the University of Fukui proceeded to lessen the invasiveness of the 15O-O2 injection method in the next step. In fact, all of the manuscripts using the 15O-O2 injection method described above adopted continuous arterial blood sampling during the PET scans for estimation of the input function to analyze cerebral metabolic parameters [13–16, 18]. Since the total volume of blood sampling is limited in small animals such as rats, they applied a steady-state method they originally developed for CBF measurement using 15O-water PET in rats [20] to the 15O-O2-HbV PET to decrease the injection and blood sampling volumes [21]. They prepared 15O-water, 15O-O2-HbV, and 15O-CO-HbV obtained in a similar manner as the 15O-O2-HbV, and PET scans were performed with continuous intravenous administration of 15O-CO-HbV, 15O-water, and 15O-O2-HbV through a multiprogrammed syringe pump with gradual changes in the injection speed. They reported that the injection and sampling blood volumes were 1.65 and 0.65 mL in 15O-water PET and 1.65 and 1.40 mL in 15O-O2-HbV PET, respectively, and achieved the measurement of CBF, OEF, CMRO2, and cerebral blood volume (CBV) values in several cerebral regions using a high resolution PET system (SHR-41000; Hamamatsu Photonics, Hamamatsu, Japan). In addition, the usefulness of the steady-state method was confirmed in a rat model of brain infarction. As such, in combination with the improvement in small animal PET systems and experimental procedures, the 15O-O2 intravenous administration method made possible cerebral oxygen metabolism measurement of rats in normal and pathological conditions, with minimal invasiveness.
Figure 3.
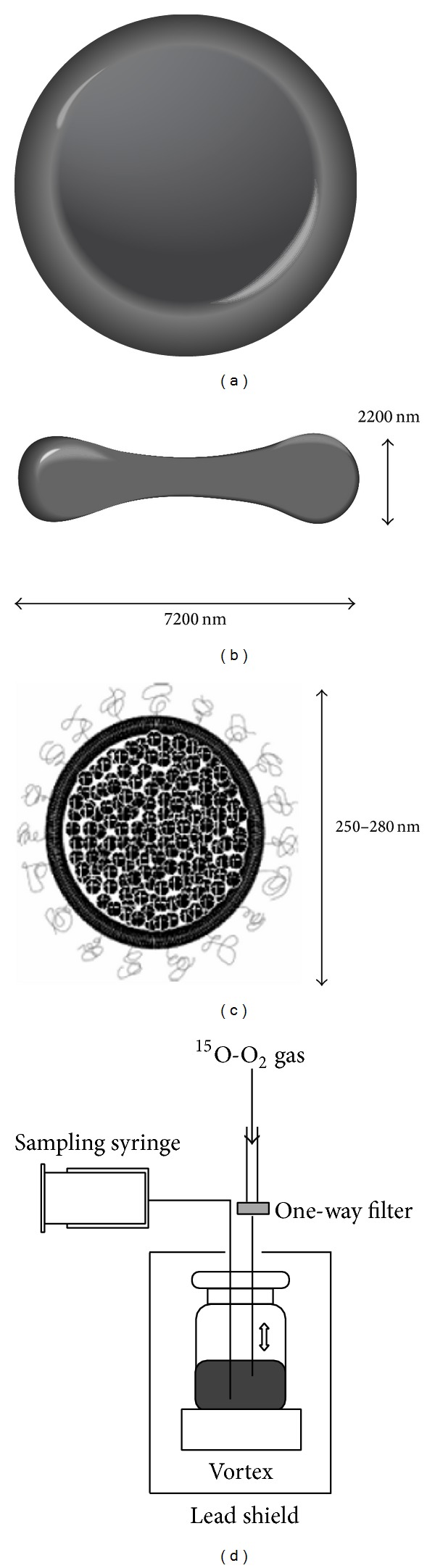
A schematic diagram of the 15O-O2-HbV preparation system. Normal human red blood cells (RBC) (a, b), hemoglobin-vesicle (HbV) structure (c) with shape and approximate diameters, and the final labeling setup with a lead shield for injectable 15O-O2-HbV preparation (d) are shown (courtesy of Dr. Kiyono, University of Fukui, Fukui, Japan).
3. Inhalation Method
Aside from the intravenous administration method, researchers have also tried to develop an 15O-O2 gas inhalation method for small animals such as rats. Yee et al. first performed a micro-PET experiment using normal rats with briefly inhaled 15O-O2 gas [22]. In this report, the authors applied the one-step method using single inhalation of 15O-O2 gas [23] to rats, and the 15O-O2 gas contained in a syringe was administered by a bolus insufflation into the lung through a cannula surgically placed in the trachea. In addition, they omitted arterial blood sampling in consideration of the limited blood volume of rats. Instead, for the estimation of input function, the field of view (FOV) of a PET scan was positioned to cover the brain and the heart at the same time. The time activity curve data from the heart was corrected using the volume ratio of the pure arterial space inside the ROI as the arterial input function [24]. As a result, 5.00 ± 0.36 mL/min/100 g of CMRO2 was calculated in 10 normal rats under α-chloralose anesthesia with continuous infusion. The study was successfully performed to achieve rat CMRO2 measurement with only one PET scan and without arterial blood sampling; however, a tracheotomy for tracer administration, animal size restriction for simultaneous brain-heart scan, and poor signal to noise ratio were mentioned as limitations.
Recently, Watabe et al. reported the application of a steady-state 15O-O2 gas inhalation method for normal rats [25]. Namely, they performed a tracheotomy and placed a flexible tube into the trachea to serve as an administration route for the 15O-gas tracers. They performed three serial PET scans using 15O-CO2, 15O-O2, and 15O-CO gas, respectively, and measured CBF, OEF, CMRO2, and CBV values in the normal brains of rats under anesthesia according to the original 15O gas steady-state inhalation method used in clinical settings [26–28]. A clinical PET camera (Headtome-V PET scanner; Shimadzu Corp.) was used and the feasibility of using the camera for small animal studies was evaluated by phantom experiments. After precise evaluation of partial volume effects, scatter correction from the high radioactivity in the pleural cavity, and application of a cross-calibration factor, the authors succeeded in obtaining quantitative and comparable values and functional images of CBF, OEF, CMRO2, and CBV in normal rats. In addition, they tested the applicability of the method to a small number of ischemia model rats (n = 2) and successfully showed decreased CBF and CMRO2 values and increased OEF value in the ipsilateral hemisphere. The total time was about 73 min for the entire PET experiment in each rat. The results clearly indicated that the steady-state 15O-gas inhalation method used in clinical settings could be applied to rats with consideration of the appropriate care to avoid possible errors. However, tracheotomy was still required for gas tracer administration and the rats underwent arterial blood sampling during the PET scan, which might be considered a limitation in the above study.
On this basis, we are now developing an 15O gas administration technique that uses the spontaneous respiration of rats under isoflurane anesthesia for micro-PET measurement of cerebral metabolic function without arterial blood sampling. As shown in Figure 4 (unpublished data), we can provide “pseudo” functional images of a rat brain under both normal and pathological conditions. We expect to successfully perform this technique in the near future.
Figure 4.
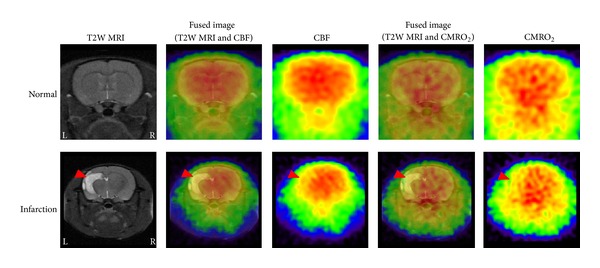
Functional images (“pseudo” CBF and CMRO2) of normal and infarction rat brains (Wistar rats, male, 8 weeks old). T2 weighted MR images are shown as a position reference. PET scans were performed during continuous administration of 15O-CO2 and 15O-O2 gases by spontaneous respiration of rats under isoflurane anesthesia.
Finally, regardless of the administration route of 15O-O2, recirculating 15O labeled water, which is a metabolic product of 15O-O2, should be taken into consideration for estimating quantitative CMRO2 and OEF in small animals. The recirculating 15O-water could have a crucial impact on these parameters due to more rapid appearance after 15O-O2 administration in small animals than in humans. In fact, most of the studies described above measured the contribution of recirculating 15O-water as an input function by separating the plasma from the whole blood samples [13–15, 21, 25]. However, this procedure requires repetitive blood sampling during a PET study, which may alter physiological function due to the limited total blood volume in small animals. Recently, an alternative approach has been applied, in which the time activity curve of recirculating 15O-water could be predicted from a whole blood radioactivity concentration curve by modeling the kinetics of the metabolic process of oxygen molecules in the whole body [29]. Thus, the labor intensive procedure of frequent arterial blood sampling with centrifugation can be avoided, making the protocol applicable to many studies using clinical patients as well as experimental animals. It is of note that this method was shown to be applicable to a wide range of species from human to rats. Therefore, using the simplified method to predict the contribution of recirculating 15O-water, in combination with less invasive techniques to obtain the time activity curve such as an online scintillation detector coupled to an arteriovenous shunt [30] or ROI analysis of the cardiac ventricle in PET images [22], the 15O PET technique could be more widely applied to small animals under a broad range of conditions.
4. Conclusion
Since oxygen is a key molecule for energy production in living brains, the measurement of cerebral oxygen metabolism is important to understand brain function in normal and pathological conditions. With some technological innovations including the development of injectable 15O-O2 preparations and the successful application of an 15O-O2 gas inhalation method with appropriate corrections, measurement of cerebral oxygen metabolism (OEF and CMRO2) has become possible in living rats, as compared to the difficult challenges faced more than a decade ago. However, there are several issues that remain unresolved for the ideal achievement of noninvasive quantification of OEF and CMRO2 in living rats by PET using 15O gas tracers; these include tracheotomy, arterial blood sampling, and long experimental time. In contrast, the total examination time in clinical settings has been dramatically reduced from more than 40 minutes [31] to about 10 minutes by recent technical innovations [9, 32]. Therefore, experiments involving small animal models would also benefit from further methodological progress including faster and less invasive measurement (e.g., 15O gas administration by spontaneous respiration, input function estimation from the heart or large arteries) with improvement of resolution and sensitivity by dedicated PET scanners for small animals and the development of a fully automated rapid measurement system for animal 15O gas experiments. With such innovation, the 15O PET technique could be more widely applied to studies in model animals including not only ischemia and infarction but also neurodegenerative and psychiatric diseases.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Yao H, Nabika T. Standards and pitfalls of focal ischemia models in spontaneously hypertensive rats: with a systematic review of recent articles. Journal of Translational Medicine. 2012;10(1, article 139) doi: 10.1186/1479-5876-10-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver J, Jalal FY, Yang Y, Thompson J, Rosenberg GA, Liu KJ. Tissue oxygen is reduced in white matter of spontaneously hypertensive-stroke prone rats: a longitudinal study with electron paramagnetic resonance. Journal of Cerebral Blood Flow & Metabolism. 2014;34(5):890–896. doi: 10.1038/jcbfm.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Y, Huang Y, Tang C, et al. Brain areas involved in the acupuncture treatment of AD model rats: a PET study. BMC Complementary and Alternative Medicine. 2014;14, article 178 doi: 10.1186/1472-6882-14-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C, Yuan K, Schluesener H. Impact of minocycline on neurodegenerative diseases in rodents: a meta-analysis. Reviews in the Neurosciences. 2013;24:553–562. doi: 10.1515/revneuro-2013-0040. [DOI] [PubMed] [Google Scholar]
- 5.Morris GP, Clark IA, Zinn R, Vissel B. Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiology of Learning and Memory. 2013;105:40–53. doi: 10.1016/j.nlm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Cone JJ, McCutcheon JE, Roitman MF. Ghrelin acts as an interface between physiological state and phasic dopamine signaling. The Journal of Neuroscience. 2014;34:4905–4913. doi: 10.1523/JNEUROSCI.4404-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Baradaran H, Schweitzer AD, et al. Oxygen extraction fraction and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. American Journal of Neuroradiology. 2014;35:250–255. doi: 10.3174/ajnr.A3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hokari M, Kuroda S, Shiga T, Nakayama N, Tamaki N, Iwasaki Y. Impact of oxygen extraction fraction on long-term prognosis in patients with reduced blood flow and vasoreactivity because of occlusive carotid artery disease. Surgical Neurology. 2009;71(5):532–538. doi: 10.1016/j.surneu.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 9.Kudomi N, Hirano Y, Koshino K, et al. Rapid quantitative CBF and CMRO2 measurements from a single PET scan with sequential administration of dual 15O-labeled tracers. Journal of Cerebral Blood Flow and Metabolism. 2013;33(3):440–448. doi: 10.1038/jcbfm.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nezu T, Yokota C, Uehara T, et al. Preserved acetazolamide reactivity in lacunar patients with severe white-matter lesions: 15O-labeled gas and H2O positron emission tomography studies. Journal of Cerebral Blood Flow and Metabolism. 2012;32(5):844–850. doi: 10.1038/jcbfm.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka M, Shimosegawa E, Kajimoto K, et al. Chronic middle cerebral artery occlusion: a hemodynamic and metabolic study with positron-emission tomography. The American Journal of Neuroradiology. 2008;29(10):1841–1846. doi: 10.3174/ajnr.A1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baron J, Jones T. Oxygen metabolism, oxygen extraction and positron emission tomography: historical perspective and impact on basic and clinical neuroscience. NeuroImage. 2012;61(2):492–504. doi: 10.1016/j.neuroimage.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 13.Magata Y, Temma T, Iida H, et al. Development of injectable O-15 oxygen and estimation of rat OEF. Journal of Cerebral Blood Flow and Metabolism. 2003;23(6):671–676. doi: 10.1097/01.WCB.0000066792.97069.B3. [DOI] [PubMed] [Google Scholar]
- 14.Temma T, Magata Y, Kuge Y, et al. Estimation of oxygen metabolism in a rat model of permanent ischemia using positron emission tomography with injectable15O-O2 . Journal of Cerebral Blood Flow and Metabolism. 2006;26(12):1577–1583. doi: 10.1038/sj.jcbfm.9600302. [DOI] [PubMed] [Google Scholar]
- 15.Temma T, Kuge Y, Sano K, et al. PET O-15 cerebral blood flow and metabolism after acute stroke in spontaneously hypertensive rats. Brain Research. 2008;1212:18–24. doi: 10.1016/j.brainres.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Temma T, Magata Y, Iida H, et al. Development of injectable O-15 oxygen and its application for estimation of OEF. International Congress Series. 2004;1265:262–265. [Google Scholar]
- 17.Temma T, Iida H, Hayashi T, et al. Quantification of regional myocardial oxygen metabolism in normal pigs using positron emission tomography with injectable 15O-O2 . European Journal of Nuclear Medicine and Molecular Imaging. 2010;37(2):377–385. doi: 10.1007/s00259-009-1262-2. [DOI] [PubMed] [Google Scholar]
- 18.Tiwari VN, Kiyono Y, Kobayashi M, et al. Automatic labeling method for injectable 15O-oxygen using hemoglobin-containing liposome vesicles and its application for measurement of brain oxygen consumption by PET. Nuclear Medicine and Biology. 2010;37(1):77–83. doi: 10.1016/j.nucmedbio.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Ogata Y. Characteristics and function of human hemoglobin vesicles as an oxygen carrier. Polymers for Advanced Technologies. 2000;11(5):205–209. [Google Scholar]
- 20.Kobayashi M, Kiyono Y, Maruyama R, Mori T, Kawai K, Okazawa H. Development of an H2 15O steady-state method combining a bolus and slow increasing injection with a multiprogramming syringe pump. Journal of Cerebral Blood Flow and Metabolism. 2011;31(2):527–534. doi: 10.1038/jcbfm.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi M, Mori T, Kiyono Y, et al. Cerebral oxygen metabolism of rats using injectable 15O-oxygen with a steady-state method. Journal of Cerebral Blood Flow and Metabolism. 2012;32(1):33–40. doi: 10.1038/jcbfm.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yee SH, Lee K, Jerabek PA, Fox PT. Quantitative measurement of oxygen metabolic rate in the rat brain using microPET imaging of briefly inhaled 15O-labelled oxygen gas. Nuclear Medicine Communications. 2006;27(7):573–581. doi: 10.1097/01.mnm.0000220586.02591.fd. [DOI] [PubMed] [Google Scholar]
- 23.Ohta S, Meyer E, Thompson CJ, Gjedde A. Oxygen consumption of the living human brain measured after a single inhalation of positron emitting oxygen. Journal of Cerebral Blood Flow and Metabolism. 1992;12(2):179–192. doi: 10.1038/jcbfm.1992.28. [DOI] [PubMed] [Google Scholar]
- 24.Yee S, Jerabek PA, Fox PT. Non-invasive quantification of cerebral blood flow for rats by microPET imaging of 15O labelled water: the application of a cardiac time-activity curve for the tracer arterial input function. Nuclear Medicine Communications. 2005;26(10):903–911. doi: 10.1097/00006231-200510000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Watabe T, Shimosegawa E, Watabe H, et al. Quantitative evaluation of cerebral blood flow and oxygen metabolism in normal anesthetized rats: 15O-Labeled gas inhalation PET with MRI fusion. Journal of Nuclear Medicine. 2013;54(2):283–290. doi: 10.2967/jnumed.112.109751. [DOI] [PubMed] [Google Scholar]
- 26.Jones T, Chesler DA, Ter Pogossian MM. The continuous inhalation of Oxygen-15 for assessing regional oxygen extraction in the brain of man. The British Journal of Radiology. 1976;49(580):339–343. doi: 10.1259/0007-1285-49-580-339. [DOI] [PubMed] [Google Scholar]
- 27.Frackowiak RSJ, Lenzi GL, Jones T, Heather JD. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. Journal of Computer Assisted Tomography. 1980;4(6):727–736. doi: 10.1097/00004728-198012000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Lammertsma AA, Jones T. Correction for the presence of intravascular oxygen-15 in the steady-state technique for measuring regional oxygen extraction ratio in the brain. I. Description of the method. Journal of Cerebral Blood Flow and Metabolism. 1983;3(4):416–424. doi: 10.1038/jcbfm.1983.67. [DOI] [PubMed] [Google Scholar]
- 29.Kudomi N, Hayashi T, Watabe H, et al. A physiologic model for recirculation water correction in CMRO2 assessment with 15O2 inhalation PET. Journal of Cerebral Blood Flow and Metabolism. 2009;29(2):355–364. doi: 10.1038/jcbfm.2008.132. [DOI] [PubMed] [Google Scholar]
- 30.Ose T, Watabe H, Hayashi T, et al. Quantification of regional cerebral blood flow in rats using an arteriovenous shunt and micro-PET. Nuclear Medicine and Biology. 2012;39(5):730–741. doi: 10.1016/j.nucmedbio.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Mintun MA, Raichie ME, Martin WRW, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. Journal of Nuclear Medicine. 1984;25(2):177–187. [PubMed] [Google Scholar]
- 32.Kudomi N, Hayashi T, Teramoto N, et al. Rapid quantitative measurement of CMRO2 and CBF by dual administration of 15O-labeled oxygen and water during a single PET scan - A validation study and error analysis in anesthetized monkeys. Journal of Cerebral Blood Flow and Metabolism. 2005;25(9):1209–1224. doi: 10.1038/sj.jcbfm.9600118. [DOI] [PubMed] [Google Scholar]