Abstract
Background
Preeclampsia, a new-onset hypertensive disorder of pregnancy, is associated with lifetime cardiovascular disease risk, but less is known about risk after other pregnancy-related hypertension.
Methods and Results
The Northern Finland Birth Cohort 1966 included all expected births from 1 year (N=12 055 women). Blood pressure measurements and other prospective data were determined from prenatal care records and questionnaires for 10 314 women. Subsequent diagnoses were ascertained from Finnish registries (average follow-up, 39.4 years). Adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) estimate risks in hypertensive women compared with normotensive women. Hypertension during pregnancy was associated with increased risk of subsequent cardiovascular disease and arterial hypertension. Women with chronic hypertension and superimposed preeclampsia/eclampsia had high risk for future diseases. Gestational hypertension was associated with increased risk of ischemic heart disease (HR, 1.44 [95% CI, 1.24–1.68]), myocardial infarcts (HR, 1.75 [95% CI, 1.40–2.19]), myocardial infarct death (HR, 3.00 [95% CI, 1.98–4.55]), heart failure (HR, 1.78 [95% CI, 1.43–2.21]), ischemic stroke (HR, 1.59 [95% CI, 1.24–2.04]), kidney disease (HR, 1.91 [95% CI, 1.18–3.09]), and diabetes mellitus (HR, 1.52 [95% CI, 1.21–1.89]). Isolated systolic hypertension was associated with increased risk of myocardial infarct death (HR, 2.15 [95% CI, 1.35–3.41]), heart failure (HR, 1.43 [95% CI, 1.13–1.82]), and diabetes mellitus (HR, 1.42 [95% CI, 1.13–1.78]), whereas isolated diastolic hypertension was associated with increased risk of ischemic heart disease (HR, 1.26 [95% CI, 1.05–1.50]). Results were similar in nonsmoking women aged <35 years with normal weight and no diabetes mellitus during pregnancy.
Conclusions
Elevated blood pressure during pregnancy, regardless of type and even without known risk factors, signals high risk of later cardiovascular disease, chronic kidney disease, and diabetes mellitus. Clinical monitoring, risk factor evaluation, and early intervention could benefit women with hypertension in pregnancy.
Keywords: epidemiology, hypertension, myocardial infarction, pregnancy, prevention, stroke
New-onset hypertensive disorders in pregnancy, like gestational hypertension and preeclampsia, are common complications that can lead to increased maternal and fetal morbidity and mortality during pregnancy and can affect the future health of both the mother and child.1 Isolated newonset elevation in either systolic or diastolic blood pressure is probably quite common during pregnancy, but they currently have no diagnostic criteria, and isolated hypertensions are not independently noted in administrative data or discharge summaries.2
During the past 20 years, the incidence of hypertensive disorders during pregnancy has increased,3 probably at least partly because of increasing obesity and maternal age.4,5 Pregnancy requires complex adaptation of the cardiovascular system with decreased vascular resistance, increased blood volume, and other metabolic changes.2 As a physiological stressor, pregnancy may uncover susceptibility to subsequent chronic disease, particularly of a vascular or metabolic origin.1 Notably, ≤60% of reproductive-aged women have ≥1 cardiovascular risk factor,6 which is associated with an increased cardiovascular disease risk during the life course.7 Women with preeclampsia have clustering of metabolic syndrome traits before and after pregnancy,8–13 with an antiangiogenic state during pregnancy,14–16 as well as persistent endothelial dysfunction,17 all of which may increase their risk for cardiovascular diseases.13,18
Well-conducted studies have shown that preeclampsia associates with the risk for end-stage renal disease,19 cardiovascular diseases, and death.20–25 Increased risk for cardiovascular disease has also been observed among women with gestational hypertension,21,22,24,26,27 but contradicting results have also been published.28 Women with preeclampsia and gestational hypertension more often have diabetes mellitus13,26,29 and chronic hypertension later in life,20,28,30 which may modify the subsequent cardiovascular risks. However, no previous studies have long-term follow-up sufficient in time and clinical detail to comprehensively examine long-term health risks for several chronic disease end points among women who experienced hypertension in pregnancy. In addition, previous studies generally rely on administrative data or hospital discharge summaries and have not had prospective data, including clinical blood pressure measures from pregnancy, that allow for examination of elevated blood pressure in the absence of a diagnosed condition.
Our aim was to evaluate the risk to women for subsequent cardiovascular, cerebrovascular, and kidney disease, as well as diabetes mellitus morbidity and cardiovascular mortality associated with a full spectrum of hypertension during pregnancy with a long-term follow-up in the prospectively collected Northern Finland Birth Cohort 1966.
Methods
Northern Finland Birth Cohort 1966
The prospective Northern Finland Birth Cohort (NFBC) 1966 was composed of all expected births for the year 1966, in the 2 northernmost provinces of Finland (N=12 055 women). NFBC 1966 covers all live-born and stillborn infants of >28 gestational weeks or who had a birth weight of ≥600 g (96.3% of all births in the area). Demographic and clinical information on the women was collected by local midwives during routine visits in free-of-charge communal maternity welfare clinics (MWCs), with participation rate >90%. Women generally began the visits between 10 and 16 weeks’ gestation (the onset of NFBC 1966 follow-up) and visited the clinics on average 7 times during pregnancy (93% had >5 visits). The final recruitment happened at the 28th gestational week if they still were pregnant. Questionnaire data on the women were collected at 24 to 28 weeks with 10.1% completing it later during pregnancy or after delivery. Health and sociodemographic data were obtained from antenatal cards filled in during routine MWC visits and/or from the questionnaire.31 Informed consent was obtained from all participants, and this study was approved by the ethical committee of the Northern Ostrobothnia Hospital District.
Definition of Hypertension and Study Population
Blood pressure was measured in sitting position, and urinary dip stick tests were performed during every visit at MWCs (average of 7 visits with measurements per woman) by experienced nurses following standardized instructions by the Finnish National Board of Health. The highest blood pressure values in the first trimester of pregnancy and during 5 to 10 pregnancy months were documented in cohort databases at the time of initial data collection of NFBC 1966. These data and the complete clinical blood pressure data from each MWC visit were evaluated by 2 experienced obstetricians (A.-.L.H. and A.P.). The diagnoses of hypertensive disorders were based on the guidelines of the National Heart, Lung, and Blood Institute,32 with 2 modifications: in the 1960s, clinical blood pressure values were rounded up or down to the nearest 5 mm Hg; thus, blood pressure values ≥145 mm Hg systolic and ≥95 mm Hg diastolic were selected to represent hypertension instead of the current cutoffs of ≥140 mm Hg systolic and ≥90 mm Hg diastolic, and we required women to have both systolic and diastolic blood pressure elevations for the diagnoses of gestational hypertension, preeclampsia/eclampsia, chronic hypertension, and superimposed preeclampsia/eclampsia. Women with elevations isolated to systolic or diastolic blood pressure were categorized as separate groups. Because hypertensive disorders are a continuum of disorders where gestational hypertension may progress to preeclampsia or to chronic hypertension as pregnancy progresses, our longitudinally collected data allowed diagnoses to be based on blood pressure elevation and proteinuria over the course of pregnancy, but single high values were sufficient for the diagnoses. Positive urinary dip stick tests were confirmed by boiling test and quantitated by the Esbach test to confirm proteinuria ≥0.3 g/L.
Women were grouped into mutually exclusive categories as described below.
Normotensive
Blood pressure <145/95 mm Hg throughout pregnancy (N=6552).
New-Onset Hypertension During Pregnancy
-
1
Isolated systolic hypertension described systolic blood pressure ≥145 mm Hg any time during pregnancy with no proteinuria (N=866).
-
2
Isolated diastolic hypertension described diastolic blood pressure ≥95 mm Hg any time during pregnancy with no proteinuria (N=742).
-
3
Isolated hypertension with proteinuria described either isolated systolic or diastolic hypertension (but not both) and proteinuria in ≥1 sample at any time during pregnancy (N=137).
Most (72.1%) women with isolated hypertension had hypertension only after midgestation, but 27.9% had either isolated hypertension in early pregnancy or high isolated hypertensive values recorded throughout pregnancy, but not postpartum.
-
4
Gestational hypertension included women who were normotensive before the 20th gestational week but hypertensive after the 20th gestational week, with no proteinuria during pregnancy (N=991).
-
5
Preeclampsia/eclampsia included women who were normotensive before the 20th gestational week but hypertensive after the 20th gestational week with proteinuria in ≥1 sample (N=242).
Chronic Hypertension
-
6
Chronic hypertension included those with hypertension before the 20th gestational week, continuing throughout the pregnancy and/or up to 6 weeks after pregnancy. Alternatively, it included those having chronic hypertension and/or antihypertensive medication recorded in the cohort questionnaire and/or MWC files and no proteinuria during pregnancy (N=668).
-
7
Superimposed preeclampsia/eclampsia included chronic hypertension with proteinuria (N=116).
Our analyses are based on 10 314 women with singleton pregnancies. We excluded 1554 women from the original cohort who could not be classified because of missing blood pressure measurements, 11 women who died within 1 year of pregnancy, 163 women with multiple gestations, and the second pregnancy of 13 women who delivered twice during the study year. Women with missing blood pressure measurements had similar age and prepregnancy body mass index (weight in kilograms/height2 in meters) but were more often nulliparous, had lower socioeconomic status and visited the MWCs less often than women with available data. Their overall rate of chronic diseases was similar to women with available data (45.7% versus 46.3%).
Because our hypertension data were from 2 sources, we did a sensitivity analysis among women with gestational hypertension, preeclampsia/eclampsia, and isolated hypertension who could have been solely diagnosed using blood pressure values recorded in the NFBC 1966 database during the original data collection (women who had data on the highest systolic and diastolic blood pressure values in 2 to 4 months and in 5 to 10 months of pregnancy, composing 80.1% of the groups). Sensitivity analyses were also conducted restricting data to nulliparous women and the groups with gestational hypertension, preeclampsia/eclampsia, and isolated hypertension to women who were presumed to be low risk (age <35, normal weight [body mass index, 18.5–24.9 kg/m2], and no smoking or diabetes mellitus) at the beginning of pregnancy. These analyses were further restricted to nulliparous women.
Follow-up Data on Disease Outcomes
Average follow-up time was 39.4 years (range, 3.0–43.6 years) and the studied outcomes were as follows (see the online only Data Supplement Methods section for specific definitions): total cardiovascular disease (first episode of any cardiovascular or cerebrovascular disease), ischemic heart disease, myocardial infarct (MI) and MI deaths, heart failure, ischemic cerebrovascular disease, chronic kidney disease, diabetes mellitus, and arterial hypertension.
The first year of follow-up was excluded from analyses to reduce the immediate effect of pregnancy complications on the outcomes. An outcome was considered to have occurred if women had the disease-specific International Classification of Diseases code recorded in ≥1 of the following Finnish registers; the Special Refund Entitlement Register (from 1967–2000) has medically verified diagnoses for chronic diseases, required to receive medication reimbursement for said disease. As the verification of diagnoses requires evidence based on examination and approval of a medical certificate by a specialist physician,33 we believe that the register has a high accuracy, as currently shown for heart failure (with positive predictive value of 93.4%).34 The Hospital Discharge Register (from 1972–2008) includes all discharge diagnoses with International Classification of Diseases codes from hospital wards or outpatient clinics and has a positive predictive value of 87% to 94% for stroke and MI, 73% for heart failure, and 94% for endocrine diseases.35 The Population Register and Register of Causes of Death (from 1962–2006) includes all deaths and causes of death with International Classification of Diseases codes, with positive predictive values comparable to the Hospital Discharge Register36,37 and a 7.7% false-positive rate of ischemic heart disease.38 Women who moved abroad (N=529) were identified by the Population Register, and they provided follow-up data until the year of their move.
The diagnosis of arterial hypertension after pregnancy was based on reimbursement for hypertensive medication, hospitalization, or death with hypertension noted, so women treated with diet and lifestyle modification will not be captured. The register-based data were combined with the NFBC 1966 data using Finnish individual social security numbers by personnel uninvolved in this study. The researchers had no access to identifiable data concerning the participants.
Statistical Methods
Student t tests, Fisher exact probability tests, or χ2 tests were used to compare unadjusted differences between the characteristics of women with and without hypertension during pregnancy. A small amount of missing covariate data were imputed (0.1% of maternal age and socioeconomic status, 0.2% of parity, 2.1% of smoking, 7.1% of number of MWC visits, and 5.0% and 5.1% of weight and height) using multiple imputation (5 imputations with 100 iterations) with the aforementioned variables as covariates. The original and pooled results with imputations were similar, and only the pooled results are presented.
Cox regression analysis assessed the impact of hypertension on subsequent morbidity and mortality using age at the beginning of follow-up and age at event/censoring as indicators of time to account for maternal age during pregnancy and length of follow-up. Proportional hazard assumption was verified graphically and using linear regression to estimate slope of partial residuals over time. The results are presented as hazard ratios with 95% confidence intervals and as 1-survival curves for selected outcomes. Adjusted models also include maternal prepregnancy body mass index, smoking, parity (nulliparous/multiparous), diabetes mellitus before or during pregnancy, and socioeconomic status (manager or office worker/worker or farmer/housewife). Crude and adjusted hazards were fairly similar, and only the adjusted models are presented. The risk of arterial hypertension was not estimated for women with chronic hypertension or superimposed preeclampsia/eclampsia in pregnancy, as per definition these women were already affected with the outcome. All of the statistical analyses and graphics were performed with SPSS 19.0 (IBM SPSS Statistics).
Results
Population Characteristics
Hypertensive women were generally older, heavier, and visited MWCs more often than normotensive women (Table 1). There were significantly more nulliparas among most hypertensive groups than among normotensive women with the exception of superimposed preeclampsia/eclampsia and isolated systolic hypertension. Women with gestational hypertension, preeclampsia/eclampsia, isolated diastolic hypertension, and chronic hypertension were less likely to be smokers, and women with chronic hypertension, isolated systolic hypertension, and superimposed preeclampsia/eclampsia were less likely to be managers or office workers than normotensive women. At the end of the follow-up, women were, on average, 66.7 years old (interquartile range, 62.6– 72.6 years).
Table 1.
Clinical Characteristics of Women With and Without Hypertension During Pregnancy in Northern Finland Birth Cohort 1966
| Characteristic | Normotensive (N=6552) |
Isolated Systolic Hypertension (N=866) |
Isolated Diastolic Hypertension (N=742) |
Isolated Systolic or Diastolic Hypertension With Proteinuria (N=137) |
Gestational Hypertension (N=991) |
Preeclampsia/ Eclampsia (N=242) |
Chronic Hypertension (N=668) |
Superimposed Hypertension Preeclampsia/ Eclampsia (N=116) |
|---|---|---|---|---|---|---|---|---|
| Mean age at birth (SD), y | 26.6 (6.2) | 28.3 (7.0)§ | 26.9 (2.7) | 26.8 (6.6) | 27.8 (7.3)§ | 26.7 (6.9) | 31.5 (7.1)§ | 32.1 (7.1)§ |
| Mean prepregnancy BMI (SD), kg/m2 | 22.6 (3.0) | 23.8 (3.8)§ | 23.3 (3.5)§ | 23.7 (3.3)§ | 23.6 (3.5)§ | 23.5 (3.6)§ | 25.4 (4.0)§ | 25.9 (4.5)§ |
| Nullipara, n (%) | 2028 (30.9) | 259 (29.9) | 273 (36.8)‡ | 56 (40.9)* | 402 (40.6)§ | 133 (55.0)§ | 142 (21.3)§ | 33 (28.4) |
| Smoking before pregnancy, n (%) | 1559 (23.8) | 196 (22.6) | 133 (17.9)§ | 31 (22.6) | 183 (18.5)§ | 44 (18.2)* | 97 (14.5)§ | 20 (17.2) |
| ≥5 visits during pregnancy, n (%) | 6077 (92.7) | 830 (95.7)§ | 717 (96.6)§ | 136 (99.3)§ | 939 (94.5)* | 229 (95.0) | 639 (95.7)‡ | 112 (96.6) |
| Socioeconomic status at 1966, n (%) Managerial and office workers | 876 (13.4) | 78 (9.0)† | 110 (14.8) | 14 (10.2) | 132 (13.3) | 37 (15.3) | 64 (9.6)* | 6 (5.2)* |
| Workers and farmers | 3333 (50.9) | 473 (54.6) | 372 (50.1) | 72 (52.6) | 527 (53.2) | 131 (54.1) | 369 (55.2) | 68 (58.6) |
| Housewives | 2343 (35.8) | 315 (36.4) | 260 (35.0) | 51 (37.2) | 332 (33.5) | 74 (30.6) | 235 (35.2) | 42 (36.2) |
BMI indicates body mass index. BMI is the weight in kilograms divided by the square of the height in meters. Statistical difference in demographics was analyzed with the Student t test or Fisher exact test when comparing the hypertensive group individually with the normotensive group.
P<0.05.
P<0.01.
P<0.001.
P<0.0001.
Cardiovascular and Ischemic Heart Disease
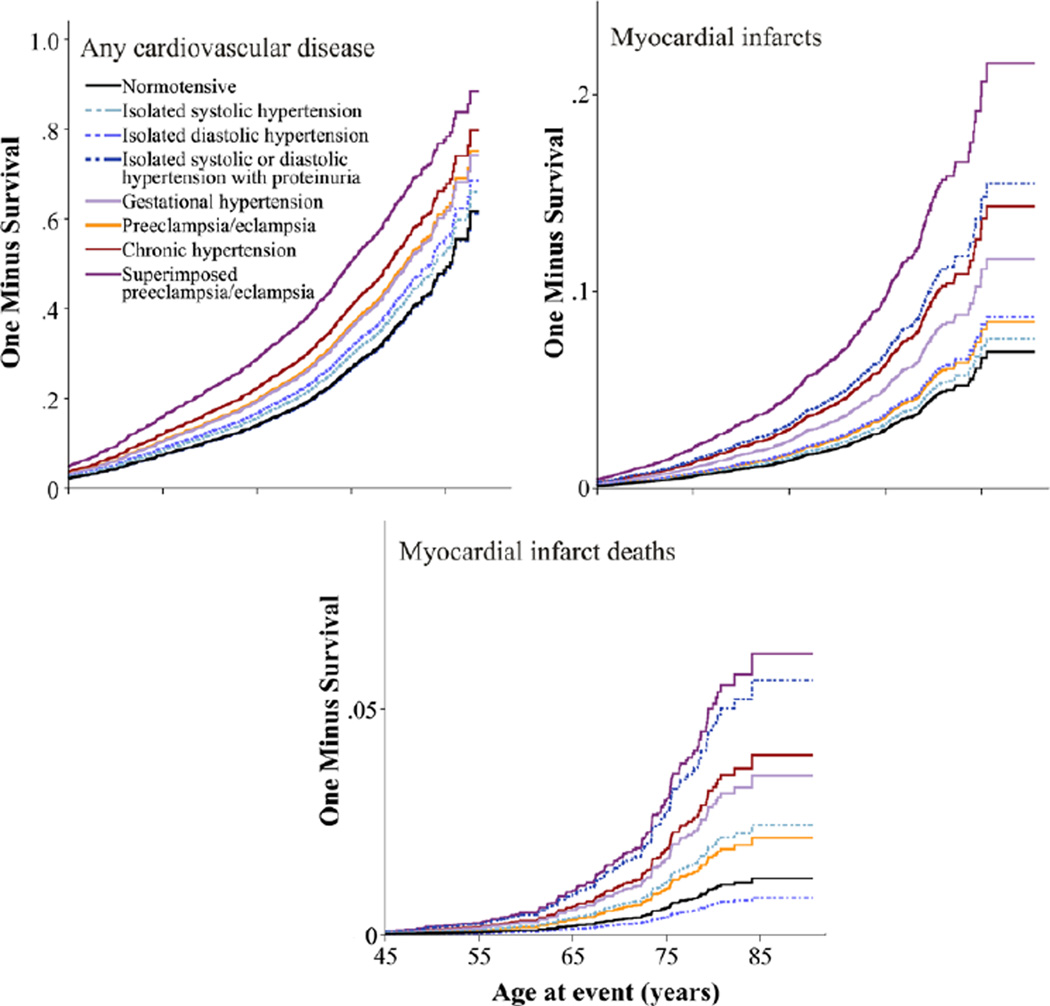
Increased risk for subsequent cardiovascular disease was observed among all of the hypertensive groups except among women with isolated hypertension and proteinuria (Table 2 and Figure). Isolated diastolic hypertension, gestational hypertension, preeclampsia/eclampsia, chronic hypertension, and superimposed preeclampsia/eclampsia were associated with increased risk for ischemic heart disease.
Table 2.
Cardiovascular, Cerebrovascular, and Chronic Kidney Disease and Diabetes Mellitus Risks of Women of Northern Finland Birth Cohort 1966 During ≈40 Years of Follow-up
| Outcome | Normotensive (N=6552) |
Isolated Systolic Hypertension (N=866) |
Isolated Diastolic Hypertension (N=742) |
Isolated Systolic or Diastolic Hypertension With Proteinuria (N=137) |
Gestational Hypertension (N=991) |
Preeclampsia/Eclampsia (N=242) |
Chronic Hypertension (N=668) |
Superimposed Preeclampsia/Eclampsia (N=116) |
|---|---|---|---|---|---|---|---|---|
| Cardiovascular disease | ||||||||
| No. of cases (%) | 1633 (24.9) | 284 (32.8) | 219 (29.6) | 34 (24.8) | 357 (36.1) | 77 (31.8) | 337 (50.4) | 62 (54.4) |
| Mean age at first event (SD), y | 59.0 (11.0) | 58.7 (12.0) | 58.2 (10.3) | 57.7 (10.3) | 58.1 (11.6) | 58.3 (11.7) | 59.1 (12.0) | 59.4 (11.3) |
| HR (95% CI) | 1.00 | 1.14 (1.00–1.30) | 1.18 (1.02–1.36) | 0.96 (0.67–1.37) | 1.45 (1.29–1.63) | 1.40 (1.11–1.76) | 1.66 (1.46–1.88) | 2.06 (1.61–2.65) |
| Ischemic heart disease | ||||||||
| No. of cases (%) | 1000 (15.3) | 173 (20.0) | 145 (19.5) | 23 (16.8) | 225 (22.7) | 46 (19.0) | 223 (33.4) | 40 (34.5) |
| Mean age at first event (SD), y | 60.5 (9.9) | 60.7 (11.2) | 60.4 (10.1) | 59.9 (9.4) | 61.1 (9.5) | 61.5 (10.8) | 63.0 (10.2)† | 61.8 (10.6) |
| HR (95% CI) | 1.00 | 1.12 (0.95–1.32) | 1.26 (1.05–1.50) | 1.13 (0.74–1.72) | 1.44 (1.24–1.68) | 1.36 (1.01–1.83) | 1.63 (1.40–1.90) | 1.86 (1.35–2.56) |
| Myocardial infarcts | ||||||||
| No. of cases (%) | 366 (5.6) | 74 (8.5) | 54 (7.3) | 17 (12.4) | 105 (10.6) | 16 (6.6) | 118 (17.7) | 28 (24.1) |
| Mean age at first event (SD), y | 61.5 (10.8) | 62.7 (11.4) | 59.9 (10.4) | 59.9 (8.3) | 62.4 (10.3) | 62.1 (11.9) | 64.7 (10.0)† | 65.2 (10.8) |
| HR (95% CI) | 1.00 | 1.19 (0.92–1.55) | 1.26 (0.94–1.69) | 2.26 (1.36–3.76) | 1.75 (1.40–2.19) | 1.22 (0.73–2.05) | 2.09 (1.67–2.61) | 3.18 (2.18–4.64) |
| Died of myocardial infarcts | ||||||||
| No. of cases (%) | 67 (1.0) | 29 (3.3) | 7 (0.9) | 7 (5.1) | 38 (3.8) | 4 (1.7) | 42 (6.3) | 12 (10.3) |
| Mean age at first event (SD), y | 66.3 (9.8) | 66.9 (10.2) | 66.8 (4.2) | 64.0 (6.5) | 66.5 (10.4) | 61.0 (6.6) | 68.3 (9.7) | 69.9 (7.0) |
| HR (95% CI) | 1.00 | 2.15 (1.35–3.41) | 0.79 (0.35–1.77) | 4.83 (2.13–10.94) | 3.00 (1.98–4.08) | 1.44 (0.51–4.08) | 3.11 (2.05–4.74) | 5.12 (2.77–9.46) |
| Heart failure | ||||||||
| No. of cases (%) | 371 (5.7) | 95 (11.0) | 53 (7.2) | 12 (8.8) | 115 (11.6) | 23 (9.5) | 132 (19.8) | 30 (26.3) |
| Mean age at first event (SD), y | 59.0 (12.9) | 59.3 (14.6) | 60.7 (11.7) | 60.4 (12.3) | 57.6 (13.0) | 59.9 (13.0) | 58.9 (13.7) | 58.8 (13.0) |
| HR (95% CI) | 1.00 | 1.43 (1.13–1.82) | 1.16 (0.87–1.55) | 1.43 (0.79–2.58) | 1.79 (1.43–2.21) | 1.69 (1.12–2.56) | 2.04 (1.64–2.55) | 3.32 (2.30–4.80) |
| Ischemic cerebrovascular disease | ||||||||
| No. of cases (%) | 300 (4.6) | 62 (7.2) | 33 (4.4) | 4 (2.9) | 84 (8.5) | 14 (5.8) | 86 (12.9) | 12 (10.3) |
| Mean age at first event (SD), y | 65.7 (10.3) | 66.5 (11.1) | 63.1 (11.1) | 60.0 (3.1) | 67.3 (9.1) | 61.3 (10.1) | 66.1 (10.0) | 65.0 (9.3) |
| HR (95% CI) | 1.00 | 1.24 (0.94–1.64) | 0.92 (0.64–1.33) | 0.61 (0.22–1.72) | 1.59 (1.24–2.04) | 1.19 (0.68–2.09) | 1.80 (1.39–2.34) | 1.51 (0.83–2.76) |
| Chronic kidney disease | ||||||||
| No. of cases (%) | 73 (1.1) | 15 (1.7) | 14 (1.9) | 4 (2.9) | 22 (2.2) | 2 (0.8) | 12 (1.8) | 2 (1.7) |
| Mean age at first event (SD), y | 62.2 (11.7) | 66.9 (12.5) | 66.5 (10.5) | 50.6 (13.1) | 65.8 (11.2) | 60.9 (16.2) | 64.0 (13.2) | 49.2 (22.8) |
| HR (95% CI) | 1.00 | 1.27 (0.72–2.25) | 1.77 (0.99–3.14) | 2.83 (1.02–7.90) | 1.91 (1.18–3.09) | 0.75 (0.17–3.38) | 1.23 (0.67–2.24) | 1.24 (0.28–5.44) |
| Arterial hypertension | ||||||||
| No. of cases (%) | 1374 (21.0) | 327 (37.7) | 243 (32.7) | 46 (33.6) | 423 (42.7) | 94 (38.8) | 415 (62.1) | 72 (62.1) |
| Mean age at first event (SD), y | 55.0 (10.8) | 52.7 (11.3)‡ | 51.2 (10.9)‡ | 51.3 (11.4)* | 49.8 (9.9)‡ | 51.0 (11.3)† | Not estimated | Not estimated |
| HR (95% CI) | 1.00 | 1.89 (1.67–2.13) | 1.64 (1.42–1.88) | 1.77 (1.31–2.39) | 2.53 (2.25–2.84) | 2.33 (1.88–2.88) | Not estimated | Not estimated |
| Diabetes mellitus | ||||||||
| No. of cases (%) | 388 (5.9) | 95 (11.0) | 55 (7.4) | 11 (8.0) | 104 (10.5) | 22 (9.2) | 113 (17.0) | 24 (21.1) |
| Mean age at first event (SD), y | 60.4 (10.7) | 60.5 (10.7) | 57.2 (12.4)* | 50.8 (11.5)† | 59.9 (11.7) | 59.5 (11.3) | 59.8 (10.1) | 56.6 (11.0) |
| HR (95% CI)§ | 1.00 | 1.42 (1.13–1.78) | 1.10 (0.82–1.47) | 1.26 (0.69–2.33) | 1.52 (1.21–1.89) | 1.42 (0.92–2.19) | 1.65 (1.31–2.07) | 2.22 (1.43–3.45) |
BMI indicates body mass index; CI, confidence interval; and HR, hazard ratio. Difference in age at first event was compared among women with observed outcomes during follow-up with the Student t test; censored women did not contribute to these analyses. Hypertensive groups were individually compared with the normotensive group. HRs were calculated using Cox regression, and the model was adjusted with prepregnancy BMI, smoking before pregnancy, parity, and diabetes mellitus before/during pregnancy and socioeconomic status. Age was used as time scale.
P<0.05.
P<0.01.
P<0.0001.
Model for diabetes mellitus was adjusted with prepregnancy BMI, smoking before pregnancy, parity, and socioeconomic status. Age was used as time scale. Subjects with diabetes mellitus before and within 1 year of pregnancy were excluded.
Figure 1.
The 1-survival curves for total cardiovascular disease, myocardial infarcts, and myocardial infarct deaths among women with hypertension during pregnancy.
MI and Heart Failure
Women with chronic hypertension and superimposed preeclampsia/eclampsia experienced high risks for MI, MI death, and heart failure. Similarly high risk for MI and MI death was observed among women with isolated hypertension and proteinuria. Gestational hypertension, preeclampsia/eclampsia, and isolated systolic hypertension were associated with increased risk for heart failure, gestational hypertension with increased risk for MI and MI death, and isolated systolic hypertension with increased risk for MI death (Table 2 and Figure).
Ischemic Cerebrovascular Disease
Women with chronic hypertension and gestational hypertension had similar risks for ischemic cerebrovascular disease with hazard ratios of 1.8 and 1.6, respectively (Table 2).
Chronic Kidney Disease
Gestational hypertension and isolated hypertension with proteinuria were associated with higher risks for chronic kidney disease compared with normotensive mothers (Table 2).
Arterial Hypertension and Diabetes Mellitus
All of the women with transient hypertension during pregnancy had higher risks of subsequent arterial hypertension compared with normotensive women and were also significantly younger at arterial hypertension diagnosis than normotensive women. Women with isolated systolic hypertension, gestational hypertension, chronic hypertension, and superimposed preeclampsia/eclampsia had increased risks of subsequent diabetes mellitus compared with normotensive women (Table 2).
Sensitivity Analyses
We examined long-term risks restricting the analyses to women without known risk factors for cardiovascular diseases, such as smoking, overweight/obesity, advanced age, and diabetes mellitus (Table 3). Elevated risks remained significant for all of the outcomes associated with gestational hypertension. Arterial hypertension risk remained significant among those with isolated hypertensions, gestational hypertension, and preeclampsia/eclampsia in this low-risk group. Analyses restricted to nulliparas and those restricted to women with hypertension diagnoses based solely on original cohort data yielded generally similar findings to those reported, although with a loss of statistical power and precision because of the smaller sample size (data not shown).
Table 3.
Cardiovascular, Cerebrovascular, and Kidney Disease and Diabetes Mellitus Risks of Women Without Traditional Cardiovascular Risk Factors in Northern Finland Birth Cohort 1966.
| Outcome | Normotensive (N=4283) |
Isolated Systolic Hypertension (N=511) |
Isolated Diastolic Hypertension (N=523) |
Isolated Systolic or Diastolic Hypertension With Proteinuria (N=90) |
Gestational Hypertension (N=620) |
Preeclampsia/ Eclampsia (N=162) |
|---|---|---|---|---|---|---|
| Cardiovascular disease | ||||||
| No. of cases (%) | 893 (20.8) | 125 (24.5) | 142 (27.2) | 19 (21.1) | 182 (29.4) | 39 (24.1) |
| Mean age at first event (SD), y | 57.2 (10.0) | 55.0 (11.1)* | 56.3 (9.7) | 57.4 (9.9) | 55.5 (10.5)* | 57.1 (10.7) |
| HR (95% CI) | 1.00 | 1.14 (0.94–1.38) | 1.36 (1.13–1.62) | 1.02 (0.64–1.60) | 1.59 (1.35–1.88) | 1.39 (1.01–1.92) |
| Ischemic heart disease | ||||||
| No. of cases (%) | 535 (12.5) | 79 (15.5) | 99 (18.9) | 11 (12.2) | 116 (18.8) | 21 (13.0) |
| Mean age at first event (SD), y | 58.3 (8.7) | 57.0 (9.3) | 59.0 (9.9) | 59.9 (10.6) | 58.8 (7.8) | 60.9 (9.9) |
| HR (95% CI) | 1.00 | 1.18 (0.93–1.51) | 1.56 (1.26–1.93) | 1.03 (0.57–1.84) | 1.67 (1.36–2.04) | 1.27 (0.82–1.95) |
| Myocardial infarcts | ||||||
| No. of cases (%) | 165 (3.9) | 30 (5.9) | 30 (5.7) | 8 (8.9) | 40 (6.5) | 7 (4.3) |
| Mean age at first event (SD), y | 58.3 (9.4) | 60.6 (10.5) | 56.7 (10.8) | 60.6 (9.6) | 59.6 (8.9) | 60.2 (11.2) |
| HR (95% CI) | 1.00 | 1.41 (0.95–2.08) | 1.49 (1.00–2.21) | 2.53 (1.26–5.07) | 1.85 (1.30–2.62) | 1.46 (0.69–3.09) |
| Died of myocardial infarcts | ||||||
| No. of cases (%) | 17 (0.4) | 9 (1.8) | 5 (1.0) | 2 (2.2) | 10 (1.6) | 1 (0.6) |
| Mean age at first event (SD), y | 58.9 (7.9) | 65.6 (4.8)* | 65.8 (4.1) | 59.9 (5.0) | 61.6 (7.6) | 65.0 (NA) |
| Adjusted HR (95% CI) | 1.00 | 4.04 (1.78–9.22) | 2.34 (0.85–6.46) | 6.46 (1.45–28.8) | 4.49 (2.05–9.86) | 2.06 (0.29–14.9) |
| Heart failure | ||||||
| No. of cases (%) | 148 (3.5) | 30 (5.9) | 22 (4.2) | 5 (5.6) | 48 (7.7) | 7 (4.3) |
| Mean age at first event (SD), y | 56.4 (12.1) | 51.5 (13.8)* | 57.1 (11.5) | 58.0 (9.9) | 55.1 (12.4) | 53.3 (12.5) |
| HR (95% CI) | 1.00 | 1.62 (1.08–2.42) | 1.24 (0.79–1.96) | 1.71 (0.71–4.13) | 2.47 (1.77–3.45) | 1.60 (0.73–3.49) |
| Ischemic cerebrovascular disease | ||||||
| No. of cases (%) | 144 (3.4) | 23 (4.5) | 15 (2.9) | 1 (1.1) | 33 (5.3) | 7 (4.3) |
| Mean age at first event (SD), y | 62.7 (9.2) | 60.0 (11.7) | 56.3 (10.1)* | 61.2 (NA) | 62.5 (7.4) | 56.2 (7.1) |
| HR (95% CI) | 1.00 | 1.24 (0.79–1.95) | 0.80 (0.47–1.38) | 0.34 (0.05–2.48) | 1.67 (1.13–2.45) | 1.40 (0.64–3.09) |
| Chronic kidney disease | ||||||
| No. of cases (%) | 29 (0.7) | 4 (0.8) | 6 (1.1) | 1 (1.1) | 12 (1.9) | 1 (0.6) |
| Mean age at first event (SD), y | 60.0 (10.1) | 68.0 (8.5) | 66.3 (11.3) | 63.0 (NA) | 61.0 (10.7) | 49.4 (NA) |
| HR (95% CI) | 1.00 | 1.01 (0.35–2.97) | 1.66 (0.69–3.99) | 1.76 (0.24–13.0) | 2.97 (1.50–5.87) | 1.06 (0.14–8.30) |
| Arterial hypertension | ||||||
| No. of cases (%) | 862 (20.1) | 169 (33.1) | 163 (31.2) | 32 (35.6) | 254 (41.0) | 58 (35.8) |
| Mean age at first event (SD), y | 53.2 (9.5) | 50.2 (10.5)† | 49.9 (9.7)† | 49.8 (10.2)* | 47.9 (9.4)† | 50.3 (10.9)* |
| HR (95% CI) | 1.00 | 1.78 (1.51–2.11) | 1.70 (1.44–2.02) | 2.08 (1.46–2.97) | 2.70 (2.34–3.13) | 2.33 (1.78–3.05) |
| Diabetes mellitus | ||||||
| No. of cases (%) | 206 (4.8) | 42 (8.2) | 23 (4.4) | 5 (5.6) | 50 (8.1) | 9 (5.6) |
| Mean age at first event (SD), y | 58.3 (9.8) | 56.0 (8.9) | 53.0 (11.4)* | 45.3 (9.7)* | 53.7 (10.1)* | 56.3 (9.9) |
| HR (95% CI) | 1.00 | 1.68 (1.20–2.35) | 0.90 (0.58–1.39) | 1.24 (0.50–3.07) | 1.83 (1.33–2.52) | 1.37 (0.70–2.69) |
BMI indicates body mass index; CI, confidence interval; HR, hazard ratio; and NA, not applicable. Traditional cardiovascular risk factors included smoking, age ≥35 y, overweight/obesity (prepregnancy BMI ≥25 kg/m2), and diabetes mellitus before/during pregnancy. Only nonsmoking, nondiabetic women with BMI between 18.5 and 24.9 kg/m2 and age <35 y are included in the analyses. Difference in age at first event was compared among women with observed outcomes during follow-up with Student t test; censored women did not contribute to these analyses. Hypertensive groups were individually compared with the normotensive group. HRs were calculated using Cox regression, and the model was adjusted for parity and socioeconomic status. Age was used as a time scale.
P<0.05.
P<0.0001.
Discussion
New-onset hypertension is common during pregnancy, and its possible consequences on the future health of the mother must be carefully evaluated. This large, population-based prospective cohort with a long-term follow-up shows that new-onset gestational hypertension is a more important risk factor for ischemic cardiovascular and cerebrovascular diseases than reported previously and shows for the first time that it was associated with increased risk for kidney disease. Even in the absence of risk factors such as smoking, over-weight/obesity, and advanced age, women with gestational hypertension have high risk for future disease, particularly for fatal MIs, compared with normotensive women. We present novel findings that new-onset isolated systolic or diastolic hypertension was associated with significantly increased cardiovascular and kidney disease risk later in life. We also observed the anticipated results that women with chronic hypertension with and without superimposed preeclampsia/eclampsia during pregnancy have high risk for later cardiovascular diseases.
Our findings regarding new-onset gestational hypertension or preeclampsia/eclampsia are generally consistent with previous studies, with increasing risk for arterial hypertension,26,28,30 all-cause cardiovascular morbidity and mortality,13,21,22,26,27 ischemic heart disease,21,26 heart failure,25,26 and diabetes mellitus,26 although we generally find higher disease risks among women with gestational hypertension than among those with preeclampsia, in contrast to other studies.22,24 In addition to lower statistical power for preeclampsia analysis, we also recognize potential differences attributed to the underlying pathophysiology, with preeclampsia more likely to have an immunologic or placental origin14–16 whereas women with gestational hypertension have poorer metabolic health.8–12 Many reproductive-aged women already have cardiovascular risk factors,6 and pregnancy as a stressor may reveal their vulnerability as new-onset or worsening hypertension occurs during pregnancy. This might suggest that their poor metabolic health,8–12 not hypertension, leads to increased chronic disease risk. However, we also found increased risks among women with elevated blood pressure but no known risk factors in the sensitivity analyses, suggesting that hypertension during pregnancy has an independent effect on long-term risk. As the proportion of pregnancies complicated with hypertension increases,3 these long-term health risks merit attention in the obstetrician’s office, as well as in primary and specialty care as women age.
Our study is the first to show that de novo gestational hypertension is associated with increased risk for ischemic cerebrovascular disease and for kidney disease. Previous studies have examined total cerebrovascular disease, which can include cerebral hemorrhages and other events resulting from trauma that are unrelated to hypertension.28 Others have reported kidney disease after preeclampsia,19 whereas ours is the first to show increased risk associated with gestational hypertension, contradicting results of one previous study.28
We recognize that our study may have some misclassification, because current diagnostic criteria for gestational hypertension and preeclampsia suggest that elevations in either systolic or diastolic blood pressure after midgestation are diagnostic for the disorders.32 Therefore, some of our cases of isolated hypertension might be classifiable as having preeclampsia or gestational hypertension. This difference in classification might explain why other studies have shown stronger association with cardiovascular diseases and preeclampsia than seen in our study. Also, our cases of gestational hypertension probably represent more severe cases, which might explain the stronger association with subsequent diseases than reported in previous studies.
Our study is the first one to report the association with new-onset isolated systolic or diastolic hypertension during pregnancy and subsequent cardiovascular and kidney diseases and diabetes mellitus. As isolated elevations in either systolic and diastolic blood pressure in adulthood increase the risk of cardiovascular events,39 we wanted to specifically study the effects of isolated hypertension during pregnancy. We also had a subgroup of women with isolated systolic or diastolic blood pressure in early pregnancy only, and this group would not fit any current diagnostic criteria.
We observed new-onset isolated elevation in systolic or diastolic blood pressure during pregnancy in ≈17% of all women in our cohort, and ≈30% of them had a cardiovascular event before their late 60s and 3% died of MIs. If we were to extrapolate our results to the population of United States with ≈4 million births each year,4 >200 000 women per year could have isolated hypertension during pregnancy, indicating a substantially increased risk for later cardiovascular events that currently is not well recognized. Our results indicate that any history of isolated hypertension during pregnancy merits comprehensive cardiovascular risk assessment during later life.
The strength of this study is the prospectively collected blood pressure and proteinuria data, which allowed us to reliably categorize mothers as hypertensive or normotensive during their index pregnancy and separate cases with chronic hypertension. To our knowledge this is the first study with prospective clinical blood pressure data during pregnancy and a follow-up of ≈40 years. Previous studies have lacked clinical data,21,22,24,26,27 with the potential of misclassification of hypertensive disorders of pregnancy, difficulty in separating cases of chronic hypertension from de novo hypertension, and no data on isolated systolic or diastolic hypertension. All of the clinical measurements in our study were obtained following standardized instructions, and repeated longitudinal data collection should limit the error in our diagnoses. Any potential misclassification of pregnancy hypertension would also be nondifferential with respect to subsequent registry assessment of chronic disease. Most previous studies exclude women with preexisting hypertension,21,23,24,28 but our rich data allow us to observe meaningful differences in later life risk for MI, MI death, and heart failure between women with superimposed preeclampsia/eclampsia compared with women with chronic hypertension in pregnancy. Linkage with comprehensive Finnish registers allowed us to evaluate several chronic disease outcomes.
Although the prospective clinical data are rich, we do not have the ability to evaluate changes in cardiovascular risk factors during follow-up. Finns generally have a higher cardiovascular disease morbidity and mortality rate than populations of Southern European countries but a comparable rate with the United States.40 However, it is noteworthy that, during the follow-up period of this study, several population-wide interventions for cardiovascular risk factors have been done in Finland, and the coronary disease mortality has decreased 80% during that time.41 We were also able to adjust for prepregnancy body mass index and smoking, which is a significant improvement over previous analyses. We do not know how intensely hypertensive mothers were treated during and after pregnancy, and those with the most severe cases of hypertensive disorders during pregnancy may have received more extensive treatment, which impacted future disease risk. Given that those women still experienced high risk for chronic disease, if they received intensive treatment, our estimates of risk would be conservative. The prevalence of arterial hypertension and diabetes mellitus is likely underestimated, because these conditions can be treated without medication or hospitalization, and some will not show up in the registers.
Conclusions and Recommendations
Elevated blood pressure during pregnancy signaled higher risk of cardiovascular, cerebrovascular, and kidney disorders, as well as diabetes mellitus, later in life. Significantly increased risks were seen for women with new-onset isolated systolic or diastolic hypertension and among women with de novo gestational hypertension even without other known risk factors. Current obstetric practice does not separate isolated hypertension with or without proteinuria from other hypertensive disorders of pregnancy or fails to diagnose them completely. We find the long-term risks of these isolated blood pressure elevations are striking. Primary and cardiac care for women should include an assessment of any history of elevated blood pressure during pregnancy, and additional monitoring and cardiovascular risk factor evaluation may be warranted in women with such history to prevent poor outcomes in later life.
Supplementary Material
Clinical PERSPECTIVE.
Hypertension during pregnancy affects ≥10% of all women, and the incidence of hypertensive disorders in pregnancy is increasing. The current study followed women with diagnosed hypertensive disorders during pregnancy for ≈40 years. We observed that ≤33% of all pregnant women had some form of hypertension, with 6.5% having chronic hypertension before or during pregnancy. Any elevation in blood pressure during pregnancy, even isolated systolic or diastolic blood pressure elevations that resolved during or after pregnancy, was associated with increased risk of subsequent cardiovascular diseases. Highest risks were associated with chronic hypertension (63% increase) and superimposed preeclampsia/eclampsia (98% increase) compared with normotensive women. Especially high risks were observed for fatal myocardial infarcts, with risks 100% to 400% higher in hypertensive women compared with normotensive women. We also observed that gestational hypertension was associated with 49% higher risk of subsequent diabetes mellitus and 90% higher risk of chronic kidney disease. All of the women who had transient hypertension during pregnancy were at higher risk (64% to 153%) of developing chronic hypertension. History of blood pressure elevations during pregnancy could be used to identify at-risk individuals for cardiovascular events. Detection of hypertension during pregnancy warrants further cardiovascular disease risk factor screening and at least counseling on lifestyle factors to reduce disease risk.
Acknowledgment
We thank the staff working with the Northern Finland Birth Cohort 1966 and the cohort subjects for making this study possible.
Sources of Funding
This research was supported in part by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and by the Academy of Finland.
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.112.128751/-/DC1.
Disclosure
None.
References
- 1.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Regitz-Zagrosek V, Blomstrom LC, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L, Bax J, Auricchio A, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Knuuti J, Kolh P, McDonagh T, Moulin C, Poldermans D, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Torbicki A, Vahanian A, Windecker S, Baumgartner H, Deaton C, Aguiar C, Al-Attar N, Garcia AA, Antoniou A, Coman I, Elkayam U, Gomez-Sanchez MA, Gotcheva N, Hilfiker-Kleiner D, Kiss RG, Kitsiou A, Konings KT, Lip GY, Manolis A, Mebaaza A, Mintale I, Morice MC, Mulder BJ, Pasquet A, Price S, Priori SG, Salvador MJ, Shotan A, Silversides CK, Skouby SO, Stein JI, Tornos P, Vejlstrup N, Walker F, Warnes C. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 3.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21:521–526. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton BE, Miniño AM, Martin JA, Kochanek KD, Strobino DM, Guyer B. Annual summary of vital statistics: 2005. Pediatrics. 2007;119:345–360. doi: 10.1542/peds.2006-3226. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Daviglus ML, Stamler J, Pirzada A, Yan LL, Garside DB, Liu K, Wang R, Dyer AR, Lloyd-Jones DM, Greenland P. Favorable cardiovascular risk profile in young women and long-term risk of cardiovascular and all-cause mortality. JAMA. 2004;292:1588–1592. doi: 10.1001/jama.292.13.1588. [DOI] [PubMed] [Google Scholar]
- 7.Pencina MJ, D’Agostino RB, Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation. 2009;119:3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrenthal DB, Jurkovitz C, Hoffman M, Jiang X, Weintraub WS. Prepregnancy body mass index as an independent risk factor for pregnancyinduced hypertension. J Womens Health (Larchmt) 2011;20:67–72. doi: 10.1089/jwh.2010.1970. [DOI] [PubMed] [Google Scholar]
- 9.Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335:978. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation. 2010;122:579–584. doi: 10.1161/CIRCULATIONAHA.110.943407. [DOI] [PubMed] [Google Scholar]
- 11.Seely EW, Solomon CG. Insulin resistance and its potential role in pregnancy-induced hypertension. J Clin Endocrinol Metab. 2003;88:2393–2398. doi: 10.1210/jc.2003-030241. [DOI] [PubMed] [Google Scholar]
- 12.Pouta A, Hartikainen AL, Sovio U, Gissler M, Laitinen J, McCarthy MI, Ruokonen A, Elliott P, Järvelin MR. Manifestations of metabolic syndrome after hypertensive pregnancy. Hypertension. 2004;43:825–831. doi: 10.1161/01.HYP.0000120122.39231.88. [DOI] [PubMed] [Google Scholar]
- 13.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, Lawlor DA. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125:1367–1380. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 15.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA. CPEP Study Group. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 16.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285:1607–1612. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 18.Williams D. Long-term complications of preeclampsia. Semin Nephrol. 2011;31:111–122. doi: 10.1016/j.semnephrol.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359:800–809. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 20.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kestenbaum B, Seliger SL, Easterling TR, Gillen DL, Critchlow CW, Stehman-Breen CO, Schwartz SM. Cardiovascular and thromboembolic events following hypertensive pregnancy. Am J Kidney Dis. 2003;42:982–989. doi: 10.1016/j.ajkd.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366:1797–1803. doi: 10.1016/S0140-6736(05)67726-4. [DOI] [PubMed] [Google Scholar]
- 23.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357:2002–2006. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 24.Wikström AK, Haglund B, Olovsson M, Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG. 2005;112:1486–1491. doi: 10.1111/j.1471-0528.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 25.Ray JG, Schull MJ, Kingdom JC, Vermeulen MJ. Heart failure and dysrhythmias after maternal placental syndromes: HAD MPS Study. Heart. 2012;98:1136–1141. doi: 10.1136/heartjnl-2011-301548. [DOI] [PubMed] [Google Scholar]
- 26.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 27.Lykke JA, Langhoff-Roos J, Lockwood CJ, Triche EW, Paidas MJ. Mortality of mothers from cardiovascular and non-cardiovascular causes following pregnancy complications in first delivery. Paediatr Perinat Epidemiol. 2010;24:323–330. doi: 10.1111/j.1365-3016.2010.01120.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WC. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326:845. doi: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libby G, Murphy DJ, McEwan NF, Greene SA, Forsyth JS, Chien PW, Morris AD for the DARTS/MEMO Collaboration. Pre-eclampsia and the later development of type 2 diabetes in mothers and their children: an intergenerational study from the Walker cohort. Diabetologia. 2007;50:523–530. doi: 10.1007/s00125-006-0558-z. [DOI] [PubMed] [Google Scholar]
- 30.Marín R, Gorostidi M, Portal CG, Sánchez M, Sánchez E, Alvarez J. Long-term prognosis of hypertension in pregnancy. Hypertens Pregnancy. 2000;19:199–209. doi: 10.1081/prg-100100136. [DOI] [PubMed] [Google Scholar]
- 31.Rantakallio P. Groups at risk in low birth weight infants and perinatal mortality. Acta Paediatr Scand. 1969;193(Suppl 193) 1+. [PubMed] [Google Scholar]
- 32.Report of the National High Blood Pressure Education Program Working Group. High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 33.Kruuti J, Harsia-Alatalo J. National Agency of Medicines, Social Insurance Institute. Finnish Statistics on Medicines 2007. Edita Prima Oy: Helsinki, Finland; 2008. Medicine reimbursement system and approval of medicine prices; pp. 75–77. [Google Scholar]
- 34.Mähönen M, Jula A, Harald K, Antikainen R, Tuomilehto J, Zeller T, Blankenberg S, Salomaa V. The validity of heart failure diagnoses obtained from administrative registers. [Accessed January 16, 2013];Eur J Prev Cardiol. 2012 Feb 6; doi: 10.1177/2047487312438979. [DOI] [PubMed] [Google Scholar]
- 35.Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health. 2012;40:505–515. doi: 10.1177/1403494812456637. [DOI] [PubMed] [Google Scholar]
- 36.Pajunen P, Koukkunen H, Ketonen M, Jerkkola T, Immonen-Räihä P, Kärjä-Koskenkari P, Mähönen M, Niemelä M, Kuulasmaa K, Palomäki P, Mustonen J, Lehtonen A, Arstila M, Vuorenmaa T, Lehto S, Miettinen H, Torppa J, Tuomilehto J, Kesäniemi YA, Pyörälä K, Salomaa V. The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:132–137. doi: 10.1097/00149831-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Tolonen H, Salomaa V, Torppa J, Sivenius J, Immonen-Räihä P, Lehtonen A for the FINSTROKE register. The validation of the Finnish Hospital Discharge Register and Causes of Death Register data on stroke diagnoses. Eur J Cardiovasc Prev Rehabil. 2007;14:380–385. doi: 10.1097/01.hjr.0000239466.26132.f2. [DOI] [PubMed] [Google Scholar]
- 38.Lahti RA, Penttilä A. The validity of death certificates: routine validation of death certification and its effects on mortality statistics. Forensic Sci Int. 2001;115:15–32. doi: 10.1016/s0379-0738(00)00300-5. [DOI] [PubMed] [Google Scholar]
- 39.Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens. 2000;13(1 Pt 2):3S–10S. doi: 10.1016/s0895-7061(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 40.Menotti A, Puddu PE, Lanti M, Kromhout D, Blackburn H, Nissinen A. Twenty-five-year coronary mortality trends in the seven countries study using the accelerated failure time model. Eur J Epidemiol. 2003;18:113–122. doi: 10.1023/a:1023092415353. [DOI] [PubMed] [Google Scholar]
- 41.Vartiainen E, Laatikainen T, Peltonen M, Juolevi A, Männistö S, Sundvall J, Jousilahti P, Salomaa V, Valsta L, Puska P. Thirty-five-year trends in cardiovascular risk factors in Finland. Int J Epidemiol. 2010;39:504–518. doi: 10.1093/ije/dyp330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.