Abstract
One of the hallmarks of highly proliferative adult tissues is the presence of a stem cell population that produces progenitor cells bound for differentiation. Progenitor cells undergo multiple transit amplifying (TA) divisions before initiating terminal differentiation. In the adult male germline, daughter cells arising from the spermatogonial stem cells (SSCs) undergo multiple rounds of TA divisions to produce undifferentiated clones of interconnected 2, 4, 8 and 16 cells, collectively termed Aundifferentiated (Aundiff) spermatogonia, before entering a stereotypic differentiation cascade. Although the number of TA divisions markedly affects the tissue output both at steady state and during regeneration, mechanisms regulating the expansion of the TA cell population are poorly understood in mammals. Here, we show that mice with a conditional deletion of Lin28a in the adult male germline, display impaired clonal expansion of the progenitor transit amplifying Aundiff spermatogonia. The in vivo proliferative activity of Aundiff spermatogonial cells as indicated by BrdU incorporation during S phase was reduced in the absence of LIN28A. Thus contrary to the role of LIN28A as a key determinant of cell fate signals in multiple stem cell lineages, in the adult male germline it functions as an intrinsic regulator of proliferation in the population of Aundiff TA spermatogonia. In addition, neither precocious differentiation nor diminished capacity for self-renewal potential as assessed by transplantation was observed, suggesting that neither LIN28A itself, nor the pool of Aal progenitor cells, substantially contribute to the functional stem cell compartment.
Keywords: SSC, Progenitor, LIN28A, germ cells, spermatogonia, transit amplifying cells, spermatogenesis, spermatogonial stem cell, germ line stem cell
Introduction
A fundamental subpopulation within many adult stem cell lineages are progenitor cells that undergo several rounds of mitotic transit amplifying divisions before initiating terminal differentiation. These transit-amplifying (TA) progenitor cells differ from the fewer and often more infrequently dividing stem cells in both proliferation rate and pluripotency [1, 2]. Mechanisms regulating the size of the TA progenitor cell compartment are key to maintaining highly proliferative adult tissues, enabling the few slowly dividing stem cells to give rise to many differentiated progeny. The TA progenitor population also provides regenerative capacity to tissues following stress. However, the normally slowly dividing stem cells can switch to a more rapid proliferation mode and increase their self-renewal rate when necessary so as to replenish the stem cell pool and restore tissue capacity for generating differentiated progeny [3, 4]. The TA population is a critical determinate of the size of a tissue and the numbers of terminally differentiated cells. Yet, the molecular factors and their mechanisms that regulate TA proliferation are notably unknown [5].
Like in other organ systems, the TA progenitor population plays an integral role in the adult male germline. Within a given species there are a fixed number of TA divisions that occur prior to meiotic entry. This number is crucial for the sperm production capacity of the resident stem cell population. For instance, in monkeys, where 3% of the germ cell population is supposed to consist of spermatogonial stem cells (SSCs), the TA progenitor cells undergo 4 rounds of mitotic divisions prior to meiosis [6]. In mice however, that reportedly have fewer SSCs (0.03% of the germ cell population), the TA clones undergo up to 10 rounds of transit-amplifying divisions [7–10]. Although several gene products have been shown to regulate the transit amplification in the Drosophila male germline, no analogous regulatory mechanisms in mammals have been reported [11–13].
The spermatogonial TA population in rodents includes the undifferentiated spermatogonia and all of the differentiating spermatogonia [14]. Undifferentiated spermatogonia consist of syncytial clones of 2, 4, 8 and 16 cells interconnected by cytoplasmic bridges, which arise from mitoses of Asingle (As) spermatogonial cells and are referred to as Apaired (Apr), Aaligned-4 (Aal-4), Aal-8, and Aal-16. Collectively the As, Apr and Aal cells are termed Aundifferentiated (Aundiff) spermatogonia. Aal differentiate into type A1 spermatogonia without going through mitosis, and then undergo additional rounds of transit amplification to form A2, A3, A4, Intermediate, and B spermatogonia; collectively referred to as differentiating spermatogonia. Following mitotic amplification, spermatogonia undergo meiosis to generate haploid spermatids [9, 14].
Historical data support the notion that Aundiff spermatogonia differentiate in a linear and non-reversible manner through spermatogenesis [9]. However, it has recently been suggested that the TA Aundiff spermatogonia may constitute an expanded subset of the SSC population. Lineage-tracing experiments in mice can be interpreted to suggest that clones of Aundiff spermatogonia are not irreversibly committed to differentiation, but can undergo clonal fragmentation and regain their stem cell potential during regeneration after tissue damage [15]. Thus, the TA Aundiff subpopulation of cells would not only help in obtaining large numbers of differentiated progeny from the very few resident stem cells but may aid the regenerative process after a tissue insult. Both of these features are critical for maintaining male fertility.
Here, we assess the role of LIN28A, a critical determinant of cell fate and proliferation, which is expressed largely in the undifferentiated spermatogonia in both mice and humans and has been suggested to mark spermatogonial stem cells [16, 17]. Lin-28 was originally identified in Caenorhabditis elegans (C. elegans) as a component of the heterochronic gene pathway that regulates developmental timing [18]. Unlike C. elegans, the mammalian genome encodes two Lin28 paralogs, Lin28a and Lin28b [19, 20]. In vitro studies have indicated LIN28A can play a multitude of roles; mediating proliferation, differentiation, or cell fate selection in a cell-context manner [21–28]. The most characterized molecular mechanism underlying these processes involves the let-7 miRNA biogenesis pathway. LIN28 has been shown to block the processing of let-7 into its mature form by binding to the terminal loops of the let-7 miRNA precursor [29–37].
Transgenic mice overexpressing Lin28a display a delayed onset of puberty and enhanced glucose metabolism resulting in increased body size [38, 39]. Conversely, Lin28a KO males are reported to have a runted growth phenotype, altered levels of FSH and testosterone and a marked reduction in the germ cell population at birth due to reduced PGC proliferation [40]. Neither model has addressed a function for LIN28A in spermatogonial cells. Using conditional knockout of Lin28a in adult germline stem cells, we show that the loss of LIN28A dampens the proliferative capacity of the TA spermatogonial population without compromising their differentiation capacity. In addition, LIN28A does not seem to have a role in SSC self-renewal.
Results
LIN28A is expressed in undifferentiated and differentiating spermatogonia
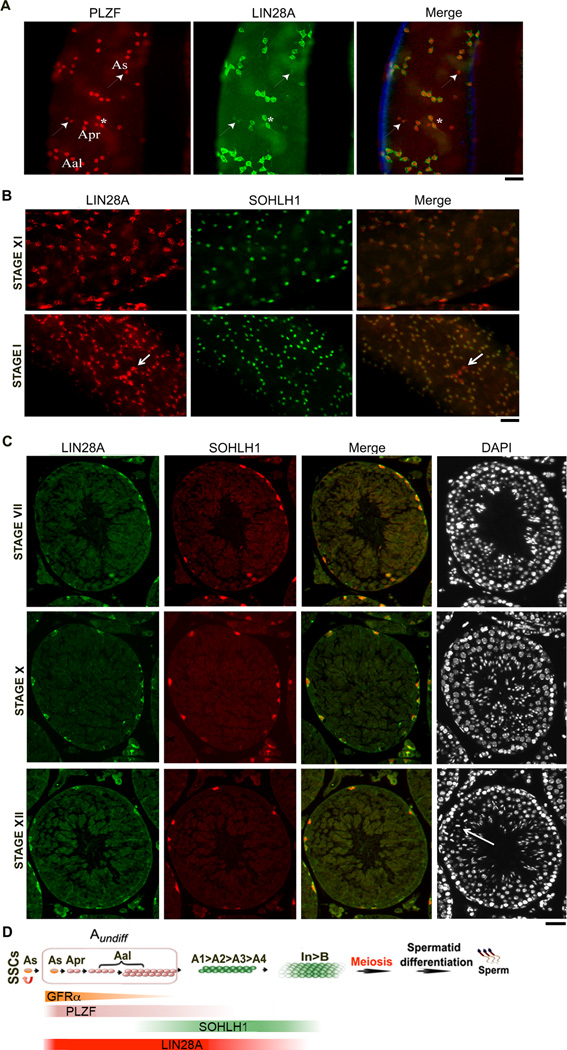
Previously published reports on the expression of LIN28A in subpopulations of TA spermatogonial cells have been inconsistent. LIN28A has been reported to specifically mark As, Apr and Aal spermatogonia [17, 41]; others have observed its continued presence in A1–A4 spermatogonia [42]. We evaluated LIN28A expression in the TA subpopulations using markers of both Aundiff (PLZF) and differentiating spermatogonia (SOHLH1) by immunofluorescence [43, 44]. Consistent with previous studies we found that LIN28A was co-expressed with PLZF in As, Apr and Aal - Aundiff spermatogonia (Fig. 1A). Intriguingly, we also observed As and Aal PLZF positive cells that were weakly LIN28A positive (Fig. 1A, arrowheads) or completely devoid of LIN28A staining (Fig. 2B, boxed area).
Figure 1. LIN28A is expressed in Aundiff progenitor and differentiating A1–A4 spermatogonia.
Whole mount or immunofluorescence staining of seminiferous tubules from adult mice was performed with a combination of anti-LIN28A and PLZF or SOHLH1 antibodies. (A) Immunofluorescence localization of LIN28A in Aundiff spermatogonia marked by PLZF is shown. PLZF+ As and Apr spermatogonia expressing low levels of LIN28A (white arrowhead) and Apr spermatogonia with high levels of LIN28A (asterisks) are shown. (B) Co-expression of LIN28A and SOHLH1 is depicted in differentiating Type A spermatogonia in stages XI and I. Arrow shows a chain of undifferentiated Aal-4 cells. (C) LIN28A and SOHLH1 is shown in differentiating A1, A2, A3 spermatogonia in stages VII, X and XII. Dividing spermatocytes in stage XII are depicted with an arrow. (D) Schematics of spermatogenesis showing the different stages of germ cell differentiation. The transit TA cells are marked in pink and green. The Aundiff spermatogonial population addressed in this study is enclosed in pink. Colored bars indicate the extent of marker expression in the different spermatogonial cell-populations. LIN28A marks a large fraction of the Aundiff spermatogonial population. Scale bar = 20 um.
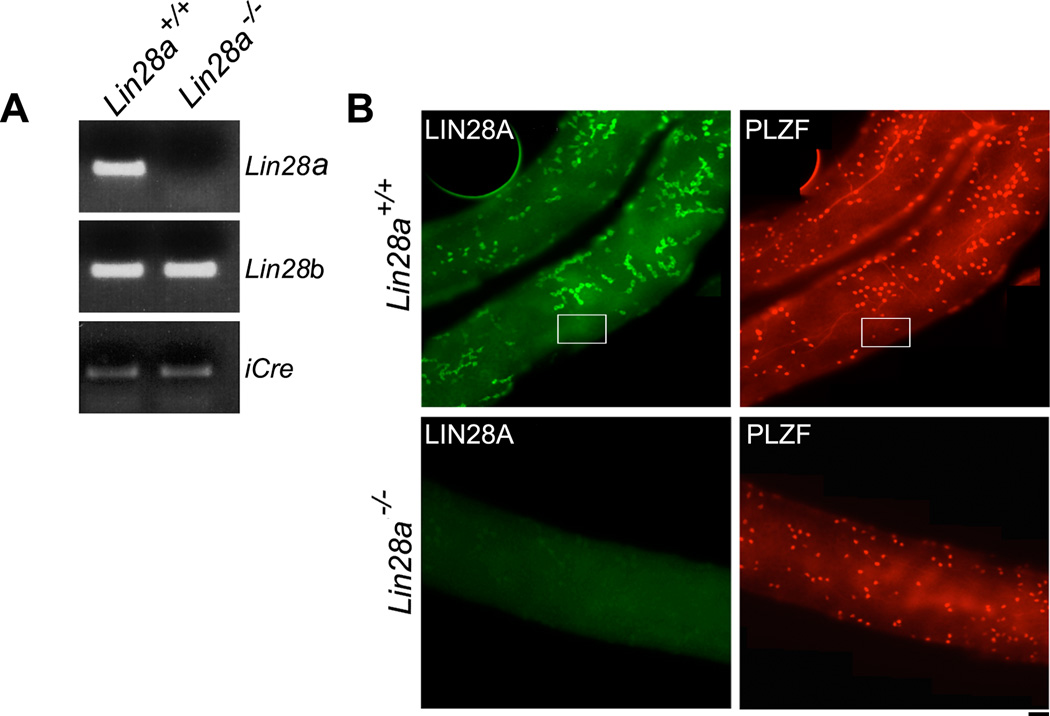
Figure 2. Levels of Lin28a transcript and LIN28A protein in Lin28a mutant testis samples are significantly reduced in adult mice.
(A) RT-PCR analysis of Lin28a, Lin28b and iCre transcript in control and Lin28a mutant testis showing loss of Lin28a while Lin28b and iCre expression remains unaltered. (B) Whole mount immunostaining of isolated seminiferous tubules from control (top panels) and Lin28a mutant samples (bottom panels) stained with LIN28A and PLZF antibodies confirming the absence of LIN28A in spermatogonia of mutant mice. Boxed area shows PLZF+LIN28A− As cells. Scale bar = 20 um.
We then interrogated LIN28A expression in differentiating Type A spermatogonia The seminiferous cycle in mice can be divided into 12 stages (I through XII) that contain germ cells at all stages of differentiation; however, each individual stage contains only a specific set of differentiating germ cell types, including subpopulations of A1–B spermatogonia. These stages can be identified on whole mount immunostaining using specific features observed by DAPI staining and DIC microscopy (see Materials and methods for details) [45]. We observed LIN28A in differentiating Type A spermatogonia (marked by SOHLH1) in stage XI and I tubules (Fig. 1B). Expression of LIN28A in the A1–A4 spermatogonial population was however reduced compared to the Aundiff spermatogonial cells (Fig. 1B, lower panel, compare SOHLH1+ spermatogonia to SOHLH1− chain of Aal-4 spermatogonia, shown by arrow). To further validate this observation, specific stages were identified based on the characteristic DAPI staining pattern on tubular cross-sections [45]. As expected, LIN28A was present in A1 (stage VII), A2 (stage X) and A3 (stage XII) spermatogonia (Fig. 1C). Together, these results demonstrate that LIN28A marks a large proportion of the TA spermatogonial population in adult mice. Fig. 1D summarizes the results of the immunostaining and depicts the subsets of spermatogonia marked by GFRα1, PLZF, SOHLH 1 and LIN28A [46–48].
Conditional knockout of Lin28a reduces testis mass and sperm number
To explore the role of LIN28A in spermatogenesis, we conditionally inactivated Lin28a in the male germ line. Lin28a was excised in adult germ cells using a gene cassette containing Cre recombinase driven by the promoter of germ cell-specific Stra8. In this model Cre is first expressed at postnatal day 3 and in adults it is expressed in Aundiff spermatogonia [(Stra8-icre; formally Tg(Stra8-cre1Reb)][36] [49].
Real time PCR analysis on whole testis RNA confirmed that the expression of Lin28a transcript was significantly diminished in the testes of adult Lin28a mutant mice, while transcript levels of Lin28b, a gene paralog of Lin28a, were unchanged (Fig. 2A). Consistent with this finding, whole mount immunostaining of mutant seminiferous tubules indicated an absence of LIN28A expression in all germ cells, including As cells, confirming successful Cre-mediated deletion of Lin28a in spermatogonia and loss of LIN28A protein (Fig. 2B).
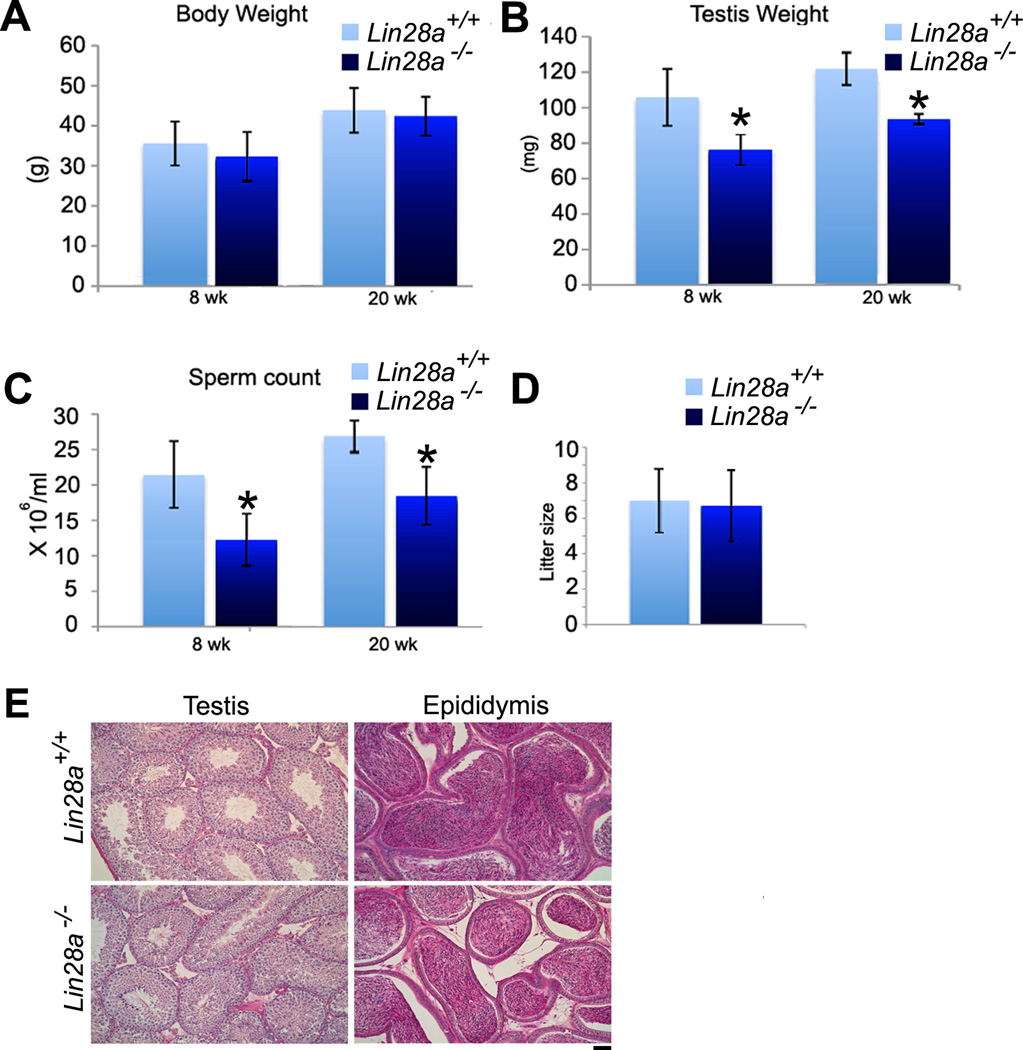
Lin28a mutant mice grew normally to adulthood as evidenced by similar body weights at 8 and 20 weeks of age in comparison to controls (Fig. 3A). However, a significant reduction in testis weight was observed in Lin28a mutant males by 8 weeks and was still present in 20-week mutants (Fig. 3B). The effect of Lin28a deficiency on the overall spermatogenic process was assessed histologically using adult testis cross-sections. Both control and Lin28a mutant tubules contained germ cells in all stages of differentiation with the expected distribution of pre and post-meiotic cells observed within the seminiferous epithelium (Fig. 3E). Compared with controls, sperm numbers in the epididymal ducts of Lin28a mutant mice were significantly reduced at 8 and 20 weeks (Fig. 3C). Overall, we did not observe any age-dependence in weight loss of the testes or reductions in epidydimal sperm counts in Lin28a mutant mice. Despite the reduced sperm counts, mutant male mice exhibited similar fertility at 20 weeks with Lin28a mutant males siring litters of comparable size and frequency compared to controls (Fig. 3D).
Figure 3. Lin28a mutant mice show reduced testis weight and sperm number.
(A and B) Body and testis weights were measured in control and Lin28a mutant males at 8 and 20 weeks of age. In comparison to control, Lin28a mutant males exhibited no body weight difference at 8 or 20 weeks of age, yet showed significant reductions in testis weight and sperm number at these time points. (C) Lin28a mutant epididymal sperm counts revealed significant reductions in sperm number by 8 and 20 weeks of age. (n=5). (D) Fertility testing showed that 5–6 month old mutant Lin28a males were able to sire litters of comparable sizes to controls when mated with 12-week old fertile FVB/N females. (n=3). Values represent the mean ± SEM. * Indicates P≤0.01. (E) PAS-stained histological cross-sections of 20-week-old Lin28a control and mutant testes showed no severe abnormalities. Scale bar = 20 um.
Spermatogonial numbers and clonal size are reduced in Lin28a mutant mice
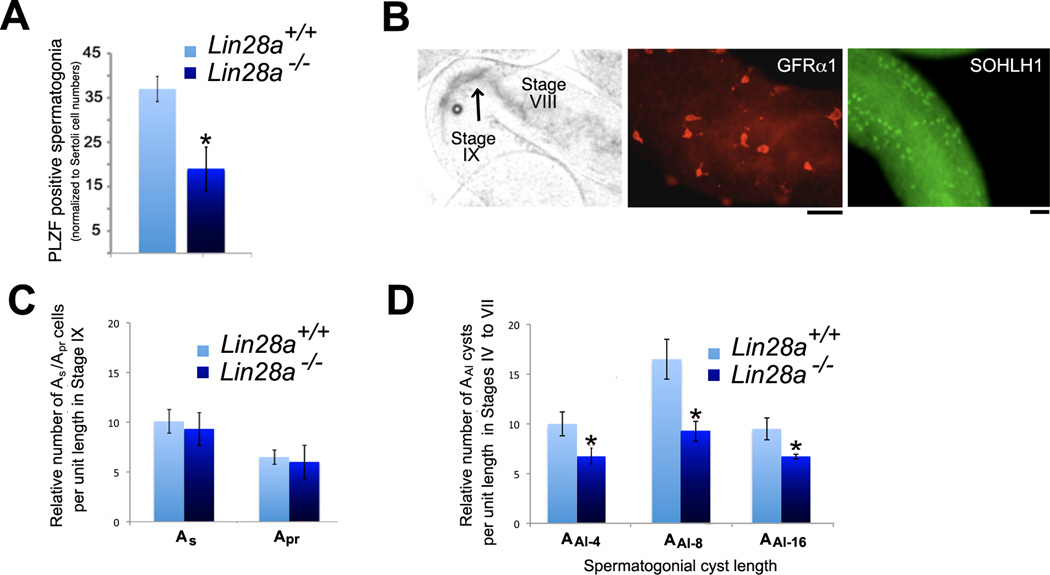
Because Lin28a mutant mice exhibited lowered sperm counts, we next evaluated alterations of the TA Aundiff spermatogonial pool that expresses LIN28A during steady state spermatogenesis. To minimize experimental fluctuation due to cell proliferation, we chose to analyze the Aundiff population during stages VI and VII when proliferation has ceased and Aal spermatogonia are poised to differentiate in response to retinoic acid signaling [50, 51]. Immunohistochemical studies were performed on 12-week-old mice with an antibody against PLZF to assess the Aundiff spermatogonial population. Cell counts were normalized to Sertoli cells identified by the characteristic nuclear staining pattern with DAPI. We observed a significant reduction in PLZF positive spermatogonia in mutant mice in stages VI and VII indicating a loss in the numbers of Aundiff spermatogonia (Fig. 4A).
Figure 4. Transit amplifying Aundiff chain length and numbers are reduced in Lin28a mutant mice.
(A) Quantification of PLZF positive cells on histological cross sections of adult testis detected by immunofluorescence in epithelial stages VI and VII. PLZF positive cells were reduced in mutants when normalized by Sertoli cell number. (B) Images representative of the criteria for identifying GFRα1 positive As/Apr spermatogonia in stages IX (post sperm release front, indicated with a black arrow) and the SOHLH1 positive Aal-4, Aal-8, and Aal-16 cells in stages VI and VII (pre-sperm release front) on whole mount seminiferous tubules isolated from adult mice. (C) Plot shows no change in number of GFRα1 positive As/Apr spermatogonia in epithelial stages IX (as depicted in B) when comparing control and Lin28a mutant samples. (D) Plot shows significant changes in number of SOHLH1 positive chains in epithelial stages VII and VIII (as depicted in B) when comparing control and Lin28a mutant samples. (n=5). Values represent the mean ± SEM. * Indicates P≤0.01. Scale bar = 20 um.
During stages VII and VIII of the seminiferous cycle, clones of Aal-4, Aal-8, and Aal-16 cells differentiate into A1 spermatogonia leaving behind mostly As, Apr and a few chains of Aal-4 spermatogonia. These As, Apr and Aal-4 cells proliferate over the next seminiferous cycle to generate new clones of expanded Aal spermatogonia [52]. As only As, Apr and a few clones of Aal spermatogonia escape differentiation into A1 spermatogonia, the total number of Aundiff is lowest in stage IX [53]. Thus a decrease in Aundiff spermatogonia in Lin28a mutants could arise due to reduced numbers of As and Apr spermatogonia at stage IX, the predecessors to the next cycle of Aal spermatogonia. To test this, we determined As and Apr spermatogonial numbers for a fixed tubule length in both control and mutant mice at this stage. Using the transillumination technique [54], stage IX can be identified as a region adjacent to the sperm release front and is an easily identifiable region to assess the number of As and Apr cells (see Fig. 4B, left panel). An estimate of the As and Apr spermatogonial cell numbers was obtained by whole mount immunostaining for GFRα1 (representative image shown in Fig. 4B, middle panel). Seminiferous tubules from 12-week control and mutant testes were used for the analysis. After normalizing cell counts to the tubule length, no significant differences in GFRα1 positive As and Apr cell numbers were observed in control and mutant mice (Fig. 4C). Therefore, at the onset of a new proliferative cycle of Aundiff, Lin28a mutants have equivalent numbers of As and Apr spermatogonia as controls.
Next, we assessed the impact of LIN28A loss on longer clones of Aal-4, Aal-8, and Aal-16 spermatogonia. Previous studies have shown that Aundiff spermatogonia proliferate in stages XI-II and then become largely quiescent in stages III–VII. Thus the longest chains of undifferentiated spermatogonia poised to differentiate can be observed in stage VI–VII of the seminiferous cycle [10]. These cells are marked by SOHLH1, a molecule known to coordinate spermatogonial differentiation (representative image shown in Fig. 4B, right panel). Thus, whole mount seminiferous tubules were treated with an anti-SOHLH1 antibody and the numbers of Aal-4, Aal-8, and Aal-16 clones of spermatogonia were scored in stages VI–VII of the seminiferous cycle. On comparing the clonal numbers from both mutant and control adult seminiferous tubules, significant reductions in the number and clonal sizes of Aal-4, Aal-8, and Aal-16 spermatogonia were observed in mutant mice when compared to controls (Fig. 4D).
Loss of LIN28A does not affect viability or capacity of spermatogonial cells to differentiate
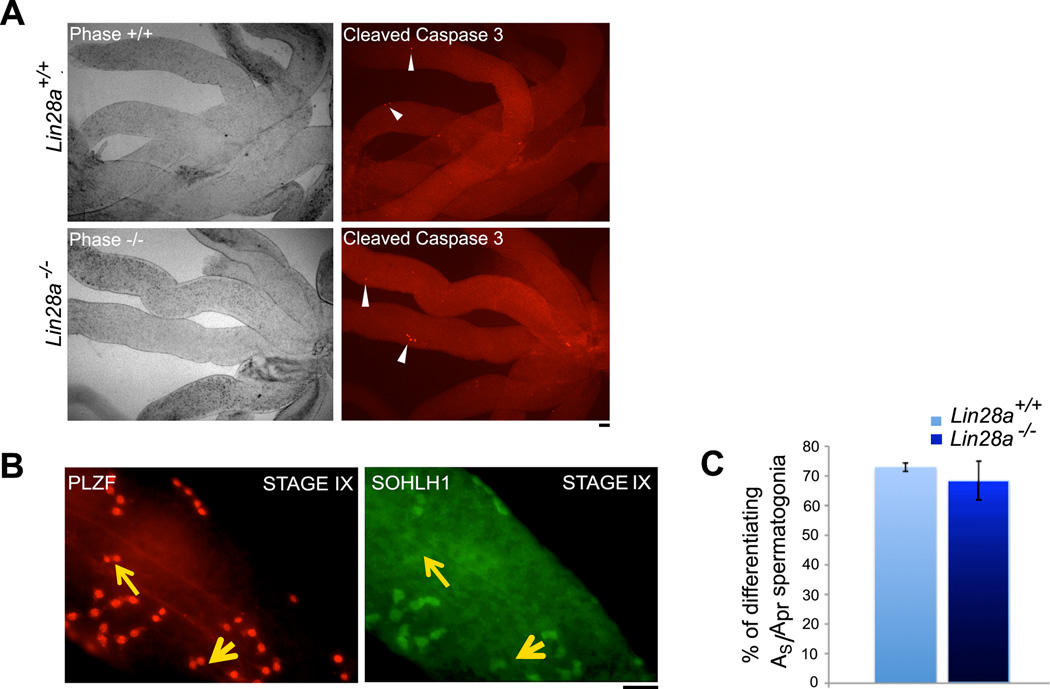
A decrease in spermatogonial clone size and numbers of Aal spermatogonia could arise from apoptosis of the expanding Aal-4, Aal-8, and Aal-16 spermatogonial clones, or from precocious differentiation of the undifferentiated As and Apr spermatogonia that are precursors to the longer clones of Aal spermatogonia. Therefore, we first performed whole mount immunostaining on seminiferous tubules with antibody against cleaved caspase 3, a marker of cells undergoing apoptosis [55], to assess spermatogonial cell death in Lin28a mutant mice. All stages of the seminiferous cycle were scrutinized for presence of apoptotic cells. In comparison to control mice, no significant differences in cell death were observed in mutant tubules (Fig. 5A).
Figure 5. Loss of Lin28a does not affect viability or differentiation potential of spermatogonial cells.
(A) Whole mount seminiferous tubules were immunostained with antibody against cleaved caspase-3 to identify apoptotic germ cells. Minimal cell death was observed in both control and Lin28a mutant testes. White arrowheads indicate the rare apoptotic cells in control and mutant testes (n=3) (B) Images represent the criteria used to identify differentiating AS and Apr spermatogonia in stages VIII and IX. PLZF positive AS and Apr spermatogonia that were also marked by SOHLH1 were scored as differentiating (small yellow arrowhead), while PLZF positive Apr spermatogonia negative for SOHLH1 were scored as undifferentiated spermatogonia (large yellow arrowhead). (C) Percentages of differentiating As and Apr spermatogonia in Stages VIII and IX were not significantly different for control and Lin28a mutant animals. Scale bar = 20 um.
Next, we assessed if Lin28a null spermatogonia were undergoing premature differentiation. Previous reports have suggested that while nearly all Aal cells differentiate into A1 cells by stage IX, 33% of the Apr cell population evade differentiation along with the As cells and “seed” fresh rounds of transit-amplification [10]. Thus premature differentiation of these remaining As and Apr undifferentiated cells at stages VIII and IX would result in fewer and shorter chains of Aal spermatogonia in the subsequent cycle. To assess premature differentiation of As and Apr spermatogonia, whole mount seminiferous tubules co-labeled with anti-PLZF (marker for undifferentiated spermatogonia) and anti-SOHLH1 (marker for differentiated spermatogonia) were evaluated [43, 44, 56]. Examination of the stages VIII and IX tubules allowed us to identify As and Apr undifferentiated (PLZF positive) and differentiating (SOHLH1 positive) spermatogonia (Fig. 5B). The ratio of SOHLH1 positive cells over PLZF positive spermatogonia was calculated. No differences were observed between control and Lin28a mutant mice for numbers of differentiating As and Apr spermatogonia (Fig. 5C). Premature differentiation of As and Apr spermatogonia into A1 spermatogonia in stage IX would also alter the normal stage-dependent associations of differentiating germs cells; something we have not observed. Together, these data suggest that absence of LIN28A did not cause premature differentiation of these As and Apr spermatogonia.
Stem cell potential of LIN28A depleted spermatogonial cells is unaltered
To determine the functional stem cell potential in Lin28a mutants, we performed transplantation experiments wherein the capacity of donor cells to recolonize and initiate spermatogenesis in a germ cell depleted recipient testis was assessed [57]. LIN28A is expressed in some As and all Apr cells, which together are thought to represent a portion of the SSC population [58]. If LIN28A functions in SSC self-renewal, the size of the stem cell pool in mutant mice should be reduced. In addition, lineage-tracing experiments have suggested that Ngn3-GFP positive spermatogonia (primarily Apr and chains of Aal that are marked by LIN28A) can contribute to colonization of the stem cell compartment following transplantation [15]. Given that Lin28a mutant mice have decreased numbers of PLZF-positive Aundiff spermatogonia, and if clones of Aal spermatogonia contribute substantially to colonization potential, a decrease in the Aundiff numbers is predicted to reduce the quantity of functional stem cells resulting in fewer colonies.
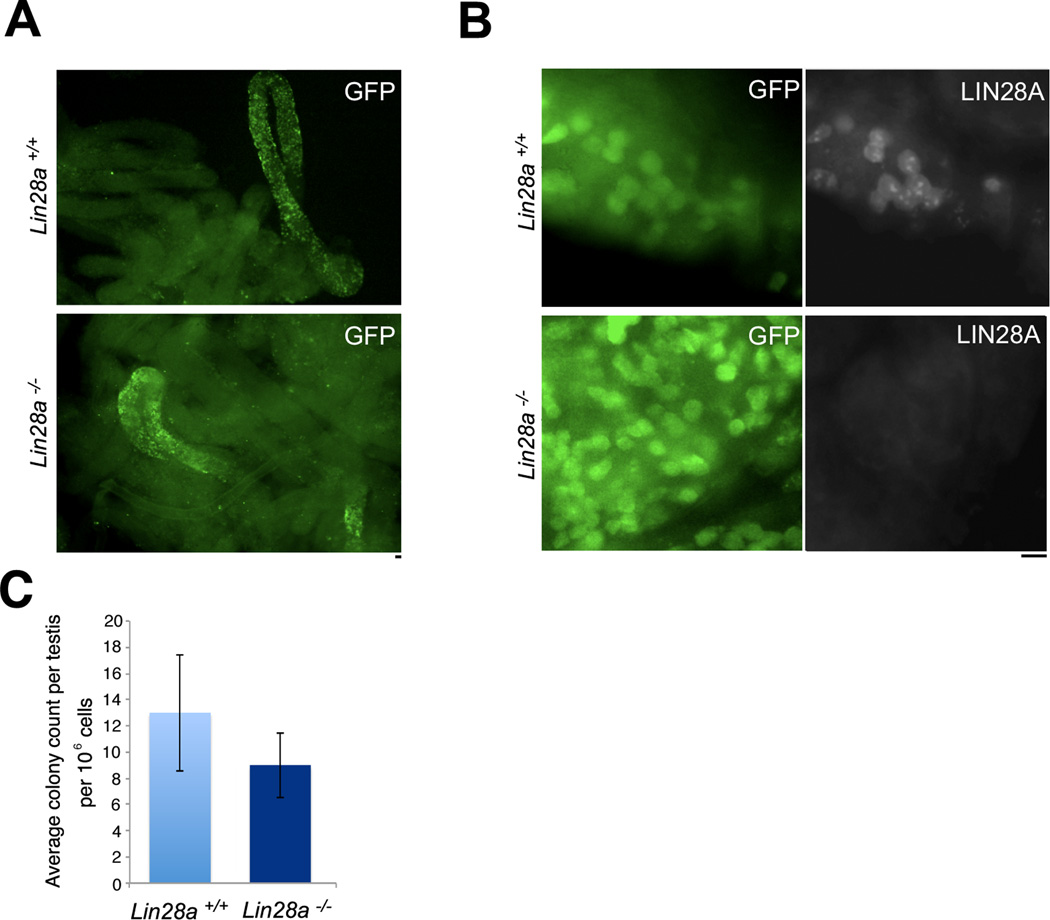
To identify donor-derived germ cells following transplantation experiments, Lin28a mutant mice were first crossed to a transgenic GFP reporter strain (Z/EG referred hereafter as ZEG) [59]. This reporter strain carries a GFP gene cassette preceded by a floxed transcriptional termination sequence. Cells that activate the Stra8 promoter express Cre recombinase, resulting in removal of the transcriptional stop sequences resulting in permanent GFP expression in the germ cell population. Germ cell suspensions prepared from 3-week-old Lin28a-Cre/ZEG and control animals were transplanted into busulfan treated (germ cell depleted) NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ recipient mice [60]. This immune deficient strain of mouse proved to be an ideal recipient for the Lin28a-Cre/ZEG mutant stem cells, which were derived from a mixed genetic background containing segregating C57BL/6, 129Sv and FVB/N alleles. Germ cells from Lin28a mutant males successfully colonized recipient testes (Fig. 6A). Because Stra8-iCre is not expressed in all As cells [49], we confirmed that colonies arising from Lin28a-Cre/ZEG mutants cells were depleted of LIN28A.Tubules from recipient mice were subjected to whole mount immunostaining with anti-GFP and LIN28A antibodies. The fact that the colonies generated by LIN28A deficient cells were indeed depleted of the LIN28A protein demonstrated that lack of LIN28A did not annihilate the self-renewing capacity of spermatogonial cells (Fig. 6B). Additionally, the number of colonies formed by donor cells from control and Lin28a mutant mice were not significantly altered (Fig. 6C) indicating that lack of Lin28a is not required for the self-renewal potential of the Aundiff spermatogonial compartment.
Figure 6. Stem cell potential of Lin28a-depleted spermatogonial cells is unaltered.
(A) Spermatogonial transplantation of control and Lin28a mutant donor testis. Panels on left shows microscopic views of seminiferous tubules from control and Lin28a mutant testes at low magnification. The fluorescent green stretches of cells in the tubules represent colonies of control (top) and Lin28a mutant (bottom) donor germ cells expressing a GFP reporter at 4 weeks post transplantation. (B) Whole mount immunostaining of tubules obtained from recipient testis stained with antibodies against LIN28A protein. Transplanted colonies obtained from donor Lin28a mutant mice showed an absence of LIN28A protein, while LIN28A positive spermatogonia were observed in transplanted colonies in control samples. (C) GFP positive colonies visualized under the microscope were scored and average colony count per testis per 105 cells was plotted. Three donors and three recipients were used in three independent experiments, and an average of colony numbers in two testes of a recipient mouse were taken in each experiment. No significant differences were observed in transplantation efficiencies of the control and Lin28a mutant donor cells. Scale bar = 20 um.
Loss of LIN28A dampens proliferation of Aundiff spermatogonia
Loss of LIN28A did not cause premature differentiation or apoptosis of spermatogonia, we therefore hypothesized that absence of LIN28A could dampen expansion of spermatogonial clones resulting in fewer and shorter syncytial chains. The mitotic amplification of the Aundiff population occurs during stages X to III of the spermatogenic cycle [50]. Cues extrinsic to cells arising from the local microenvironment in the seminiferous epithelium influence spermatogonial cell cycle kinetics. Interestingly, proliferation of the Aundiff spermatogonia extends beyond stage III when the numbers of A4, Intermediate and B spermatogonia are 50% lower than usual, suggesting that critical cell density-dependent regulation acts in concert with cell intrinsic factors to drive the cell cycle [50, 51, 61]. Previous studies have postulated the presence of unidentified chalone-like diffusible factors secreted by the differentiated spermatogenic cells that inhibit proliferation of Aundiff spermatogonia [62–64]. However, As cells may be less sensitive than Apr and Aal spermatogonia to these extrinsic cues and are known to proliferate in stages where Aal spermatogonia are mostly poised [53], indicating that cell intrinsic factors regulating proliferation are dominant in the As cell population. Thus, to eliminate these cell extrinsic cues and solely assess the cell intrinsic proliferative capacity of Lin28a null Aundiff spermatogonia, we determined their proliferative index during regeneration after busulfan insult [65]. Between days 8 to 16 post-busulfan insult, differentiating spermatogonia and maturing germ cells in an adult testes are greatly reduced thereby minimizing the impact of cell density dependent inhibitory feedback mechanisms as well as the chalone producing cell populations [66].
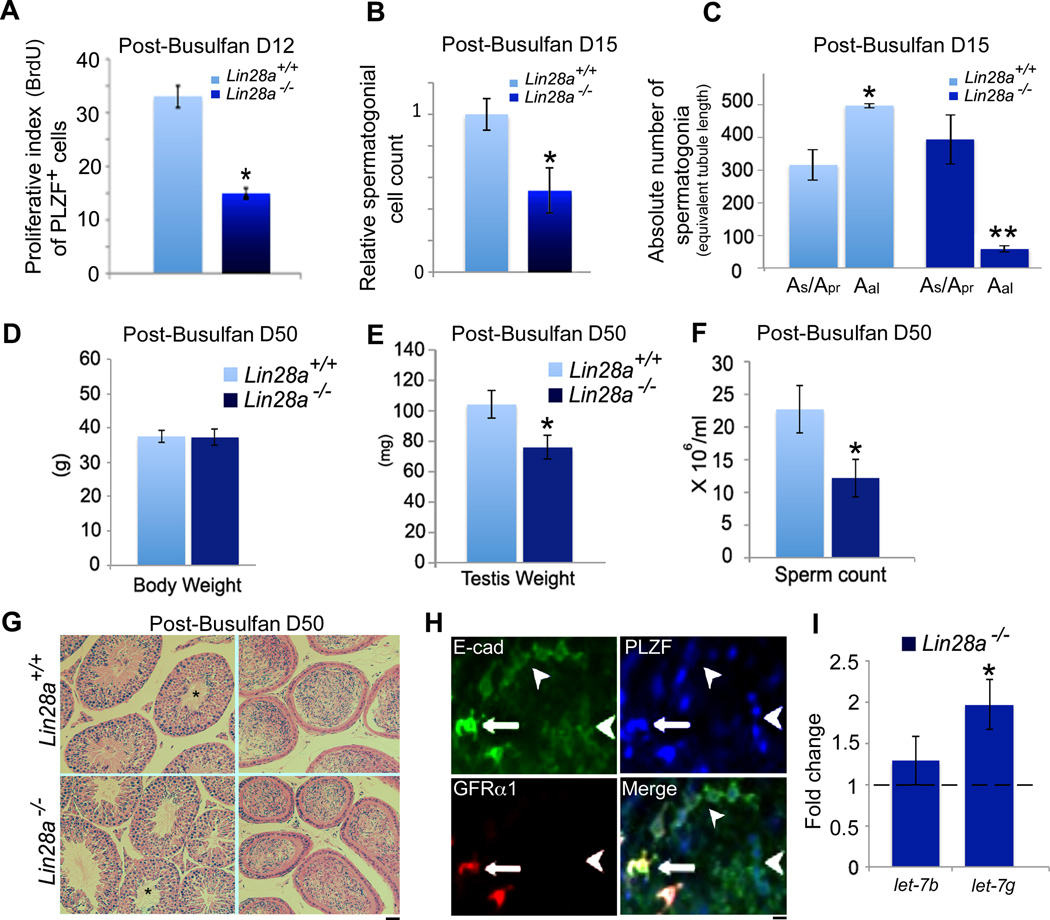
Previous reports have shown that by 9-days post-treatment with a single dose (10mg/kg) of busulfan, the stem cell pool is replenished [15]. Following this phase, between days 12 through 16, the TA progenitor cells enter a highly proliferative state to reinitiate spermatogenesis resulting in a rapid increase in Aundiff cell numbers [15, 61, 66]. We therefore compared the proliferative indices of Aundiff spermatogonial cells during this time-period when the influence of cell cycle altering extrinsic factors from the differentiating and differentiated cell types were minimal. In vivo BrdU labeling was performed at day 12-post busulfan treatment and the labeling indices of the Aundiff spermatogonia identified by PLZF on whole mount seminiferous tubules were scored. A 50% reduction in proliferation was observed in Lin28a mutant animals compared to controls (Fig. 7A). As a proxy of this reduction in proliferative index of Lin28a mutant spermatogonia at day 12, cell counts were also performed at day 15-post drug treatment. Whole mount seminiferous tubules co-labeled with GFRα1 and PLZF were scored at day 15-post busulfan treatment. A significant decrease in Aundiff spermatogonial numbers was observed in mutant animals at this time-point (Fig. 7B), primarily due to a marked reduction in the longer Aal undifferentiated spermatogonia clonal chains (Fig. 7C). To further assess the long-term effect of busulfan treatment on spermatogenesis in the absence of Lin28a, we analyzed control and mutant mice at day 50- post busulfan treatment. Both control and Lin28a mutant testes weights and sperm count levels were comparable and returned to pre-busulfan levels (compare Fig. 7D, E, F and Fig. 3 A, B, C). PAS stained sections showed spermiation fronts in both control and Lin28a mutants and accumulation of sperm in the epididymal ducts (Fig. 7G). Together, these data suggest that loss of LIN28A impairs expansion of Aundiff spermatogonial clones leading to a proportional reduction in sperm output, and that this condition reaches an equilibrium with no long-term effects on fertility.
Figure 7. Loss of LIN28A dampens spermatogonial cell proliferation.
(A) Analysis of BrdU labeling index of PLZF positive spermatogonia at day-12 post busulfan treatment. Seminiferous tubules from 8–10 week old adult control and Lin28a mutant mice were injected with BrdU and subjected to whole mount immunostaining with anti-BrdU and anti-PLZF antibodies. Percentage of nuclei double-positive for PLZF and BrdU were plotted. A significant decrease in the proliferative index of PLZF positive spermatogonia was observed (n=5). (B) 15 days post-busulfan treatment, testes samples were processed for whole mount immunostaining with GFRα1 and PLZF antibodies. PLZF/ GFRα1 double-positive spermatogonia were counted for equivalent tubule length in both control and Lin28a mutant mice. Both As/ Apr cells expressing high levels of GFRα1 as well as Aal spermatogonia with greatly reduced GFRα1 signal intensity were taken into account. Bar graph shows a significant decrease in PLZF/ GFRα1 double-positive spermatogonia in Lin28a mutant mice. (C) Bar graph represents absolute numbers of Aal and As/ Apr spermatogonia in control and Lin28a mutant mice for equivalent tubule length. A significant decrease in Aal spermatogonia was observed in Lin28a mutant mice in comparison to controls. (n=5). (D, E, F) Body weight, testis weight and epididymal sperm numbers were obtained at day-50 post-busulfan treatment. Both mutant and control mice recovered to steady state spermatogenesis (compare to Fig. 3A, B, C) (n=3). (G) PAS stained testes sections at day-50 post-busulfan treatment showed distinct spermiation fronts (left panel, asterisks) and mature sperm in the epididymal sections. (H) Whole mount immunostaining on adult seminiferous tubules revealed high levels of E-cadherin in GFRα1/PLZF positive As/ Apr spermatogonia and a moderate expression in GFRα1 negative PLZF positive Aal spermatogonia. (I) Plot represents levels of let-7b and let-7g in MACS sorted E-cadherin positive spermatogonia by qRT-PCR. In comparison to control, Lin28a mutants displayed a two-fold increase in let-7g miRNA expression whereas let-7b levels were not altered (n=4). * Indicates P≤0.01. ** Indicates P≤0.001. Values represent the mean ± SEM. Scale bar = 20 um.
The most intensely studied LIN28 molecular mechanism is its impact on the miRNA biogenesis of the let-7 gene family [36, 67]. The regulation of let-7g, in particular by LIN28A, has been well characterized [17, 68–70]. We assessed levels of mature let-7b and let-7g in spermatogonial cells from control and Lin28a mutant mice. Enrichment of spermatogonial cells was obtained by magnetic activated cell sorting (MACS) for E-cadherin, which is expressed at high levels in As and Apr cells marked by GFRα1, but is also expressed at moderate levels on the PLZF positive Aal spermatogonia (Fig. 7H). Real-time qRT-PCR analysis revealed a two-fold increase in let-7g levels in mutant spermatogonia, whereas the family member let-7b levels remained unaltered.
Discussion
A large transit amplifying stem progenitor pool is a hallmark of adult tissues that maintain high cellular output. Perturbation of the TA population can dramatically affect the pool of differentiated cells, and in the testis where some stem cell potential is thought to reside in the TA population, reduction of the progenitor population could have adverse effects on the size of the functional stem cell compartment. Our data show that Lin28a acts cell autonomously in germ cells to regulate Aundiff TA divisions and that it is a critical determinant of the progenitor pool size. However, contrary to expectations, loss of LIN28A did not significantly affect the size of the functional stem cell compartment, suggesting that neither LIN28A itself, nor the bulk of the Aal spermatogonia, substantially contribute to the stem cell compartment.
Other studies have not observed an adverse impact of LIN28A loss on spermatogonial proliferation. In one study, Zheng et al. showed that siRNA knockdown of Lin28a in SSCs cultured in vitro did not impact their proliferative capacity [17]. It is likely that the SSC population assessed in this study did not recapitulate the clones of undifferentiated Aal spermatogonia since they were still positive for GFRα1, a marker that largely represents the As and Apr cell population in vivo. In a second study, LIN28A function in the adult germline was assessed in a mouse model following total body deletion of the gene. Since Lin28a is expressed in several embryonic and adult tissues, whole body knockout (KO) of Lin28a produces a perinatal lethal phenotype in most inbred strains of mice with the exception of BALB/c, which yielded smaller litters [40, 42]. BALB/c Lin28a null mice presented a runted growth phenotype and alterations of FSH levels [40], which have been shown to impact the adult spermatogonial cell population in addition to the overall spermatogenic process [71–73]. Though the Lin28a KO adult males displayed reduced sperm counts, which were attributed to reduced PGC numbers, the numerous systemic effects in the BALB/c model make it difficult to interpret the direct impact of the loss of Lin28a on the TA Aundiff population [40].
SSC self-renewal
LIN28A does not appear to have a significant role in maintaining the SSC pool size. Lin28a mutant mice did not show an age-dependent decrease in testis weight or in sperm count. In addition, although mutant mice displayed a reduction in the progenitor pool of Aal-4, Aal-8 and Aal-16 clones, the loss of LIN28A did not significantly impact the size of the functional stem cell compartment as assayed by transplantation. This was surprising given that LIN28A has been associated with maintaining self-renewing capacity in embryonic cells [20, 74, 75], and lineage tracing studies suggest that some of the stem cell potential lies within the NGN3-positive population of germline progenitor cells [15]. Given that we primarily observed a reduction in longer chains of Aal spermatogonia (Aal-4, Aal-8 and Aal-16), our data suggest that stem cell potential is limited to As cells and clones no larger than Apr. We also propose (see below) that the As cells with the highest regenerating capacity are LIN28A negative.
Differentiation
Several studies have suggested a role of LIN28A in regulating cell fate selection. Constitutive overexpression of LIN28A during neurogliogenesis, where gliogenic neural progenitors divide and differentiate to first form neurons and then the supporting glial cells, causes reiteration of neural fates at the expense of glia [28]. In addition, overexpression of Lin28a induces differentiated fates in both muscle and hematopoietic cell lineages [26, 76]. In contrast, neural differentiation from neurogenic progenitors requires suppression of LIN28A function [16, 27]. We found that loss of Lin28a in the male germ line did not alter differentiation of As and Apr spermatogonia as assayed by SOHLH1 expression. We also failed to detect prematurely differentiating c-KIT positive chains of Aal spermatogonia in stages prior to VII and VIII. Therefore, in the testis, LIN28A does not appear to regulate spermatogonial differentiation.
Our experiments also show that loss of Lin28a does not impact viability of the Aal spermatogonial cells. Though Lin28a knockdown in human embryonic stem cells by siRNA causes cell apoptosis [22], depletion of Lin28a from spermatogonia did not lead to an increase in programmed cell death.
A possible conserved function of LIN28A in expansion of TA cell populations in adult stem cell compartments
The role of LIN28A as a regulator of cell proliferation is not unique to male germ cells. LIN28A regulates proliferation of both ES cells as well as primordial germ cells (PGC) in vitro and in vivo [21, 22, 40, 77, 78]. During induced pluripotent stem cell formation (iPSC), ectopic expression of Lin28a accelerates cell division rates and thus improves the kinetics of the process [24].
Like the murine male germline, which generates close to 4 million sperm on a daily basis [79], transit amplification of the progenitor cells in the adult intestine generates approximately 300 new cells/crypt/day to replenish its epithelial surface every 24–60 hours depending on the villi length [80, 81]. A commonality in both tissues is LIN28A expression in their TA cell populations [74]. Although the functional relevance of LIN28A expression in the TA cell population of the intestinal villi has not yet been elucidated, in light of our findings, it is tempting to speculate that LIN28A acts as a proliferative factor in this cell population as well. In support of this notion, overexpression of Lin28a in transgenic mice increases the numbers of the TA cells in the intestinal crypts [38]. It would be interesting to assess the presence and role of LIN28A in other adult stem cell systems such as the skin and blood to discern if it plays a similar function in the TA cell population in adult stem cell compartments that support a high cellular output.
LIN28A expression has also been observed in germ cell tumors, suggesting that enhanced levels of LIN28A in combination with additional genetic hits can alter TA cell kinetics resulting in neoplastic growth [82]. These findings are further bolstered by the observation that knocking down Lin28a expression in cancer cell lines causes growth inhibition and reduces cancer cell viability [83–86]. These proliferative effects are partly mediated by the let-7 family of miRNAs, levels of which are regulated by LIN28A [87]. For example, LIN28A has been shown to bind pre-let-7g and prevent the biogenesis of mature let-7g. The increased levels of let-7g in Lin28a mutant undifferentiated spermatogonia are consistent with it having a function in let-7 biogenesis. Intriguingly, we failed to detect a change in the family member let-7b, suggesting LIN28A does not regulate all let-7 family members equally. Overexpression of let-7g in murine lung cancer cells lead to cell cycle arrest and cell death [88]. It is possible an analogous mechanism involving let-7g might be at play in dampening proliferation of Aundiff spermatogonia in the absence of LIN28A.
LIN28A heterogeneity in the As population may distinguish stem cells from progenitor cells
Our data show that LIN28A is present in the Apr, Aal-4, Aal-8, and Aal-16 undifferentiated spermatogonia and in the differentiating A1–A4 spermatogonia, which together with the Intermediate and B spermatogonia, constitute the transit-amplifying compartment. However, we observed both LIN28A positive and LIN28A negative cells in the pool of PLZF-positive As spermatogonia, suggesting functional heterogeneity in the As cell population. We propose that this observed heterogeneity demarcates a PLZF+LIN28A− As stem cell population from As spermatogonia that are subjected to factors that induce their differentiation and will produce Apr at their next division. The latter cells express LIN28A and have entered a transit amplifying As cell population. In cells in which LIN28A is expressed, clonal growth becomes severely depressed when this factor is not available. Indeed, LIN28A may constitute a very early marker of stem cell differentiation. The fact that the As population does not get depleted with age, the ongoing production of Apr spermatogonia and the full development of repopulating colonies by transplanted LIN28A deficient SSCs indicate that the role of LIN28A in the regulation of SSC behavior is at best minimal. This is further corroborated by data obtained from a transgenic mouse model in which GDNF is overexpressed and SSCs are amplified (unpublished). The SSCs in these mice occur as clusters of GFRα1+/PLZF+/LIN28A− cells, indicating that LIN28A does not mark the As SSC population.
Finally, we propose that during the normal cycle of the seminiferous tubule the bulk of the Aal spermatogonia arise from the PLZF+ LIN28A+ As and Apr spermatogonia that escape differentiation into A1 spermatogonia during stages VII and VIII. Furthermore, the PLZF+/ LIN28A− As population represent cells that are in the SSC niche where self-renewal of these cells is stimulated [89]. When their daughter cells migrate out of the niche and come under the influence of differentiation promoting factors they express LIN28A and advance along the differentiation pathway as TA progenitor cells. These findings are a step forward in characterizing the bona fide SSCs that lie within the GFRA1+ / PLZF+ / LIN28A− As spermatogonial population.
Materials and methods
Animals
Mice were bred and maintained in a high barrier facility at The Jackson Laboratory. All experimental protocols were approved by The Jackson Laboratory Institutional Animal Care and Use Committee (ACUC) (permit #07007) and were in accordance with accepted institutional and government policies outlined in the NIH Guide for the Care and Use Laboratory Animals (1996; revised 2011).
Generation of Conditional Lin28a-Deleted Mice
For conditional ablation of Lin28a in adult spermatogonial stem cells, hemizygous FVB/N-Tg(Stra8-cre)1Reb/J (JAX Stock Number 008208) referred to here as Stra8-icre mice were mated with homozygous “floxed” B6;129-Lin28atm1Egm mice (kindly provided by Dr Eric G. Moss, Dept of Mol Biology, UMDNJ, New Jersey) to generate Stra8-icre;Lin28afl/+ offspring. F1 animals were intercrossed to obtain Stra8-icre; Lin28afl/fl mutant males.
Genotyping
Conditional Lin28a mice were genotyped using previously described primer sets [40]. Stra8-icre mice were genotyped using the primers (seq 5'-GTGCAAGCTGAACAACAGGA-3’) and ic381R (seq 5'-AGG GACACAGCATTGGAGTC-3’). Z/EG transgenic GFP mice were genotyped using the following primer sets: EGFP D1 (seq 5'-GCA AGCTGACCCTGAAGTTCA-3') and EGFP R1 (seq 5'-TCA CCT TGA TGC CGT TCT TCT-3').
Transplant Experiments
Hemizygous B6 Z/EG transgenic GFP reporter mice [Tg (CAG-Bgeo/GFP) 21Lbe/J], (JAX Stock Number 004178) were bred to Lin28afl/fl mice to obtain Lin28afl/+; Z/EG mice, that were then intercrossed with Stra8-icre;Lin28afl/+ mice to obtain donor cells for transplantation experiments. Stra8-icre;Lin28afl/−; Z/EG mutant and control (Stra8-icre;Lin28a+/+;Z/EG) mice were euthanized at 3 weeks and testicular single cell suspensions was made by a two-step digestion procedure as described previously [57]. For transplantation, cells were suspended in DMEM media containing 0.5mM pyruvate, 6mM L-glutamine, 100 units of penicillin, 100mg/ml of streptomycin, 2% of Fetal Calf Serum and trypan blue to aid in visualization the transplant. 108 cells/ml were injected into the efferent ducts of germ cell depleted recipient mice testes (NOD.CgPrkdcscid Il2rgtm1Wjl/SzJ, JAX Stock number 005557). 3-week-old NOD.CgPrkdcscid Il2rgtm1Wjl/SzJ recipients underwent germ cell depletion via treatment with 40mg/kg body weight of busulfan (Sigma, St Louis) dissolved in 1:1 volume ratio of DMSO and water injected intraperitoneally. Injections were performed in Biological Safety Laboratory level 2 designated rooms. Mice were housed separately and monitored for a 3–5 week period prior to performing transplants. Transplantation experiments were carried out under isoflurane inhalation anesthesia. Seminiferous tubules from mice containing transplanted germ cells were analyzed after 6 weeks for identification of donor colonies, as detected by the expression of GFP.
Epididymal sperm counts and fertility testing
Epididymal sperm counts were performed as mentioned previously [54]. For fertility testing, 6 unique matings of 10–12 week old FVB/N females were set up with either control or Lin28a mutant males and the number of produced progeny was recorded for each mating pair.
Immunofluorescence and whole mount immunostaining
Histology was performed as described previously [54]. Testes from control and mutant mice were weighed, fixed overnight at 4°C in Bouin's fixative or neutral-buffered formalin (NBF) prior to dehydration and embedding. For histological analysis, 5 µm sections were cut and mounted on glass slides and stained with Periodic Acid / Schiff’s (PAS) reagent using standard protocols. Whole mount immunostaining was performed as previously described [45] with tubules were fixed in 2% paraformaldehyde for 4 h prior to processing. Antibodies were purchased from the following companies. Polyclonal anti-PLZF, monoclonal anti-PLZF (Santa Cruz Biotech Inc., Santa Cruz, CA); monoclonal anti-GFP (BD Biosciences); polyclonal anti-GFRα-1 (R&D systems, Minneapolis, MN); polyclonal anti-LIN28A, cleaved Caspase 3, E-Cadherin (24E10) (Cell Signaling, Danvers, MA); polyclonal anti-SOHLH1 from (Abcam, Cambridge, MA); monoclonal anti-BrdU (G3G4 monoclonal, Hybridoma Shared Research Facility (Mount Sinai School of Medicine, New York, NY) and rat anti-LIN28A antibody (gift from Dr. Eric G. Moss, Dept of Mol. Biology, UMDNJ, New Jersey).
Epithelial stages were identified by characteristic differential interference contrast (DIC) and DAPI staining, allowing us to identify cell types at the basal membrane and also deeper in the tubules. In whole mounts, Stage VI can be distinguished by the presence of late B spermatogonia that are dividing into preleptotene spermatocytes. In addition, the elongated spermatids move to the lumen of the tubules in this stage. In Stage XI there are two generations of spermatocytes (zygotene and diplotene spermatocytes) and the elongating spermatids have contracted to such an extent that they have become flat cells. Stage I areas can be found by first looking for spermatocytes in meiotic divisions, indicating stage XII. Thanks to the spermatogenic wave, stage I is the bordering area in which meiotic divisions have been completed and round spermatids have been formed. Stage IV is characterized by the presence of late In spermatogonia that in this stage divide into B spermatogonia.
Quantitative Real Time RT-PCR
Total RNA was prepared from testes using the RiboPureTM Kit (Ambion). RNA was then reverse transcribed using SuperScript® III First-Strand synthesis system (Invitrogen, Camarillo, CA). Quantitative PCR was performed on the cDNA using SYBR® Green Master Mix (Applied Biosystems, Foster City, CA), and analyzed on an ABI 7500 Real Time PCR Machine (Applied Biosystems, Carlsbad, California). Primer sequences are as follows; Lin28a: 5'-GGTGGACGTCTTTGTGCACCAGAG-3' and 5'-CGCTCACTCCCAATACAGAACACAC-3', Lin28b: 5'-CGGGTAACAGGCCCAGG-3' and 5'-CGTCTCCACCTATCTCCCTTTG-3', 5'-CCAGTTCGCCATGGATGACGATAT-3' and 5'-GTCAGGATACCTCTCTTGCTCTG-3'. The PCR products were separated on a 2% agarose gel and visualized by ethidium bromide staining.
MACS sort and assessment of miRNA expression
Single cell suspensions from control and mutant testis were made by a two-step digestion procedure as described previously [57]. Cells from the testes were resuspended in 550 ul of PBS containing 0.5% BSA/ 2mM EDTA and incubated with 10 ul of E-cadherin antibody on ice for 30 min. E-cadherin positive cells were MACS sorted using anti-rabbit IgG microbeads as per manufacturers instructions (Miltenyi Biotec, Auburn, CA). Total RNA isolated from MACS sorted cells was obtained using the miRNeasy kit (Qiagen). RNA quality was analyzed on the Agilent 2100 Bioanalyzer. cDNA was prepared from 10 ng total RNA using the TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems) following manufacturer’s suggestions. Briefly, 10 ng of total RNA were reverse transcribed using primers specific to miRNAs hsa-let-7b, hsa-let-7g) and U6 snRNA. The resulting products were amplified and quantified using TaqMan Universal PCR Master Mix II, No UNG, (Applied Biosystems) and target specific primers on an ABI 7500 Real Time PCR System. Expression changes were determined using the standard ddCt method.
BrdU Proliferation assay
Mice were injected intraperitoneally with busulfan (10 mg/kg, Sigma, St Louis). Testes were processed for whole mount immunofluorescence at day 12 and day 15 post-busulfan treatments. Mice were injected intraperitoneally with 100 ug/ml of BrdU (Sigma, St. Louis, MO) 12 h prior to sample collection. Seminiferous tubules were dissected out and fixed in 4% paraformaldehyde for 6 h, washed three times in PBS and dehydrated in a methanol gradient (25%, 50%, 75%, in PBS) on ice for 5 min each. Tubules were then rehydrated in the same methanol gradient in the reverse order. Samples were washed in PBS two times for 5 min prior to blocking in 3% goat serum for 1 h at RT. Tubules were then incubated with polyclonal anti-PLZF over night at room-temperature. Following three PBS washes, samples were incubated for 1 h in the dark at room temperature with fluorescence-conjugated secondary antibodies Alexa Fluor®568 or Alexa Fluor®488 diluted in PBS + 3% goat serum, washed in PBS, and placed in 250 ul of cold 0.15 M NaCl. 600 ul of ice-cold ethanol was added dropwise while vortexing the samples. Tubules were then placed on ice for 30 min. Prior to refixing samples in 1% PFA (in PBS-T, 0.05% Tween 10 in PBS) for 30 min, samples were washed with 3% goat serum for 5 min at RT. Tubules were then incubated on ice for 30 min. 100 Kunitz units/ml of DNase (in 0.15M NaCl + 4.2mM MgCl) was added to the tubules and incubated for 30 min at RT. Samples were blocked with 3% goat serum for 15 min on ice and then reacted with an anti-BrdU antibody for 30 min on ice. Samples were washed with PBS-T, three times for 5 min and incubated with appropriate secondary antibodies prior to visualization.
Acknowledgements
The authors would like to thank Andrea Dearth, Adrienne Burton and Nichelle Gray for providing assistance with mouse colony maintenance, Dr Anne Greenlee for critically reviewing our manuscript and the Histology Core at The Jackson laboratory for their invaluable service.
Grant support: This research was supported by a grant to R.E.B. from NICHD/NIH (HD042454UW), and from NCI to The Jackson Laboratory (CA34196) in support of The Jackson Laboratory's shared services. P.C. was supported by a training grant from NICHD (HD07065).
Abbreviations used in this paper
- TA
transit amplifying
- SSCs
spermatogonial stem cells
- As
Asingle
- Apr
Apaired
- Aal
Aaligned
- Aundiff
Aundifferentiated
Footnotes
Disclaimer: There are no potential financial conflicts of interest.
Author Contribution: P.C.: conception and design, data analysis and interpretation, collection and assembly of data, manuscript writing, financial support; F.W.B.: provision of study materials, collection and assembly of data, manuscript writing; M.S.: provision of study materials, collection and assembly of data, manuscript writing; E.S.: collection and assembly of data, manuscript writing; D.G.D.R.: data analysis and interpretation, manuscript writing; R.E.B.: conception and design, data analysis and interpretation, manuscript writing, financial support.
Contributor Information
P Chakraborty, Email: Papia.Chakraborty@jax.org.
F.W. Buaas, Email: Bill.Buaas@jax.org.
M Sharma, Email: Manju.Sharma@jax.org.
E Snyder, Email: Elizabeth.Snyder@jax.org.
D.G. de Rooij, Email: D.G.deRooij@uu.nl.
R.E. Braun, Email: bob.braun@jax.org.
References
- 1.Jaks V, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature genetics. 2008;40(11):1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 2.Mascre G, et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489(7415):257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- 3.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 4.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303(5656):359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberts B, et al. Molecular Biology of the Cell. 2007;Chapter 23:83–88. [Google Scholar]
- 6.Hermann BP, et al. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques. Human reproduction. 2009;24(7):1704–1716. doi: 10.1093/humrep/dep073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franca, R.A..H.a.L.R.d. Molecular mechanisms of Spermatogensis. Chapter 1. Molecular mechanisms of Spermatogensis; 2008. pp. 1–15. [Google Scholar]
- 8.Davies EL, Fuller MT. Regulation of self-renewal and differentiation in adult stem cell lineages: lessons from the Drosophila male germ line. Cold Spring Harbor symposia on quantitative biology. 2008;73:137–145. doi: 10.1101/sqb.2008.73.063. [DOI] [PubMed] [Google Scholar]
- 9.Oakberg EF. Spermatogonial stem-cell renewal in the mouse. The Anatomical record. 1971;169(3):515–531. doi: 10.1002/ar.1091690305. [DOI] [PubMed] [Google Scholar]
- 10.Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutation research. 1993;290(2):193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 11.Insco ML, et al. Accumulation of a differentiation regulator specifies transit amplifying division number in an adult stem cell lineage. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22311–22316. doi: 10.1073/pnas.0912454106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parrott BB, et al. Nucleoporin98-96 function is required for transit amplification divisions in the germ line of Drosophila melanogaster. PloS one. 2011;6(9):e25087. doi: 10.1371/journal.pone.0025087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivdasani AA, Ingham PW. Regulation of stem cell maintenance and transit amplifying cell proliferation by tgf-beta signaling in Drosophila spermatogenesis. Current biology : CB. 2003;13(23):2065–2072. doi: 10.1016/j.cub.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 14.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. Journal of andrology. 2000;21(6):776–798. [PubMed] [Google Scholar]
- 15.Nakagawa T, et al. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328(5974):62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aeckerle N, et al. The pluripotency factor LIN28 in monkey and human testes: a marker for spermatogonial stem cells? Molecular human reproduction. 2012;18(10):477–488. doi: 10.1093/molehr/gas025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng K, et al. The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC developmental biology. 2009;9:38. doi: 10.1186/1471-213X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss EG, Lee RC, Ambros V. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell. 1997;88(5):637–646. doi: 10.1016/s0092-8674(00)81906-6. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y, et al. Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene. 2006;384:51–61. doi: 10.1016/j.gene.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Moss EG, Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Developmental biology. 2003;258(2):432–442. doi: 10.1016/s0012-1606(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 21.Darr H, Benvenisty N. Genetic analysis of the role of the reprogramming gene LIN-28 in human embryonic stem cells. Stem cells. 2009;27(2):352–362. doi: 10.1634/stemcells.2008-0720. [DOI] [PubMed] [Google Scholar]
- 22.Peng S, et al. Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells. 2011;29(3):496–504. doi: 10.1002/stem.591. [DOI] [PubMed] [Google Scholar]
- 23.Xu B, Zhang K, Huang Y. Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells. RNA. 2009;15(3):357–361. doi: 10.1261/rna.1368009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanna J, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462(7273):595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li N, et al. Lin-28 homologue A (LIN28A) promotes cell cycle progression via regulation of cyclin-dependent kinase 2 (CDK2), cyclin D1 (CCND1), and cell division cycle 25 homolog A (CDC25A) expression in cancer. The Journal of biological chemistry. 2012;287(21):17386–17397. doi: 10.1074/jbc.M111.321158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polesskaya A, et al. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev. 2007;21(9):1125–1138. doi: 10.1101/gad.415007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawahara H, et al. Musashi1 cooperates in abnormal cell lineage protein 28 (Lin28)-mediated let-7 family microRNA biogenesis in early neural differentiation. J Biol Chem. 286(18):16121–16130. doi: 10.1074/jbc.M110.199166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balzer E, et al. LIN28 alters cell fate succession and acts independently of the let-7 microRNA during neurogliogenesis in vitro. Development. 2010;137(6):891–900. doi: 10.1242/dev.042895. [DOI] [PubMed] [Google Scholar]
- 29.Piskounova E, et al. Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J Biol Chem. 2008;283(31):21310–21314. doi: 10.1074/jbc.C800108200. [DOI] [PubMed] [Google Scholar]
- 30.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320(5872):97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heo I, et al. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell. 2008;32(2):276–284. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Heo I, et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell. 2009;138(4):696–708. doi: 10.1016/j.cell.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Lehrbach NJ, et al. LIN-28 and the poly(U) polymerase PUP-2 regulate let-7 microRNA processing in Caenorhabditis elegans. Nat Struct Mol Biol. 2009;16(10):1016–1020. doi: 10.1038/nsmb.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagan JP, Piskounova E, Gregory RI. Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nature structural & molecular biology. 2009;16(10):1021–1025. doi: 10.1038/nsmb.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y. A mirror of two faces: Lin28 as a master regulator of both miRNA and mRNA. Wiley interdisciplinary reviews. RNA. 2012;3(4):483–494. doi: 10.1002/wrna.1112. [DOI] [PubMed] [Google Scholar]
- 36.Nam Y, et al. Molecular basis for interaction of let-7 microRNAs with Lin28. Cell. 2011;147(5):1080–1091. doi: 10.1016/j.cell.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viswanathan SR, Daley GQ. Lin28: A microRNA regulator with a macro role. Cell. 2010;140(4):445–449. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H, et al. Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nature genetics. 2010;42(7):626–630. doi: 10.1038/ng.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu H, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147(1):81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shinoda G, et al. Lin28a Regulates Germ Cell Pool Size and Fertility. Stem cells. 2013 doi: 10.1002/stem.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu J, et al. Mouse ZAR1-like (XM_359149) colocalizes with mRNA processing components and its dominant-negative mutant caused two-cell-stage embryonic arrest. Dev Dyn. 239(2):407–424. doi: 10.1002/dvdy.22170. [DOI] [PubMed] [Google Scholar]
- 42.Gaytan F, et al. Distinct Expression Patterns Predict Differential Roles of the miRNA-Binding Proteins, Lin28 and Lin28b, in the Mouse Testis: Studies During Postnatal Development and in a Model of Hypogonadotropic Hypogonadism. Endocrinology. 2013;154(3):1321–1336. doi: 10.1210/en.2012-1745. [DOI] [PubMed] [Google Scholar]
- 43.Ballow D, et al. Sohlh1 is essential for spermatogonial differentiation. Developmental biology. 2006;294(1):161–167. doi: 10.1016/j.ydbio.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 44.Buaas FW, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nature genetics. 2004;36(6):647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 45.Smith BE, Braun RE. Germ cell migration across Sertoli cell tight junctions. Science. 2012;338(6108):798–802. doi: 10.1126/science.1219969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buageaw A, et al. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biology of reproduction. 2005;73(5):1011–1016. doi: 10.1095/biolreprod.105.043810. [DOI] [PubMed] [Google Scholar]
- 47.Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Developmental biology. 2005;279(1):114–124. doi: 10.1016/j.ydbio.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng X, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287(5457):1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 49.Sadate-Ngatchou PI, et al. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis. 2008;46(12):738–742. doi: 10.1002/dvg.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121(3):347–354. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- 51.De Rooij DG, Janssen JM. Regulation of the density of spermatogonia in the seminiferous epithelium of the Chinese hamster: I. Undifferentiated spermatogonia. The Anatomical record. 1987;217(2):124–130. doi: 10.1002/ar.1092170203. [DOI] [PubMed] [Google Scholar]
- 52.de Rooij DG. Spermatogonial stem cell renewal in the mouse. I. Normal situation. Cell and tissue kinetics. 1973;6(3):281–287. doi: 10.1111/j.1365-2184.1973.tb01617.x. [DOI] [PubMed] [Google Scholar]
- 53.De Rooij DG. Regulation of the proliferation of spermatogonial stem cells. Journal of cell science. Supplement. 1988;10:181–194. doi: 10.1242/jcs.1988.supplement_10.14. [DOI] [PubMed] [Google Scholar]
- 54.Gallagher SJ, et al. Distinct requirements for Sin3a in perinatal male gonocytes and differentiating spermatogonia. Developmental biology. 2013;373(1):83–94. doi: 10.1016/j.ydbio.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends in biochemical sciences. 1997;22(8):299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki H, et al. SOHLH1 and SOHLH2 coordinate spermatogonial differentiation. Developmental biology. 2012;361(2):301–312. doi: 10.1016/j.ydbio.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(24):11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aponte PM, et al. Spermatogonial stem cells: characteristics and experimental possibilities. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2005;113(11–12):727–742. doi: 10.1111/j.1600-0463.2005.apm_302.x. [DOI] [PubMed] [Google Scholar]
- 59.Novak A, et al. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28(3–4):147–155. [PubMed] [Google Scholar]
- 60.Shultz LD, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. Journal of immunology. 2005;174(10):6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 61.van Keulen CJ, de Rooij DG. The recovery from various gradations of cell loss in the mouse seminiferous epithelium and its implications for the spermatogonial stem cell renewal theory. Cell and tissue kinetics. 1974;7(6):549–558. doi: 10.1111/j.1365-2184.1974.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 62.Bustos-Obregon E. [G1 spermatogonial chalone] Archivos de biologia y medicina experimentales. 1989;22(1):15–23. [PubMed] [Google Scholar]
- 63.Clermont Y, Mauger A. Effect of a spermatogonial chalone on the growing rat testis. Cell and tissue kinetics. 1976;9(1):99–104. doi: 10.1111/j.1365-2184.1976.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 64.de Rooij DG. Effect of testicular extracts on proliferation of spermatogonia in the mouse. Virchows Archiv. B, Cell pathology including molecular pathology. 1980;33(1):67–75. doi: 10.1007/BF02899171. [DOI] [PubMed] [Google Scholar]
- 65.Bucci LR, Meistrich ML. Effects of busulfan on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities, and dominant lethal mutations. Mutation research. 1987;176(2):259–268. doi: 10.1016/0027-5107(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 66.van Keulen CJ, de Rooij DG. Spermatogonial stem cell renewal in the mouse. II. After cell loss. Cell and tissue kinetics. 1973;6(4):337–345. doi: 10.1111/j.1365-2184.1973.tb01622.x. [DOI] [PubMed] [Google Scholar]
- 67.Mayr F, et al. The Lin28 cold-shock domain remodels pre-let-7 microRNA. Nucleic acids research. 2012;40(15):7492–7506. doi: 10.1093/nar/gks355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ali PS, et al. Recognition of the let-7g miRNA precursor by human Lin28B. FEBS letters. 2012;586(22):3986–3990. doi: 10.1016/j.febslet.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 69.Desjardins A, et al. Importance of the NCp7-like domain in the recognition of pre-let-7g by the pluripotency factor Lin28. Nucleic acids research. 2012;40(4):1767–1777. doi: 10.1093/nar/gkr808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lightfoot HL, et al. A LIN28-dependent structural change in pre-let-7g directly inhibits dicer processing. Biochemistry. 2011;50(35):7514–7521. doi: 10.1021/bi200851d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia-Galiano D, et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153(1):316–328. doi: 10.1210/en.2011-1260. [DOI] [PubMed] [Google Scholar]
- 72.Sairam MR, Krishnamurthy H. The role of follicle-stimulating hormone in spermatogenesis: lessons from knockout animal models. Archives of medical research. 2001;32(6):601–608. doi: 10.1016/s0188-4409(01)00328-9. [DOI] [PubMed] [Google Scholar]
- 73.Zhang FP, et al. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Molecular endocrinology. 2001;15(1):172–183. doi: 10.1210/mend.15.1.0582. [DOI] [PubMed] [Google Scholar]
- 74.Yang DH, Moss EG. Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expr Patterns. 2003;3(6):719–726. doi: 10.1016/s1567-133x(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 75.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463(7281):621–626. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X, et al. MiR-181 mediates cell differentiation by interrupting the Lin28 and let-7 feedback circuit. Cell death and differentiation. 2012;19(3):378–386. doi: 10.1038/cdd.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Du L, Lin G, Lu G. Generation and identification of pluripotent stem cells from human embryonic fibroblast cells by 4 defined factors. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34(12):1157–1165. [PubMed] [Google Scholar]
- 78.Viswanathan SR, Daley GQ. Lin28: A microRNA regulator with a macro role. Cell. 140(4):445–449. doi: 10.1016/j.cell.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 79.Thayer KA, et al. Altered prostate growth and daily sperm production in male mice exposed prenatally to subclinical doses of 17alpha-ethinyl oestradiol. Human reproduction. 2001;16(5):988–996. doi: 10.1093/humrep/16.5.988. [DOI] [PubMed] [Google Scholar]
- 80.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110(4):1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 81.Creamer B, Shorter RG, Bamforth J. The turnover and shedding of epithelial cells. I. The turnover in the gastro-intestinal tract. Gut. 1961;2:110–118. doi: 10.1136/gut.2.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cao D, et al. RNA-binding protein LIN28 is a marker for testicular germ cell tumors. Human pathology. 2011;42(5):710–718. doi: 10.1016/j.humpath.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 83.Viswanathan SR, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41(7):843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peng S, Maihle NJ, Huang Y. Pluripotency factors Lin28 and Oct4 identify a sub-population of stem cell-like cells in ovarian cancer. Oncogene. 29(14):2153–2159. doi: 10.1038/onc.2009.500. [DOI] [PubMed] [Google Scholar]
- 85.Pan L, et al. Lin-28 reactivation is required for let-7 repression and proliferation in human small cell lung cancer cells. Molecular and cellular biochemistry. 2011;355(1–2):257–263. doi: 10.1007/s11010-011-0862-x. [DOI] [PubMed] [Google Scholar]
- 86.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson CD, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer research. 2007;67(16):7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 88.Kumar MS, et al. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(10):3903–3908. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Rooij DG, van Beek ME. Computer simulation of the rodent spermatogonial stem cell niche. Biology of reproduction. 2013;88(5):131. doi: 10.1095/biolreprod.113.108639. [DOI] [PubMed] [Google Scholar]