Abstract
Highly active antiretroviral therapy (HAART) has been successful in reducing human immunodeficiency virus (HIV)-1-associated morbidity and mortality since its introduction in 1996. However, it fails to eradicate HIV-1 infection. The high cost of life-long highly active antiretroviral therapy and the emergence of drug resistance among HIV-1-infected individuals have brought renewed pressure for the discovery of novel antivirals and alternative medicines. Traditional Chinese medicine (TCM) is a complementary and alternative medicine, and serves as a rich resource for new drug development. Despite the almost 100 plant-derived compounds that are in clinical trials, few target HIV-1 infection. In this study, we discovered that Sanguisorba officinalis extract (SOE) has anti-HIV-1 properties. Using a cell-based assay and single-cycle luciferase reporter viruses pseudotyped with envelopes from HIV-1 or control viruses, we found that SOE exhibited significant inhibitory ability against both CCR5 and CXCR4 tropic HIV-1 (ADA and HXB2), with respective IC50 values of 1.91 ± 0.16 μg/mL and 3.70 ± 0.53 μg/mL. SOE also inhibited simian immunodeficiency virus infection but failed to block vesicular stomatitis virus, severe acute respiratory syndrome coronavirus, and influenza H5N1 pseudoviruses. Furthermore, we showed that SOE had no effect on postentry events of HIV-1 replication. Because SOE pretreatment with the virus but not with cell lines expressing viral receptors showed the maximal inhibitory activity, we can state that SOE probably blocks entry by acting on the viral envelope directly. In addition, SOE was able to inhibit reverse transcriptase inhibitor resistant viruses (K103N, Y188L, and K103N/Y188L/G190A) and a protease inhibitor resistant strain (PI-2840). Our findings demonstrate SOE as a novel and specific entry inhibitor, which sheds light on the discovery of anti-HIV-1 drugs from traditional herbal medicines.
Keywords: Entry inhibitor, Highly active antiretroviral therapy, Human immunodeficiency virus, Sanguisorba officinalis, Traditional Chinese medicine
1. Introduction
Traditional Chinese medicine (TCM) includes various forms of herbal medicine, acupuncture, moxibustion, massage, Qi exercise, meditation, and dietary therapy. TCM was developed in China as a personalized medical practice based on a tradition of more than 5000 years. As a result, thousands of plants have been used as herbal medicines for treating different types of diseases. For example, one of the most famous drugs, artemisinin, was originally extracted from the plant Artemisia annua and is now a standard treatment worldwide for Plasmodium falciparum malaria [1]. During the past decade, the use of TCM has increased globally as one of the mainstreams of complementary and alternative medicine. It also serves as a rich resource for new drug development.
Prior to the availability of highly active antiretroviral therapy (HAART), people with human immunodeficiency virus (HIV)/AIDS often sought herbal therapy in China. This situation continues today because HAART is still not readily accessible or affordable especially in rural areas, where the majority of patients reside. Even in the era of HAART, HIV-infected people who used herbal therapy at a high rate sought more frequent visits to TCM providers and reported helpful improvement with the treatment [2], [3]. In most cases, however, it remains unknown whether the herbs used had any anti-HIV activities, and therefore it is unknown if they were regarded as enhancing patients' immune function, as the treatment of HIV-related symptoms, or as the management of HAART-related side effects [4]. In recent years, studies have demonstrated that evidence-based research on compounds extracted from herbal plants can elucidate their biochemical activity, revealing antitumor activity in some cases and anti-HIV activity in others [5], [6], [7], [8], [9]. The study of some herbal compounds has been moved forward into clinical trials [6], [7], [10]. Prior to this study, however, it was unclear if Sanguisorba officinalis had any anti-HIV activity.
Sanguisorba officinalis (also called Great Burnet) is a plant in the family Rosaceae, subfamily Rosoideae. It is easily found in the northern regions of China and has been used as TCM for thousands of years to treat hemostasis and inflammation [11]. Some other functions that have been discovered in recent years include its antioxidant and antitumor properties [12], [13], [14].To the best of our knowledge, we are the first group to study the anti-HIV-1 activities of the extract of S. officinalis (SOE). Our findings have implications for exploring TCM for new antiviral discoveries.
2. Materials and methods
2.1. Preparation of SOE
The stem of S. officinalis was cut into small pieces and immersed in distilled water. The mixture was treated by ultrasound for 1 hour, followed by boiling twice at 100°C for 30 minutes. After filtration, the supernatant was concentrated by a rotary evaporator under reduced pressure, followed by lyophilization for 48 hours to obtain the aqueous extract powder (SOE). The yield of SOE was 9.9–11.2%. High-performance liquid chromatography analysis of SOE was conducted on an Ultimate AQ-C18 column (250 mm × 4.6 mm, 5 μm)(Welch, Shanghai, China). The mobile phase consisted of acetonitrile (A) and 0.2% formic acid (B) in water, using a gradient elution of 5–15% A at 0–10 minutes, 15%–25% A at 10–25 minutes, 25%–30% A at 25–35 minutes, 30%–70% A at 35–45 minutes, 70%–95% A at 45–50 minutes, and 95% A at 50–60 minutes. The solvent velocity of flow was 1.0 mL/minute and the column was at room temperature. The detection wavelength was set at 254 nm. The contents of each component were controlled between 1.25% and 1.35% and 1.69% and 1.80%, respectively. The extract powder was dissolved in phosphate buffered saline and passed through 0.45 μm filter for sterilization. SOE was dissolved in dimethyl sulfoxide at a concentration of 40 mg/mL, followed by gentle vortex at room temperature. The supernatants were carefully collected after centrifugation at 3000g for 5 minutes and filtered through a 0.22 μm Millipore syringe filter for experiments.
2.2. Cell culture and virus generation
The cell lines 293T, MDCK, and HEK293T-ACE2 were cultured in Dulbecco's modified Eagle medium with 10% inactivated fetal bovine serum (Invitrogen), 100 units/mL penicillin, and 100 μg/mL streptomycin sulfate (Invitrogen). GHOST(3)-CD4-CCR5/CXCR4 cells were obtained from the US National Institutes of Health (NIH, Bethesda, MA, USA) AIDS Research and Reference Reagent Program and cultured in Dulbecco's modified Eagle medium with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin sulfate, 100 μg/mL hygromycin B, 500 μg/mL G418, and 1 μg/mL puromycin (Sigma Aldrich). Single-cycle luciferase HIVADA, HIVHxB2, simian immunodeficiency virus (SIV)mac239, SIVmac1a11 vesicular stomatitis virus (VSV), H5N1anhui, and severe acute respiratory syndrome coronavirus (SARS-CoV) pseudoviruses were constructed as described previously [9], [15], [16], [17]. Briefly, pseudoviruses were generated by co-transfection of 293T cells (using polyethylenimine; Polysciences Inc., Warrington, PA, USA) with NL4-3 E–V– Luc+ and envelope protein from different strains of viruses. All non-nucleoside reverse-transcriptase inhibitor (NNRTI) mutant constructs were generated based on a molecular clone backbone NL4-3 E–V– Luc+, as we previously described [8]. The protease-inhibitor resistant strain (PI-2840) was obtained from NIH (resistant to multiple anti-HIV protease drugs; carrying L10R, M46I, L63P, V82T, and I84V). Cell-free supernatant was collected 48 hours post-transfection and frozen at −80°C. The 50% tissue culture infective dose was calculated as described previously [8].
2.3. Cell viability assay
GHOST(3)-CD4-CCR5/CXCR4 cells were incubated in the presence or absence of serially diluted SOE at 37°C in 5% CO2 for 48 hours. Cell viability was then measured using a commercially available kit (CellTiter-Glo Luminescent Cell Viability Assay kit; Promega, USA).
2.4. Antiviral assay
The inhibitory activities of the SOE against viruses were evaluated as described previously [8]. Briefly, a serially diluted drug was tested against virus infection at 50% tissue culture infective dose. GHOST(3)-CD4-CCR5/CXCR4 were used for HIVADA, HIVHxB2, SIVmac239 and SIVmac1a11, whereas MDCK and HEK293T-ACE2 were used for H5N1 and SARS-CoV pseudovirus infection, respectively. The viral infection was determined on Day 3 by measuring the reporter luciferase activity in target cells postinfection using commercially available kits. Antiviral data are reported as the quantity of drug required to inhibit viral production by 50% (EC50).
2.5. Fractionation of aqueous SOE
Different polar solvents were used to obtain subfractions from SOE yielding petroleum–ether-partitioned extract (PE), ethyl-acetate-partitioned extract (EtOAc), n-butanol-partitioned extract (BuOH), and water residues. These subfractions were then concentrated in a vacuum dryer for later testing.
3. Results
3.1. Anti-viral activity of aqueous SOE
To determine the antiviral activity of SOE, GHOST-CCR5 and GHOST-CXCR4 cells were infected with CCR5-tropic HIV-1ADA and CXCR4-tropic HIV-1HxB2, respectively, in the presence of serial diluted SOE. As a control, VSV pseudovirus was included in parallel experiments. As shown in Fig. 1 A and B, SOE displayed an EC50 of 1.91 ± 0.16 μg/mL and 3.70 ± 0.53 μg/mL in the inhibition of HIV-1 replication of both coreceptor tropisms without observed cell toxicity (Fig. 1C and D). By contrast, when SOE was tested against VSV pseudovirus infection, no inhibition was observed. Because ADA, HXB2, and VSV pseudoviruses share the same HIV backbone and differ only in the glycoproteins expressed on the viral surface, this result suggests that SOE acts specifically in inhibiting HIV-1 replication at an early stage of viral entry.
Fig. 1.
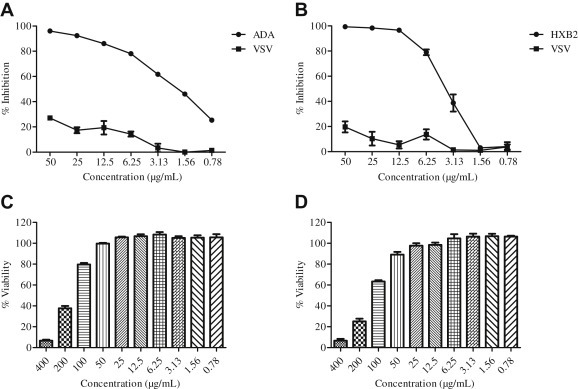
Activity of Sanguisorba officinalis extract (SOE) against HIV-1ADA, HIVHxB2, and HIVVSV. Serially diluted SOE was added to GHOST-CCR5 and GHOST-CXCR4 infected with (A) HIV-1ADA and (B) HIVHxB2, respectively. HIVVSV was tested as a negative control. The luciferase level was measured 2 days postinfection. All results are means ± standard errors of the means from three independent experiments. To test SOE cytotoxicity, (C) GHOST(3)-CD4-CCR5 and (D) GHOST(3)-CD4-CXCR4 cells were cultured in serially diluted SOE at 37°C for 48 hours in 5% CO2. Cell viability was then measured using the Promega CellTiter-Glo Luminescent Cell Viability Assay kit. The data represent the mean ± standard deviation of triplicate experiments.
3.2. No effect of SOE on HIV-1 postentry
To confirm that SOE antagonizes viral entry rather than postentry events, we tested the same panel of viruses but with a modified experimental schedule. Target cells were first co-incubated with pseudovirus for 2 hours, washed, and then treated with the presence of 50 μg/mL SOE or 1 μM AZT, which is a potent NNRTI, for 48 hours. As shown in Fig. 2 A, with this experimental schedule, SOE does not strongly inhibit HIV-1 anymore, as compared with AZT. This result supports the finding that inhibition of SOE on HIV-1 replication is not due to the blockade at the postentry step (e.g., reverse transcription) but rather at an entry step.
Fig. 2.
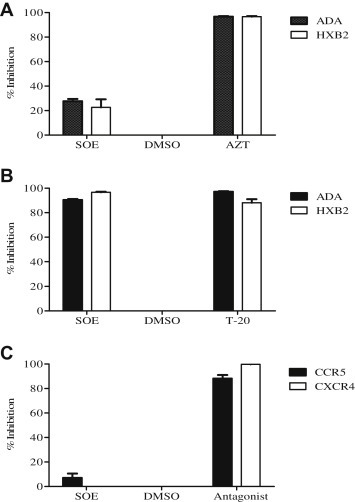
Sanguisorba officinalis extract (SOE) inhibits HIV-1 infection by binding to the viral envelope and blocking entry of the virus. (A) Post entry assay. GHOST-CD4-CCR5 or CXCR4 cells were coincubated with pseudovirus for 2 hours, washed, and then treated with the presence of 50 μg/mL SOE, dimethyl sulfoxide (DMSO) as negative control and 1 μM AZT as positive control for 48 hours. SOE does not inhibit either HIV-1ADA or HIVHxB2 virus gene replication after viral entry is achieved. (B) SOE-virus interaction assay. HIV-1ADA and HIVHxB2 pseudovirus pre-treated with 50 μg/mL SOE or DMSO as a negative control and entry inhibitor enfuvirtide (T-20) as a positive control. SOE pretreatment inhibited both HIVADA and HIVHxB2 pseudovirus infection to a similar degree as T-20. (C) SOE-cell binding assay. GHOST cells were pretreated with SOE or the CCR5 antagonist maraviroc and the CXCR4 antagonist JM2987 as positive controls, and DMSO as negative control for 1 hour at 37°C prior to being infected with HIV-1ADA or HIV-1HxB2. SOE pretreatment with the cells had no antiviral effect, whereas maraviroc and JM2987 pretreatment showed strong inhibition against HIV-1 ADA and HXB2 pseudoviruses at 1 μM, as expected. The data represent the mean ± standard deviation of triplicate experiments.
3.3. SOE inhibits HIV-1 directly
To determine whether SOE inhibits virus entry by directly inactivating HIV-1 or binding to its receptor on target cells, SOE-virus binding and SOE-cell binding assays were performed. In the SOE-virus binding assay, pseudovirus was pretreated with 50 μg/mL SOE first. In the meantime, dilution solution dimethyl sulfoxide was used as a negative control and entry inhibitor enfuvirtide (T-20) as a positive control. The viruses were then recovered by ultracentrifugation and used to infect target cells. We found that SOE inhibited both ADA and HXB2 pseudovirus infection to a similar degree as T-20, which blocks HIV-1 fusion (Fig. 2B). Subsequently, the SOE-cell binding assays were performed by pretreating the target GHOST cells with SOE or with the CCR5 antagonist maraviroc (MVC) and CXCR4 antagonist JM2987 as positive controls for 1 hour at 37 °C. After washing with phosphate buffered saline, cells were incubated with HIV-1ADA or HIV-1HxB2 for 2 hours, washed, and cultured at 37°C for 48 hours [18]. We found that pretreating the target cells with SOE had virtually no antiviral effect, whereas MVC and JM2987 showed strong inhibition against the respective ADA and HXB2 pseudoviruses at 1μM as expected (Fig. 2C). This demonstrates that SOE inhibits HIV-1 infection by acting on the viral envelope glycoprotein gp160, which mediates viral entry into the host target cells.
3.4. Antiviral activity of SOE against SIV, influenza H5N1, and SARS-CoV pseudoviruses
To further confirm that SOE is a HIV-1-specific entry inhibitor, we tested two SIV, one influenza H5N1 and one SARS-CoV pseudoviral strain. A previous study indicated that residue peptides were found to be potent inhibitors of virus entry against both HIV-1 and SIV envelope glycoproteins [19]. Interestingly, SOE inhibited both SIV strains mac239 and mac1A11, but neither influenza H5N1 nor SARS-CoV (Fig. 3 ). These results suggest that SOE has a broad and specific reactivity against diverse AIDS viruses.
Fig. 3.
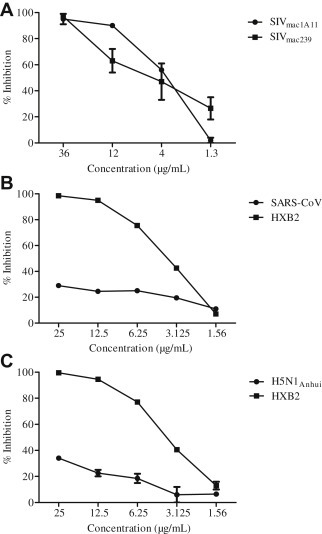
Activity of Sanguisorba officinalis extract (SOE) against simian immunodeficiency virus (SIV), influenza, and severe acute respiratory syndrome coronavirus (SARS-CoV). Serially diluted SOE was added to (A) GHOST-CCR5 infected with SIVmac239, and SIVmac1A11, (B) 293T-ACE2 infected with SARS-CoV, and (C) MDCK cells infected with H5N1Anhui. (B,C) HIVHxB2 was tested as a positive control in GHOST-CXCR4 cells. The luciferase level was measured 2 days postinfection. All results are means ± standard errors of the means from three independent experiments.
3.5. Antiviral activity of SOE against drug resistant HIV-1 strains
The rapid emergence of drug-resistant strains of HIV-1 in patients is one of the major obstacles to successful antiretroviral treatment. Therefore, an experiment involving anti-HIV resistant viruses was carried out in which four HIV-1 drug-resistant strains were tested, including three NNRTI resistant viruses (K103N, Y188L, and K103N/Y188L/G190A) and one protease inhibitor resistant virus (PI-2840) [8]. As expected, SOE inhibited all four drug-resistant strains (Fig. 4 A), demonstrating greater breadth of inhibition when compared to nevirapine, which is a United States Food and Drug Administration-approved NNRTI. Consistent with previous findings, nevirapine did not inhibit HIV-1 resistant strains harboring Y188L or K103N/Y188L/G190A mutations (Fig. 4B). Because viral entry precedes reverse transcription, these results provide further support for SOE as an entry inhibitor against live replicating HIV-1 strains.
Fig. 4.
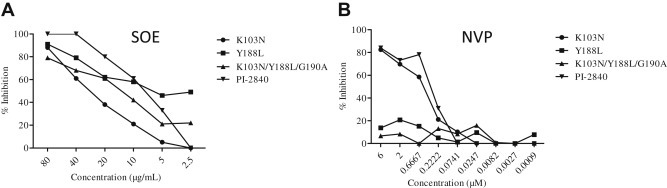
Activity of Sanguisorba officinalis extract (SOE) against non-nucleoside reverse-transcriptase inhibitor (NNRTI) mutant pseudovirus and a protease-inhibitor resistant strain. (A) Serially diluted SOE was added to GHOST-CCR5 and GHOST-CXCR4 infected with NNRTI mutant pseudoviruses (K103N, Y188L and K103N/Y188L/G190A) and a protease-inhibitor resistant strain (PI-2840), respectively. (B) Nevirapine (NVP) was included as control. All NNRTI mutant constructs were generated based on a molecular clone backbone, pNL4-3LucEnvVpr. The PI-2840 strain (resistant to multiple anti-HIV protease drugs) carries L10R, M46I, L63P, V82T, and I84V. The luciferase level was measured 2 days postinfection. All results are means ± standard errors of the means from three independent experiments.
3.6. Anti-HIV activities fractionation of SOE
To further characterize the anti-HIV activity of aqueous SOE, the extracts were subjected to different polar solvents to obtain PE, EtOAc, BuOH, and water residues. Each fraction was vacuum dried and then screened for anti-HIV-1 activity. The EtOAc and BuUOH subfractions strongly diminished HIV-1 infection of target cell lines similar to SOE (Figs. 1 and 5A–D). However, the PE subfraction did not show any inhibition of HIV-1. These results indicate that the solubility of SOE is dependent on the polarity of the solvent, which provides direction for future isolation of small molecule HIV-1 entry inhibitors from these subfractions.
Fig. 5.
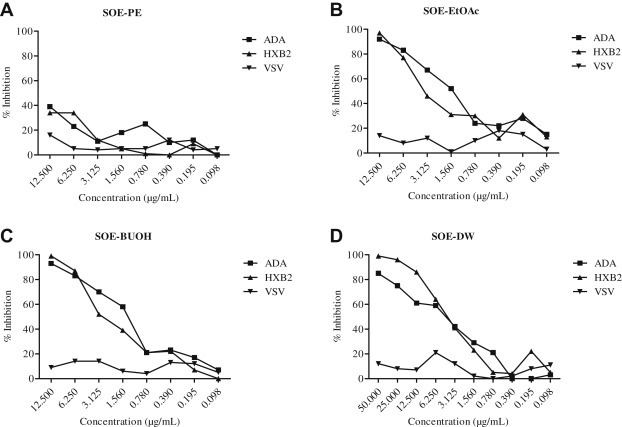
Activity of fractions of Sanguisorba officinalis extract against HIV-1ADA, HIVHxB2, and HIVVSV. Serially diluted (A) petroleum–ether-partitioned extract (PE), (B) ethyl-acetate-partitioned extract (EtOAc), (C) n-butanol-partitioned extract (BuOH), or (D) distilled water (DW) fractions were added to GHOST-CCR5 and GHOST-CXCR4 infected with HIV-1ADA, HIVHxB2, and HIVVSV. The luciferase level was measured 2 days postinfection. All results are means ± standard errors of the means from three independent experiments.
4. Discussion
In this study, we demonstrated that SOE blocks the entry of both CCR5-tropic and CXCR4-tropic strains of HIV-1. This blockade is HIV-1 specific because SOE does not exhibit any activities against HIV-Luc+ viruses pseudotyped with envelopes of VSV, SARS-CoV, and influenza. Interestingly, SOE acts directly on HIV-1 instead of cellular receptors or coreceptors. It also exhibits potency against replication competent HIV-1 strains resistant to specific drugs targeting protease and reverse transcriptase (RT) activities. Our findings therefore provide scientific evidence for the potential use of SOE in the prevention and treatment of HIV-1 infection and the search for small molecular compounds as specific entry inhibitors.
Stopping HIV-1 from entering target cells is a critical strategy for both prevention and treatment [20], [21]. Until now, there have been only two drugs in this category approved by the FDA for clinical use in the market: MVC and T-20. MVC binds to the host CCR5 coreceptor to prevent binding with gp120, whereas T-20 interacts with gp41 to interrupt membrane fusion between HIV-1 and the host target cell plasma membrane. T-20 is part of the repertoire of antiretroviral drugs that have been used in combination therapy to treat HIV-1 infection [22]. Although effective, T-20 has to be administrated by injection, whereas MVC requires the screening of patients to exclude strains of CXCR4-tropism. It is therefore useful to identify new types of HIV-1 entry inhibitors. To this end, it is of great interest to discover that SOE is capable of inhibiting entry of both CCR5- and CXCR4-tropic HIV-1 ADA and HXB2 strains, as well as of SIVmac1A11 and SIVmac239, by binding viral envelope glycoproteins directly. It has previously been shown that despite a significant level of sequence diversity, HIV-1 and SIV share a series of events during virus entry including binding of gp120 to CD4 and chemokine coreceptor, and fusion between viral and cellular membranes [20], [23], [24], [25]. SOE probably acts upon a highly conserved element of HIV-1 and SIV envelope glycoproteins, an issue that still requires future investigation [23], [24], [26], [27], [28]. Because SIVmac1A11 enters target cells in a CD4-independent way [16], SOE probably inhibits virus entry through other mechanisms rather than by blocking the CD4 binding site directly. Critically, the proven anti-HIV-1 specificity, together with the lack of antiviral effects against VSV, SARS-CoV, and influenza, also rule out the possibility that SOE simply inactivates the HIV-1 virus by acting on viral lipids like a disinfectant. In support of our findings, several small molecules isolated from plants have been reported to have some moderate inhibitory ability against HIV-1 entry. For example, polyphenols found in green tea probably interrupt membrane fusion between HIV-1 and the host cell plasma membrane [29]. Further investigations, are necessary to reveal the mode of SOE action, which may lead to the identification of a new drug targeting the HIV-1 envelope.
With the enhanced efforts in treatment as prevention, there is also the issue of rapid emergence of drug-resistant strains of HIV-1, especially in developing countries [30]. Although several extracts of herbal medicines have been investigated for their anti-HIV activities, inhibition of anti-HIV drug-resistant strains was rarely studied [7], [31], [32], [33]. We recently reported that plant-derived calanolide A and their analogues have unique advantages in overcoming the existing drug resistant viruses [8], [9]. Using the same methods as in this study, we showed that SOE inhibited not only RT inhibitor resistant viruses (K103N, Y188L, and K103N/Y188L/G190A) but also a protease inhibitor resistant primary strain (PI-2840). Because RT and protease act after HIV entry, these results are consistent with SOE as a new entry inhibitor. To explore the potential of SOE for treating AIDS patients, a future well-designed clinical trial should be conducted for in vivo efficacy evaluation. It is also necessary to purify the small molecule compound from SOE with anti-HIV-1 activity.
In conclusion, in this study, we demonstrated that SOE blocks entry by CCR5-tropic and CXCR4-tropic strains of HIV-1. This blockade is HIV-1-specific because SOE does not exhibit any activities against HIV-Luc+ viruses pseudotyped with envelopes of VSV, SARS-CoV, and influenza. Interestingly, SOE acts directly on HIV-1 instead of cellular receptors or coreceptors. It also exhibits potency against replication-competent HIV-1 strains resistant to specific drugs targeting protease and RT activities. Our findings therefore provide evidence for the potential use of SOE in the prevention and treatment of HIV-1 infection, as well as in the search for small molecular compounds as specific entry inhibitors.
Acknowledgements
This work was supported by Hong Kong RFCID 11100702, and HKU-UDF/LKSFM MATCHING FUND to AIDS Institute. We thank NIDA grant R13DA035084 for conference support.
References
- 1.White N.J. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zou W., Liu Y., Wang J. Traditional Chinese herbal medicines for treating HIV infections and AIDS. Evid Based Complement Alternat Med. 2012;2012:950757. doi: 10.1155/2012/950757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J.P. The use of herbal medicines in early drug development for the treatment of HIV infections and AIDS. Expert Opin Investig Drugs. 2007;16:1355–1364. doi: 10.1517/13543784.16.9.1355. [DOI] [PubMed] [Google Scholar]
- 4.Cui M., Li J., Li H. Herbal compatibility of traditional Chinese medical formulas for acquired immunodeficiency syndrome. J Tradit Chinese Med. 2012;32:329–334. doi: 10.1016/s0254-6272(13)60033-3. [DOI] [PubMed] [Google Scholar]
- 5.Man S., Gao W., Wei C. Anticancer drugs from traditional toxic Chinese medicines. Phytother Res. 2012;26:1449–1465. doi: 10.1002/ptr.4609. [DOI] [PubMed] [Google Scholar]
- 6.Wang J., Zou W. Practices, challenges, and opportunities: HIV/AIDS treatment with traditional Chinese medicine in China. Front Med. 2011;5:123–126. doi: 10.1007/s11684-011-0124-z. [DOI] [PubMed] [Google Scholar]
- 7.Chu Y., Liu H. Advances of research on anti-HIV agents from traditional Chinese herbs. Adv Dent Res. 2011;23:67–75. doi: 10.1177/0022034511399912. [DOI] [PubMed] [Google Scholar]
- 8.Lu X., Liu L., Zhang X. F18, a novel small-molecule nonnucleoside reverse transcriptase inhibitor, inhibits HIV-1 replication using distinct binding motifs as demonstrated by resistance selection and docking analysis. Antimicrob Agents Chemother. 2012;56:341–351. doi: 10.1128/AAC.05537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma T., Liu L., Xue H. Chemical library and structure-activity relationships of 11-demethyl-12-oxo calanolide A analogues as anti-HIV-1 agents. J Med Chem. 2008;51:1432–1446. doi: 10.1021/jm701405p. [DOI] [PubMed] [Google Scholar]
- 10.Wang J., Zou W. Recent advances of HIV/AIDS treatment with traditional Chinese medicine in China. J Tradit Chinese Med. 2010;30:305–308. doi: 10.1016/s0254-6272(10)60062-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S., Liu X., Zhang Z.L. Isolation and identification of the phenolic compounds from the roots of Sanguisorba officinalis L. and their antioxidant activities. Molecules. 2012;17:13917–13922. doi: 10.3390/molecules171213917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goun E.A., Petrichenko V.M., Solodnikov S.U. Anticancer and antithrombin activity of Russian plants. J Ethnopharmacol. 2002;81:337–342. doi: 10.1016/s0378-8741(02)00116-2. [DOI] [PubMed] [Google Scholar]
- 13.Zberts V.L., Plakhova N.B. Application of burnet (Sanguisorba officinalis) in treatment of dysentery in children. Sovetskaia Meditsina. 1951;4:27–29. [Article in Russian] [PubMed] [Google Scholar]
- 14.Wang Z., Loo W.T., Wang N. Effect of Sanguisorba officinalis L on breast cancer growth and angiogenesis. Expert Opin Ther Targets. 2012;16(Suppl. 1):S79–S89. doi: 10.1517/14728222.2011.642371. [DOI] [PubMed] [Google Scholar]
- 15.Liu L., Fang Q., Deng F. Natural mutations in the receptor binding domain of spike glycoprotein determine the reactivity of cross-neutralization between palm civet coronavirus and severe acute respiratory syndrome coronavirus. J Virol. 2007;81:4694–4700. doi: 10.1128/JVI.02389-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun C., Chen Z., Tang X. Mucosal priming with a replicating-vaccinia virus-based vaccine elicits protective immunity to simian immunodeficiency virus challenge in rhesus monkeys. J Virol. 2013;87:5669–5677. doi: 10.1128/JVI.03247-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M.W., Cheng T.J., Huang Y. A consensus-hemagglutinin-based DNA vaccine that protects mice against divergent H5N1 influenza viruses. Proc Natl Acad Sci U S A. 2008;105:13538–13543. doi: 10.1073/pnas.0806901105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rapista A., Ding J., Benito B. Human defensins 5 and 6 enhance HIV-1 infectivity through promoting HIV attachment. Retrovirology. 2011;8:45. doi: 10.1186/1742-4690-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustchina E., Hummer G., Bewley C.A. Differential inhibition of HIV-1 and SIV envelope-mediated cell fusion by C34 peptides derived from the C-terminal heptad repeat of gp41 from diverse strains of HIV-1, HIV-2, and SIV. J Med Chem. 2005;48:3036–3044. doi: 10.1021/jm049026h. [DOI] [PubMed] [Google Scholar]
- 20.Kang Y., Wu Z., Lau T.C. CCR5 antagonist TD-0680 uses a novel mechanism for enhanced potency against HIV-1 entry, cell-mediated infection, and a resistant variant. J Biol Chem. 2012;287:16499–16509. doi: 10.1074/jbc.M112.354084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang Y., Guo J., Chen Z. Closing the door to human immunodeficiency virus. Protein Cell. 2013;4:86–102. doi: 10.1007/s13238-012-2111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biswas P., Tambussi G., Lazzarin A. Access denied? The status of co-receptor inhibition to counter HIV entry. Expert Opin Pharmacother. 2007;8:923–933. doi: 10.1517/14656566.8.7.923. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z., Telfer P., Reed P. Isolation and characterization of the first simian immunodeficiency virus from a feral sooty mangabey (Cercocebus atys) in West Africa. J Med Primatol. 1995;24:108–115. doi: 10.1111/j.1600-0684.1995.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z., Zhou P., Ho D.D. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol. 1997;71:2705–2714. doi: 10.1128/jvi.71.4.2705-2714.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L., He T., Huang Y. Chemokine coreceptor usage by diverse primary isolates of human immunodeficiency virus type 1. J Virol. 1998;72:9307–9312. doi: 10.1128/jvi.72.11.9307-9312.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moriuchi M., Moriuchi H., Turner W. Cloning and analysis of the promoter region of CXCR4, a coreceptor for HIV-1 entry. J Immunol. 1997;159:4322–4329. [PubMed] [Google Scholar]
- 27.Samson M., Labbe O., Mollereau C. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry. 1996;35:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z., Telfier P., Gettie A. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J Virol. 1996;70:3617–3627. doi: 10.1128/jvi.70.6.3617-3627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S., Lu H., Zhao Q. Theaflavin derivatives in black tea and catechin derivatives in green tea inhibit HIV-1 entry by targeting gp41. Biochim Biophys Acta. 2005;1723:270–281. doi: 10.1016/j.bbagen.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Perelson A.S., Ribeiro R.M. Estimating drug efficacy and viral dynamic parameters: HIV and HCV. Stat Med. 2008;27:4647–4657. doi: 10.1002/sim.3116. [DOI] [PubMed] [Google Scholar]
- 31.Han H., He W., Wang W. Inhibitory effect of aqueous Dandelion extract on HIV-1 replication and reverse transcriptase activity. BMC Complement Altern Med. 2011;11:112. doi: 10.1186/1472-6882-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park I.W., Han C., Song X. Inhibition of HIV-1 entry by extracts derived from traditional Chinese medicinal herbal plants. BMC Complement Altern Med. 2009;9:29. doi: 10.1186/1472-6882-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Au T.K., Lam T.L., Ng T.B. A comparison of HIV-1 integrase inhibition by aqueous and methanol extracts of Chinese medicinal herbs. Life Sci. 2001;68:1687–1694. doi: 10.1016/s0024-3205(01)00945-6. [DOI] [PubMed] [Google Scholar]


