Abstract
Objective
Cross-talk between inflammation and angiogenesis pathways has been recently reported. The objective of this study was to: 1) examine whether amniotic fluid (AF) concentrations of soluble endoglin (sEng), a protein with anti-angiogenic properties, changes during pregnancy, parturition or intraamniotic infection and/or inflammation (IAI); 2) determine whether an elevation of sEng in the AF of patients with preterm labor (PTL) and preterm prelabor rupture of membranes (PROM) is associated with adverse neonatal outcomes; and 3) investigate potential sources of sEng in AF.
Study Design
A cross-sectional study was conducted to include patients in the following groups: 1) midtrimester (n=20); 2) PTL with term delivery (n=95); 3) PTL leading to preterm delivery with (n=40) and without IAI (n=46); 4) preterm PROM with (n=37) and without IAI (n=37); 5) term in labor (n=48) and not in labor (n=44). AF concentrations of sEng were determined by ELISA. Chorioamniotic membranes, umbilical cord blood and AF macrophages were examined for the expression of endoglin.
Results
1) Patients with IAI had a higher median AF concentration of sEng than those without IAI (p=0.02 for PTL; and 0.06 for preterm PROM); 2) AF concentrations of sEng in the 3rd and 4th quartiles were associated with IAI (OR 2.5 and 7.9 respectively); 3) an AF sEng concentration ≥779.5 pg/ml was associated with bronchopulmonary dysplasia (BPD) (OR 7.9); 4) endoglin was co-localized with CD14+ macrophages in AF pellets of patients with IAI by immunofluorescence and flow cytometry; and 5) the concentration of sEng in the supernatant was significantly increased after treatment of macrophages with endotoxin or TNF-α.
Conclusions
sEng participates in the host response against IAI. Activated macrophages may be a source of sEng concentrations in the AF of patients with IAI. An increase of sEng in the AF is associated with BPD and adverse neonatal outcomes.
Keywords: preterm labor, preterm prelabor rupture of membranes, angiogenesis, adverse neonatal outcomes, intraamniotic infection, intraamniotic inflammation
INTRODUCTION
Preterm birth accounts for 75% of perinatal mortality and more than half of the long- term morbidity of survivors.1-8 Preterm neonates are at increased risk for the development of short-term complications such as respiratory distress syndrome,9-11 sepsis,12-16 intraventricular hemorrhage,17-19 periventricular leukomalacia,20-22 and necrotizing enterocolitis,23-25 as well as long-term disabilities, such as cerebral palsy,26-34 bronchopulmonary dysplasia (BPD),35-41 and retinopathy of prematurity.42,43 Intra-amniotic infection and/or inflammation (IAI) has emerged as an important pathologic process for which a firm causal link to preterm parturition is proven.1,2,44-51
Infection can occurs in different sites such as the amniotic cavity or placenta. A role for microbial invasion of the amniotic cavity with bacteria in the etiology of complications of pregnancy, such as preterm labor with intact membranes,52-65 preterm premature rupture of membranes,66-71 and cervical insufficiency72-78is well-established. Human chorioamniotic membranes79-82 and trophoblasts83-90are bestowed with pattern recognition receptors that allow them to recognize a wide range of microorganisms. Infection of the placenta with viruses has been recently demonstrated to elicit an inflammatory response in the fetus even though the organisms have not been recovered from amniotic fluid or fetal tissues91. Such infection of the placenta can lead to fetal inflammation and sensitization to bacterial products predisposing to preterm labor.92 Whatever the precise locations of intrauterine infection are, it can elicit an inflammatory response in the fetus,5,12,13,19,93-95 chorioamniotic membranes,81,96-99 amniotic cavity12,19,47,93,94,100 and placenta.101-103
Angiogenesis104 and inflammation105 are two distinct processes. Yet, cross-talk between these two processes has been identified in the mechanisms responsible for wound healing,106 cancer107 and sepsis.108,109 Inflammation is a mechanism of host defense to control endogenous or exogenous damage and restore homeostasis in response to infection, bacteria, or tissue injury.110 This complex process can stimulate angiogenesis, which, in turn, plays an important role in inflammation107 and its resolution.110
Endoglin, also known as CD105, is a co-receptor of transforming growth factor (TGF)-ß and exists in two forms: membrane-bound and soluble.111-114 Soluble endoglin (sEng) can inhibit TGF-ß activity and has an anti-angiogenic effect on endothelial cells.114 An elevation of sEng concentration in the maternal circulation114-120 and in amniotic fluid121 has been reported in patients with preeclampsia, an obstetrical syndrome proposed to be an anti-angiogenic state.114,122 Macrophages and neutrophils from patients with a deficiency in endoglin (e.g. hereditary hemorrhagic telangiectasia, a genetic disorder characterized by a mutation in the endoglin gene and reduced expression of endoglin), have a deficit in phagocytosis and oxidative burst, suggesting a role for endoglin in the regulation of the innate immune response.123
One of the most important long-term neonatal complications of preterm birth is BPD and intraamniotic inflammation has been associated with the subsequent development of BPD.35,40,93,124-127 Recent studies also suggest that BPD is associated with dysregulation of angiogenesis in the pulmonary vasculature.128-137 Of note, a study reported that the pulmonary microvasculature of ventilated preterm infants, compared to age-matched non-ventilated lungs of control infants, showed significant up-regulation of endoglin mRNA and protein expression.138 The authors proposed that BPD is associated with a shift in the balance of angiogenic (vascular endothelial growth factor, VEGF, and angiopoietin-1), to alternative regulators such as endoglin, which may contribute to BPD-associated microvascular abnormalities.138
The objectives of this study were to: 1) examine whether amniotic fluid concentrations of sEng, a protein with anti-angiogenic activity, change during pregnancy, parturition or IAI; 2) determine if there was a change, whether an elevation of sEng concentration in the amniotic fluid of patients with preterm labor (PTL) and preterm prelabor rupture of membranes (PROM) was associated with adverse neonatal outcomes especially for BPD; and 3) to investigate potential sources of sEng in AF.
MATERIALS AND METHODS
Study design and population
A cross-sectional study was conducted by searching our clinical database and bank of biological specimens, including 367 patients in the following groups: 1) women in the midtrimester of pregnancy who underwent amniocentesis for genetic indications and delivered a normal neonate at term (n=20); 2) patients with an episode of spontaneous PTL and intact membranes who were classified as: a) PTL without IAI who delivered at term (n=95); b) PTL without IAI who delivered preterm (<37 weeks gestation; n=46); and c) PTL with IAI who delivered preterm (n=40); 3) patients with preterm PROM with (n=37) and without IAI (n=37); and 4) normal pregnant women at term with (n=48) and without spontaneous labor (n=44). Patients with multiple pregnancies, preeclampsia, maternal medical disease, fetal death, and fetal congenital or chromosomal abnormalities were excluded.
All patients provided written informed consent upon enrollment. The Institutional Review Boards of both Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NICHD/NIH/DHHS) approved the collection of samples for research purposes. Many of the biological materials of patients who were enrolled in this study have previously been used for studies of inflammation and growth factor concentrations in normal pregnant women and those with pregnancy complications.
Clinical definitions
Spontaneous PTL was defined by the presence of regular uterine contractions (at least four in 20 minutes) associated with cervical changes between 20 and 36 6/7 weeks of gestation and required hospitalization.139 Preterm PROM was diagnosed by visualization of pooling of amniotic fluid in the vagina in association with positive nitrazine and/or ferning tests or by a positive amniocentesis-dye test before 37 weeks of gestation. Women at term (≥ 37 weeks) not in labor underwent amniocentesis for the assessment of fetal lung maturity prior to cesarean section. Women at term in labor consisted of those admitted for suspected PTL because of uncertain dates and had an amniocentesis for the assessment of fetal lung maturity and microbial invasion of the amniotic cavity. However, those who delivered a neonate ≥2500 g without complications of prematurity were considered likely to represent patients in spontaneous labor at term.60 IAI was defined as a positive culture for microorganisms and/or an elevated amniotic fluid interleukin (IL)-6 concentration (≥2.6 ng/mL).94
Composite neonatal morbidity was defined as the presence of one or more of the following complications: sepsis or suspected sepsis, respiratory distress syndrome, persistent ductus arteriosus, BPD, intraventricular hemorrhage, necrotizing enterocolitis and retinopathy of prematurity. BPD was diagnosed if the neonate required oxygen and ventilatory therapy for >28 days during the first 2 months of life, had typical radiographic changes and/or dysplasia of the bronchopulmonary tree at autopsy.140,141 The definition of other neonatal complications has been described in previous publications. 11,13,19
Amniotic fluid collection
Amniotic fluid samples were obtained by transabdominal amniocentesis under ultrasonographic guidance. Samples of amniotic fluid were cultured for the presence of microorganisms, including aerobic and anaerobic bacteria as well as genital Mycoplasmas. White blood cell (WBC) count,142 glucose concentration,143,144 and Gram stain145 were also performed. The results of these tests were used for clinical management. Amniotic fluid not required for clinical assessment was centrifuged for 10 min at 4°C, and the supernatant was aliquoted and stored at -70°C until analysis. Among patients with spontaneous PTL and those with preterm PROM who delivered preterm within 72 hours of amniocentesis, the placenta, umbilical cord and chorioamniotic membranes were collected and the presence or absence of histologic chorioamnionitis and/or funisitis was assessed. This interval was chosen to preserve a meaningful temporal relationship between amniotic fluid sEng concentration and placental histopathologic findings.
Determination of sEng concentrations in amniotic fluid and culture supernatants
sEng concentration was measured by a commercial ELISA kit (R & D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. The amniotic fluid concentrations of sEng were determined by interpolation from individual standard curves generated from known concentrations of sEng. The assay was validated for use in amniotic fluid prior to its use in this study. The calculated inter-assay and intra-assay coefficients of variation for sEng in our laboratory were 2.88% and 4.76%, respectively, and the sensitivity was 32 pg/mL.
Flow cytometry
Flow cytometry analysis of amniotic fluid cell pellets was performed to identify if these cells were a source of endoglin. We used a BDTM LSR II flow cytometer (BD Biosciences, San Jose, CA, USA). After incubation with mouse monoclonal anti-human CD14-APC (BD Biosciences, San Jose, CA, USA) and anti-human endoglin-PE (R & D Systems), macrophages were gated as CD14 positive cells. An isotype IgG1 antibody (BD Biosciences, San Jose, CA, USA) matched by concentration was used as the control. The endoglin expression of macrophages (CD14+) in amniotic fluid was analyzed using the FlowJo software (Tree Star, San Carlos, CA, USA).
Immunofluorescence microscopy
Double label immunofluorescence staining on amniotic fluid cell pellets was conducted using a panel of antibodies to endoglin (mouse monoclonal; DakoCytomation Inc., Carpinteria, CA, USA) and CD14 (rabbit polyclonal; Abcam, Cambridge, MA, USA). After fixation with 4% paraformaldehyde and blocking with 5% BSA in PBS, the slides were incubated with a primary antibody to endoglin, followed by incubation with Alexa 594 goat anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) and Alexa 488 donkey anti-rabbit IgG (Invitrogen). The stained slides were mounted in ProLong® Gold antifade reagent with DAPI (4′,6-diamidino-2-phenylindole, dihydrochloride) (Invitrogen), and images were taken using an Olympus BX-60 digital microscope (Olympus Optical Co., Hamburg, Germany). The antibody for the marker of neutrophils was not used because neutrophils can be recognized easily by morphology.
Immunohistochemistry to detect endoglin in the chorioamniotic membranes
Immunohistochemical staining was performed using a mouse monoclonal anti-endoglin antibody (DakoCytomation Inc.) on 5 μm thick paraffin-embedded sections of the chorioamniotic membranes with an automatic immunostainer (Ventana Benchmark; Ventana Medical Systems, Inc., Tucson, AZ, USA).
Immunoblotting and densitometry to examine the expression of endoglin in chorioamniotic membranes
Immunoblotting was performed using chorioamniotic membranes obtained from PTL with (n=8) and without (n=8) histologic chorioamnionitis. Total protein was obtained from liquid nitrogen-pulverized fetal membranes using a RIPA (radio-immunoprecipitation assay) lysis buffer (Sigma, St Louis, MO, USA) containing a proteinase inhibitor cocktail (Roche, Indianapolis, IN, USA). Thirty micrograms of protein were electrophoresed in a 12% SDS-PAGE gel (Bio-Rad, Hercules, CA, USA) and electro-blotted to polyvinylidene difluoride membranes (Hybond™-P; GE Healthcare Life Sciences, Piscataway, NJ, USA). After blocking with 5% blotting grade blocker non-fat dry milk (Bio-Rad), the membranes were probed with a goat polyclonal anti-endoglin (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) or a rabbit polyclonal anti-HPRT antibody (Santa Cruz Biotechnology Inc.). The chemiluminescent signals were detected using a ChemiGlow West kit (Alpha Innotech, San Leandro, CA, USA). Densitometric analyses were carried out using AlphaEase®FC software version 4.1.0 of the FluorChemTM SP densitometer (Alpha Innotech).
Expression of endoglin by a macrophage cell line
U937 cells (ATCC, Manassas, VA, USA), a human promonocytic cell line, were used as a model for macrophage-like cells. We cultured at 37°C in a 5% CO2 atmosphere in RPMI-1640 medium supplemented with 2.5g/L glucose, 10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid ), 1 mM sodium pyruvate, 10% fetal bovine serum and 100 μg/mL of penicillin-streptomycin. U937 cells were incubated with 10 ng/mL of phorbol myristic acid (PMA) for 48 hours to induce differentiation into adherent macrophage-like cells. Following the PMA treatment, the medium was replaced with a fresh one, and the differentiated cells were incubated for an additional 24 hours prior to the conduction of the studies.
To determine whether microbial products and pro-inflammatory cytokines can induce the release of sEng by macrophage-like cells, we incubated U937-derived macrophages with 1 μg/mL of lipopoylsaccharide (LPS; Sigma) or 0.1 μg/mL recombinant human tumor necrosis factor (TNF)-α (R & D Systems). Endotoxin and TNF-α have been previously found in the amniotic fluid of women in preterm labor.47,146,147 The supernatants and the cells were collected after 24 hours of incubation. sEng was determined by an ELISA previously described above.
Real-time quantitative reverse transcription-polymerase chain reaction
To examine the effect of sEng on the production of cytokines by macrophages, U937-derived macrophages were treated in the presence or absence of recombinant human sEng (R & D Systems; at final concentrations of 0.1, 1 and 2 μg/mL). The supernatants and cells were collected after 24 hours of incubation. Total RNA from the cells was isolated using Trizol (Invitrogen). Reverse transcription of the DNase-treated total RNA was performed using a SuperScript III reverse transcriptase (Invitrogen) and oligo (dT) primers according to the manufacturer’s instructions. qRT-PCR analysis using TaqMan® Gene Expression Assays (Applied Biosystems, Foster City, CA, USA) was performed on U937-derived macrophages to determine the mRNA expression of IL-1ß (Hs00174097_m1) and TNF-α (Hs00174128_m1) with RPLPO (4326314E) as an internal control. The concentrations of IL-1ß and TNF-α in culture supernatants were measured by commercially available ELISA (R & D Systems).
Statistical analysis
The distribution of the data was tested using the Shapiro-Wilk test. Non-parametric statistics were used for analyses. Proportions were compared using Chi-square or Fisher’s exact tests. A Kruskal-Wallis with post-hoc Mann-Whitney U test was utilized for comparisons among and between continuous variables. Correlations between continuous variables were assessed by the Spearman’s rank correlation test. Associations between the amniotic fluid concentration of sEng and the presence of IAI, or neonatal outcomes (BPD and composite neonatal morbidity) were determined by logistic regression (backward-stepwise) adjustment for potential confounders. A p-value of <0.05 was considered statistically significant. The statistical analyses were performed using SPSS version 12.0 (SPSS Inc, Chicago, IL, USA).
RESULTS
Demographic and clinical characteristics of the study population
Table I, II, and III present the demographic and clinical characteristics of patients included in this study. There was no significant difference in the median gestational age at amniocentesis among subgroups of patients with spontaneous PTL (Table II) or preterm PROM (Table III).
Table I.
Demographic and clinical characteristics of women in the midtrimester, those at term not in labor, and those in spontaneous labor at term
| Midtrimester (n=20) | p a | Term no labor (n=44) | Term in labor (n=48) | p b | |
|---|---|---|---|---|---|
| Maternal age (years) | 37.0 (35.0-38.8) | <0.001 | 27.0 (21.8-32.8) | 22.0 (19.3-26.8) | 0.005 |
| GA at amniocentesis (weeks) | 16.1 (16.0-17.0) | <0.001 | 38.5 (38.0-39.0) | 38.5 (37.6-39.3) | NS |
| GA at delivery (weeks) | 40.0 (39.0-40.0) | 0.001 | 38.5 (38.0-39.0) | 38.5 (37.6-39.3) | NS |
| Birthweight (g) | 3445 (3261-3824) | NS | 3250 (3065-3563) | 3355 (3078-3548) | NS |
Values are expressed as median (interquartile range).
GA, gestational age; NS, not significant.
p, comparison between patients in the midtrimester and those at term not in labor.
p, comparison between patients at term not in labor and those at term in labor.
Table II.
Demographic and clinical characteristics of patients presenting with spontaneous preterm labor with intact membranes.
| PTL without IAI | PTL without IAI | PTL with IAI | ||||
|---|---|---|---|---|---|---|
| term delivery (n=95) | p | preterm delivery (n=46) | p a | preterm delivery (n=40) | p b | |
| Maternal age (years) | 22.0 (19.0-27.0) | NS | 23.3 (19.0-30.0) | NS | 23.5 (18.8-29.3) | NS |
| Smoking | 15.8 (15/95) | NS | 10.9 (5/46) | NS | 26.3 (10/38) | NS |
| Nulliparity | 45.3 (43/95) | NS | 39.1 (18/46) | 0.02 | 65.0 (26/40) | 0.04 |
| GA at amniocentesis (weeks) | 31.9 (29.1-33.3) | NS | 31.1 (27.5-33.1) | NS | 30.6 (27.0-32.9) | NS |
| GA at delivery (weeks) | 38.7 (37.9-39.9) | <0.001 | 34.3 (32.7-35.6) | <0.001 | 31.1 (27.3-33.3) | <0.001 |
| Birthweight (g) | 3240 (2970-3550) | <0.001 | 2370 (1783-2700) | <0.001 | 1655 (910-2118) | <0.001 |
| Birthweight <10th percentile | 3.2 (3/95) | NS | 6.5 (3/49) | NS | 5.0 (2/40) | NS |
Values are expressed as percentage (number) or median (interquartile range).
PTL, preterm labor; GA, gestational age; IAI, intra-amniotic infection/inflammation; NS, not significant.
p, comparison between patients PTL who delivered at term and PTL without IAI.
p, comparison between patients PTL who delivered preterm without IAI and PTL with IAI.
p, comparison between patients PTL who delivered at term and PTL with IAI.
Table III.
Demographic and clinical characteristics of patients presenting with preterm prelabor rupture of membranes.
| Preterm PROM without IAI (n=37) | Preterm PROM with IAI (n=37) | p | |
|---|---|---|---|
| Maternal age (years) | 24.0 (20.0-32.5) | 30.0 (24.3-37.0) | 0.009 |
| Smoking | 21.6 (8/37) | 22.2 (8/36) | NS |
| Nulliparity | 37.8 (14/37) | 16.2 (6/37) | NS |
| GA at amniocentesis (weeks) | 31.6 (28.9-33.8) | 30.6 (28.5-32.7) | NS |
| GA at delivery (weeks) | 33.0 (31.1-34.5) | 30.9 (28.7-33.5) | 0.002 |
| Birthweight (g) | 2010 (1655-2265) | 1740 (1380-2380) | NS |
| Birthweight <10th percentile | 0 (0/37) | 2.8 (1/36) | NS |
Values are expressed as percentage (number) or median (interquartile range).
PROM, prelabor rupture of membranes; GA, gestational age; IAI, intra-amniotic infection/inflammation; NS, not significant.
Intraamniotic infection/inflammation is associated with a change in the amniotic fluid concentrations of sEng
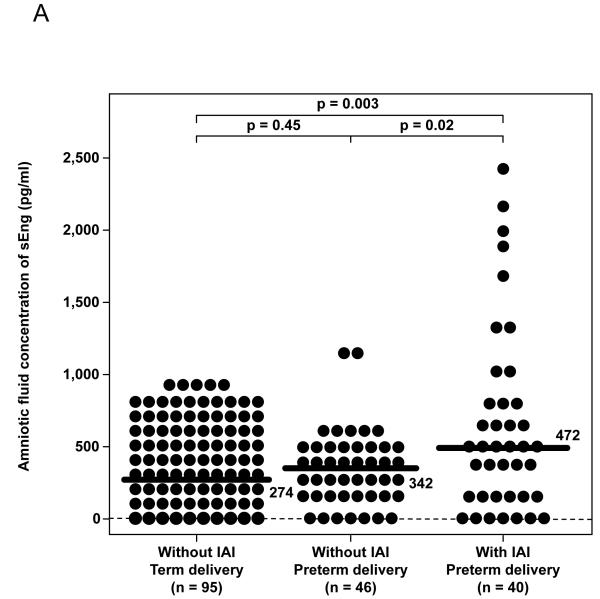
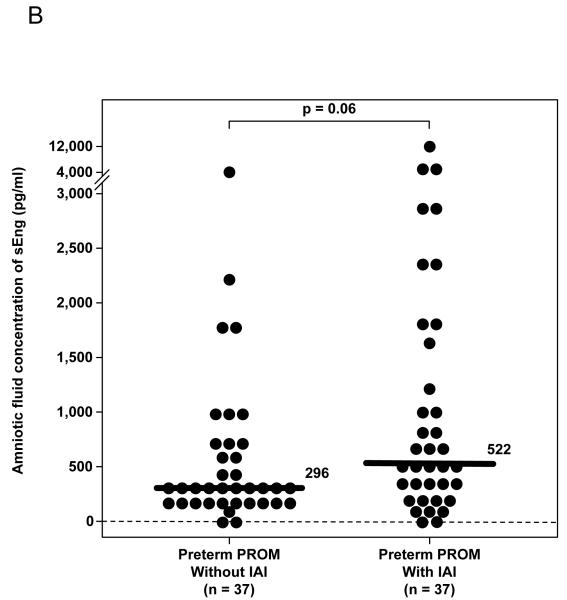
Patients with spontaneous PTL with IAI had a significantly higher median amniotic fluid concentration of sEng than those who delivered preterm without IAI (p=0.02) and those who delivered at term (p=0.003; Figure 1A). Similarly, among patients with preterm PROM, the median amniotic fluid concentration of sEng was higher in patients with IAI than in those without IAI, but the difference did not reach statistical significance (p=0.06; Figure 1B). The amniotic fluid concentration of sEng was positively correlated with the amniotic fluid concentration of IL-6 and the amniotic fluid WBC count (Spearman’s rho 0.4 and 0.3; respectively, each p<0.001).
Figure 1. Amniotic fluid concentrations of soluble endoglin (sEng).
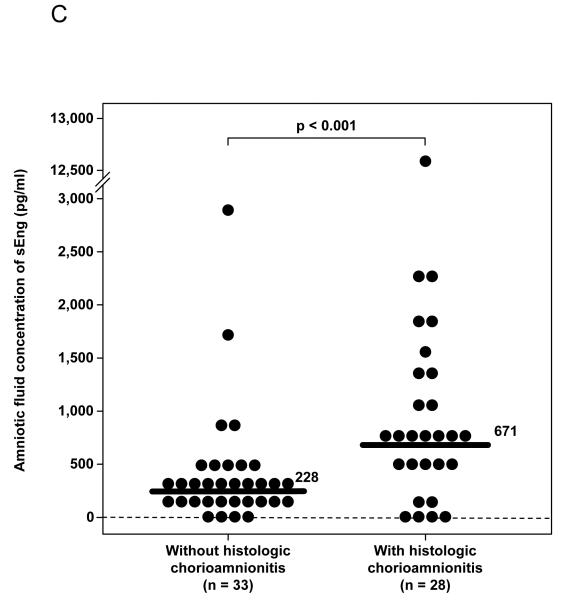
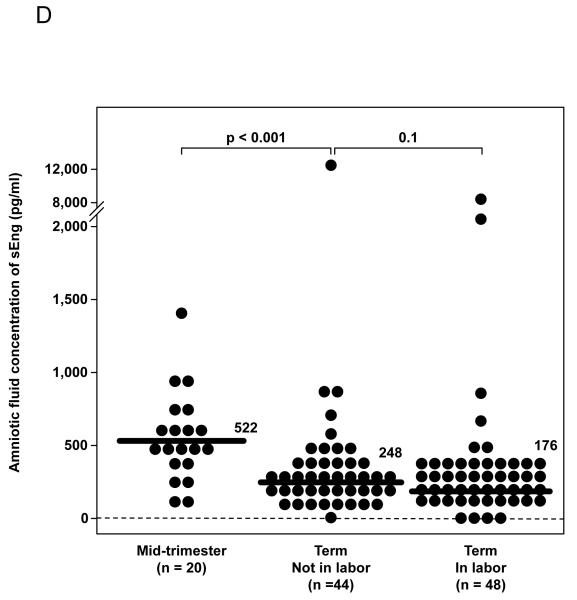
(A) Patients with preterm labor (PTL) with intra-amniotic infection/inflammation (IAI) had a significantly higher median amniotic fluid concentration of sEng than those who delivered preterm without IAI [PTL with IAI: 472 pg/mL, interquartile range (IQR) 213-795 pg/mL vs. PTL without IAI: 342 pg/mL, IQR 166-468 pg/mL; p=0.02] and those who delivered at term (PTL delivered at term: 274 pg/mL, IQR 157-405 pg/mL; P=0.003). Among patients with PTL without IAI, there was no significant difference in the median AF concentration of sEng between those who delivered preterm and those who delivered at term (p=0.45). (B) The median amniotic fluid concentration of sEng was higher in patients with IAI than in those without IAI, but the difference did not reach statistical significance (522 pg/mL, IQR 239-1687 vs. 296 pg/mL, IQR 211-626, respectively; p=0.06). (C) Patients with histologic chorioamnionitis had higher median amniotic fluid concentration of sEng than those without histologic chorioamnionitis (671 pg/mL, IQR 419-1334 vs. 228 pg/mL, IQR 128-435, respectively; p<0.001). (D) The median amniotic fluid concentration of sEng was significantly higher in pregnancies in the midtrimester than in those with at term not in labor (522 pg/mL, IQR 351-685 vs. 248 pg/mL, IQR 144-357, respectively; p<0.001). In contrast, no significant differences were observed in the median amniotic fluid sEng concentration between women with spontaneous labor at term and those at term not in labor (176 pg/mL, IQR 124-322 vs. 248 pg/mL, IQR 144-357, respectively; p=0.1).
Logistic regression analysis indicated that amniotic fluid concentrations of sEng in the 3rd (336-545 pg/mL) and 4th quartiles (>545 pg/mL) were associated with IAI after adjusting for gestational age at amniocentesis, the presence of preterm PROM, the presence of SGA, smoking, nulliparity, maternal age and sample storage time [odds ratio (OR) 2.5; 95% confidence interval (CI) 1.1-5.5 and OR 7.9; 95% CI 3.7-16.9, respectively].
Among patients with spontaneous PTL and those with preterm PROM who delivered preterm within 72 hours of amniocentesis, placental pathology was available in 73.5% (61/83) of cases. Patients with histologic chorioamnionitis had a higher median amniotic fluid concentration of sEng than those without histologic chorioamnionitis (p<0.001; Figure 1C).
Amniotic fluid concentrations of sEng in normal pregnancies
Women in the midtrimester of pregnancy had a significantly higher median amniotic fluid concentration of sEng than those at term who were not in labor (p<0.001; Figure 1D). There was no significant difference in the median amniotic fluid concentration of sEng between women with spontaneous labor at term and those not in labor at term (p=0.1; Figure 1D).
Neonatal outcomes and amniotic fluid concentrations of sEng in patients with PTL and preterm PROM
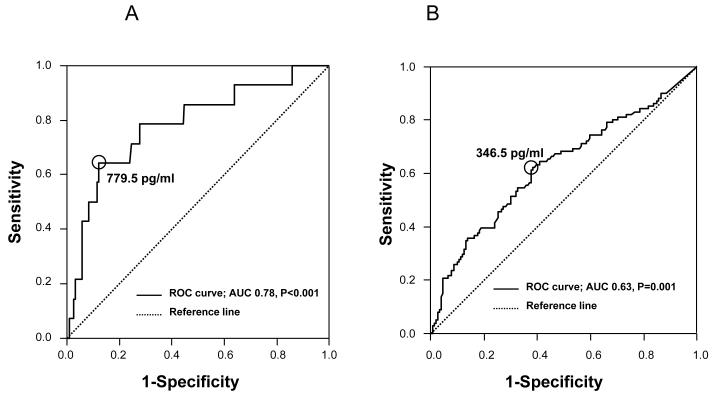
The prevalence of BPD and composite neonatal morbidity in patients with PTL and preterm PROM was 5.5% (14/255) and 39.6% (101/255), respectively. Receiver-operating characteristic (ROC) curves of the amniotic fluid concentrations of sEng for the identification of BPD and composite neonatal morbidity are displayed in Figures 2A and 2B. Amniotic fluid sEng concentrations ≥ 779.5 pg/mL in patients with PTL and preterm PROM were associated with BPD (OR 7.9; 95% CI 2.4-26.0, p<0.001) after adjustment for gestational age at delivery, antenatal steroid administration, the presence of preterm PROM, the presence of SGA, smoking, nulliparity, maternal age and sample storage time. Similarly, amniotic fluid sEng concentrations ≥ 346.5 pg/mL were associated with composite neonatal morbidity (OR 2.2; 95% CI 1.1-4.3, p=0.02).
Figure 2.
Receiver-operating characteristic (ROC) curves of amniotic fluid concentrations of soluble endoglin for the identification of bronchopulmonary dysplasia (a; AUC, 0.78; P < 0.001) and composite neonatal morbidity (b; AUC, 0.63; P = 0.001) in patients with preterm labor and preterm prelabor rupture of membranes. AUC = area under the curve.
Amniotic fluid macrophages as a potential source of sEng in the amniotic fluid of patients with intraamniotic infection/inflammation
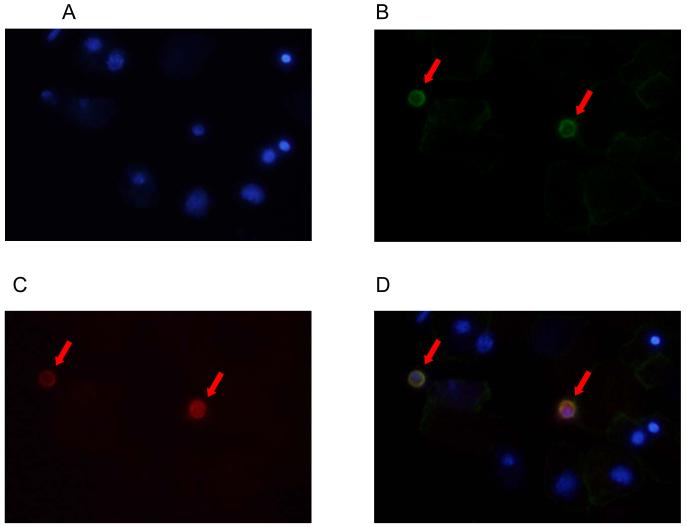
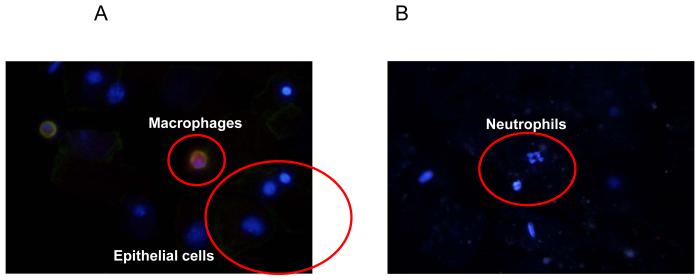
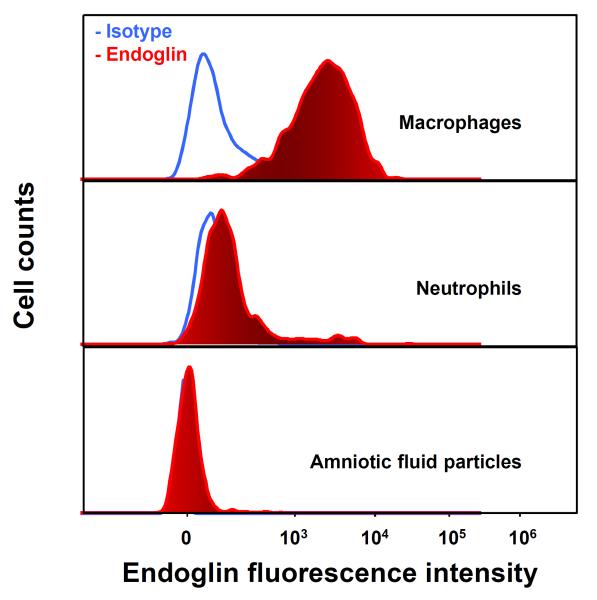
The potentially ubiquitous nature of endoglin expression and previous observation of endoglin expression in macrophages148 led us to examine whether amniotic fluid leukocytes express endoglin. Immunofluorescence staining was performed on amniotic fluid cell pellets of patients with IAI, which demonstrated that endoglin was expressed only in CD14+ macrophages (Figure 3), but not in epithelial cells or neutrophils (Figure 4). Furthermore, endoglin was not expressed on the neutrophils in the flow cytometry experiment (Figure 5).
Figure 3. Double-label immunofluorescence staining of an amniotic fluid cell pellet from a patient with intra-amniotic infection/inflammation using antibodies to endoglin and CD14.
(A) DAPI (4′,6-Diamidino-2-Phenylindole, Dihydrochloride) staining of nuclei (blue). (B) CD14 expression on macrophages (green; Alexa Fluor 488). (C) Endoglin expression on macrophages (red; Alexa Fluor 594). (D) The merged image showing that macrophages expressed both CD14 (green) and endoglin (red).
Figure 4. Double-label immunofluorescence staining of an amniotic fluid cell pellet from a patient with intra-amniotic infection/inflammation using antibodies to endoglin and CD14.
Endoglin was expressed on CD14+ macrophages (A), but not on epithelial cells (A) or neutrophils (B).
Figure 5. Flow cytometry analysis of endoglin expression on different amniotic fluid (AF) cell subsets from an AF cell pellet.
Endoglin was expressed on macrophages (CD14+), but not on neutrophils or AF particles.
Using flow cytometry, CD14+ macrophages were identified in the amniotic fluid of patients with IAI (Figure 6). In accordance with the results of immunofluorescence staining, CD14+ macrophages expressed endoglin if they were obtained from patients with IAI (Figure 6C). In contrast, CD14+ macrophages were rarely detected in the amniotic fluid of patients without IAI (Figure 6A), and endoglin expression was not detected by flow cytometry.
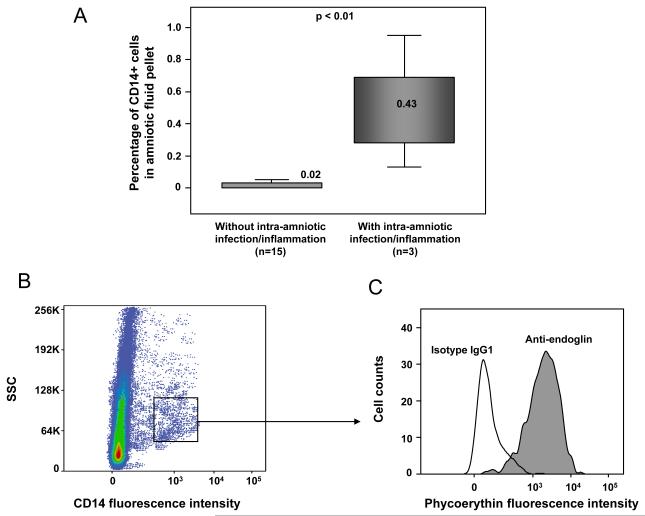
Figure 6. Flow cytometry analysis of endoglin and macrophages (CD14+) in amniotic fluid cell pellets of patients with and without intra-amniotic infection/inflammation (IAI).
The median percentage of CD14+ cells was significantly higher in patients with IAI than in those without IAI (median 0.43 range 0.13-0.95 vs. median 0.02 range 0-0.11; p<0.01; see panel A). A subset of macrophages or CD 14+ cells expressed endoglin (see panel B and C).
To confirm that macrophages can release sEng, U937-derived macrophages were treated with either LPS or recombinant TNF-α. The concentration of sEng in the supernatant was significantly increased after treatment with LPS or TNF-α (LPS vs. control: p=0.002; TNF-α vs. control: p=0.03; Figure 7A).
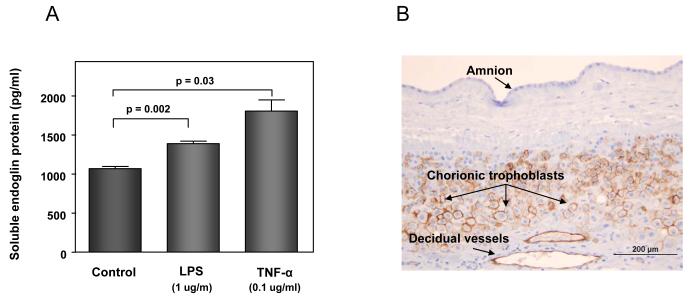
Figure 7. Potential sources of an increased soluble endoglin (sEng) in the amniotic fluid of patients with intra-amniotic infection/inflammation.
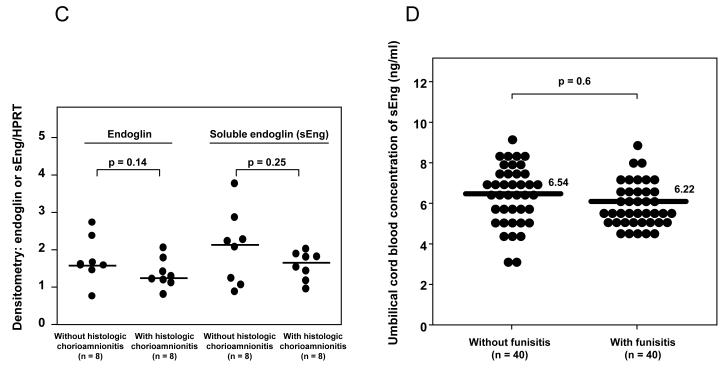
(A) amniotic fluid macrophages: the mean concentration of sEng in supernatant of U937-derived macrophages was significantly higher than in the control after treatment with LPS (p=0.002) or TNF-α (p=0.03). Data represents mean ± SEM. All the experiments were done in triplicate. The p-values were calculated by Student’s t-tests. (B) chorioamniotic membranes: immunohistochemical staining of chorioamniotic membranes demonstrated that endoglin was expressed in chorionic trophoblasts and endothelial cells of decidual vessels, but not in amnion cells. (C) chorioamniotic membranes: densitometric analysis of chorioamniotic membranes for the expression of endoglin and sEng over HPRT (hypoxanthine-guanine phosphoribosyltransferase) between patients with preterm labor (PTL) with (n=8) and without (n=8) histologic chorioamnionitis. There was no significant difference in the median endoglin density ratio and sEng density ratio between patients with and without histologic chorioamnionitis [endoglin density ratio: median 1.24, interquartile range (IQR) 1.14–1.58 vs. median 1.58, IQR 1.49-2.0, p=0.14; sEng density ratio: median 1.66, IQR 1.29–1.83 vs. median 2.13, IQR 1.14–2.55, p=0.25]. (D) umbilical cord blood: there was no significant difference in the median plasma concentration of sEng in the umbilical cord blood of neonates born to mothers with PTL with (n=40) and without (n=40) histologic chorioamnionitis/funisitis [median 6.22 ng/mL, IQR 5.65-7.33 ng/mL vs. median 6.54 ng/mL, IQR 5.62-7.51 ng/mL; p=0.6].
The expression of endoglin and sEng in chorioamniotic membranes and umbilical cord blood
Immunohistochemical staining of the chorioamniotic membranes demonstrated that endoglin was expressed in chorionic trophoblasts and in the endothelial cells of decidual vessels, but not in amnion cells (Figure 7B). A comparison of chorioamniotic membranes obtained from the placentas of patients with PTL with (n=8), and without (n=8) histologic chorioamnionitis showed no significant difference in endoglin or sEng expression on densitometric analysis (endoglin: p=0.14 and sEng: p=0.25; Figure 7C). Moreover, there was no significant difference in the median plasma concentrations of sEng in umbilical cord blood from patients who delivered preterm with (n=40) and without (n=40) histologic chorioamnionitis and funisitis, matched (within 2 weeks) for gestational age at delivery (p=0.6; Figure 7D).
The effect of endoglin on IL-1β and TNF-α production by macrophages
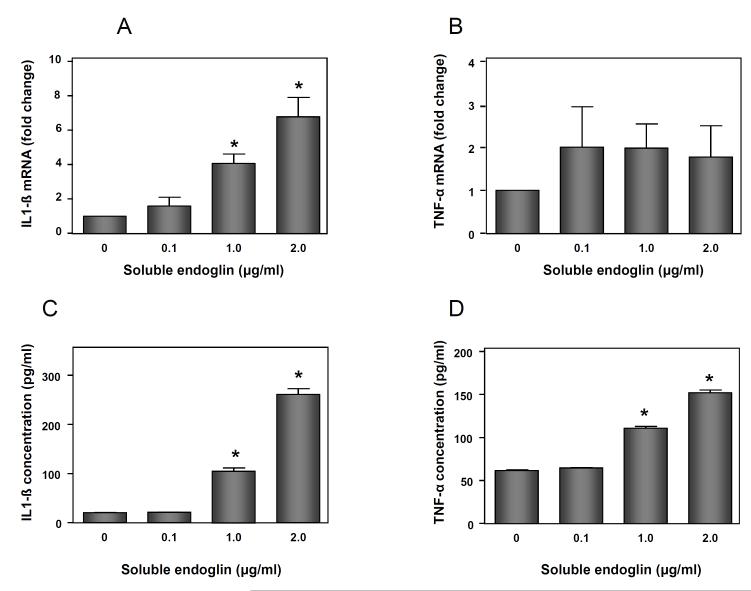
U937-derived macrophages were treated in the presence or absence of recombinant endoglin (0.1, 1 and 2 μg/mL). The mean mRNA expression of IL-1β was significantly increased after treatment with recombinant endoglin at concentrations of 1 and 2 μg/mL (p<0.05 for each; Figure 8A). The mean mRNA expression of TNF-α was also increased after adding recombinant endoglin, but the difference did not reach statistical significance (Figure 8B). Treatment of U937-derived macrophages with recombinant endoglin increased the mean concentration of IL-1β and TNF-α in supernatant in a dose-dependent manner (p<0.05 for each; Figures 8C and 8D).
Figure 8. Effects of soluble endoglin (sEng) on U937-derived macrophages.
(A) The mean mRNA expression of IL-1β on macrophages was significantly increased after treatment with recombinant sEng at 1 and 2 μg/mL (p<0.05 for each). (B) The mean mRNA expression of TNF-α was also increased after treatment with recombinant sEng, but the difference did not reach statistical significance. (C, D) The mean concentration of IL-1β and TNF-α protein in supernatant was significantly increased after treatment of macrophages with recombinant sEng at 1 and 2μg/mL. Data represents mean ± SEM. All the experiments were done in triplicate. The p-values were calculated by Student’s t-tests. (*)= p<0.05.
DISCUSSION
Principal findings of the study
1) IAI was associated with an increase in the amniotic fluid concentrations of sEng; 2) an increase in the amniotic fluid concentrations of sEng was associated with histologic chorioamnionitis, BPD and composite neonatal morbidity in patients with PTL and preterm PROM; 3) endoglin was expressed in chorionic trophoblasts and on amniotic fluid macrophages; 4) treatment of macrophages with endotoxin or TNF-α increases sEng concentrations in the culture supernatant; 5) sEng increased mRNA and protein IL-1β and TNF-α production by macrophages; and 6) amniotic fluid concentrations of sEng decreased as a function of gestational age and did not change with spontaneous labor at term.
Intraamniotic infection/inflammation is associated with an increase in the amniotic fluid concentrations of sEng
This is the first study to demonstrate that the amniotic fluid concentration of sEng is higher in patients with IAI than in those without IAI. This finding is in contrast with that of soluble vascular endothelial growth factor receptor (sVEGFR)-1, another important anti-angiogenic factor, which does not increase in the AF of patients with IAI.149 Furthermore, the amniotic fluid concentration of sEng was positively correlated with indirect markers of IAI, including IL-6 concentration and WBC count in amniotic fluid. These findings are consistent with the results of previous observations, in which endoglin expression was up-regulated in inflammatory conditions. For example: 1) endoglin is strongly expressed in inflamed skin, and this is accompanied by infiltration of macrophage and T cells;150 and 2) endoglin is highly expressed in the vascular endothelium of cirrhotic livers, and inflamed bowel and lung.150
Endoglin and the innate immune response: bridging the gap between inflammation and angiogenesis
An emerging theme in biology is that inflammation is linked to angiogenesis.107,151 Indeed, inflammation can stimulate angiogenesis: nutrients and oxygen are supplied from the formation of new vessels to allow the transport of inflammatory cells during inflammatory processes. Of note, changes in angiogenic and anti-angiogenic factors expression have been observed in patients with sepsis or those with localized infection. For example, circulating concentrations of angiogenic factors such as VEGF and placental growth factor (PlGF) were increased in patients with sepsis.109,152 The serum concentration of soluble VEGF receptor-1, which has an anti-angiogenic effect on endothelial cells, correlated with disease severity in sepsis.153 Moreover, we have demonstrated that IAI is also associated with elevated amniotic fluid concentrations of angiopoietin-2, one of the major regulators of angiogenesis,154 and acute pyelonephritis during pregnancy changes the balance of angiogenic and anti-angiogenic factors in maternal plasma.155
Endoglin has been implicated in the pathophysiology of anti-angiogenic states of pregnancy, such as preeclampsia,114-116,119,156 SGA,116 fetal death,157 preterm labor,158 twin to twin transfusion syndrome,159 mirror syndrome,160 and other conditions such as tumors161 and hereditary hemorrhagic telangiectasia.162 The latter is a rare genetic disease, characterized by mutations in the endoglin gene, resulting in low endoglin expression and abnormalities of the blood vessels leading to epistaxis, telangiectasia, and visceral arteriovenous malformations.163 Although the disease is characterized mainly by abnormal vascular structures, severe infections (such as cerebral abscesses, extra-cerebral infections, hepatic, renal and splenic abscesses) have been reported.163 Indeed, polymorphonuclear leukocytes and monocytes from patients with hereditary hemorrhagic telangiectasia had reduced phagocytosis and oxidative burst activity.123 Thus, it is possible that the decreased production of endoglin may be associated with impairment in the innate immune response.
sEng and Bronchopulmonary dysplasia
The findings of this study demonstrate, for the first time, that high concentrations of sEng in amniotic fluid are associated with subsequent development of BPD in neonates. sEng has an anti-angiogenic effect by inhibition of TGF-ß activity.114 There is evidence that BPD is associated with dysregulation of angiogenesis in the pulmonary vasculature. Evidence in support of this includes: (1) angiogenesis in the lung promotes active alveolarization;134,137 (2) VEGF and TGF-β signaling are disrupted in BPD;128,131,164-166 (3) in animal experiments, recombinant VEGF treatment and adenovirus-mediated VEGF gene therapy prevent alveolar injury in BPD;132,135,136 (4) neonatal treatment with VEGF inhibitor (SU5416) impairs lung growth and decreases nitric oxide production in neonatal rat lungs:133 in contrast, treatment with inhaled nitric oxide restores lung structure in eNOS-deficient mice;129 (5) similarly, intraamniotic administration of sVEGFR-1, a potent anti-angiogenic factor, to mice in preterm gestations can decrease the alveolar number, reduce pulmonary vessel density, suppress activation of lung VEGF receptor-2 and increased apoptosis in endothelial and mesenchymal cells in the newborn lung, suggesting that an elevation of anti-angiogenic factors in the amniotic fluid may result in impaired alveolarization and pulmonary vascular growth and contribute to the increased risk of BPD;167 (6) a high concentration of the anti-angiogenic factor endostatin in umbilical cord blood is associated with the development of BPD in very low birth weight infants;130 and (7) a significant increase in the expression of endoglin transcript and protein was observed more frequently in the lungs of ventilated preterm infants than in age-matched non-ventilated control lungs.138
What are the potential sources of elevated amniotic fluid concentration of sEng in patients with intraamniotic infection/inflammation?
Our findings that endoglin was expressed on amniotic fluid macrophages are consistent with previous studies which identified endoglin expression on the surface of interstitial macrophages from the red pulp of the spleen and on differentiated monocytes.148,168 Of note, endoglin is weakly expressed or absent on freshly isolated monocytes, while it is readily detectable on tissue and in vitro-cultured macrophages.148,168 Indeed, endoglin expression of maternal monocytes was very weak compared to that of amniotic fluid macrophages obtained from the same patients using flow cytometry (data not shown). Collectively, the results of previous studies coupled with our findings indicate that endoglin is expressed on CD14+ cells in amniotic fluid, and that these cells are generally present only in patients with IAI (but not in those without IAI).
Other potential sources of sEng in amniotic fluid examined in this study are chorioamniotic membranes and umbilical cord blood. Endoglin expression was observed on chorionic trophoblasts and on the endothelial cells of decidual vessels, but not on amnion epithelial cells, by immunohistochemical staining. However, there was no significant difference (as determined by immunoblotting) in the mean endoglin expression in the chorioamniotic membranes from placentas with and without histologic chorioamnionitis. In addition, there was no significant difference in the median plasma concentration of sEng in umbilical cord blood from preterm neonates with and without histologic chorioamnionitis and funisitis. Therefore, these findings suggest that amniotic fluid macrophages, but not chorioamniotic membranes or umbilical cord blood, may be a source of elevated sEng concentrations in the amniotic fluid of patients with IAI.
Macrophages can release sEng in response to microbial products or pro-inflammatory cytokines
The mechanisms involved in the generation of the soluble form of endoglin are incompletely understood. However, sEng is thought to be released by proteolytic cleavage of the membrane-bound endoglin.114 The concentrations of sEng in the supernatant of U937-derived macrophages were increased after stimulation with LPS or TNF-α. The findings of this study are novel, and provide evidence that macrophages can release sEng in response to a microbial product or pro-inflammatory cytokines.
sEng increases IL-1β and TNF-α production by macrophages
There is a paucity of information about the role of sEng in the inflammatory response, although several studies have shown its anti-angiogenic effects in the pathophysiology of preeclampsia.114,115 Our findings demonstrate a novel observation, that sEng increases the production of IL-1β and TNF-α by macrophages. This suggests that the increased sEng concentrations observed in inflamed amniotic fluid may participate in the inflammatory response by stimulating the production of pro-inflammatory cytokines by macrophages. Although the precise mechanisms of cytokine production by sEng were not examined in this study, one plausible mechanism involves TGF-β.
TGF-β has anti-inflammatory properties. Previous studies have supported an important role for TGF-β in the suppression of macrophage cytokine production.169,170 Werner et al.171 have demonstrated that deactivation of macrophages by TGF-β1 is mediated via Smad3 signaling through inhibiting the activities of pro-inflammatory transcriptional factors such as NFκB and activating protein-1. sEng can inhibit TGF-β activity, which attenuates eNOS activation and, therefore, has an anti-angiogenic effect on endothelial cells.114 Further studies are required to elucidate the interactions of endoglin and other pro-inflammatory or anti-inflammatory cytokines.
sEng concentration in amniotic fluid decreases with advancing gestational age in normal human pregnancy
The changes of sEng concentration in amniotic fluid with advancing gestational age in normal pregnancy have not been previously reported. Vascular remodeling and angiogenesis are required for fetal development and normal pregnancy outcome.172,173 The finding that the amniotic fluid concentration of sEng in the midtrimester was higher than in term gestation suggests a role for sEng during early pregnancy and fetal development. Similar changes have been observed for PlGF174 and angiopoietin-2.154 As normal pregnancy approaches term, angiogenesis in the uterus may decrease.
Conclusions
sEng concentrations in amniotic fluid are higher in patients with IAI than in those without IAI. A potential source of sEng in IAI is amniotic fluid macrophages, which release sEng in response to microbial products. sEng, in turn, can stimulate IL-1β and TNF-α production by macrophages. An elevation of sEng in the amniotic fluid is associated with the subsequent development of BPD in neonates. Given these observational and experimental findings, we propose that sEng participates in the intraamniotic inflammatory response in preterm parturition and may contribute to the development of BPD in infants who delivered preterm.
Acknowledgements
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
Conflicts of Interest The authors have no financial conflicts of interest
Reference List
- 1.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. In: Critchely H, Bennett P, Thomton S, editors. Preterm birth. RCOG press; London: [Google Scholar]; Critchely H, Bennett P, Thomton S, editors. Preterm birth. ROCG Press; London: 2004. pp. 28–60. [Google Scholar]
- 3.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med. 2006;19:773–782. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- 4.Lopez BA. Overview. Preterm labour: mechanisms and management. BMC Pregnancy Childbirth. 2007;7(Suppl 1):S2. doi: 10.1186/1471-2393-7-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 6.Iacovidou N, Varsami M, Syggellou A. Neonatal outcome of preterm delivery. Ann N Y Acad Sci. 2010;1205:130–134. doi: 10.1111/j.1749-6632.2010.05657.x. [DOI] [PubMed] [Google Scholar]
- 7.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, III, Watterberg KL, Saha S, Das A, Higgins RD. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, Mathews TJ, Wilson EC. Births: final data for 2009. Natl Vital Stat Rep. 2011;60:1–70. [PubMed] [Google Scholar]
- 9.Graham BD. Neonatal pediatrics. Part IV. Respiratory distress syndrome. Am Pract Dig Treat. 1960;11:362–365. [PubMed] [Google Scholar]
- 10.Hjalmarson O. Epidemiology and classification of acute, neonatal respiratory disorders. A prospective study. Acta Paediatr Scand. 1981;70:773–783. doi: 10.1111/j.1651-2227.1981.tb06228.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Seong HS, Kim BJ, Jun JK, Romero R, Yoon BH. Evidence to support that spontaneous preterm labor is adaptive in nature: neonatal RDS is more common in “indicated” than in “spontaneous” preterm birth. J Perinat Med. 2009;37:53–58. doi: 10.1515/JPM.2009.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 13.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 14.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, Jun JK. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–1129. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 15.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 16.Baltimore RS. Neonatal sepsis: epidemiology and management. Paediatr Drugs. 2003;5:723–740. doi: 10.2165/00148581-200305110-00002. [DOI] [PubMed] [Google Scholar]
- 17.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 18.Kuperman AA, Kenet G, Papadakis E, Brenner B. Intraventricular hemorrhage in preterm infants: coagulation perspectives. Semin Thromb Hemost. 2011;37:730–736. doi: 10.1055/s-0031-1297163. [DOI] [PubMed] [Google Scholar]
- 19.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 20.Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, Chi JG. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997;177:797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- 21.Martinez E, Figueroa R, Garry D, Visintainer P, Patel K, Verma U, Sehgal PB, Tejani N. Elevated Amniotic Fluid Interleukin-6 as a Predictor of Neonatal Periventricular Leukomalacia and Intraventricular Hemorrhage. J Matern Fetal Investig. 1998;8:101–107. [PubMed] [Google Scholar]
- 22.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, Syn HC. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 23.Kosloske AM. Epidemiology of necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:2–7. doi: 10.1111/j.1651-2227.1994.tb13232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caplan MS, Jilling T. New concepts in necrotizing enterocolitis. Curr Opin Pediatr. 2001;13:111–115. doi: 10.1097/00008480-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, Neu J. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One. 2011;6:e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon JB, Kim JC, Yoon BH, Romero R, Kim G, Oh SY, Kim M, Shim SS. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med. 2002;30:301–306. doi: 10.1515/JPM.2002.044. [DOI] [PubMed] [Google Scholar]
- 27.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003;110(Suppl 20):124–127. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 28.Willoughby RE, Jr., Nelson KB. Chorioamnionitis and brain injury. Clin Perinatol. 2002;29:603–621. doi: 10.1016/s0095-5108(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 29.Nelson KB. Infection in pregnancy and cerebral palsy. Dev Med Child Neurol. 2009;51:253–254. doi: 10.1111/j.1469-8749.2008.03256.x. [DOI] [PubMed] [Google Scholar]
- 30.Nelson KB, Grether JK, Dambrosia JM, Walsh E, Kohler S, Satyanarayana G, Nelson PG, Dickens BF, Phillips TM. Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res. 2003;53:600–607. doi: 10.1203/01.PDR.0000056802.22454.AB. [DOI] [PubMed] [Google Scholar]
- 31.Kalpakidou AK, Allin MP, Walshe M, Giampietro V, Nam KW, McGuire P, Rifkin L, Murray RM, Nosarti C. Neonatal brain injury and neuroanatomy of memory processing following very preterm birth in adulthood: an fMRI study. PLoS One. 2012;7:e34858. doi: 10.1371/journal.pone.0034858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allin MP, Kontis D, Walshe M, Wyatt J, Barker GJ, Kanaan RA, McGuire P, Rifkin L, Murray RM, Nosarti C. White matter and cognition in adults who were born preterm. PLoS One. 2011;6:e24525. doi: 10.1371/journal.pone.0024525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leviton A, Allred EN, Kuban KC, Hecht JL, Onderdonk AB, O’shea TM, Paneth N. Microbiologic and histologic characteristics of the extremely preterm infant’s placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatr Res. 2010;67:95–101. doi: 10.1203/PDR.0b013e3181bf5fab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Shea TM, Allred EN, Kuban KC, Hirtz D, Specter B, Durfee S, Paneth N, Leviton A. Intraventricular hemorrhage and developmental outcomes at 24 months of age in extremely preterm infants. J Child Neurol. 2012;27:22–29. doi: 10.1177/0883073811424462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, Janisse J, Mazor M. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol. 1998;78:5–10. doi: 10.1016/s0301-2115(97)00236-4. [DOI] [PubMed] [Google Scholar]
- 36.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 37.Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, Martin C. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140:171–176. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 38.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 39.Cutz E, Chiasson D. Chronic lung disease after premature birth. N Engl J Med. 2008;358:743–745. doi: 10.1056/NEJMc073362. [DOI] [PubMed] [Google Scholar]
- 40.Lee J, Oh KJ, Yang HJ, Park JS, Romero R, Yoon BH. The importance of intra-amniotic inflammation in the subsequent development of atypical chronic lung disease. J Matern Fetal Neonatal Med. 2009;22:917–923. doi: 10.1080/14767050902994705. [DOI] [PubMed] [Google Scholar]
- 41.Grisaru-Granovsky S, Reichman B, Lerner-Geva L, Boyko V, Hammerman C, Samueloff A, Schimmel MS. Mortality and morbidity in preterm small-for-gestational-age infants: a population-based study. Am J Obstet Gynecol. 2012;206:150–157. doi: 10.1016/j.ajog.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 43.Harrell SN, Brandon DH. Retinopathy of prematurity: the disease process, classifications, screening, treatment, and outcomes. Neonatal Netw. 2007;26:371–378. doi: 10.1891/0730-0832.26.6.371. [DOI] [PubMed] [Google Scholar]
- 44.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165:969–971. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- 45.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, Dinarello CA. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–123. doi: 10.1111/j.1600-0897.1992.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 46.Fidel PL, Jr., Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, Cotton DB. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 47.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trivedi S, Joachim M, McElrath T, Kliman HJ, Allred EN, Fichorova RN, Onderdonk A, Heitor F, Chaychi L, Leviton A, Majzoub JA. Fetal-placental inflammation, but not adrenal activation, is associated with extreme preterm delivery. Am J Obstet Gynecol. 2012;206:236–238. doi: 10.1016/j.ajog.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiefer DG, Keeler SM, Rust O, Chow SS, Craig ME, Peltier MR, Vintzileos AM, Rawlinson WD, Hanna N. Amniotic fluid inflammatory score is associated with pregnancy outcome in patients with mid trimester short cervix. Am J Obstet Gynecol. 2012;206:68–6. doi: 10.1016/j.ajog.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Romero R, Velez Edwards DR, Kusanovic JP, Hassan SS, Mazaki-Tovi S, Vaisbuch E, Kim CJ, Chaiworapongsa T, Pearce BD, Friel LA, Bartlett J, Anant MK, Salisbury BA, Vovis GF, Lee MS, Gomez R, Behnke E, Oyarzun E, Tromp G, Williams SM, Menon R. Identification of fetal and maternal single nucleotide polymorphisms in candidate genes that predispose to spontaneous preterm labor with intact membranes. Am J Obstet Gynecol. 2010;202:431–434. doi: 10.1016/j.ajog.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalan AM, Simhan HN. Mid-trimester cervical inflammatory milieu and sonographic cervical length. Am J Obstet Gynecol. 2010;203:126–5. doi: 10.1016/j.ajog.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol. 1981;140:947–952. doi: 10.1016/0002-9378(81)90090-9. [DOI] [PubMed] [Google Scholar]
- 53.Garite TJ, Freeman RK. Chorioamnionitis in the preterm gestation. Obstet Gynecol. 1982;59:539–545. [PubMed] [Google Scholar]
- 54.Romero R, Roslansky P, Oyarzun E, Wan M, Emamian M, Novitsky TJ, Gould MJ, Hobbins JC. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol. 1988;158:1044–1049. doi: 10.1016/0002-9378(88)90216-5. [DOI] [PubMed] [Google Scholar]
- 55.Toth M, Witkin SS, Ledger W, Thaler H. The role of infection in the etiology of preterm birth. Obstet Gynecol. 1988;71:723–726. [PubMed] [Google Scholar]
- 56.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 57.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 58.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 59.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351–357. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, Insunza A, Montiel F, Behnke E, Cassell GH. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med. 1993;38:543–548. [PubMed] [Google Scholar]
- 61.Yost NP, Cox SM. Infection and preterm labor. Clin Obstet Gynecol. 2000;43:759–767. doi: 10.1097/00003081-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Marconi C, de Andrade Ramos BR, Peracoli JC, Donders GG, da Silva MG. Amniotic fluid interleukin-1 beta and interleukin-6, but not interleukin-8 correlate with microbial invasion of the amniotic cavity in preterm labor. Am J Reprod Immunol. 2011;65:549–556. doi: 10.1111/j.1600-0897.2010.00940.x. [DOI] [PubMed] [Google Scholar]
- 63.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol. 2002;7:259–274. doi: 10.1016/s1084-2756(02)90121-1. [DOI] [PubMed] [Google Scholar]
- 64.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Wennerholm UB, Hagberg H. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand. 2003;82:120–128. doi: 10.1034/j.1600-0412.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 65.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, Erez O, Edwin S, Relman DA. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, Hobbins JC. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:661–666. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 67.Papiernik E. Preterm labor, preterm delivery, intrauterine infection, and preterm rupture of membranes. Curr Opin Obstet Gynecol. 1990;2:8–12. [PubMed] [Google Scholar]
- 68.Carroll SG, Ville Y, Greenough A, Gamsu H, Patel B, Philpott-Howard J, Nicolaides KH. Preterm prelabour amniorrhexis: intrauterine infection and interval between membrane rupture and delivery. Arch Dis Child Fetal Neonatal Ed. 1995;72:F43–F46. doi: 10.1136/fn.72.1.f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greig PC. The diagnosis of intrauterine infection in women with preterm premature rupture of the membranes (PPROM) Clin Obstet Gynecol. 1998;41:849–863. doi: 10.1097/00003081-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 70.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Nikolaitchouk N, Wennerholm UB, Hagberg H. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82:423–431. doi: 10.1034/j.1600-0412.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 71.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, Bik EM, Chaiworapongsa T, Oyarzun E, Relman DA. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. doi: 10.1111/j.1600-0897.2010.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomez R, Romero R, Nien JK, Chaiworapongsa T, Medina L, Kim YM, Yoon BH, Carstens M, Espinoza J, Iams JD, Gonzalez R. A short cervix in women with preterm labor and intact membranes: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2005;192:678–689. doi: 10.1016/j.ajog.2004.10.624. [DOI] [PubMed] [Google Scholar]
- 73.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Nien JK, Berry SM, Bujold E, Camacho N, Sorokin Y. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kusanovic JP, Espinoza J, Romero R, Goncalves LF, Nien JK, Soto E, Khalek N, Camacho N, Hendler I, Mittal P, Friel LA, Gotsch F, Erez O, Than NG, Mazaki-Tovi S, Schoen ML, Hassan SS. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30:706–714. doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hong JS, Park KH, Noh JH, Suh YH. Cervical length and the risk of microbial invasion of the amniotic cavity in women with preterm premature rupture of membranes. J Korean Med Sci. 2007;22:713–717. doi: 10.3346/jkms.2007.22.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38:261–268. doi: 10.1515/JPM.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vaisbuch E, Romero R, Erez O, Kusanovic JP, Mazaki-Tovi S, Gotsch F, Romero V, Ward C, Chaiworapongsa T, Mittal P, Sorokin Y, Hassan SS. Clinical significance of early (< 20 weeks) vs. late (20-24 weeks) detection of sonographic short cervix in asymptomatic women in the mid-trimester. Ultrasound Obstet Gynecol. 2010;36:471–481. doi: 10.1002/uog.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, Dong Z, Yeo L, Mittal P, Yoon BH, Romero R. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433–438. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim YM, Romero R, Chaiworapongsa T, Kim GJ, Kim MR, Kuivaniemi H, Tromp G, Espinoza J, Bujold E, Abrahams VM, Mor G. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191:1346–1355. doi: 10.1016/j.ajog.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 80.Sverremark-Ekstrom E, Holmlund U. Immnohistochemical distribution of Toll-like receptor 4 (TLR4) in term and preterm human placentas. Hum Pathol. 2006;37:121–122. doi: 10.1016/j.humpath.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 81.Abrahams VM, Potter JA, Bhat G, Peltier MR, Saade G, Menon R. Bacterial Modulation of Human Fetal Membrane Toll-like Receptor Expression. Am J Reprod Immunol. 2012 Sep 11; doi: 10.1111/aji.12016. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi SJ, Jung SH, Eom M, Han KH, Chung IB, Kim SK. Immunohistochemical distribution of toll-like receptor 4 in preterm human fetal membrane. J Obstet Gynaecol Res. 2012;38:108–112. doi: 10.1111/j.1447-0756.2011.01626.x. [DOI] [PubMed] [Google Scholar]
- 83.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 84.Koga K, Cardenas I, Aldo P, Abrahams VM, Peng B, Fill S, Romero R, Mor G. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61:196–212. doi: 10.1111/j.1600-0897.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol. 2005;175:8096–8104. doi: 10.4049/jimmunol.175.12.8096. [DOI] [PubMed] [Google Scholar]
- 86.Abrahams VM, Schaefer TM, Fahey JV, Visintin I, Wright JA, Aldo PB, Romero R, Wira CR, Mor G. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I : C) Hum Reprod. 2006;21:2432–2439. doi: 10.1093/humrep/del178. [DOI] [PubMed] [Google Scholar]
- 87.Beijar EC, Mallard C, Powell TL. Expression and subcellular localization of TLR-4 in term and first trimester human placenta. Placenta. 2006;27:322–326. doi: 10.1016/j.placenta.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 88.Abrahams VM, Aldo PB, Murphy SP, Visintin I, Koga K, Wilson G, Romero R, Sharma S, Mor G. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J Immunol. 2008;180:6035–6043. doi: 10.4049/jimmunol.180.9.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aldo PB, Mulla MJ, Romero R, Mor G, Abrahams VM. Viral ssRNA induces first trimester trophoblast apoptosis through an inflammatory mechanism. Am J Reprod Immunol. 2010;64:27–37. doi: 10.1111/j.1600-0897.2010.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abrahams VM, Mor G. Toll-like receptors and their role in the trophoblast. Placenta. 2005;26:540–547. doi: 10.1016/j.placenta.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 91.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cardenas I, Mor G, Aldo P, Lang SM, Stabach P, Sharp A, Romero R, Mazaki-Tovi S, Gervasi M, Means RE. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol. 2011;65:110–117. doi: 10.1111/j.1600-0897.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F, Kim BI. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177:825–830. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 94.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 95.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, Kim JS, Edwin SS, Gomez R, Draghici S. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63:73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gillaux C, Mehats C, Vaiman D, Cabrol D, Breuiller-Fouche M. Functional screening of TLRs in human amniotic epithelial cells. J Immunol. 2011;187:2766–2774. doi: 10.4049/jimmunol.1100217. [DOI] [PubMed] [Google Scholar]
- 97.Adams KM, Lucas J, Kapur RP, Stevens AM. LPS induces translocation of TLR4 in amniotic epithelium. Placenta. 2007;28:477–481. doi: 10.1016/j.placenta.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Torricelli M, Voltolini C, Novembri R, Bocchi C, Di TM, Severi FM, Petraglia F. Activin A and its Regulatory Molecules in Placenta and Fetal Membranes of Women with Preterm Premature Rupture of the Membranes Associated with Acute Chorioamnionitis. Am J Reprod Immunol. 2012 doi: 10.1111/j.1600-0897.2012.01180.x. [DOI] [PubMed] [Google Scholar]
- 99.Zaga-Clavellina V, Martha RV, Flores-Espinosa P. In vitro secretion profile of pro-inflammatory cytokines IL-1beta, TNF-alpha, IL-6, and of human beta-defensins (HBD)-1, HBD-2, and HBD-3 from human chorioamniotic membranes after selective stimulation with Gardnerella vaginalis. Am J Reprod Immunol. 2012;67:34–43. doi: 10.1111/j.1600-0897.2011.01054.x. [DOI] [PubMed] [Google Scholar]
- 100.Gulati S, Bhatnagar S, Raghunandan C, Bhattacharjee J. Interleukin-6 as a predictor of subclinical chorioamnionitis in preterm premature rupture of membranes. Am J Reprod Immunol. 2012;67:235–240. doi: 10.1111/j.1600-0897.2011.01084.x. [DOI] [PubMed] [Google Scholar]
- 101.Mor G, Romero R, Aldo PB, Abrahams VM. Is the trophoblast an immune regulator? The role of Toll-like receptors during pregnancy. Crit Rev Immunol. 2005;25:375–388. doi: 10.1615/critrevimmunol.v25.i5.30. [DOI] [PubMed] [Google Scholar]
- 102.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y, Luo XP, Lim CE, Wong WS, Zhong G. Regulatory Effect of Peptidoglycan on the Expression of Toll-Like Receptor 2 mRNA and Proteins in Trophoblast Cell Line TEV-1 Cells. ISRN Obstet Gynecol. 2011;2011:692858. doi: 10.5402/2011/692858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 105.Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lingen MW. Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch Pathol Lab Med. 2001;125:67–71. doi: 10.5858/2001-125-0067-ROLAEC. [DOI] [PubMed] [Google Scholar]
- 107.Imhof BA, urrand-Lions M. Angiogenesis and inflammation face off. Nat Med. 2006;12:171–172. doi: 10.1038/nm0206-171. [DOI] [PubMed] [Google Scholar]
- 108.Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, Simms BT, Mizgerd JP, Carmeliet P, Karumanchi SA, Aird WC. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med. 2006;203:1447–1458. doi: 10.1084/jem.20060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yano K, Okada Y, Beldi G, Shih SC, Bodyak N, Okada H, Kang PM, Luscinskas W, Robson SC, Carmeliet P, Karumanchi SA, Aird WC. Elevated levels of placental growth factor represent an adaptive host response in sepsis. J Exp Med. 2008;205:2623–2631. doi: 10.1084/jem.20080398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 111.Quackenbush EJ, Gougos A, Baumal R, Letarte M. Differential localization within human kidney of five membrane proteins expressed on acute lymphoblastic leukemia cells. J Immunol. 1986;136:118–124. [PubMed] [Google Scholar]
- 112.Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 113.Fonsatti E, Maio M. Highlights on endoglin (CD105): from basic findings towards clinical applications in human cancer. J Transl Med. 2004;2:18. doi: 10.1186/1479-5876-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D’Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 115.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 116.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reddy A, Suri S, Sargent IL, Redman CW, Muttukrishna S. Maternal circulating levels of activin A, inhibin A, sFlt-1 and endoglin at parturition in normal pregnancy and pre eclampsia. PLoS One. 2009;4:e4453. doi: 10.1371/journal.pone.0004453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Edwin SS, Gomez R, Yeo L, Conde-Agudelo A, Hassan SS. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–1038. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chaiworapongsa T, Romero R, Kusanovic JP, Mittal P, Kim SK, Gotsch F, Than NG, Mazaki-Tovi S, Vaisbuch E, Erez O, Yeo L, Hassan SS, Sorokin Y. Plasma soluble endoglin concentration in pre-eclampsia is associated with an increased impedance to flow in the maternal and fetal circulations. Ultrasound Obstet Gynecol. 2010;35:155–162. doi: 10.1002/uog.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chaiworapongsa T, Romero R, Savasan ZA, Kusanovic JP, Ogge G, Soto E, Dong Z, Tarca A, Gaurav B, Hassan SS. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24:1187–1207. doi: 10.3109/14767058.2011.589932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Staff AC, Braekke K, Johnsen GM, Karumanchi SA, Harsem NK. Circulating concentrations of soluble endoglin (CD105) in fetal and maternal serum and in amniotic fluid in preeclampsia. Am J Obstet Gynecol. 2007;197:176. doi: 10.1016/j.ajog.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 122.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cirulli A, Loria MP, Dambra P, Di SF, Ventura MT, Amati L, Jirillo E, Sabba C. Patients with Hereditary Hemorrhagic Telangectasia (HHT) exhibit a deficit of polymorphonuclear cell and monocyte oxidative burst and phagocytosis: a possible correlation with altered adaptive immune responsiveness in HHT. Curr Pharm Des. 2006;12:1209–1215. doi: 10.2174/138161206776361336. [DOI] [PubMed] [Google Scholar]
- 124.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- 125.Kallapur SG, Jobe AH. Contribution of inflammation to lung injury and development. Arch Dis Child Fetal Neonatal Ed. 2006;91:F132–F135. doi: 10.1136/adc.2004.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schmidt B, Cao L, kensen-Haen S, Kendziorra H, Klingel K, Speer CP. Chorioamnionitis and inflammation of the fetal lung. Am J Obstet Gynecol. 2001;185:173–177. doi: 10.1067/mob.2001.113321. [DOI] [PubMed] [Google Scholar]
- 127.Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol. 2003;8:63–71. doi: 10.1016/s1084-2756(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 128.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 129.Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L119–L127. doi: 10.1152/ajplung.00395.2005. [DOI] [PubMed] [Google Scholar]
- 130.Janer J, Andersson S, Kajantie E, Lassus P. Endostatin concentration in cord plasma predicts the development of bronchopulmonary dysplasia in very low birth weight infants. Pediatrics. 2009;123:1142–1146. doi: 10.1542/peds.2008-1339. [DOI] [PubMed] [Google Scholar]
- 131.Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, Andersson S. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med. 2001;164:1981–1987. doi: 10.1164/ajrccm.164.10.2012036. [DOI] [PubMed] [Google Scholar]
- 132.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 133.Tang JR, Seedorf G, Balasubramaniam V, Maxey A, Markham N, Abman SH. Early inhaled nitric oxide treatment decreases apoptosis of endothelial cells in neonatal rat lungs after vascular endothelial growth factor inhibition. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1271–L1280. doi: 10.1152/ajplung.00224.2007. [DOI] [PubMed] [Google Scholar]
- 134.Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med. 2001;164:1755–1756. doi: 10.1164/ajrccm.164.10.2109111c. [DOI] [PubMed] [Google Scholar]
- 135.Kunig AM, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1068–L1078. doi: 10.1152/ajplung.00093.2006. [DOI] [PubMed] [Google Scholar]
- 136.Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2005;289:L529–L535. doi: 10.1152/ajplung.00336.2004. [DOI] [PubMed] [Google Scholar]
- 137.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 138.De Paepe ME, Patel C, Tsai A, Gundavarapu S, Mao Q. Endoglin (CD105) up-regulation in pulmonary microvasculature of ventilated preterm infants. Am J Respir Crit Care Med. 2008;178:180–187. doi: 10.1164/rccm.200608-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guinn DA, Goldenberg RL, Hauth JC, Andrews WW, Thom E, Romero R. Risk factors for the development of preterm premature rupture of the membranes after arrest of preterm labor. Am J Obstet Gynecol. 1995;173:1310–1315. doi: 10.1016/0002-9378(95)91377-7. [DOI] [PubMed] [Google Scholar]
- 140.Bancalari E, Abdenour GE, Feller R, Gannon J. Bronchopulmonary dysplasia: clinical presentation. J Pediatr. 1979;95:819–823. doi: 10.1016/s0022-3476(79)80442-4. [DOI] [PubMed] [Google Scholar]
- 141.Andrews WW, Goldenberg RL, Faye-Peterson O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195:803–808. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 142.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 143.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, Baumann P, Araneda H, Kenney JS, Cotton DB. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839–851. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 144.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, Ramirez M, Fidel PL, Sorokin Y, Cotton D. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169:805–816. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 145.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, Edberg S. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–119. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 146.MacDonald PC, Koga S, Casey ML. Decidual activation in parturition: examination of amniotic fluid for mediators of the inflammatory response. Ann N Y Acad Sci. 1991;622:315–330. doi: 10.1111/j.1749-6632.1991.tb37877.x. [DOI] [PubMed] [Google Scholar]
- 147.Menon R, Peltier MR, Eckardt J, Fortunato SJ. Diversity in cytokine response to bacteria associated with preterm birth by fetal membranes. Am J Obstet Gynecol. 2009;201:306, e1–6. doi: 10.1016/j.ajog.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 148.Lastres P, Bellon T, Cabanas C, Sanchez-Madrid F, Acevedo A, Gougos A, Letarte M, Bernabeu C. Regulated expression on human macrophages of endoglin, an Arg-Gly-Asp-containing surface antigen. Eur J Immunol. 1992;22:393–397. doi: 10.1002/eji.1830220216. [DOI] [PubMed] [Google Scholar]
- 149.Alpay Savasan Z, Romero R, Chaiworapongsa T, Kusanovic JP, Kim SK, Mazaki-Tovi S, Vaisbuch E, Mittal P, Ogge G, Madan I, Dong Z, Yeo L, Hassan SS. Evidence in support of a role for anti-angiogenic factors in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2010;23:828–841. doi: 10.3109/14767050903440471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Torsney E, Charlton R, Parums D, Collis M, Arthur HM. Inducible expression of human endoglin during inflammation and wound healing in vivo. Inflamm Res. 2002;51:464–470. doi: 10.1007/pl00012413. [DOI] [PubMed] [Google Scholar]
- 151.Sookhai S, Wang JH, McCourt M, O’Connell D, Redmond HP. Dopamine induces neutrophil apoptosis through a dopamine D-1 receptor-independent mechanism. Surgery. 1999;126:314–322. [PubMed] [Google Scholar]
- 152.Pickkers P, Sprong T, Eijk L, Hoeven H, Smits P, Deuren M. Vascular endothelial growth factor is increased during the first 48 hours of human septic shock and correlates with vascular permeability. Shock. 2005;24:508–512. doi: 10.1097/01.shk.0000190827.36406.6e. [DOI] [PubMed] [Google Scholar]
- 153.Shapiro NI, Yano K, Okada H, Fischer C, Howell M, Spokes KC, Ngo L, Angus DC, Aird WC. A prospective, observational study of soluble FLT-1 and vascular endothelial growth factor in sepsis. Shock. 2008;29:452–457. doi: 10.1097/shk.0b013e31815072c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pacora P, Romero R, Chaiworapongsa T, Kusanovic JP, Erez O, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Jai KC, Than NG, Yeo L, Mittal P, Hassan SS. Amniotic fluid angiopoietin-2 in term and preterm parturition, and intra-amniotic infection/inflammation. J Perinat Med. 2009;37:503–511. doi: 10.1515/JPM.2009.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chaiworapongsa T, Romero R, Gotsch F, Kusanovic JP, Mittal P, Kim SK, Erez O, Vaisbuch E, Mazaki-Tovi S, Kim CJ, Dong Z, Yeo L, Hassan SS. Acute pyelonephritis during pregnancy changes the balance of angiogenic and anti-angiogenic factors in maternal plasma. J Matern Fetal Neonatal Med. 2009:1–12. doi: 10.3109/14767050903067378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stepan H, Geipel A, Schwarz F, Kramer T, Wessel N, Faber R. Circulatory soluble endoglin and its predictive value for preeclampsia in second-trimester pregnancies with abnormal uterine perfusion. Am J Obstet Gynecol. 2008;198:175–176. doi: 10.1016/j.ajog.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 157.Romero R, Chaiworapongsa T, Erez O, Tarca AL, Gervasi MT, Kusanovic JP, Mittal P, Ogge G, Vaisbuch E, Mazaki-Tovi S, Dong Z, Kim SK, Yeo L, Hassan SS. An imbalance between angiogenic and anti-angiogenic factors precedes fetal death in a subset of patients: results of a longitudinal study. J Matern Fetal Neonatal Med. 2010;23:1384–1399. doi: 10.3109/14767051003681121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chaiworapongsa T, Romero R, Tarca A, Kusanovic JP, Mittal P, Kim SK, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S, Pacora P, Ogge G, Dong Z, Kim CJ, Yeo L, Hassan SS. A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: Evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J Matern Fetal Neonatal Med. 2009:1–18. doi: 10.3109/14767050902994838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kusanovic JP, Romero R, Espinoza J, Nien JK, Kim CJ, Mittal P, Edwin S, Erez O, Gotsch F, Mazaki-Tovi S, Than NG, Soto E, Camacho N, Gomez R, Quintero R, Hassan SS. Twin-to-twin transfusion syndrome: an antiangiogenic state? Am J Obstet Gynecol. 2008;198:382–388. doi: 10.1016/j.ajog.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rana S, Venkatesha S, DePaepe M, Chien EK, Paglia M, Karumanchi SA. Cytomegalovirus-induced mirror syndrome associated with elevated levels of circulating antiangiogenic factors. Obstet Gynecol. 2007;109:549–552. doi: 10.1097/01.AOG.0000248538.03280.cf. [DOI] [PubMed] [Google Scholar]
- 161.Romani AA, Borghetti AF, Del RP, Sianesi M, Soliani P. The risk of developing metastatic disease in colorectal cancer is related to CD105-positive vessel count. J Surg Oncol. 2006;93:446–455. doi: 10.1002/jso.20456. [DOI] [PubMed] [Google Scholar]
- 162.McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 1994;8:345–351. doi: 10.1038/ng1294-345. [DOI] [PubMed] [Google Scholar]
- 163.Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome) Am J Med Genet. 2000;91:66–67. doi: 10.1002/(sici)1096-8628(20000306)91:1<66::aid-ajmg12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 164.Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1178–L1185. doi: 10.1152/ajplung.00049.2004. [DOI] [PubMed] [Google Scholar]
- 165.Kallapur SG, Nitsos I, Moss TJ, Kramer BW, Newnham JP, Ikegami M, Jobe AH. Chronic endotoxin exposure does not cause sustained structural abnormalities in the fetal sheep lungs. Am J Physiol Lung Cell Mol Physiol. 2005;288:L966–L974. doi: 10.1152/ajplung.00389.2004. [DOI] [PubMed] [Google Scholar]
- 166.Kunzmann S, Speer CP, Jobe AH, Kramer BW. Antenatal inflammation induced TGF-beta1 but suppressed CTGF in preterm lungs. Am J Physiol Lung Cell Mol Physiol. 2007;292:L223–L231. doi: 10.1152/ajplung.00159.2006. [DOI] [PubMed] [Google Scholar]
- 167.Tang JR, Karumanchi SA, Seedorf G, Markham N, Abman SH. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302:L36–L46. doi: 10.1152/ajplung.00294.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.O’Connell PJ, McKenzie A, Fisicaro N, Rockman SP, Pearse MJ, d’Apice AJ. Endoglin: a 180-kD endothelial cell and macrophage restricted differentiation molecule. Clin Exp Immunol. 1992;90:154–159. doi: 10.1111/j.1365-2249.1992.tb05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 170.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 171.Werner F, Jain MK, Feinberg MW, Sibinga NE, Pellacani A, Wiesel P, Chin MT, Topper JN, Perrella MA, Lee ME. Transforming growth factor-beta 1 inhibition of macrophage activation is mediated via Smad3. J Biol Chem. 2000;275:36653–36658. doi: 10.1074/jbc.M004536200. [DOI] [PubMed] [Google Scholar]
- 172.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 173.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 174.Seubert DE, Maymon E, Pacora P, Gervasi MT, Berry SM, Torry DS, Romero R. A study of the relationship between placenta growth factor and gestational age, parturition, rupture of membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;182:1633–1637. doi: 10.1067/mob.2000.107437. [DOI] [PubMed] [Google Scholar]