Abstract
In Drosophila, males absent on the first (MOF) acetylates histone H4 at lysine 16 (H4K16ac). This acetylation mark is highly enriched on the male X chromosome and is required for dosage compensation in Drosophila but not utilized for such in mammals. Recently, we and others reported that mammalian MOF, through H4K16ac, has a critical role at multiple stages in the DNA damage response (DDR) and double-strand break repair pathways. The goal of this study was to test whether mof is similarly required for the response to ionizing radiation (IR) in Drosophila. We report that Drosophila mof mutations in males and females, as well as mof knockdown in SL-2 cells, reduce post-irradiation survival. MOF depletion in SL-2 cells also results in an elevated frequency of metaphases with chromosomal aberrations, suggesting that MOF is involved in DDR. Mutation in Drosophila mof also results in a defective mitotic checkpoint, enhanced apoptosis, and a defective p53 response post-irradiation. In addition, IR exposure enhanced H4K16ac levels in Drosophila as it also does in mammals. These results are the first to demonstrate a requirement for MOF in the whole animal IR response and suggest that the role of MOF in the response to IR is conserved between Drosophila and mammals.
Introduction
Males absent on the first (MOF), a member of the MYST family of histone acetyl transferases, is a core component of the dosage compensation mechanism that regulates hyper-activation of X-linked genes by increasing H4K16ac levels on Drosophila male X chromosome (Neal et al. 2000). MOF was identified in a screen for ethyl methane sulfonate-induced male-specific lethal mutations (Hilfiker et al. 1997) and was shown to directly acetylate histone H4 at lysine 16. Males carrying a loss-of-function mof mutation do not survive because they lack the X-chromosome enrichment for H4K16ac; this epigenetic mark is thought to contribute to dosage compensation by increasing the transcription of the X-chromosome in males (Akhtar and Becker 2000; Smith et al. 2000). The MOF H4K16 acetylation function is conserved in mammalian cells (Gupta et al. 2005, 2008; Neal et al. 2000; Smith et al. 2005; Taipale et al. 2005), and mammalian MOF has high sequence similarity to the Drosophila MOF protein. The dosage compensation role of MOF in Drosophila is not observed in mammals, but recent studies have found that in mammals H4K16 acetylation is also an epigenetic signature of cellular proliferation during both embryogenesis and oncogenesis (Gupta et al. 2008). In addition, mammalian MOF has a critical role at multiple points in the cellular DDR and double-strand break (DSB) repair pathways (Sharma et al. 2010). Whether MOF has an analogous role in the IR response required for cell survival and chromosome damage repair in Drosophila is unknown. Overall, DDR is conserved among mammals and Drosophila: Signaling pathways elicited by Drosophila DDR are observed as the intra-S-phase checkpoint, G2/M arrest, metaphase arrest, centrosome inactivation, and apoptosis that occur in different developmental contexts (Brodsky et al. 2004; Jaklevic and Su 2004). Mutations in Drosophila mei-41 (Drosophila ATR orthologue), grp (grapes: Drosophila Chk1 orthologue), mus304 (Drosophila ATRIP orthologue), mei-41, mre11, rad50, Chk2, p53, and 14-3-3 show defects in DNA damage checkpoint responses similar to those caused by the mammalian gene mutations (Brodsky et al. 2004; Jaklevic and Su 2004; Song et al. 2004). During Drosophila larval development, the central nervous system cells and imaginal discs undergo an archetypal mitotic cell cycle that has been exploited to study the DDR (Wolff and Ready 1991a, b).
Although a role for MOF in the IR response has been established in a variety of cell-based models, it is unknown whether MOF contributes to the IR response in whole animals. MOF knockout mice die during embryogenesis, so we turned to Drosophila to test whether MOF contributes to IR during development. mof mutant males can survive to the end of third instar, and mof is not required at all for female development. In this study, we exploit the genetic opportunities of Drosophila to show that post-irradiation DDR in Drosophila as in mammalian cells is MOF-dependent as determined by both in vitro and in vivo assays.
Experimental procedures
MOF knockdown by RNAi
SL-2 cells were cultured in Schnieder’s insect medium supplemented with 10% fetal bovine serum at 25°C. Depletion of MOF by RNAi was performed in SL-2 cells by incubation with 50 μg dsRNA by the described procedure (Worby et al. 2001). For all time points, GFP control RNAi experiments were performed in parallel.
Immunostaining of polytene chromosomes
Third instar larvae from wild-type Drosophila were irradiated at a dose rate of 10 Grays (Gy) per min. Salivary gland chromosomes were prepared, fixed, and processed for immunostaining by a previously described procedure (Pal-Bhadra et al. 2004). Chromosomes were immunostained with anti-MOF (1:200) and H4K16 acetylation antibodies. For confocal microscopy, Cy-5-conjugated goat anti-rabbit (1:200), and fluorescein isothiocyanate (FITC)-conjugated goat anti-rat (1:200) antibodies were used. After incubation, the chromosomes were thoroughly washed with buffer (400 mM NaCl, 0.2% Tween 20, 0.2% NP40 in phosphate-buffered saline (PBS) to remove background. Slides were incubated with RNase I (20 mg/ml) for 30 min to digest chromosomal RNA and mounted with 4′,6-diamidino-2-phenylindole (DAPI) or propidium iodide containing antifade Vectashield mounting media. Cells were examined with an Olympus FluoView 1000 confocal microscope using ×60 oil lens.
Acridine orange staining
Eye discs from third instar larvae were dissected in PBS and stained with freshly diluted acridine orange solution (1 mg/ml). The discs were then washed, mounted in PBS, and immediately viewed under a confocal microscope in the rhodamine red channel.
Imaginal discs staining
Eye discs were dissected from well-fed third instar larvae, fixed for 30 min (4% formaldehyde, 0.1 M piperazine-N, N′-bis (2-ethanesulfonic acid) (PIPES) (pH 6.9), 0.3% Triton X-100, 2 mM ethylene glycol tetraacetic acid, 1 mM MgSO4) and then washed in 0.3% Triton X-100 in PBS for 15 min. The discs were treated with RNase A, blocked in 2% animal serum for 1 h, and stained with anti-H3S10ph (1:30) antibody followed by FITC-conjugated rabbit secondary antibody (1:50). The discs were mounted in Vectashield mounting media (Vector Laboratories, USA) containing DAPI or propidium iodide.
Metaphase preparations
Cells with or without depletion of MOF were irradiated with gamma rays and then incubated for different time periods before adding colcemid. Cells were harvested 3 h after irradiation and metaphases made as described previously (Gupta et al. 2005; Pandita et al. 2006). For each experiment, 200 metaphases were analyzed for chromosomal aberrations.
To determine the segregation of chromatids of X chromosomes, cells were irradiated and fixed at the indicated time points. Immunostaining for MOF and H4K16ac using the specific antibodies was performed by the described procedure (Gupta et al. 2008; Hunt et al. 2007; Kumar et al. 2011).
Western analysis
Cell lysates were prepared according to the described procedure (Kind et al. 2008; Pandita et al. 2000). Immunoblots and detection of MOF, H4K16ac, p53, chk1, and mei-41 were done as described previously (Kind et al. 2008; Pandita et al. 2000; Sharma et al. 2003).
Generation of MOF mutant female Drosophila
All crosses were maintained at 25°C. y w mof3; [mof t6.8] 18H1, and y w mof6; [mof t6.8]18H1 females were crossed to FM7, y w B males. [mof t6.8]18H1 is a third chromosome transgene insert that expresses wild-type mof and rescues male lethality of mof mutations (Kageyama et al. 2001). From progeny, y w mof3; [mof t6.8]18H1/+ males were crossed to y w mof6/FM7, y w B; [mof t6.8] 18H1/+ females and y w mof6; [mof t6.8]18H1/+ males were crossed to y w mof3/FM7, y w B; [mof t6.8]18H1/+ females. Note that in this cross, Mendelian segregation would predict that 25% of the resulting B+ female progeny will lack a functional mof gene. Embryos from each of these crosses were collected over a 4 h interval on apple juice-agar plates, and then aged 24 h before irradiation. After irradiation with X-rays, the irradiated first instar larvae were transferred to standard cornmeal–agar–molasses food and allowed to develop to adulthood. Note that y w mof3/y w mof6;+/+ show markedly delayed development compared with their [mof t6.8]18H1-bearing sibs, so care was taken to allow all the adults time to eclose.
Histone isolation from larvae and Western blot analysis
Approximately 0.5 g of larvae were collected and ground in liquid nitrogen. The material was transferred to a Dounce homogenizer and homogenized (20 strokes on ice) thoroughly in 5 ml of lysis buffer (20 mM Hepes, 1 mM EDTA (ethylenediaminetetraacetic acid), 10 mM NaCl, 2 mM MgCl2, 20 mM sodium β-glycerophosphate, 2.5% NP-40 supplemented with protease inhibitors). The solution was passed through a 26 G syringe ten times. The nuclei were washed with 500 μl of lysis buffer, further dissolved in 0.4 N HCl/10% glycerol, and then incubated in a cold room for 30 min with moderate rotation. After centrifugation at 12,000 rpm for 10 min at 4°C, the supernatant was transferred to a new tube; 120 μl of trichloroacetic acid (TCA) was added and incubated for 1 h on ice to precipitate the histone proteins. After complete removal of supernatant, the pellet was air-dried and dissolved in 1× sample buffer. The nuclear extracts were divided into several aliquots and stored at −70°C. The protein was quantified, and Western blot analyses were carried out using acetylated anti-histone H3 lysine 16 antibody at 1:1,000 dilution (Millipore, USA).
Results and discussion
Chromatin, the physiological packaging structure of histones and DNA, is now gaining appreciation as a relevant target of signaling pathways (Fischle et al. 2003; Legube and Trouche 2003). Chromatin structure is an important factor for determining protein–DNA interactions, with consequences for DNA metabolism and transcription control (Otten and Tapscott 1995; Pruss et al. 1994; Wallrath et al. 1994). After DNA damage, eukaryotic cells orchestrate a complex array of responses (Hoeijmakers 2001). These responses occur within the context of chromatin, and its structure is altered post-DSB induction. Chromatin structure creates a natural barrier to damaged DNA sites, suggesting that histone modifications play an important role in the DDR by facilitating repair protein access to DNA breaks (Misri et al. 2008; Pandita 2001, 2003; Pandita and Richardson 2009; van Attikum and Gasser 2005, 2009). Recent studies have revealed that mammalian MOF, through acetylation at H4K16, has a critical role in the DDR (Li et al. 2010; Sharma et al. 2010). Mammalian cells depleted of MOF or expressing a catalytically dead mutant MOF protein show higher cell killing following exposure to IR (Gupta et al. 2005). We examined whether MOF has a similar protective role in the IR response of Drosophila in both cultured cells and whole animals. MOF was knocked down in Drosophila SL-2 cultured cells by specific RNAi (Fig. 1a) as described previously (Worby et al. 2001). Cell density was quantified for cells with and without MOF depletion at various times post-IR exposure (Fig. 1b). Consistent with mammalian cell data, MOF-depleted SL-2 cells had a reduced cell number post-irradiation, suggesting that MOF contributes to survival of irradiated cells (Gupta et al. 2005, 2008; Li et al. 2010).
Fig. 1.
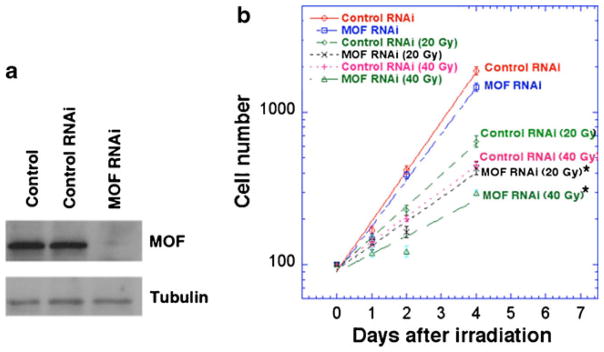
MOF depletion results in decreased cell numbers post-irradiation. a Western blot analysis of MOF levels in SL-2 cells 72 h after transfection with MOF–RNAi or control GFP-RNAi. b SL-2 cells with and without depletion of MOF were irradiated with 20 or 40 Gy and the cell numbers determined at the indicated time points, means of three independent experiments. MOF-depleted cells exposed to IR had significantly reduced cell number than control irradiated cells. (Differences from control are statistically significant according to Student’s t test, *P<0.05)
Since we found that MOF is important for survival after IR in SL-2, we tested the role of MOF in the IR response in whole animals by employing two approaches. In the first, we tested radiation resistance in mof mutant males, since SL-2 cells have a male genotype (X; autosome ratio of 0.5; Zhang et al. 2010). In male cells, MOF is found in two protein complexes, the male-specific lethal (MSL) complex where it is required in males for dosage compensation, and the non-specific lethal (NSL) complex, which is not essential under normal growth conditions in either sex. Drosophila eggs were collected overnight on apple juice-agar Petri plates from a y1 w67c23 mof4/FM7 stock. The collected embryos were irradiated at various doses while still on the plates. After irradiation, the embryos were allowed to hatch and develop at 25°C until third instar larvae were visible, but before any pupae had formed. Third instar larvae were collected and sexed based on gonad size. Male larvae were then scored as mof4 or mof+(FM7) based on mouth hook pigmentation; mof4 male larvae have yellow-brown mouth hooks, due to the linked y1 allele, while mof+ larvae have black mouth hooks due to the linked y31d allele. Multiple irradiations were conducted, together with controls, and survival numbers for each experiment were pooled for each genotype at a given treatment for several experiments (Fig. 2). Consistent with the SL-2 cell survival data, we found that Drosophila mof mutant embryos displayed significantly increased sensitivity to IR compared with their irradiated mof+ male sibs.
Fig. 2.
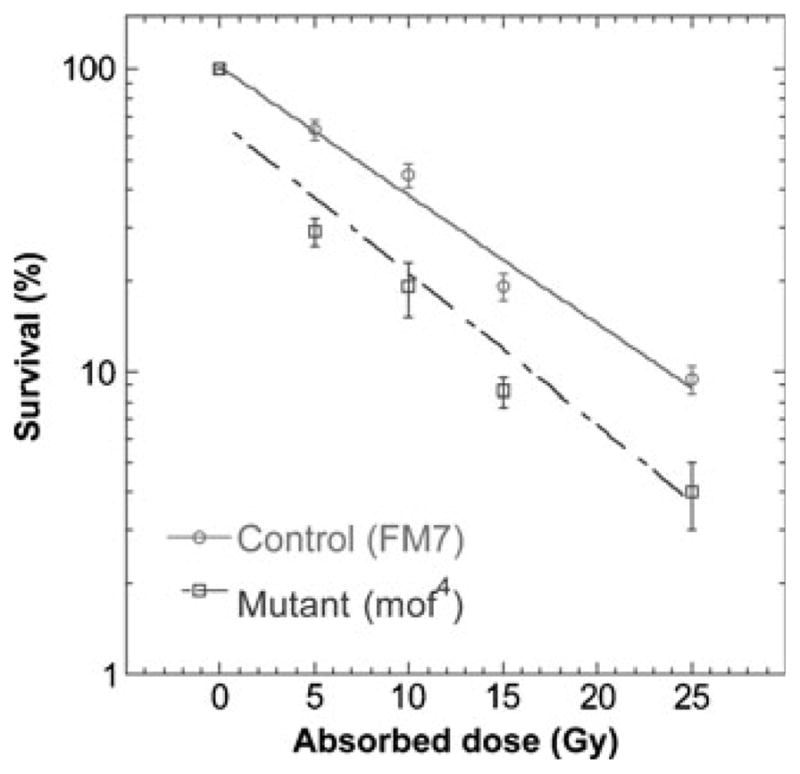
Comparison of wild-type and mof mutant male Drosophila survival post-irradiation. Male third instar larvae, irradiated as embryos, were scored as mof4 or mof+(FM7) at the third instar larval stage. Mean survival from three to four experiments is presented for each dose point
To test the role of MOF in DNA repair independent of its essential role in male dosage compensation, we examined X-ray resistance in developing female flies with and without MOF. In female flies, MOF is only present as a subunit of the NSL complex that is found throughout all the chromosomes (Raja et al. 2010). As mof mutations, we used the mof3 and mof6 alleles (J.C. Lucchesi, unpublished). Sequence analyses revealed that mof3 results from a nonsense mutation at amino acid 151 (Q151X) while mof6 results from a nonsense mutation at amino acid 28 (Q28X). MOF is an 827 amino acid protein, so both mutations produce severe truncations well before the catalytic domain and are thus both likely to be null alleles. By testing females that are heteroallelic for these alleles, we minimize the possibility that recessive mutations elsewhere on each X chromosome would contribute to a radiation-resistant phenotype. Sibling females of the same heteroallelic genotype but also carrying one or two doses of the wild-type mof transgene [mof t6.8]18H1 were used as controls.
We collected embryos over a 4 h time interval from crosses of females and males bearing a wild-type mof transgene (see methods for details on the crosses). These embryos were then aged for 24 h to deplete maternally loaded MOF prior to irradiation. The resulting first instar larvae were irradiated with 5, 10, or 15 Gy, and the numbers of adult B+ females were compared with siblings from the same crosses without irradiation (mof is essential in males, independent of irradiation). Note that, among the B+ females scored in this experiment, Mendelian segregation predicts that 25% of these females will lack a functional mof transgene. Non-irradiated control females ± mof survived in proportions not significantly different from those predicted by Mendelian segregation, demonstrating that there is no intrinsic semi-lethality associated with mof null females. For all genotypes, survival to adulthood fell off dramatically at 15 Gy, and no flies survived to adulthood after 25 Gy. Most of the lethality occurred during pupation. Preferential sensitivity to IR in mof3/mof6 females lacking the mof+ transgene was most dramatic at 15 Gy, the highest survivable dosage tested (Table 1). While overall viability is reduced at 15 Gy, as expected, there is clear and highly significant preferential sensitivity of mof− females relative to their irradiated mof+ female sibs. These experiments are the first to show a role for MOF in IR resistance in a whole animal. Additionally, they demonstrate that Drosophila MOF is required for the DDR to IR exposure in both male (in which MOF is present in both the MSL and NSL complexes) and female cells (in which MOF is in the NSL complex).
Table 1.
Preferential sensitivity to IR in mof3/mof6 females
| Dose (Gy) | mof3/mof6; 18H1a | mof3/mof6 | Significanceb |
|---|---|---|---|
| 0 | 366 | 97 | |
| 5 | 383 | 75 | No (P=0.0765) |
| 10 | 396 | 95 | No (P=0.572) |
| 15 | 50 | 0 | Yes (P<0.0001) |
Combination of flies with one and two doses of the 18H1 mof+ transgene
Compared with 0 Gy control, using Fishers Exact Test, two-tailed
Cell sensitivity to IR as demonstrated by enhanced cell killing has been linked to higher levels of chromosomal damage when analyzed either directly at G0/G1 or G2 phase by premature chromosome condensation technology (Pandita and Hittelman 1992a, b) or at metaphase (Pandita and Geard 2006). Mammalian MOF-deficient cells exhibit delayed appearance of γ-H2AX foci formation as well as defective responses for other DDR components post-irradiation, e.g., Rad51, MDC1, etc. (Li et al. 2010; Sharma et al. 2010; Taipale et al. 2005). In addition, immunofluorescent localization shows that the MOF protein paints mouse meiotic prophase 1 chromosomes, suggesting a role in recombinational repair: This result is consistent with the observation that MOF depletion, in an in vitro assay, leads to decreased repair of I-Sce-I-induced DNA DSB by homologous recombinational repair (Sharma et al. 2010). To test whether Drosophila MOF is specifically required for IR-induced chromosome damage repair, metaphases were analyzed for chromosome aberrations at different time points post-irradiation. Cell cycle phase-specific chromosome aberrations post-irradiation will reveal whether MOF is involved in repair of DNA DSB by non-homologous end-joining (NHEJ), predominantly a G0 and G1-phase event, or homologous recombination (HR) which is predominant in the S-phase and G2-phase of the cell cycle (Pandita and Richardson 2009; Scott and Pandita 2006). Although NHEJ and HR assays were not performed in SL-2 cells with and without MOF depletion, we examined chromosome aberrations at metaphase collected after different time points of post-irradiation in SL-2 cells with and without MOF depletion. G1-specific chromosome aberrations detected at metaphase are mostly of the chromosomal type (breaks + gaps) and include a high frequency of dicentrics (Gupta et al. 2005; Hunt et al. 2004; Pandita 2006; Pandita and Geard 1996). S-phase-type aberrations detected at metaphase are chromatid and chromosomal (breaks + gaps + exchanges) (Gupta et al. 2005), whereas G2-type chromosome aberrations detected at metaphase are predominantly of the chromatid type (breaks + gaps + exchanges; Gupta et al. 2005). To determine the influence of MOF depletion, cells were exposed to three different doses of ionizing radiation, and metaphases were collected at different time points post-irradiation. SL-2 cells with and without MOF depletion exposed to 3 Gy and metaphase cells collected 12 h post-irradiation had aberrations primarily of the chromosome type, which included dicentrics and chromosome fragments in addition to chromosome breaks and gaps. Cells deficient for MOF had a higher frequency of metaphases with aberrations in comparison to control cells (Fig. 3a). Cells exposed to 2.5 Gy and collected 5 h post-irradiation displayed a statistically significant increase in metaphases with chromosomal aberrations in MOF-deficient SL-2 cells (Fig. 3b), results that are consistent with those observed in mammalian cells deficient for MOF (Gupta et al. 2005). Furthermore, when cells were exposed to 2 Gy and metaphases aberrations were analyzed after 2 h post-irradiation (Fig. 3c), there was a higher frequency of aberrant metaphases in MOF-depleted cells as compared with control cells (Fig. 3c). Thus, MOF is required for repair of DNA damage post-irradiation in Drosophila, similar to the MOF requirement for DNA damage repair in mammalian cells (Gupta et al. 2005, 2008; Sharma et al. 2010).
Fig. 3.
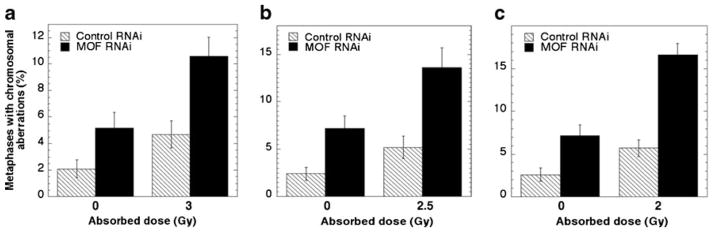
Frequency of metaphases with chromosome aberrations at different time points of post irradiation. SL-2 cells, with or without MOF knockdown, were irradiated with gamma rays and the metaphases were analyzed. a 12 h after exposure to 3 Gy, b 5 h after irradiation with 2.5 Gy, and c 2 h after irradiation with 2 Gy. For each experiment, 200 metaphases were analyzed for chromosome aberrations and each experiment was repeated at least three times. Mean of three independent experiments is presented
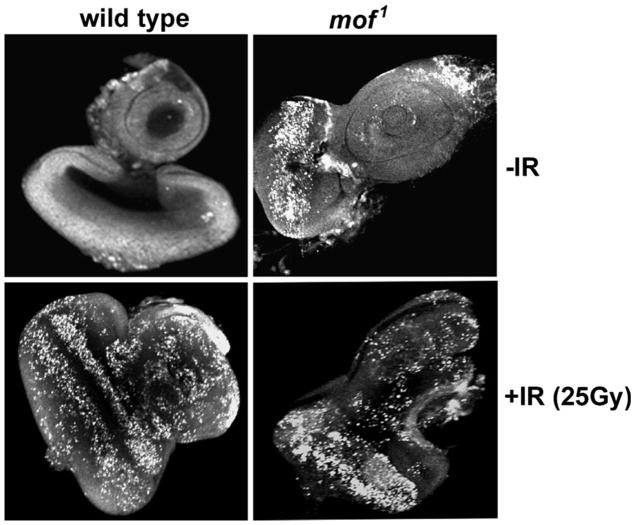
To test whether MOF is required for IR-induced apoptosis, the eye imaginal discs of third instar larvae from wild-type and mof mutants were exposed to 25 Gy, fixed 4 h post-irradiation, and stained with acridine orange to identify the apoptotic cells (Fig. 4). Larval discs without exposure to IR had very few cells stained with acridine orange while mof1 mutants showed a substantial basal level of apoptotic cells (Fig. 4). After irradiation, wild-type eye discs demonstrated a large increase in apoptotic cells while mof1 mutant eye discs had an obvious but small increase, the latter due to the high basal level of apoptotic cells.
Fig. 4.
Excessive spontaneous apoptosis is associated with mof mutation. Apoptotic cells in wild-type and mof1 mutant male larval eye imaginal discs 4 h after irradiation with 25 Gy were detected by staining with acridine orange, which stains solely the apoptotic cells
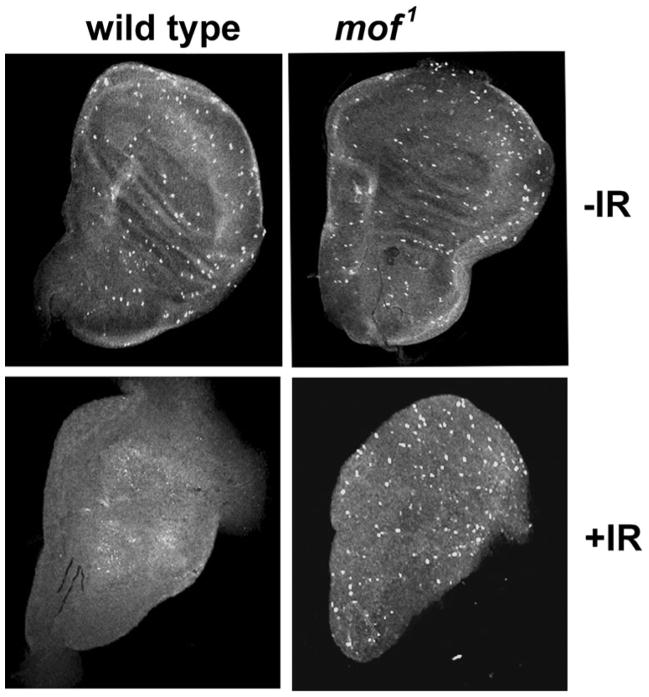
Mammalian cells deficient in MOF have a defective checkpoint response to radiation (Gupta et al. 2005). We tested whether Drosophila with mof mutation also have a similar defective checkpoint response. In Drosophila, the larval imaginal discs are actively proliferating cells that form adult organs. The DNA damage checkpoint response was assessed by immunostaining wing imaginal discs from both wild-type and mof1 mutants for the mitotic cell marker phosphorylated histone H3 (H3S10ph) before and 1 h after irradiation with 25 Gy. In wild-type imaginal discs, IR-induced DNA damage prevents cells from entering into mitosis until the damage is repaired. Staining the imaginal discs with H3S10ph antibody 1 h after irradiation detects any mitotic cells that escape the checkpoint. In the MOF wild-type discs prior to irradiation, H3S10ph-stained cells were maximum, and PH3-stained cells were also readily detected in MOF mutant imaginal discs, whereas post-irradiation MOF wild-type discs had decrease H3S10ph-stained cells and mutant mof showed a minimum decrease in the H3S10ph-stained cells, indicating that the DNA damage checkpoint is inactive in the mutant mof cells (Fig. 5). These results further indicate that the Drosophila mof gene product is required for a DNA damage response.
Fig. 5.
DNA damage checkpoint response in wing imaginal discs of wild-type and Drosophila mof mutant. Larval wing discs from wild-type and mof1 mutant male were immunostained with anti-phospho histone H3 antibody before irradiation and 1 h after irradiation with 25 Gy of gamma rays. The mitotic cells in the discs were stained brightly in the confocal images
In mammalian cells, MOF influences ATM function (Gupta et al. 2005), and cells deficient in ATM have a defective p53 response to IR (Kastan 2008; Kastan et al. 1992; Pandita 2002, 2003; Pandita et al. 2000). We therefore compared p53 levels in mutant MOF Drosophila embryos with wild type embryos before and 2 h post-irradiation. Irradiation did not induce any change in MOF levels in wild-type MOF Drosophila (data not shown), consistent with results obtained in mammals (Gupta et al. 2005). Wild-type Drosophila cells had low basal p53 that was increased markedly post-irradiation. However, mutant mof Drosophila had higher basal level p53 and therefore resulted in little obvious change following exposure to IR (Fig. 6). Thus, the overall p53 response in Drosophila was similar to that seen in mammalian cells with and without MOF depletion (Gupta et al. 2005). Furthermore, the levels of mei-41 and Chk1 before and after IR exposure were similar between the wild-type and mof1 mutant flies (Fig. 6), a response seen in mammals, suggesting once again that MOF-mediated DDR is conserved in Drosophila.
Fig. 6.
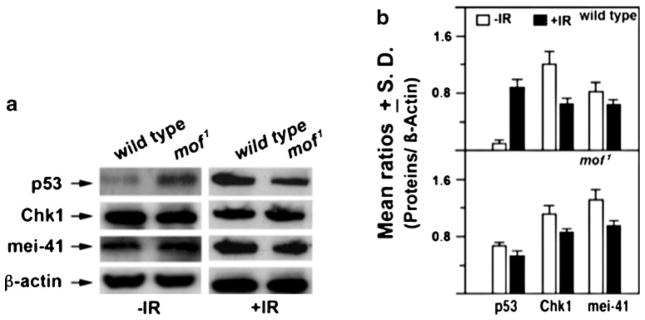
Determination of Chk1, p53, and mei-41 protein levels in larvae extracts of wild-type and mof1 mutant Drosophila male larvae with and without irradiation. Larvae were irradiated with 25 Gy of gamma rays and the proteins extracted within 1 h post-irradiation. a Western blot and b histogram representing the relative amount of proteins estimated from triplicate blots
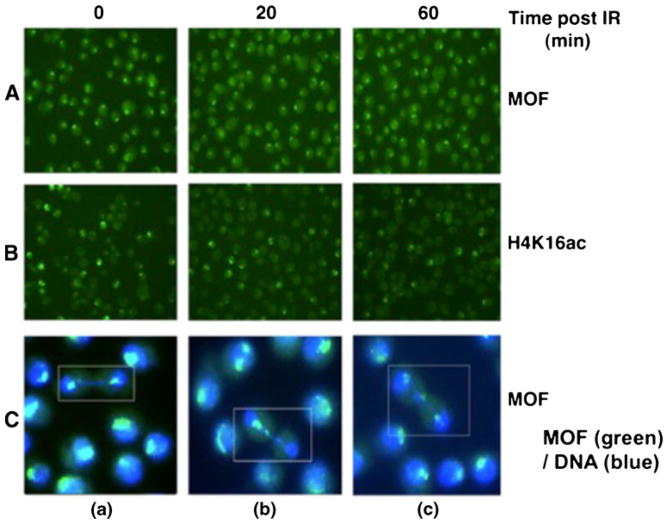
Recent studies revealed that the levels of MOF and H4K16ac remain unaltered at I-Sce-1-induced DNA DSB sites in mammalian cells (Sharma et al. 2010). In addition, when mammalian cells are exposed to IR and examined for retention of MOF with DNA, a significant overall increase in the retention of MOF with DNA is observed (Sharma et al. 2010). Since MOF, as well as H4K16ac is preferentially enriched on the Drosophila male X-chromosome, we also examined whether IR exposure induced any specific relocalization or delocalization of MOF or H4K16ac from the male X chromosome. SL-2 cells were irradiated with 7.5 Gy and examined for gain or loss of MOF signal on the X chromosome at different time points post-irradiation. To determine whether radiation-induced DNA damage could result in alteration of MOF or H4K16 acetylation on the Drosophila male X chromosome, we omitted colcemid treatment for the metaphase arrest. As expected, IR exposure resulted in a higher frequency of chromatin bridge formation in irradiated versus untreated cells as detected at anaphase and early telophase (Fig. 7A, B). Chromatin bridges are observed in the irradiated cells but not in the control sample. At 20 min post-irradiation, there was one chromatin bridge for every 250 cells. By 60 min post-irradiation, the number of chromatin bridges was reduced to one bridge per 1,000 cells. To determine whether the male X chromosome is involved in spontaneous or IR-induced chromatin bridge formation, we fixed the cells at different time points post-irradiation and immunostained with either MOF or H4K16ac antibody. We found no major differences between irradiated and unirradiated cells in MOF or H4K16ac intensity on the X chromosome as a function of the cell cycle (data not shown). The male X chromosome does not show any specificity for IR-induced damage, as cells with a normal distribution of sister chromatids of the X chromosome were identified by MOF staining in two daughter nuclei (Fig. 7C(a)). However, some dividing cells had one large signal in single daughter cells, suggesting that both sister chromatids of the X chromosome moved to one daughter nucleus (Fig. 7C(b)). In addition, some cells had three signals, one in each nucleus and a third one in the middle of the two daughter nuclei, indicating that DNA damage resulted in fragmentation, giving rise to two daughter cells having one signal each and an additional lagging chromosome fragment present between the two nuclei (Fig. 7C(c)).
Fig. 7.
Male X-chromosome staining for A MOF or B H4K16ac at different time points post-irradiation in SL-2 cells. C Cells showing the status of male X chromosomes detected by MOF staining (MOF color is green and DNA is blue): a daughter cells showing similar intensity of MOF staining of X chromosomes at late anaphase; b one daughter cell showing MOF staining whereas the other lacks MOF staining at telophase; and c two daughter cells sharing three MOF signals at telophase
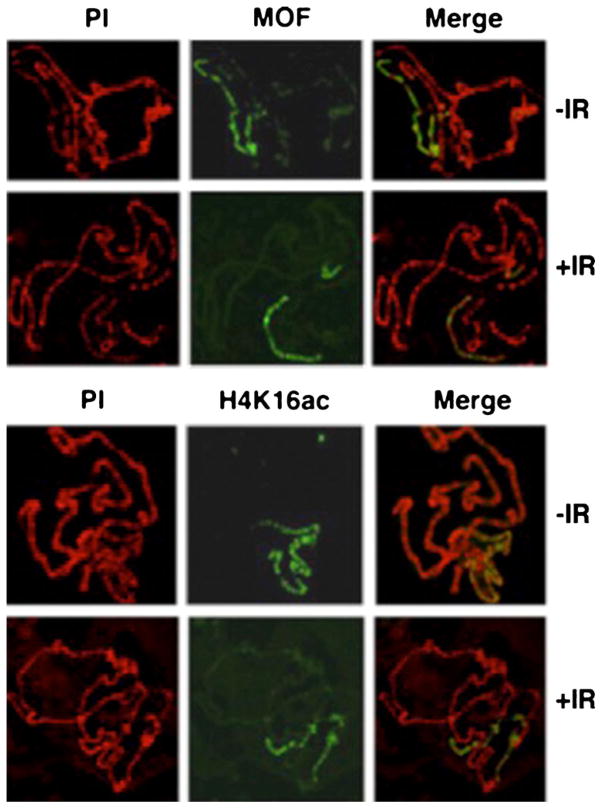
We further determined the status of MOF and H4K16ac on polytene chromosomes before and after IR exposure using higher doses of gamma radiation. Larvae were exposed to 25 Gy, and salivary gland chromosomes were analyzed at different time points post-irradiation. At this level of resolution, there appears to be minimal change in the intensity of MOF or H4K16ac immunostaining on the X chromosome (Fig. 8), suggesting that MOF is tightly associated with chromatin in Drosophila. Again, these results are consistent with previous observations that MOF does not show any major change in preferential localization on the Drosophila male X chromosome immediately post-irradiation (Sharma et al. 2010).
Fig. 8.
MOF and H4K16ac immunostained wild-type male Drosophila polytene salivary gland X-chromosomes. Drosophila were irradiated with 25 Gy and the salivary gland chromosomes, fixed and immunostained
Recent studies support a role for H4K16ac in the mammalian DDR (Sharma et al. 2010), as increased post-irradiation levels of H4K16ac have been reported in human (Gupta et al. 2005) and mouse (Li et al. 2010) cells. To determine whether Drosophila shows a similar response to IR exposure, SL-2 cells were irradiated with gamma rays and the H4K16ac levels determined. As in mammalian cells, SL-2 cells showed an increase in the levels of H4K16ac without any change in MOF levels (Fig. 9 and data not shown). To determine whether this response is also observed in vivo, third instar larvae from male and female Drosophila were irradiated (25 Gy) and histones extracted for analysis. Consistent with the in vitro SL-2 cell results, IR exposure of larvae increased the levels of H4K16 acetylation (H4K16ac) in wild-type larvae of both sexes, whereas mutant MOF larvae showed minimal increase in the levels of IR-induced H4K16ac (Fig. 10).
Fig. 9.
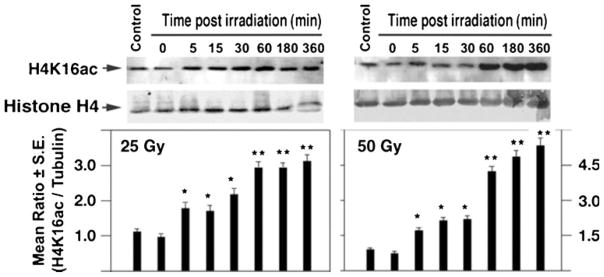
Increased cellular H4K16ac levels in SL-2 cells following irradiation. Cells were irradiated with either 25 or 50 Gy and the H4K16ac levels measured at different time points post-irradiation by Western blot analysis. Relative H4K16ac levels were normalized relative to Histone H4 levels. Differences from control (unirradiated) cells are statistically significant according to Student’s t test, *P<0.05; **P<0.001)
Fig. 10.
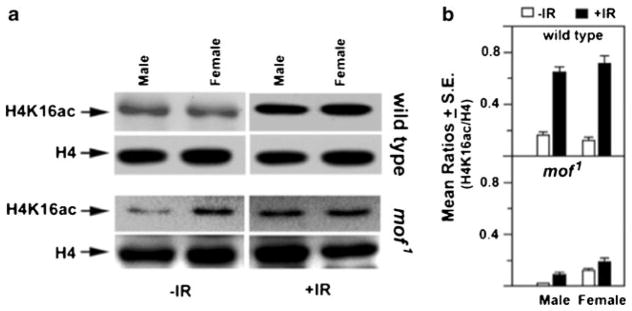
Effect of IR on MOF and H4K16ac levels in Drosophila larvae. a Wild-type and mof1 mutant third instar larvae were irradiated (25 Gy), and the histones extracted within 1 h of irradiation. Quantitative Western blot analysis was carried out with H4K16ac antibody. The same blots were re-probed with antibody to total histone H4 as a gel loading control. b Histogram representing the relative amount of H4K16ac/total histone H4 (H4K16ac/H4) as estimated from triplicate blot analysis
All of our observations obtained in studying the role of MOF in the Drosophila response to IR are consistent with those obtained in mammalian systems and therefore support the conclusion that the role of MOF in the DDR is conserved between mammals and Drosophila. Furthermore, we have shown for the first time that MOF is required for IR response in a whole animal. Previous experiments have shown that depletion of MOF results in decreased levels of H4K16ac in both mammals and Drosophila with associated alterations in gene transcription but with minimum affect on transcription of DNA repair genes (Kind et al. 2008; Sharma et al. 2010). Thus, MOF appears to be involved in the DDR primarily through the effect of H4K16 acetylation on chromatin structure. Based on this conclusion, Drosophila is an appropriate screening model for the identification of MOF inhibitors as well as inhibitors of H4K16ac deacetylases relevant to the mechanism of DDR. Furthermore, while the dosage compensation role of MOF in Drosophila is not observed in mammals, where transcriptional balance is regulated by X chromosome inactivation, the role of MOF function in the DDR is conserved in Drosophila and mammals.
Acknowledgments
Thanks are due to Edwin Smith and Peter Becker for providing regents and valuable suggestions and to Fan Zhang with help in irradiating mof3/mof6 flies. We also thank members of Pandita laboratory for helpful discussions and suggestions.
Funding This work was supported by NIH NCI grants R01CA123232, R01CA129537, RO1CA154320, and R13CA130756 (TKP); NSF grants MCB 0131414 and MCB 0615831 (JCE); NIGMS grant RO115691 (JCL); and Senior Wellcome Trust fellowship grants, GRA070065MA (UB) and (GRA076395AIA) (MPB).
Footnotes
Conflict of interest statement None.
Contributor Information
Manika P. Bhadra, Indian Institute of Chemical Technology, Hyderabad, AP 500007, India. Center for Cellular and Molecular Biology, Hyderabad, AP 500007, India
Nobuo Horikoshi, UT Southwestern Medical Center, Dallas, TX 75390, USA.
Sreerangam NCVL Pushpavallipvalli, Center for Cellular and Molecular Biology, Hyderabad, AP 500007, India.
Arpita Sarkar, Center for Cellular and Molecular Biology, Hyderabad, AP 500007, India.
Indira Bag, Center for Cellular and Molecular Biology, Hyderabad, AP 500007, India.
Anita Krishnan, Indian Institute of Chemical Technology, Hyderabad, AP 500007, India. Center for Cellular and Molecular Biology, Hyderabad, AP 500007, India.
John C. Lucchesi, Emory University, Atlanta, GA 30322, USA
Rakesh Kumar, UT Southwestern Medical Center, Dallas, TX 75390, USA. Washington University School of Medicine, St Louis, MO, USA.
Qin Yang, Washington University School of Medicine, St Louis, MO, USA.
Raj K. Pandita, UT Southwestern Medical Center, Dallas, TX 75390, USA
Mayank Singh, UT Southwestern Medical Center, Dallas, TX 75390, USA.
Utpal Bhadra, Center for Cellular and Molecular Biology, Hyderabad, AP 500007, India.
Joel C. Eissenberg, Saint Louis University School of Medicine, St Louis, MO 63104, USA
Tej K. Pandita, Email: tej.pandita@utsouthwestern.edu, UT Southwestern Medical Center, Dallas, TX 75390, USA. Washington University School of Medicine, St Louis, MO, USA
References
- Akhtar A, Becker PB. Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol Cell. 2000;5:367–375. doi: 10.1016/s1097-2765(00)80431-1. [DOI] [PubMed] [Google Scholar]
- Brodsky MH, Weinert BT, Tsang G, Rong YS, McGinnis NM, Golic KG, Rio DC, Rubin GM. Drosophila melanogaster MNK/ Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol. 2004;24:1219–1231. doi: 10.1128/MCB.24.3.1219-1231.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischle W, Wang Y, Allis CD. Histone and chromatin crosstalk. Curr Opin Cell Biol. 2003;15:172–183. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- Gupta A, Sharma GG, Young CS, Agarwal M, Smith ER, Paull TT, Lucchesi JC, Khanna KK, Ludwig T, Pandita TK. Involvement of human MOF in ATM function. Mol Cell Biol. 2005;25:5292–5305. doi: 10.1128/MCB.25.12.5292-5305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Guerin-Peyrou TG, Sharma GG, Park C, Agarwal M, Ganju RK, Pandita S, Choi K, Sukumar S, Pandita RK, et al. The mammalian ortholog of Drosophila MOF that acetylates histone H4 lysine 16 is essential for embryogenesis and oncogenesis. Mol Cell Biol. 2008;28:397–409. doi: 10.1128/MCB.01045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker A, Hilfiker-Kleiner D, Pannuti A, Lucchesi JC. mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J. 1997;16:2054–2060. doi: 10.1093/emboj/16.8.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers JH. DNA repair mechanisms. Maturitas. 2001;38:17–22. doi: 10.1016/s0378-5122(00)00188-2. discussion 22–13. [DOI] [PubMed] [Google Scholar]
- Hunt CR, Dix DJ, Sharma GG, Pandita RK, Gupta A, Funk M, Pandita TK. Genomic instability and enhanced radiosensitivity in Hsp70.1- and Hsp70.3-deficient mice. Mol Cell Biol. 2004;24:899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CR, Pandita RK, Laszlo A, Higashikubo R, Agarwal M, Kitamura T, Gupta A, Rief N, Horikoshi N, Baskaran R, et al. Hyperthermia activates a subset of ataxia-telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res. 2007;67:3010–3017. doi: 10.1158/0008-5472.CAN-06-4328. [DOI] [PubMed] [Google Scholar]
- Jaklevic BR, Su TT. Relative contribution of DNA repair, cell cycle checkpoints, and cell death to survival after DNA damage in Drosophila larvae. Curr Biol. 2004;14:23–32. doi: 10.1016/j.cub.2003.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y, Mengus G, Gilfillan G, Kennedy HG, Stuckenholz C, Kelley RL, Becker PB, Kuroda MI. Association and spreading of the Drosophila dosage compensation complex from a discrete roX1 chromatin entry site. EMBO J. 2001;20:2236–2245. doi: 10.1093/emboj/20.9.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan MB. DNA damage responses: mechanisms and roles in human disease: 2007 G.H.A. Clowes Memorial Award Lecture. Mol Cancer Res. 2008;6:517–524. doi: 10.1158/1541-7786.MCR-08-0020. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Zhan Q, el-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kind J, Vaquerizas JM, Gebhardt P, Gentzel M, Luscombe NM, Bertone P, Akhtar A. Genome-wide analysis reveals MOF as a key regulator of dosage compensation and gene expression in Drosophila. Cell. 2008;133:813–828. doi: 10.1016/j.cell.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Kumar R, Hunt CR, Gupta A, Nannepaga S, Pandita RK, Shay JW, Bachoo R, Ludwig T, Burns DK, Pandita TK. Purkinje cell-specific males absent on the first (mMof) gene deletion results in an ataxia-telangiectasia-like neurological phenotype and backward walking in mice. Proc Natl Acad Sci USA. 2011;108:3636–3641. doi: 10.1073/pnas.1016524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legube G, Trouche D. Regulating histone acetyltransferases and deacetylases. EMBO Rep. 2003;4:944–947. doi: 10.1038/sj.embor.embor941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Corsa CA, Pan PW, Wu L, Ferguson D, Yu X, Min J, Dou Y. MOF and H4 K16 acetylation play important roles in DNA damage repair by modulating recruitment of DNA damage repair protein Mdc1. Mol Cell Biol. 2010;30:5335–5347. doi: 10.1128/MCB.00350-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misri S, Pandita S, Kumar R, Pandita TK. Telomeres, histone code, and DNA damage response. Cytogenet Genome Res. 2008;122:297–307. doi: 10.1159/000167816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal KC, Pannuti A, Smith ER, Lucchesi JC. A new human member of the MYST family of histone acetyl transferases with high sequence similarity to Drosophila MOF. Biochim Biophys Acta. 2000;1490:170–174. doi: 10.1016/s0167-4781(99)00211-0. [DOI] [PubMed] [Google Scholar]
- Otten AD, Tapscott SJ. Triplet repeat expansion in myotonic dystrophy alters the adjacent chromatin structure. Proc Natl Acad Sci USA. 1995;92:5465–5469. doi: 10.1073/pnas.92.12.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal-Bhadra M, Leibovitch BA, Gandhi SG, Rao M, Bhadra U, Birchler JA, Elgin SC. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science. 2004;303:669–672. doi: 10.1126/science.1092653. [DOI] [PubMed] [Google Scholar]
- Pandita TK. The role of ATM in telomere structure and function. Radiat Res. 2001;156:642–647. doi: 10.1667/0033-7587(2001)156[0642:troait]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Pandita TK. ATM function and telomere stability. Oncogene. 2002;21:611–618. doi: 10.1038/sj.onc.1205060. [DOI] [PubMed] [Google Scholar]
- Pandita TK. A multifaceted role for ATM in genome maintenance. Expert Rev Mol Med. 2003;5:1–21. doi: 10.1017/S1462399403006318. [DOI] [PubMed] [Google Scholar]
- Pandita TK. Role of mammalian Rad9 in genomic stability and ionizing radiation response. Cell Cycle. 2006;5:1289–1291. doi: 10.4161/cc.5.12.2862. [DOI] [PubMed] [Google Scholar]
- Pandita TK, Geard CR. Chromosome aberrations in human fibroblasts induced by monoenergetic neutrons. I. Relative biological effectiveness. Radiat Res. 1996;145:730–739. [PubMed] [Google Scholar]
- Pandita TK, Hittelman WN. The contribution of DNA and chromosome repair deficiencies to the radiosensitivity of ataxia-telangiectasia. Radiat Res. 1992a;131:214–223. [PubMed] [Google Scholar]
- Pandita TK, Hittelman WN. Initial chromosome damage but not DNA damage is greater in ataxia telangiectasia cells. Radiat Res. 1992b;130:94–103. [PubMed] [Google Scholar]
- Pandita TK, Richardson C. Chromatin remodeling finds its place in the DNA double-strand break response. Nucleic Acids Res. 2009;37:1363–1377. doi: 10.1093/nar/gkn1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandita TK, Lieberman HB, Lim DS, Dhar S, Zheng W, Taya Y, Kastan MB. Ionizing radiation activates the ATM kinase throughout the cell cycle. Oncogene. 2000;19:1386–1391. doi: 10.1038/sj.onc.1203444. [DOI] [PubMed] [Google Scholar]
- Pandita RK, Sharma GG, Laszlo A, Hopkins KM, Davey S, Chakhparonian M, Gupta A, Wellinger RJ, Zhang J, Powell SN, et al. Mammalian Rad9 plays a role in telomere stability, S- and G2-phase-specific cell survival, and homologous recombinational repair. Mol Cell Biol. 2006;26:1850–1864. doi: 10.1128/MCB.26.5.1850-1864.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss D, Bushman FD, Wolffe AP. Human immunodeficiency virus integrase directs integration to sites of severe DNA distortion within the nucleosome core. Proc Natl Acad Sci USA. 1994;91:5913–5917. doi: 10.1073/pnas.91.13.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja SJ, Charapitsa I, Conrad T, Vaquerizas JM, Gebhardt P, Holz H, Kadlec J, Fraterman S, Luscombe NM, Akhtar A. The nonspecific lethal complex is a transcriptional regulator in Drosophila. Mol Cell. 2010;38:827–841. doi: 10.1016/j.molcel.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Richardson C, Horikoshi N, Pandita TK. The role of the DNA double-strand break response network in meiosis. DNA Repair (Amst) 2004;3:1149–1164. doi: 10.1016/j.dnarep.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Scott SP, Pandita TK. The cellular control of DNA double-strand breaks. J Cell Biochem. 2006;99:1463–1475. doi: 10.1002/jcb.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma GG, Hwang KK, Pandita RK, Gupta A, Dhar S, Parenteau J, Agarwal M, Worman HJ, Wellinger RJ, Pandita TK. Human heterochromatin protein 1 isoforms HP1(Hsalpha) and HP1(Hsbeta) interfere with hTERT–telomere interactions and correlate with changes in cell growth and response to ionizing radiation. Mol Cell Biol. 2003;23:8363–8376. doi: 10.1128/MCB.23.22.8363-8376.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma GG, So S, Gupta A, Kumar R, Cayrou C, Avvakumov N, Bhadra U, Pandita RK, Porteus MH, Chen DJ, et al. MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Mol Cell Biol. 2010;30:3582–3595. doi: 10.1128/MCB.01476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ER, Pannuti A, Gu W, Steurnagel A, Cook RG, Allis CD, Lucchesi JC. The Drosophila MSL complex acetylates histone H4 at lysine 16, a chromatin modification linked to dosage compensation. Mol Cell Biol. 2000;20:312–318. doi: 10.1128/mcb.20.1.312-318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ER, Cayrou C, Huang R, Lane WS, Cote J, Lucchesi JC. A human protein complex homologous to the Drosophila MSL complex is responsible for the majority of histone H4 acetylation at lysine 16. Mol Cell Biol. 2005;25:9175–9188. doi: 10.1128/MCB.25.21.9175-9188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Mirey G, Betson M, Haber DA, Settleman J. The Drosophila ATM ortholog, dATM, mediates the response to ionizing radiation and to spontaneous DNA damage during development. Curr Biol. 2004;14:1354–1359. doi: 10.1016/j.cub.2004.06.064. [DOI] [PubMed] [Google Scholar]
- Taipale M, Rea S, Richter K, Vilar A, Lichter P, Imhof A, Akhtar A. hMOF histone acetyltransferase is required for histone H4 lysine 16 acetylation in mammalian cells. Mol Cell Biol. 2005;25:6798–6810. doi: 10.1128/MCB.25.15.6798-6810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Attikum H, Gasser SM. The histone code at DNA breaks: a guide to repair? Nat Rev Mol Cell Biol. 2005;6:757–765. doi: 10.1038/nrm1737. [DOI] [PubMed] [Google Scholar]
- van Attikum H, Gasser SM. Crosstalk between histone modifications during the DNA damage response. Trends Cell Biol. 2009;19:207–217. doi: 10.1016/j.tcb.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Wallrath LL, Lu Q, Granok H, Elgin SC. Architectural variations of inducible eukaryotic promoters: preset and remodeling chromatin structures. Bioessays. 1994;16:165–170. doi: 10.1002/bies.950160306. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development. 1991a;113:841–850. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosophila. Development. 1991b;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- Worby CA, Simonson-Leff N, Dixon JE. RNA interference of gene expression (RNAi) in cultured Drosophila cells. Sci STKE. 2001;2001(95):pl1. doi: 10.1126/stke.2001.95.pl1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Malone JH, Powell SK, Periwal V, Spana E, MacAlpine DM, Oliver B. Expression in aneuploid Drosophila S2 cells. PLoS Biol. 2010;8(2):e1000320. doi: 10.1371/journal.pbio.1000320. [DOI] [PMC free article] [PubMed] [Google Scholar]