Abstract
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide, and is the most important challenge to modern obstetrics. A major obstacle has been that preterm birth is treated (implicitly or explicitly) as a single condition. Two-thirds of preterm births occur after the spontaneous onset of labor, and the remaining one-third after “indicated” preterm birth; however, the causes of spontaneous preterm labor and “indicated” preterm birth are different. Spontaneous preterm birth is a syndrome caused by multiple etiologies, one of which is a decline in progesterone action, which induces cervical ripening. A sonographic short cervix (identified in the midtrimester) is a powerful predictor of spontaneous preterm delivery. Randomized clinical trials and individual patient meta-analyses have shown that vaginal progesterone reduces the rate of preterm delivery at <33 weeks of gestation by 44%, along with the rate of admission to the neonatal intensive care unit, respiratory distress syndrome, requirement for mechanical ventilation, and a composite score of neonatal morbidity/mortality. There is no evidence that 17-alpha-hydroxyprogesterone caproate can reduce the rate of preterm delivery in women with a short cervix, and therefore, the compound of choice is natural progesterone (not the synthetic progestin). Routine assessment of the risk of preterm birth with cervical ultrasound coupled with vaginal progesterone for women with a short cervix is cost-effective, and implementation of such a policy is urgently needed. Vaginal progesterone is as effective as cervical cerclage in reducing the rate of preterm delivery in women with a singleton gestation, history of preterm birth, and a short cervix (<25mm).
Keywords: prematurity, infant mortality, respiratory distress syndrome, 17-alpha-hydroxyprogesterone caproate, 17P, 17OHP-C, cost effective analysis, cervical cerclage, pessary
Introduction
Preterm birth has been recognized as the leading cause of perinatal morbidity and mortality for decades. Yet, standard prenatal care does not include methods to predict or prevent spontaneous preterm birth.1 This situation is about to change because of two developments: 1) the realization that sonographic cervical length evaluation in the midtrimester is a simple method to identify patients at risk for spontaneous preterm delivery; and 2) the evidence derived from randomized clinical trials and meta-analyses which show that vaginal progesterone is effective in preventing preterm birth in patients with a short cervix.2,3
Why has the prevention of preterm birth been so difficult to achieve? We believe that this is due to a “cognitive trap”: a reluctance to accept the complexity of the problem. Preterm birth is often treated (implicitly or explicitly) as if it were a single condition. An important step forward would be to reframe the problem of preterm birth to make it tractable.
The obstetrical circumstances that lead to a preterm delivery are fundamentally two: 1) preterm birth that occurs after the spontaneous onset of labor (with intact or prelabor ruptured membranes); or 2) “indicated” preterm birth, which occurs because of maternal complications (such as preeclampsia) or fetal disease (such as intrauterine growth restriction [IUGR]).4 To refer to preterm birth as a single condition which could be predicted by a single test and prevented by a single intervention is a flawed concept that has resulted in unrealistic expectations and therapeutic nihilism. This article will focus on the prediction of spontaneous preterm birth with cervical length in the midtrimester, and its prevention with vaginal progesterone. The lessons learned in basic and clinical research that have led to this advance can be used as a blueprint to approach other mechanisms of disease implicated in the etiology of preterm labor.
A conceptual framework for the understanding of preterm labor
The traditional view that has governed the study of parturition is that preterm and term labor are fundamentally the same process, albeit occurring at different gestational ages.5,6 Indeed, preterm and term labor share a common terminal pathway, which we have defined as the anatomic, biochemical, endocrinologic and clinical events that occur in term and preterm parturition. For example, the uterine components of the common pathway include: 1) increased uterine contractility; 2) cervical ripening; and 3) decidual membrane activation (see Figure 1).
Figure 1.
Uterine components of the common pathway of parturition.
An important difference between term and preterm labor is that the former results from “physiologic activation of the common pathway”, while the latter results from a pathologic process (“pathologic activation that extemporaneously activates components of the common pathway”)5,6 (see Figure 2).
Figure 2.
Normal spontaneous labor at term results from physiologic activation of the common pathway of parturition. In contrast, preterm labor begins because of a pathologic insult, resulting in the initiation of labor.
Activation of the uterine components of the common pathway of parturition may be synchronous or asynchronous.7 Synchronous activation would result in clinical spontaneous preterm labor, while asynchronous would result in a different clinical presentation (referred to, by some, as a phenotype). For example, predominant activation of the membranes would lead to preterm PROM, of the cervix to cervical insufficiency, or of the myometrium to increased preterm uterine contractions (see Figure 3). The activation of each component confers a different risk for impending preterm delivery. For example, rupture of membranes is followed by the onset of labor in most cases, within a short period of time. In contrast, most patients who present with increased uterine contractility at an early gestational age deliver at term. Acute cervical insufficiency (formerly called “cervical incompetence”) may lead to a late spontaneous abortion or early preterm delivery within days or weeks after diagnosis.8–11 An isolated short cervix in the midtrimester is an example of asynchronous activation of the common pathway of parturition, because generally, patients do not have increased uterine contractility or evidence of ruptured membranes.
Figure 3.
Clinical manifestations of preterm activation of the common pathway of parturition.
Methods are available to detect the activation of each of the components of the common pathway. Increased myometrial uterine contractility is often detected by patients and documented using external tocodynamometers12–15 or electromyography.16–22 Cervical changes could lead to a sensation of vaginal pressure, and have been traditionally detected with digital examination to document effacement and dilatation of the cervix. Cervical sonography is a means to assess cervical changes, because a short cervix is often a sign of effacement in progress. Membrane/decidual activation can be detected subclinically by a positive fetal fibronectin,23 insulin growth factor binding protein-1 (IGFBP-1),24–26 placental alpha microglobulin-1 (PAMG-1),27,28 or other analytes. Degradation of extracellular matrix at the maternal-fetal interface is what allows these compounds to be detectable in cervicovaginal fluid. The most extreme form of membrane/decidual activation is ruptured membranes, which is diagnosed by speculum examination. An important concept is that activation of these processes appears clinically as an acute event, but there is now clear evidence that activation of each process has a long subclinical phase. For example, a patient with a short cervix measured at 20 weeks of gestation is at high risk (50%) for delivering a preterm neonate before 33 weeks, and the condition is clinically “silent” for weeks before the onset of preterm labor or the occurrence of preterm prelabor rupture of membranes (PPROM).29
The long subclinical phase allows the implementation of tests to predict preterm delivery (such as sonographic cervical length), and interventions to prevent it. During the last four decades, it has become clear that once a patient has preterm labor with advanced cervical dilatation or PPROM, interventions are rarely effective. For example, tocolysis can delay delivery for 2–7 days,30–35 but has not been shown to reduce the rate of preterm birth or neonatal morbidity. Antibiotics to women with PPROM can prolong the latency period,36–39 but do not reduce the rate of preterm birth, nor do they eradicate the intra-amniotic infection or prevent secondary intra-amniotic infection:40,41 hence, the need to identify the patient at risk for spontaneous preterm parturition before the clinical episode of preterm labor or ruptured membranes.
The mechanisms of disease implicated in the preterm parturition syndrome have been discussed elsewhere42; Figure 4 describes the proposed etiology of the syndrome. It is noteworthy that more than one mechanism of disease may be operative in one patient.
Figure 4.
Pathological processes implicated in the preterm parturition syndrome.
Cervical Length and the Risk of Preterm Delivery
Studies of the cervix with ultrasound began in the 1980s.43–51 Andersen et al., in 1990, published a seminal observation in which 113 patients were evaluated with digital examination of the cervix, as well as transabdominal and transvaginal sonographically-determined cervical length.52 Cervical length determined by transvaginal (but not transabdominal) ultrasound was predictive of preterm delivery. Importantly, such prediction occurred after adjusting for parity and obstetrical history. There is a curvilinear relationship between cervical length and the likelihood of preterm delivery reported originally by Anderson, and subsequently, by others.29,52–54 These findings have been confirmed by other investigators in low- and high-risk patients.10,29,53–70
An important study by Iams et al. reported the relationship between cervical length and the risk of preterm delivery in 2,915 low risk asymptomatic women who were examined at approximately 24 and then at 28 weeks of gestation.53 The 10th percentile of cervical length was 25mm, and this was used as a cutoff to calculate the diagnostic indices. Table 1 illustrates the diagnostic indices and positive predictive values in the study.
Table 1.
Diagnostic indices and predictive values of cervical length, funneling and Bishop score for prediction of preterm delivery before 35 weeks of gestation
| Cervix at 24 wk |
Cervix at 28 wk |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | ≤20 mm |
≤25 mm |
≤30 mm |
Presence of Funnel |
Bishop Score ≥6 |
Bishop Score ≥4 |
≤20 mm |
≤25 mm |
≤30 mm |
Presence of Funnel |
Bishop Score ≥6 |
Bishop Score ≥4 |
| % Sensitivity | 23.0 | 37.3 | 54.0 | 25.4 | 7.9 | 27.6 | 31.3 | 49.4 | 69.9 | 32.5 | 15.8 | 42.5 |
| % Specificity | 97.0 | 92.2 | 76.3 | 94.5 | 99.4 | 90.9 | 94.7 | 86.8 | 68.5 | 91.6 | 97.9 | 82.5 |
| % Positive predictive value | 25.7 | 17.8 | 9.3 | 17.3 | 38.5 | 12.1 | 16.7 | 11.3 | 7.0 | 11.6 | 25.6 | 9.9 |
| % Negative predictive value |
96.5 | 97.0 | 97.4 | 96.6 | 96.0 | 96.5 | 97.6 | 98.0 | 98.5 | 97.6 | 96.3 | 96.9 |
Source: Reproduced with permission from Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl I Med 1996;334(9):567-72.
Subsequently, several studies were reported from the Fetal Medicine Foundation.54,71,72 The first study included 2,567 patients at low risk for preterm delivery examined at 23 weeks of gestation.54 Patients with a history of preterm birth of Afro-Caribbean origin of low maternal age (<20 years) and low BMI had a shorter cervix than those without these risk factors. However, when logistic regression analysis was used to examine the contribution of each risk factor to the risk of preterm delivery (<32 weeks of gestation), cervical length accounted for the risk, and the other risk factors did not contribute. These findings suggest that clinical and demographic risk factors for preterm birth may operate via a short cervix. The diagnostic indices and predictive values are described in Table 2. Hassan et al. reported a cohort study of 6,877 women in which cervical sonography was performed between 14–24 weeks of gestation.29 The findings of that study also confirmed that a short cervix increased the risk of preterm delivery. Moreover, the investigators found that the later in the midtrimester the sonographic examinations were performed (closer to 24 weeks), the greater the predictive performance of cervical length for preterm birth.
Table 2.
Diagnostic Indices of Sonographic Cervical Length in Low-risk, Asymptomatic Pregnant Women According to Different Cervical Length Cutoffs.
| Author | Year | N | Gestation (wk) |
Cutoff (mm) |
Definition of PTD (wk) |
Prevalence of PTD (%) |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Andersen et al.76 | 1990 | 113 | <30 | <39 | <37 | 15 | 76 | 59 | 25 | 93 |
| Tongsong et al.81 | 1995 | 730 | 28–30 | ≤35 | <37 | 12 | 66 | 62 | 20 | 93 |
| Iams et al.49 | 1996 | 2915 | 24 | <20 | <35 | 4 | 23 | 47 | 26 | 97 |
| Taipale and Hiilesmaa67 | 1998 | 3694 | 18–22 | ≤25 | <37 | 2 | 6 | 100 | 39 | 99 |
| Heath et al.151 | 1998 | 2702 | 23 | ≤15 | ≤32 | 1.5 | 58 | 99 | 52 | 99 |
| Hassan et al.70 | 2000 | 6877 | 14–24 | ≤15 | ≤32 | 3.6 | 8 | 99 | 47 | 97 |
NPV, negative predictive value; PPV, positive predictive value.
Source: Reproduced with permission from Gervasi M–T, Romero R, Maymon E, Pacora P, Jeanty P. Ultrasound examination of the uterine cervix during pregnancy. In: Fleischer AC, Manning FA, Jeanty P, Romero R, eds. Sonography in Obstetrics and Gynecology: principles and practice, 6th ed. New York: McGraw-Hill, 2001:821–41.
Causes of a sonographic short cervix
A sonographic short cervix is a relatively new clinical “sign”, and therefore, there is limited information about its etiology. However, there is a body of literature about conditions associated with a short cervix. This section will review these conditions.
A short cervix is syndromic in nature and can be caused by multiple etiologies,42,73 such as (Figure 5):
A suspension of progesterone action: Progesterone is a key hormone for the maintenance of pregnancy, and a decline in progesterone action74 has been implicated in the control of cervical ripening75–77 and preterm labor.78–80 The evidence in support of a role for progesterone in cervical ripening includes: a) administration of a progesterone receptor antagonist (RU486 or mifepristone) to women in the midtrimester and at term ripens the cervix;75,81–85 and b) administration of progesterone receptor antagonists (RU486 or onapristone) to pregnant guinea pigs,86,87 Old World monkeys88 and Tupaja belangeri induces cervical ripening, and often labor.75 Cervical responsiveness to progesterone antagonists increases with advancing gestational age and its effect on the cervix is not always accompanied by changes in myometrial activity.75 Indeed, Stys et al.89 demonstrated a functional dissociation between the effects of progesterone in the myometrium and those in the cervix. The effect of vaginal progesterone in the prevention of preterm birth is thought to be related to a pharmacologic correction of the decline in progesterone action, which manifests itself clinically as a sonographic short cervix.
A congenital short cervix: Cervical hypoplasia after in utero exposure to diethylstilbestrol (DES) has been reported,90–97 and so has dysgenesis of the cervix (i.e fragmented cervix with separations of segments, and a thin fibrous core).98–101 These disorders are rare, and, in most cases, of unknown etiology.
Cervical surgery: The loss of connective tissue after a cervical operation such as a conization102–107 or loop electrosurgical excision procedure (LEEP).106,107
-
Intra-amniotic infection/inflammation: Hassan et al.108 showed that 9% (5 of 57) of asymptomatic women in the mid-trimester with a cervical length less than 25 mm without cervical dilation had microbiologically-proven intra-amniotic infection. The microorganisms isolated included Ureaplasma urealyticum and Fusobacterium spp. Of interest is that in this study, patients with positive cultures for Ureaplasma were treated with intravenous azithromycin for 7 days and underwent a test-of-cure amniocentesis. Three of the 4 patients had a negative amniocentesis, thus demonstrating eradication of an intra-amniotic infection, and subsequently delivered at term.
Intra-amniotic inflammation (defined as an elevation in amniotic fluid proinflammatory cytokines or chemokines) has also been observed in women with a sonographic short cervix in the midtrimester. Kiefer et al.109 reported an association between a cervical length ≤5 mm in the midtrimester and increased amniotic fluid concentrations of the chemokine monocyte chemotactic protein-1 (MCP-1) and the cytokine interleukin (IL)-6. These findings were subsequently confirmed in a study of 44 patients in the mid-trimester with a cervical length ≤25 mm, in which amniotic fluid MCP-1 concentration was predictive for spontaneous preterm delivery.110 Vaisbuch et al.111 demonstrated intra-amniotic inflammation (defined as an amniotic fluid matrix metalloproteinase-8 concentration >23 ng/mL) in 22% of asymptomatic patients (10 of 45) with a cervical length ≤15 mm in the midtrimester of pregnancy; an occurrence that was associated with adverse pregnancy outcomes. Women with intra-amniotic inflammation had a shorter median diagnosis-to-delivery interval than those without this condition. Furthermore, 40% of patients with intra-amniotic inflammation delivered within one week of the amniocentesis.111
The frequency of intra-amniotic infection/inflammation in patients with the clinical diagnosis of cervical insufficiency (which can be considered part of the spectrum of a disorder that shortens the cervix) is nearly 50%.112–114
Cervical insufficiency: this term has replaced “cervical incompetence”, which has been defined as the inability of the uterine cervix to retain a pregnancy in the absence of contractions or labor.115 This diagnosis is traditionally applied to patients with a history of recurrent midtrimester abortions and/or early preterm deliveries in which the basic process is thought to be “the failure of the cervix to remain closed during pregnancy”.116 The presenting symptom is often vaginal pressure, and patients have painless cervical dilatation. There is no objective diagnostic test to identify the patient at risk for this condition, either before pregnancy or during early pregnancy; therefore, this is a clinical diagnosis.73
History of a previous preterm birth: Several authors have documented a relationship between a previous obstetrical history of preterm birth and cervical length.10,117 Iams et al.10 reported a study of cervical length in patients with: (1) a previous history of “cervical incompetence”, (2) a previous preterm delivery at less than 26 weeks, (3) a previous preterm delivery at 27 to 32 weeks, (4) a previous preterm delivery at 33 to 35 weeks, and (5) a control group of women with previous term delivery. A strong relationship was observed between cervical length in the index pregnancy and previous obstetric history.10 Similar results were reported by Guzman et al.,117 who described a strong relationship between previous obstetric history and cervical length in the subsequent pregnancy. Specifically, the authors observed that the frequency of a short cervix (cervical length <20 mm) or progressive shortening of the cervix to a length of less than 20 mm was associated with the gestational age at delivery in the previous pregnancy.117 Collectively, these studies suggest a relationship between a history of preterm delivery and the cervical length in a subsequent pregnancy.
Other risk factors for a short cervix: Maternal age (<20 years; >35 years), a low body mass index (<19.8kg/m2) and ethnicity (African-American or Afro-Caribbean) are associated with a shorter cervical length.72 One interpretation of these observations is that the combination of genetic and environmental factors may play a role in determining cervical length. Polymorphisms in the genes encoding for collagen I (COL1A1) and transforming growth factor beta-1 (TGFB1) have been associated with cervical insufficiency.118–120
Figure 5.
Syndrome nature of a short cervix which is caused by multiple etiologies
In conclusion, a short cervix may be the result of multiple pathologic processes. The establishment of causality is a challenge, and a sonographic short cervix may evolve into the condition clinically referred to as “cervical insufficiency” or place the patient at risk for early spontaneous preterm birth.
TECHNIQUE FOR SONOGRAPHIC EXAMINATION OF THE UTERINE CERVIX
The uterine cervix can be imaged using a transabdominal, transvaginal or transperineal approach.
Transabdominal examination
This method was the first used to examine the uterine cervix in non-pregnant and pregnant patients. However, this approach is not recommended for the following reasons:
Transabdominal imaging of the cervix requires that patients have a distended bladder. Andersen reported that the cervix could only be visualized in 45% of pregnant women with an empty bladder.121 To et al. demonstrated that successful visualization of the cervix is a function of urine volume within the bladder.122 The frequency of visualization was 42% with bladder volumes of <15 ml and 73% for volumes >150 ml. Moreover, when the cervical length was <20 mm (a cervical length associated with increased risk of preterm birth), visualization with transabdominal ultrasound occurred in only 13% of cases.
A distended bladder can compress and artificially lengthen the uterine cervix (Figure 6).45,51,122,123 This seems to occur when the cervix is compliant, and therefore, may lead to the under-diagnosis of a short cervix (Figure 7).
The management of a waiting room with patients with a full bladder is a challenge for physicians and nurses.
The image quality of the uterine cervix is lower with transabdominal ultrasound than with transvaginal ultrasound because there is greater distance between the probe and the cervix (Figure 8). Occasionally, fetal parts can be close to the cervix and impair visualization of this organ.
A study comparing transabdominal with transvaginal ultrasound determined that 45% of patients with a cervical length of <25 mm would be missed if scanned transabdominally.124 Consequently, the approach of scanning patients with a transabdominal ultrasound and performing transvaginal sonography only in those who have a cervical length of <30mm would result in a high rate of false-negative diagnoses. In other words, many patients with a true short cervix would be missed if only scanned by transabdominal sonography.
Figure 6.
Transabdominal sonogram performed in a patient with a full bladder. The bladder is causing compression and artificial lengthening of the uterine cervix.
Figure 7.
Same patient as in Figure 6 but with the bladder emptied and transvaginal sonography performed. Note that the true cervical length is short (15.5 mm).
Figure 8.
Transabdominal ultrasound when the fetus is in a vertex presentation. Note that the image quality of the uterine cervix is poor, because there is greater distance between the probe and the cervix, and there is shadowing from the fetal head.
Transperineal examination
The transperineal approach was developed before the availability of transvaginal probes,125 and can be used when a transvaginal probe is not available. The technique requires the placement of a transducer covered by a sheath in the sagittal plane between the labia majora. A distended bladder is not required. The main challenge is that the presence of bowel gas can impair visualization of the cervical external os;126 therefore, it is best not to use the transperineal approach if a pelvic examination has been performed. Orientation, anatomy definition and quality of the image are better with transvaginal ultrasonography.
Transvaginal examination
This has become the “gold standard” for the performance of cervical examinations during pregnancy (Figure 9). Patients do not need to have a distended bladder, and the definition of cervical anatomy is optimal with visualization of the cervix in all cases. Studies of acceptability indicate that more than 90% of women report experiencing no or only mild discomfort or embarrassment.72,127 Indeed, transvaginal examination is preferred to digital examination by most patients. Box 1 describes the method of transvaginal sonographic examination of the uterine cervix.
Figure 9.
Transvaginal ultrasound of the uterine cervix (cervical length 39.8 mm). This is the “gold standard” for the performance of cervical examinations during pregnancy. Note that the visualization of cervical anatomy and measurement of the cervical length is optimal.
METHOD OF TRANSVAGINAL EXAMINATION.
Patients should be asked to empty the bladder immediately before the examination.
Examinations are conducted with the patient in the dorsal lithotomy position. The examining table should allow the legs to be abducted so that the transducer can be moved during the examination.
A disposable sheath should be used to cover the probe. Condoms are preferred to commercially-available probe covers. Gel is placed between the transducer and cover, and also, sterile gel is used to lubricate the surface of the sheath.
The ultrasound transducer should be of high resolution – 5 MHz or higher.
The operator introduces the vaginal transducer into the anterior vaginal fornix to ensure a sagittal view of the cervix is obtained. The probe may need to be moved laterally or in other directions in order to optimally image the cervix.
The image to measure endocervical length should include the internal os, external os, and the cervical canal.
The endocervical length measurement should not include the lower uterine segment. A useful anatomical landmark is the endocervical glands. The lower uterine segment does not display the hypoechogenic image of the cervical glands. Including the lower uterine segment would result in a falsely longer measurement than the actual endocervical length. The anterior and posterior lips of the cervix should have the same dimensions; unequal dimensions suggest that there is excessive pressure being applied during transvaginal sonography.
The image used to measure endocervical length should be magnified so that the cervix will occupy at least 75% of the image.
Excessive pressure with the probe may elongate the cervix. To avoid this pitfall, the probe is slowly withdrawn until the image blurs, and is subsequently reapplied with an amount of pressure sufficient to restore the image. This can be avoided by confirming equal cervical widths and density of the anterior and posterior lips of the endocervical canal.
The cervical length is measured by freezing the screen 3 separate times. For clinical purposes, the shortest cervical length is reported, provided the image is adequate. The examination is recorded as a video clip as well as static images.
The duration of the examination should be between 3–5 minutes.
The presence of funneling needs to be recorded. A funnel is defined as dilatation of the upper portion of the cervical canal. The width of the funnel must be at least 5mm. The funnel can only be recognized by confirming that the walls are formed by endocervical mucosa; otherwise, the covering wall of the lower uterine segment can be erroneously considered to be part of a funnel.
The presence of “dynamic changes” needs to be noted. This feature is defined as a prolapse of the membranes through the endocervical canal. The most likely explanation for a dynamic change is a uterine contraction (symptomatic or asymptomatic) in the presence of an excessively compliant cervix.
If the cervix is curved, the “trace” function can be used to measure the cervix (if not available, cervical length can be determined by adding the sum of 2 straight sections).
Observe for the presence of particulate material in the amniotic cavity (“sludge”) in proximity to the cervix, and whether such material is free-floating or attached to the cervix. Occasionally, it is possible to gently tap on the anterior uterine wall to determine the effect of pressure on the particulate material.
Vaginal progesterone to prevent preterm birth in women with a short cervix
The first randomized clinical trial to examine the effects of vaginal progesterone on the prevention of preterm birth in women with a short cervix was reported by da Fonseca et al.128 on behalf of the Fetal Medicine Foundation Second Trimester Screening Group of the United Kingdom. This was a randomized, double-blind, placebo-controlled trial in which women with a short cervix (defined as ≤15mm by transvaginal ultrasound) between 20–25 weeks of gestation were allocated to receive either vaginal progesterone (200mg of micronized progesterone) or placebo (safflower oil). The duration of treatment was from 24–34 weeks of gestation (Figure 1). The primary outcome of the trial was the frequency of spontaneous preterm delivery at <34 weeks of gestation. Patients allocated to receive vaginal progesterone had a lower rate of preterm delivery (<34 weeks) than those in the placebo group [19.2% (24/125) vs. 34.4% (43/125)]. The rate of adverse events was similar in the placebo and progesterone groups.
The trial was not designed to test whether progesterone administration could reduce neonatal morbidity, and such a reduction was not observed. It is noteworthy that twins were included in this trial, but the number of twin gestations was small (24).
The second trial to examine the effects of vaginal progesterone on the rate of preterm birth in women with a sonographic short cervix was the PREGNANT trial129, a multi-center, randomized, double-blind, placebo-controlled trial that enrolled asymptomatic women with a singleton gestation and a sonographic short cervix (10–20mm) at 19-23-6/7 weeks of gestation. Patients were randomly allocated to receive a vaginal progesterone gel (90mg) vs. placebo daily, starting between 20-23-6/7 weeks of gestation until 36-6/7 weeks of gestation, rupture of membranes or delivery, whichever occurred first. The primary endpoint was preterm birth before 33 weeks of gestation.
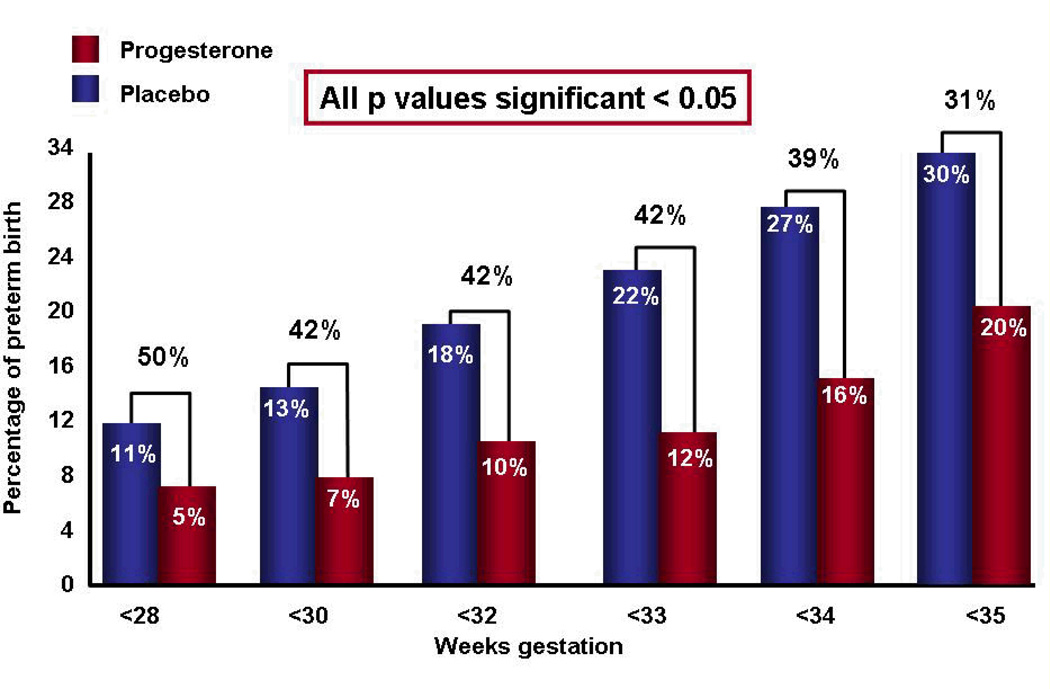
Of the 465 women randomized, 7 were lost to follow-up, and 458 were available for analysis. Patients allocated to receive vaginal progesterone had a significantly lower rate of preterm birth before 33 weeks of gestation than those allocated to placebo [8.9% vs. 61.1%; relative risk (RR) 0.55; 95% confidence interval (CI), 0.33–0.92; p=0.02 (when adjusted for pooled study site and a history of previous preterm birth, the RR was 0.54; 95% CI 0.33–0.89; p=0.01)]. It was estimated that 14 women with a cervical length between 10–20mm would need to be treated with vaginal progesterone to prevent one case of preterm birth before 33 weeks of gestation. In addition, there was a significant decrease in the rate of preterm delivery <35 and <28 weeks of gestation (see Figure 10).
Figure 10.
In women with a short cervix, those receiving vaginal progesterone (vs. placebo) had a significant decrease in the rate of preterm delivery < 28, < 33, and < 35 weeks of gestation.
Neonates born to mothers allocated to receive vaginal progesterone gel had a significantly lower frequency of RDS than those allocated to placebo (3% vs. 7.6%; RR 0.39; 95% CI 0.17–0.93; p=0.03). The number of patients needed to treat to prevent one case of RDS was 22. The reduction in RDS remains significant after adjusting for pooled study site and a history of preterm birth (RR 0.40; 95% CI 0.17–0.94; p=0.03). The frequencies of other neonatal adverse outcomes were not statistically significant. The frequency of adverse events was similar in patients allocated to progesterone and placebo, and there was no evidence of a potential safety signal.
An individual patient meta-analysis is a specific type of systematic review in which the original research data from each participant in a study are obtained directly from investigators in a trial.130 This approach is considered the “gold standard” for summarizing evidence across clinical trials, since it offers several advantages, both statistically and clinically, over conventional meta-analyses which use aggregated data.131 These advantages include standardizing and updating the data sets, verification of data quality and the appropriateness of prior analyses, improvement of consistency across trials (e.g. definition of outcomes), the ability to perform subgroup analyses that could identify groups of patients who may benefit from the intervention, and testing for interaction between patient-level covariates and treatment effects.132–134
Since there were additional studies to the two outlined above, an individual patient meta-analysis was conducted.135 The primary objective was to determine whether the use of vaginal progesterone in asymptomatic women with a short cervix (≤25mm) in the midtrimester reduces the rate of preterm birth and improves neonatal morbidity and mortality. The prespecified primary outcome was preterm birth at <33 weeks of gestation. Secondary outcomes included preterm birth at <37, <36, <35, <34, <30 and <28 weeks of gestation. Perinatal morbidity/mortality was assessed using a composite outcome (defined as the occurrence of any of the following events: RDS, intraventricular hemorrhage, necrotizing enterocolitis, proven neonatal sepsis or neonatal death); Apgar score <7 at 5 minutes; birthweight <1500g and <2500g; admission to the neonatal intensive care unit (NICU); use of mechanical ventilation; or congenital anomaly.
Five studies of high quality were included with a total of 775 women and 827 infants.128,129,136–138 Treatment with vaginal progesterone was associated with a significant reduction in the rate of preterm birth <33 weeks (RR, 0.58; 95% confidence interval [CI], 0.42–0.80), <35 weeks (RR, 0.69; 95% CI, 0.55–0.88), and <28 weeks (RR, 0.50; 95% CI, 0.30–0.81) (Figure 11); respiratory distress syndrome (RR, 0.48; 95% CI, 0.30–0.76); composite neonatal morbidity and mortality (RR, 0.57; 95% CI, 0.40–0.81); birthweight <1500 g (RR, 0.55; 95% CI, 0.38–0.80); admission to neonatal intensive care unit (RR, 0.75; 95% CI, 0.59–0.94); and requirement for mechanical ventilation (RR, 0.66; 95% CI, 0.44–0.98) (Figure 12).135
Figure 11.
Patients with a short cervix allocated to receive vaginal progesterone (vs. placebo) had a significantly lower risk in the rate of preterm birth < 28, < 33, and <35 weeks of gestation.
Figure 12.
Infants whose mothers (with a short cervix) received vaginal progesterone (vs. placebo) had a significantly lower risk of respiratory distress syndrome, composite neonatal morbidity and mortality, birthweight <1500 g, admission to neonatal intensive care unit, and requirement for mechanical ventilation
Subgroup analysis on the effect of vaginal progesterone on preventing preterm birth <33 weeks of gestation and composite neonatal morbidity/mortality yielded the following results that have clinical implications:
The daily dose of 90–100 mg of progesterone was equivalent to a 200 mg dose per day in both reduction of preterm birth and composite neonatal morbidity and mortality.
Vaginal progesterone was equally effective in women with a short cervix without a history of a previous preterm birth and those with a history of prior preterm birth in reducing preterm birth <33 weeks of gestation and composite neonatal morbidity/mortality.
No differences could be demonstrated in the effect of progesterone as a function of cervical length <25 mm in the prevention of preterm birth or reduction of neonatal morbidity (determined by a test of interaction).
Collectively, the evidence suggests that vaginal progesterone prevents preterm delivery at <33 weeks of gestation in women with a short cervix, and that this is associated with a reduction in neonatal morbidity. Moreover, this is observed in women either without or with a history of preterm birth.
Importantly, evidence has emerged that this approach identifying women at risk for preterm birth with universal risk assessment and progesterone administration to those with a short cervix is cost-effective139. The most recent estimate indicates that 19 million dollars per 100,000 patients screened can be saved when the cost of an ultrasound examination to determine cervical length is less than $184. This would represent a net saving of $500 – 750 million dollars per year in the U.S. alone. Based on these considerations, the State of Michigan has implemented universal cervical screening and progesterone treatment. In addition, some insurance companies provide reimbursement for cervical ultrasound and vaginal progesterone.
Does vaginal progesterone prevent preterm delivery in twin gestations? Randomized clinical trials of vaginal progesterone have studied twin gestations without considering cervical length: all trials have been negative thus far.137,140–143 However, a few studies included cervical length measurements, but this was not a criterion for eligibility in the trials. The individual patient meta-analysis described above135 conducted a subgroup analysis in twin gestations with a cervical length of <25mm. We have previously reported that such cervical length confers a high risk for preterm delivery.144 In the individual patient data meta-analysis, we found that vaginal progesterone administration was associated with a non-significant trend towards reduction in the rate of preterm birth <33 weeks of gestation (30.4% vs 44.8%; RR 0.70, 95% CI 0.34–1.44). However, vaginal progesterone led to a significant reduction in composite neonatal morbidity and mortality (23.9% vs 39.7%; RR 0.52, 95% CI 0.29–0.93). The number of twin gestations in this analysis was small (29 in the placebo group and 23 in the vaginal progesterone group). When neonatal morbidity was considered, the number of neonates was 58 in the placebo group and 46 in the vaginal progesterone group. Therefore, the difference between the trend towards a reduction in preterm delivery and the decrease in neonatal morbidity/mortality could reflect the number of subjects in the analysis. We believe that a randomized clinical trial of comparing vaginal progesterone vs placebo in women with a short cervix is urgently needed.
17-alpha-hydroxyprogesterone caproate does not prevent preterm birth in patients with a short cervix
A recent randomized controlled trial including nulliparous women with a singleton gestation between 16 and 22 3/7 weeks of gestation with a cervical length of <30mm (10th percentile for this gestational age) were randomized to 17-alpha-hydroxyprogesterone caproate (250 mg intramuscular injections weekly through 36 weeks or an identical appearing placebo).145 The primary outcome was preterm birth before 37 weeks. Of the 15,436 women screened, 1,588 (10.3%) had a cervical length of <30mm. The study was stopped after 657 women had been randomized (n=327 17-alpha-hydroxyprogesterone caproate and n=330 placebo) by the Data Safety Monitoring Board after a planned interim analysis that revealed that further enrollment was unlikely to demonstrate a significant difference between the study groups. There was no difference in the frequency of preterm birth between the 17-alpha-hydroxyprogesterone caproate and placebo group (25.1% vs. 24.2%; p=0.80). In addition, there was also no difference in the rate of preterm delivery of <35 weeks (13.5% vs 16.1%; p=0.35) or at <32 weeks (8.6% vs 9.7%; p=0.61). Subgroup analysis did not demonstrate any benefit from 17-alpha-hydroxyprogesterone caproate in women with a cervical length of <15mm or at 10–20mm.145 Based on the observations of this study, weekly intramuscular injections of 17-alpha-hydroxyprogesterone caproate cannot be recommended for nulliparous women with a short cervix of <30mm.
The use of 17-alpha-hydroxyprogesterone caproate to prevent preterm delivery in patients with a prior history of preterm birth
“Progestogen” is a term that includes natural and synthetic compounds with progesterone-like action.146 Such agents are now the mainstay for the prevention of preterm birth. Progesterone is a natural sex steroid produced by the corpus luteum, and, subsequently, the placenta during pregnancy. 17-alpha hydroxyprogesterone caproate is a synthetic progestogen. The human body does not make the caproate molecule; therefore, this molecule is added to 17-alpha hydroxyprogesterone in the laboratory. The primary reason to add the caproate molecule is to prolong the half-life of the compound. Yet, this modification changes the structure of the molecule and could result in modifications of the physiologic or pharmacologic properties of the drug.
A clinical trial of 17-alpha-hydroxyprogesterone caproate reported a decrease the rate of preterm birth in patients with a prior preterm delivery.147 The findings of such study have been questioned because of issues of efficacy and safety. For example, Marc Keirse has questioned the results, based on the unexpectedly high frequency of preterm birth in the placebo group (54.9%; 84/153).148 He suggested that 17-alpha-hydroxyprogesterone caproate may not have been effective because the rate of preterm birth in the active drug group was 36.3% (111/306), akin to the baseline rate of preterm birth for a similar population149 or the placebo group in another trial by the same investigators. Indeed, the power calculation of the 17-alpha-hydroxyprogesterone caproate trial was based on the observed rates of prematurity in the ‘Prediction of Prematurity’ study. The power calculation estimated that 37% of the women in the placebo group would deliver before 37 weeks.148,149 Similarly, officials of the FDA analyzing this trial indicated that the rate of preterm birth in the active arm of the study (36.3%) was very similar to that in the placebo group of a similar study (http://www.fda.gov/ohrms/dockets/ac/06/slides/2006-4227S1-index.htm; see below).
It is not well-known that there was an initial study by the Maternal-Fetal Medicine Units Network called “17P-IF-001” – a randomized placebo-controlled study with a target enrollment of 500 subjects. The study was designed to test the effectiveness and safety of 17-alpha hydroxyprogesterone caproate in preventing preterm delivery at <37 weeks. After 150 subjects had been enrolled and treated, the study was prematurely terminated because of a recall of the study drug because of quality control issues. The rate of preterm delivery in patients allocated to placebo in that trial was 38.5% (15/39), and 43.1% (28/65) in group allocated to 17 OHP-C – a non-significant result. However, the 38.5% is substantially lower than the 54% of the trial reporting positive results.
The high rate of preterm delivery in the control group has been a subject of debate and the investigators who conducted the trial have argued that the population participating in the trial was at very high risk for preterm delivery based on obstetric history, ethnic composition and willingness to be randomized to a painful injection on a weekly basis. It has been suggested that the latter would apply largely to highly motivated patients, at substantial risk for preterm delivery. However, if this is the actual explanation for the high rate of preterm delivery in the control group, this argument will erode the external validity of the trial. Randomized clinical trials are performed so that treatment can be offered to patients who did not participate in the trial and are similar to those enrolled in the trial. For example, if the explanation is that 17-alpha-hydroxyprogesterone caproate only works in African-American women with bacterial vaginosis and more than one preterm birth (which were allegedly over-represented in the control group) who are highly motivated to receive a weekly intramuscular injection, then it is legitimate to ask whether this drug should be given to women who have a previous preterm birth but do not fit the other poor prognostic factors invoked to explain the high rate of preterm birth in the control group.
The other question with 17-alpha-hydroxyprogesterone caproate is one of safety. The trial of Meis et al. reported that there was an excess of stillbirth and miscarriages in women allocated to receive 17OHP-C. However, this was not statistically significant and was not subject of commentary, neither in the paper, in the Editorial that followed, nor in the subsequent articles and opinions of professional organizations. The matter was first raised by the Medical Officer of the FDA when reviewing the trial at the Advisory Committee meeting of August 29, 2006. The FDA produced a slide indicating that women exposed to 17OHP-C in the midtrimester had a higher rate of fetal and neonatal death in the first 66 days of treatment than those allocated to placebo. This observation is what is called “a safety signal” in pharmacovigilance. The approval of the FDA of the commercial preparation of 17-alpha-hydroxyprogesterone caproate includes a warning that the administration of this agent may increase the frequency of gestational diabetes and other complications, and requires physicians to inform potential patients of the numerically non-significant increase in the rate of stillbirth and spontaneous abortions (for details, see package insert - http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021945s000lbl.pdf). The American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recommended that patients be counseled and sign an informed consent when receiving 17-alpha-hydroxyprogesterone caproate (Letter to Members, Friday, April 29, 2011).
It is noteworthy that the FDA has approved the administration of 17-alpha-hydroxyprogesterone caproate to prevent preterm birth in women with a prior history under Subpart H of the Code of Federal Regulations – this is a regulatory pathway used when the decision is made on the basis of a surrogate endpoint (delivery <37 weeks of gestation), and further studies are required. Another randomized clinical trial of 17-alpha-hydroxyprogesterone caproate is in progress in the United States in which women with a prior history of preterm delivery are being allocated to placebo or 17-alpha-hydroxyprogesterone caproate. The primary endpoint for this trial is delivery <35 weeks of gestation, and the originally-predicted date for conclusion has been moved from October 2013 to 2016. If the regulatory agency and professional organizations are really convinced of the effectiveness of 17-alpha-hydroxyprogesterone caproate, one may ask whether it is ethical to randomize women with a prior preterm birth to a placebo. The issue becomes more complex in light of recent reports that 17-alpha-hydroxyprogesterone caproate may increase perinatal mortality when administered in the context of a randomized clinical trial in triplet gestations.150 There was also a randomized clinical trial conducted in France in twins in which the rate of early preterm delivery was significantly greater in patients allocated to 17-alpha-hydroxyprogesterone caproate. If the randomized clinical trial in progress in the United States is concluded and yields negative results, the FDA has the authority to change the approval status of this agent.
The practical question is the clinical management of a patient who has begun taking weekly injections of 17-alpha-hydroxyprogesterone caproate because of a prior history of preterm delivery, and then is found to have a short cervix in the midtrimester. Should that patient continue to take 17-alpha-hydroxyprogesterone caproate, or should that agent be stopped, and the patient switched to vaginal progesterone or a cerclage? 17-alpha-hydroxyprogesterone caproate has not been shown to be effective in women with a short cervix; therefore, it would not be logical to continue to administer this agent. There is also no evidence that 17-alpha-hydroxyprogesterone caproate should be combined with vaginal progesterone. Indeed, it is prudent to use the lowest dose of any hormone or drug during pregnancy. The combined administration of 17-alpha-hydroxyprogesterone caproate and progesterone has not been studied, and therefore, cannot be recommended. In light of the safety concerns of 17-alpha-hydroxyprogesterone caproate, we believe that the best course of action is to discontinue 17-alpha-hydroxyprogesterone caproate and initiate treatment with vaginal progesterone.
Other interventions to prevent preterm delivery in women with a short cervix: cervical cerclage and a cervical pessary
Cervical cerclage was introduced in 1955 by V. N. Shirodkar, Professor of Midwifery and Gynecology at the Grand Medical College in Bombay, India.151 The procedure was developed in response to his observation that “some women abort repeatedly between the fourth and seventh months, and no amount of rest and treatment with hormones seemed to help them in retaining the product of conception.”151
Ian McDonald, from the Royal Melbourne Hospital, reported in 1957 his experience with 70 patients who had a suture of the cervix for inevitable miscarriage.152
Cerclage has also been employed to treat patients who present with the clinical condition of “acute cervical incompetence”. The term “cervical incompetence” is now discouraged in favor of the term “cervical insufficiency”. Evidence from a randomized clinical trial in which patients who presented with a dilated cervix were randomized to emergency cerclage combined with indomethacin administration versus expectant management suggests that these patients may benefit from an emergency cerclage.153 In this trial, 23 women presented with a dilated cervix and membranes at or beyond a dilated cervical external os (before 27 weeks of gestation) and were treated with antibiotics and bedrest, and randomly assigned to emergency cerclage and indomethacin (n=13) or bedrest only (n=10). Preterm delivery <34 weeks of gestation was significantly less common in patients treated with an emergency cerclage and indomethacin than in patients allocated to bedrest alone [54% (7/13) vs. 0% (0/10); p=0.02]. This is the only randomized clinical trial to test the effectiveness of cervical cerclage in patients presenting with acute cervical insufficiency.
Despite the 50 years that have elapsed since the introduction of cerclage as a procedure, there is conflicting evidence about its efficacy for standard indications (i.e. prophylactic) or for some patients with a sonographic short cervix.
Several randomized clinical trials have been conducted to date which have yielded the following clinical information:
Patients with a sonographic short cervix (defined as ≤15mm in the midtrimester) at low risk of preterm delivery by history do not benefit from a cervical cerclage to reduce the rate of preterm delivery.154 After screening a large number of patients, those with a cervical length ≤15mm were randomized to either expectant management (n=126) or cerclage (n=127). The rate of preterm delivery at <33 weeks of gestation was not significantly different between the groups [expectant management group, 26% (23/126) vs. cerclage group, 22% (28/127)].
There is little evidence that a prophylactic cerclage in patients at high risk for preterm delivery without a sonographic short cervix can prevent preterm birth.155,156 The largest trial conducted before the introduction of ultrasound (organized by the Medical Research Council of the United Kingdom)157 included patients with a prior history of one or more second trimester abortions or preterm deliveries (71% of patients) and a history of a cervical operation. The criterion for enrollment in the trial was uncertainty on the part of the obstetrician as to whether to recommend a cervical cerclage. The rate of delivery <33 weeks was significantly lower in the cervical cerclage group than in the control group (cerclage, 13% vs. control group, 17%; OR 7.72, 95% CI 0.53–0.97; p=0.03). Fever attributed to intrauterine infection was more common in patients allocated to the cerclage group (6% vs. 3%; OR 2.1; 95% CI 1.08–4.16; p=0.03). The authors called for additional research about methods to identify patients who may benefit from a cerclage.
In contrast, there is evidence that patients with a sonographic short cervix (<25mm) and a history of preterm birth may benefit from the placement of a cervical cerclage.158 This evidence is derived from a meta-analysis of randomized clinical trials of women with a short cervix determined by transvaginal sonography (performed before 24 weeks of gestation). Patients from 5 trials, which compared cerclage with expectant management, contributed to this meta-analysis.154,156,159–161 The primary outcome was preterm birth <35 weeks of gestation. Patients allocated to cerclage had a lower rate of preterm birth before 35 weeks of gestation than the no cerclage group [28.4% (71/250) vs. 41.3% (105/254); RR 0.70; 95% CI 0.55–0.89]. Cerclage also reduced preterm birth before 37, 32, 28 and 24 weeks of gestation. Composite perinatal morbidity and mortality were significantly reduced (15.6% in cerclage compared to 24.8% in no cerclage groups; RR 0.64; 95% CI 0.45–0.91).
In summary, a meta-analysis of randomized clinical trials of patients with a prior history of preterm birth and a short cervical length (<25mm) suggests that cervical cerclage is effective in reducing the rate of preterm birth and perinatal morbidity/mortality.158 A different meta-analysis has suggested that women with a prior spontaneous preterm birth and singleton gestation may be monitored safely with transvaginal sonographic cervical length measurements.162 This policy compares favorably with a universal policy of placing a cervical cerclage in all patients with a prior history.
Patients with a prior history of preterm birth and a short cervix: cervical cerclage vs. vaginal progesterone
Patients with a history of preterm birth and a cervical length of <25mm can be treated with either a cervical cerclage or vaginal progesterone. The results of an indirect patient meta-analysis of randomized clinical trials comparing vaginal progesterone vs placebo and cerclage vs expectant management in this population of patients concluded that the efficacy of both interventions are similar (Table 3).163 Therefore, considerations of cost and patient/physician preference need to be taken into account. For example, the placement of a cerclage requires anesthesia and a surgical procedure, and has been associated with some complications (e.g. rupture of membranes, bleeding, etc.). Vaginal progesterone administration requires compliance with the treatment.
Table 3.
Results of an indirect patient meta-analysis of randomized clinical trials comparing vaginal progesterone vs. placebo and cerclage vs. expectant management in this population of patients concluded that the efficacy of each intervention is similar
| Outcome | Indirect comparison Vaginal progesterone vs Cerclage |
|
|---|---|---|
| RR (95% CI) | P valueb | |
| Preterm birth <32 weeks | 0.70 (0.33–1.50) | 0.88 |
| Preterm birth <28 weeks | 0.71 (0.27–1.88) | 0.88 |
| Preterm birth <35 weeks | 0.88 (0.51–1.52) | 0.96 |
| Preterm birth <37 weeks | 1.19 (0.82–1.74) | 0.94 |
| Perinatal mortality | 1.05 (0.30–3.64) | 0.98 |
Cervical Pessary
A cervical pessary has been used by some practitioners in Europe to prevent preterm birth164. However, most of the studies have been retrospective. Recently, a prospectively open label randomized clinical trial was reported in which pregnant women with a cervical length of 25 mm or less, between 18 and 22 weeks of gestation, were randomly assigned to either a cervical pessary (n=192) or expectant management (n=193).165 The primary outcome was spontaneous delivery before 34 weeks of gestation. The rate of spontaneous preterm delivery before 34 weeks of gestation was less frequent in the pessary group than in the expectant management group (6% vs. 51%; OR: 0.18; 95% CI 0.08–0.37; p<0.0001). This was associated with a reduction in respiratory distress syndrome (3% vs. 12%; OR: 0.20; 95% CI 0.06–0.55; p<0.0003) and a reduction in neonates born with a birth weight <1500 g (5% vs. 14%; OR: 0.23; 95% CI 0.12- 0.43; p<0.0001).165 These interesting results are noteworthy and replication of these findings is desirable.
The Story of a Short Cervix and Vaginal Progesterone as a Blueprint for Further Progress to Reduce Preterm Delivery
The concept that preterm labor is not simply “labor before its time”, but rather, the result of multiple pathologic processes which activate the common pathway of parturition, has clinical and biological implications. It is now clear that the symptoms and signs of preterm labor (i.e. uterine contractions, cervical dilatation, and/or membrane rupture) are the manifestations of an underlying process, and that symptomatic treatment has not been successful. The syndromic nature of premature labor requires identification of the mechanisms of disease, biomarkers which are specific to each pathologic process, and targeted interventions. It is noteworthy that a history of preterm birth identifies patients at risk, but does not represent a mechanism of disease.
Cervical ultrasound in the midtrimester to identify women with a short cervix and treatment with vaginal progesterone represents the first step in which a logical framework has been employed to reduce the rate of preterm birth. This approach is anchored in the knowledge of the role of progesterone in the control of cervical ripening, and rigorous testing with randomized clinical trials. However, this approach is only one of the solutions to the prevention of preterm birth.
It is important to remember that the cervix was first imaged using ultrasound more than 30 years ago, and that it took decades to establish a convincing relationship between cervical length and the probability of spontaneous preterm delivery. The demonstration that progesterone is effective required a decade of rigorous clinical testing. The lessons that can be derived are that: 1) the prevention of preterm birth is possible; 2) biophysical and biochemical markers of subclinical pathologic processes need to be identified for each mechanism of disease responsible for spontaneous preterm birth. The biomarkers for one mechanism of disease (such as infection) are expected to be different from those that identify patients with maternal anti-fetal rejection,166–171 etc.; 3) interventions can only be expected to be successful if they interrupt the specific pathway leading to preterm delivery. Just as progesterone is effective in patients with a short cervix, other interventions such as antimicrobial agents may only work in patients at risk for infection-induced preterm birth;172–179 and 4) clinical trials of preventive strategies need to be designed intelligently. Progress will be achieved by testing interventions that are tailored to the specific pathophysiologic process, and administered at the right time to the subset of patients that are most likely to benefit. Testing interventions in patients who are unlikely to deliver preterm is not the way forward.
We believe that accepting the complexity of the problem of preterm birth, correctly framing the scientific questions about mechanisms of disease, and setting realistic expectations are necessary to make progress. We are confident that technological and biological developments in the 21st century will help us achieve the goal of reducing the rate of preterm birth. In summary, the lessons learned from the identification of vaginal progesterone as an effective mechanism to reduce the frequency of preterm birth can be used as a blueprint to meet the challenge posed by the complexity of this important set of syndromes.
Acknowledgments
This work was supported, in part, by the Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH/DHHS.
Footnotes
R. Romero has contributed to this work as part of his official duties as employee of the United States Federal Government.
References
- 1.Saling E. Prevention of prematurity - a complex undertaking reply. J Perinat Med. 2012;40:103. doi: 10.1515/JPM.2011.097. [DOI] [PubMed] [Google Scholar]
- 2.Romero R. Vaginal progesterone to reduce the rate of preterm birth and neonatal morbidity: a solution at last. Womens Health (Lond Engl) 2011;7:501–504. doi: 10.2217/whe.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell S. Universal cervical-length screening and vaginal progesterone prevents early preterm births, reduces neonatal morbidity and is cost saving: doing nothing is no longer an option. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2011;38:1–9. doi: 10.1002/uog.9073. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome. In: Elder M, Romero R, Lamont R, editors. Preterm Labor. New York, NY: Churchill Livingstone; 1997. pp. 29–49. [Google Scholar]
- 7.Romero R, Chaiworapongsa T, Gotsch F, Yeo L, Madan I, Hassan SS. The diagnosis and management of preterm labor with intact membranes. In: Winn HN, Chervenak FA, Romero R, editors. Clinical Maternal-Fetal Medicine Online. 2nd ed. Informa Healthcare; 2011. [Google Scholar]
- 8.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Althuisius SM, Dekker GA, van Geijn HP. Cervical incompetence: a reappraisal of an obstetric controversy. Obstet Gynecol Surv. 2002;57:377–387. doi: 10.1097/00006254-200206000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:1097–1103. doi: 10.1016/0002-9378(95)91469-2. [DOI] [PubMed] [Google Scholar]
- 11.Berghella V. Cerclage decreases preterm birth: finally the level I evidence is here. Am J Obstet Gynecol. 2011;205:89–90. doi: 10.1016/j.ajog.2011.02.079. [DOI] [PubMed] [Google Scholar]
- 12.Millar LK, DeBuque L, Wing DA. Uterine contraction frequency during treatment of pyelonephritis in pregnancy and subsequent risk of preterm birth. J Perinat Med. 2003;31:41–46. doi: 10.1515/JPM.2003.006. [DOI] [PubMed] [Google Scholar]
- 13.Germain AM, Valenzuela GJ, Ivankovic M, Ducsay CA, Gabella C, Seron-Ferre M. Relationship of circadian rhythms of uterine activity with term and preterm delivery. Am J Obstet Gynecol. 1993;168:1271–1277. doi: 10.1016/0002-9378(93)90379-w. [DOI] [PubMed] [Google Scholar]
- 14.Main DM, Grisso JA, Wold T, Snyder ES, Holmes J, Chiu G. Extended longitudinal study of uterine activity among low-risk women. Am J Obstet Gynecol. 1991;165:1317–1322. doi: 10.1016/0002-9378(91)90359-y. [DOI] [PubMed] [Google Scholar]
- 15.Kawarabayashi T, Kuriyama K, Kishikawa T, Sugimori H. Clinical features of small contraction wave recorded by an external tocodynamometer. Am J Obstet Gynecol. 1988;158:474–478. doi: 10.1016/0002-9378(88)90007-5. [DOI] [PubMed] [Google Scholar]
- 16.Maul H, Maner WL, Olson G, Saade GR, Garfield RE. Non-invasive transabdominal uterine electromyography correlates with the strength of intrauterine pressure and is predictive of labor and delivery. J Matern Fetal Neona. 2004;15:297–301. doi: 10.1080/14767050410001695301. [DOI] [PubMed] [Google Scholar]
- 17.Haran G, Elbaz M, Fejgin MD, Biron-Shental T. A comparison of surface acquired uterine electromyography and intrauterine pressure catheter to assess uterine activity. Am J Obstet Gynecol. 2012;206:412. doi: 10.1016/j.ajog.2011.12.015. e1–5. [DOI] [PubMed] [Google Scholar]
- 18.Lucovnik M, Maner WL, Chambliss LR, Blumrick R, Balducci J, Novak-Antolic Z, Garfield RE. Noninvasive uterine electromyography for prediction of preterm delivery. Am J Obstet Gynecol. 2011;204:228. doi: 10.1016/j.ajog.2010.09.024. e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucovnik M, Kuon RJ, Chambliss LR, Maner WL, Shi SQ, Shi L, Balducci J, Garfield RE. Use of uterine electromyography to diagnose term and preterm labor. Acta Obstet Gynecol Scand. 2011;90:150–157. doi: 10.1111/j.1600-0412.2010.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacod BC, Graatsma EM, Van Hagen E, Visser GH. A validation of electrohysterography for uterine activity monitoring during labour. J Matern Fetal Neona. 2010;23:17–22. doi: 10.3109/14767050903156668. [DOI] [PubMed] [Google Scholar]
- 21.Schlembach D, Maner WL, Garfield RE, Maul H. Monitoring the progress of pregnancy and labor using electromyography. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S33–S39. doi: 10.1016/j.ejogrb.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Garfield RE, Chwalisz K, Shi L, Olson G, Saade GR. Instrumentation for the diagnosis of term and preterm labour. J Perinat Med. 1998;26:413–436. doi: 10.1515/jpme.1998.26.6.413. [DOI] [PubMed] [Google Scholar]
- 23.Lockwood CJ, Dudenhausen JW. New approaches to the prediction of preterm delivery. J Perinat Med. 1993;21:441–452. doi: 10.1515/jpme.1993.21.6.441. [DOI] [PubMed] [Google Scholar]
- 24.Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for the prediction of the spontaneous preterm birth phenotype: a systematic review and meta-analysis. Bjog. 2011;118:1042–1054. doi: 10.1111/j.1471-0528.2011.02923.x. [DOI] [PubMed] [Google Scholar]
- 25.Danti L, Prefumo F, Lojacono A, Corini S, Testori A, Frusca T. The combination of short cervical length and phIGFBP-1 in the prediction of preterm delivery in symptomatic women. J Matern Fetal Neona. 2011;24:1262–1266. doi: 10.3109/14767058.2010.547962. [DOI] [PubMed] [Google Scholar]
- 26.Brik M, Hernandez AI, Pedraz CC, Perales A. Phosphorylated insulin-like growth factor binding protein-1 and cervical measurement in women with threatening preterm birth. Acta Obstet Gynecol Scand. 2010;89:268–274. doi: 10.3109/00016340903443668. [DOI] [PubMed] [Google Scholar]
- 27.Lee SM, Romero R, Park JW, Kim SM, Park CW, Korzeniewski SJ, Chaiworapongsa T, Yoon BH. The clinical significance of a positive Amnisure test in women with preterm labor and intact membranes. J Matern Fetal Neona. 2012;25:1690–1698. doi: 10.3109/14767058.2012.657279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SM, Lee J, Seong HS, Lee SE, Park JS, Romero R, Yoon BH. The clinical significance of a positive Amnisure test in women with term labor with intact membranes. J Matern Fetal Neona. 2009;22:305–310. doi: 10.1080/14767050902801694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, Wolfe HM. Patients with an ultrasonographic cervical length < or = 15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 30.Husslein P, Cabero Roura L, Dudenhausen JW, Helmer H, Frydman R, Rizzo N, Schneider D. Atosiban versus usual care for the management of preterm labor. J Perinat Med. 2007;35:305–313. doi: 10.1515/JPM.2007.078. [DOI] [PubMed] [Google Scholar]
- 31.Romero R, Sibai BM, Sanchez-Ramos L, Valenzuela GJ, Veille JC, Tabor B, Perry KG, Varner M, Goodwin TM, Lane R, Smith J, Shangold G, Creasy GW. An oxytocin receptor antagonist (atosiban) in the treatment of preterm labor: a randomized, double-blind, placebo-controlled trial with tocolytic rescue. Am J Obstet Gynecol. 2000;182:1173–1183. doi: 10.1067/mob.2000.95834. [DOI] [PubMed] [Google Scholar]
- 32.Valenzuela GJ, Sanchez-Ramos L, Romero R, Silver HM, Koltun WD, Millar L, Hobbins J, Rayburn W, Shangold G, Wang J, Smith J, Creasy GW. Maintenance treatment of preterm labor with the oxytocin antagonist atosiban. The Atosiban PTL-098 Study Group. Am J Obstet Gynecol. 2000;182:1184–1190. doi: 10.1067/mob.2000.105816. [DOI] [PubMed] [Google Scholar]
- 33.Di Renzo GC, Roura LC. Guidelines for the management of spontaneous preterm labor. J Perinat Med. 2006;34:359–366. doi: 10.1515/JPM.2006.073. [DOI] [PubMed] [Google Scholar]
- 34.Di Renzo GC, Al Saleh E, Mattei A, Koutras I, Clerici G. Use of tocolytics: what is the benefit of gaining 48 hours for the fetus? Bjog. 2006;113(Suppl 3):72–77. doi: 10.1111/j.1471-0528.2006.01127.x. [DOI] [PubMed] [Google Scholar]
- 35.Conde-Agudelo A, Romero R, Kusanovic JP. Nifedipine in the management of preterm labor: a systematic review and metaanalysis. Am J Obstet Gynecol. 2011;204:134. doi: 10.1016/j.ajog.2010.11.038. e1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercer B. Antibiotics in the management of PROM and preterm labor. Obstet Gynecol Clin North Am. 2012;39:65–76. doi: 10.1016/j.ogc.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Mercer BM, Miodovnik M, Thurnau GR, Goldenberg RL, Das AF, Ramsey RD, Rabello YA, Meis PJ, Moawad AH, Iams JD, Van Dorsten JP, Paul RH, Bottoms SF, Merenstein G, Thom EA, Roberts JM, McNellis D. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Jama. 1997;278:989–995. [PubMed] [Google Scholar]
- 38.Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD001058.pub2. CD001058. [DOI] [PubMed] [Google Scholar]
- 39.Kenyon SL, Taylor DJ, Tarnow-Mordi W. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet. 2001;357:979–988. doi: 10.1016/s0140-6736(00)04233-1. [DOI] [PubMed] [Google Scholar]
- 40.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, Hobbins JC. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:661–666. doi: 10.1016/s0002-9378(88)80030-9. [DOI] [PubMed] [Google Scholar]
- 41.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, Espinoza J, Chaiworapongsa T, Gonzalez R, Iams JD, Rojas I. Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J Matern Fetal Neona. 2007;20:167–173. doi: 10.1080/14767050601135485. [DOI] [PubMed] [Google Scholar]
- 42.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. Bjog. 2006;(113) Suppl 3:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Confino E, Mayden KL, Giglia RV, Vermesh M, Gleicher N. Pitfalls in sonographic imaging of the incompetent uterine cervix. Acta Obstet Gynecol Scand. 1986;65:593–597. doi: 10.3109/00016348609158394. [DOI] [PubMed] [Google Scholar]
- 44.Zemlyn S. The length of the uterine cervix and its significance. J Clin Ultrasound. 1981;9:267–269. doi: 10.1002/jcu.1870090603. [DOI] [PubMed] [Google Scholar]
- 45.Bowie JD, Andreotti RF, Rosenberg ER. Sonographic appearance of the uterine cervix in pregnancy: the vertical cervix. AJR American journal of roentgenology. 1983;140:737–740. doi: 10.2214/ajr.140.4.737. [DOI] [PubMed] [Google Scholar]
- 46.Varma TR, Patel RH, Pillai U. Ultrasonic assessment of cervix in normal pregnancy. Acta Obstet Gynecol Scand. 1986;65:229–233. doi: 10.3109/00016348609155176. [DOI] [PubMed] [Google Scholar]
- 47.Brook I, Feingold M, Schwartz A, Zakut H. Ultrasonography in the diagnosis of cervical incompetence in pregnancy-a new diagnostic approach. British journal of obstetrics and gynaecology. 1981;88:640–643. doi: 10.1111/j.1471-0528.1981.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 48.Vaalamo P, Kivikoski A. The incompetent cervix during pregnancy diagnosed by ultrasound. Acta Obstet Gynecol Scand. 1983;62:19–21. doi: 10.3109/00016348309155751. [DOI] [PubMed] [Google Scholar]
- 49.Varma TR, Patel RH, Pillai U. Ultrasonic assessment of cervix in 'at risk' patients. Acta Obstet Gynecol Scand. 1986;65:147–152. doi: 10.3109/00016348609158370. [DOI] [PubMed] [Google Scholar]
- 50.Ayers JW, DeGrood RM, Compton AA, Barclay M, Ansbacher R. Sonographic evaluation of cervical length in pregnancy: diagnosis and management of preterm cervical effacement in patients at risk for premature delivery. Obstet Gynecol. 1988;71(6 Pt 1):939–944. [PubMed] [Google Scholar]
- 51.Podobnik M, Bulic M, Smiljanic N, Bistricki J. Ultrasonography in the detection of cervical incompetency. J Clin Ultrasound. 1988;16:383–391. doi: 10.1002/jcu.1870160604. [DOI] [PubMed] [Google Scholar]
- 52.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859–867. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 53.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 54.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–317. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 55.Berghella V, Kuhlman K, Weiner S, Texeira L, Wapner RJ. Cervical funneling: sonographic criteria predictive of preterm delivery. Ultrasound Obstet Gynecol. 1997;10:161–166. doi: 10.1046/j.1469-0705.1997.10030161.x. [DOI] [PubMed] [Google Scholar]
- 56.Goldenberg RL, Iams JD, Mercer BM, Meis PJ, Moawad AH, Copper RL, Das A, Thom E, Johnson F, McNellis D, Miodovnik M, Van Dorsten JP, Caritis SN, Thurnau GR, Bottoms SF. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD MFMU Network. Am J Public Health. 1998;88:233–238. doi: 10.2105/ajph.88.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kushnir O, Vigil DA, Izquierdo L, Schiff M, Curet LB. Vaginal ultrasonographic assessment of cervical length changes during normal pregnancy. Am J Obstet Gynecol. 1990;162:991–993. doi: 10.1016/0002-9378(90)91302-s. [DOI] [PubMed] [Google Scholar]
- 58.Okitsu O, Mimura T, Nakayama T, Aono T. Early prediction of preterm delivery by transvaginal ultrasonography. Ultrasound Obstet Gynecol. 1992;2:402–409. doi: 10.1046/j.1469-0705.1992.02060402.x. [DOI] [PubMed] [Google Scholar]
- 59.Hasegawa I, Tanaka K, Takahashi K, Tanaka T, Aoki K, Torii Y, Okai T, Saji F, Takahashi T, Sato K, Fujimura M, Ogawa Y. Transvaginal ultrasonographic cervical assessment for the prediction of preterm delivery. J Matern Fetal Med. 1996;5:305–309. doi: 10.1002/(SICI)1520-6661(199611/12)5:6<305::AID-MFM2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 60.Guzman ER, Mellon C, Vintzileos AM, Ananth CV, Walters C, Gipson K. Longitudinal assessment of endocervical canal length between 15 and 24 weeks' gestation in women at risk for pregnancy loss or preterm birth. Obstet Gynecol. 1998;92:31–37. doi: 10.1016/s0029-7844(98)00120-3. [DOI] [PubMed] [Google Scholar]
- 61.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18–22 weeks' gestation and the risk of preterm delivery. Obstet Gynecol. 1998;92:902–907. doi: 10.1016/s0029-7844(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 62.Watson WJ, Stevens D, Welter S, Day D. Observations on the sonographic measurement of cervical length and the risk of premature birth. J Matern Fetal Med. 1999;8:17–19. doi: 10.1002/(SICI)1520-6661(199901/02)8:1<17::AID-MFM4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 63.Hibbard JU, Tart M, Moawad AH. Cervical length at 16–22 weeks' gestation and risk for preterm delivery. Obstet Gynecol. 2000;96:972–978. doi: 10.1016/s0029-7844(00)01074-7. [DOI] [PubMed] [Google Scholar]
- 64.To MS, Skentou C, Liao AW, Cacho A, Nicolaides KH. Cervical length and funneling at 23 weeks of gestation in the prediction of spontaneous early preterm delivery. Ultrasound Obstet Gynecol. 2001;18:200–203. doi: 10.1046/j.1469-0705.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 65.Matijevic R, Grgic O, Vasilj O. Is sonographic assessment of cervical length better than digital examination in screening for preterm delivery in a low-risk population? Acta Obstet Gynecol Scand. 2006;85:1342–1347. doi: 10.1080/00016340600935722. [DOI] [PubMed] [Google Scholar]
- 66.Theron G, Schabort C, Norman K, Thompson M, Geerts L. Centile charts of cervical length between 18 and 32 weeks of gestation. Int J Gynaecol Obstet. 2008;103:144–148. doi: 10.1016/j.ijgo.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 67.Riley L, Frigoletto FD, Jr, Benacerraf BR. The implications of sonographically identified cervical changes in patients not necessarily at risk for preterm birth. J Ultrasound Med. 1992;11:75–79. doi: 10.7863/jum.1992.11.3.75. [DOI] [PubMed] [Google Scholar]
- 68.de Carvalho MH, Bittar RE, Brizot ML, Bicudo C, Zugaib M. Prediction of preterm delivery in the second trimester. Obstet Gynecol. 2005;105:532–536. doi: 10.1097/01.AOG.0000154157.22500.1d. [DOI] [PubMed] [Google Scholar]
- 69.Leung TN, Pang MW, Leung TY, Poon CF, Wong SM, Lau TK. Cervical length at 18–22 weeks of gestation for prediction of spontaneous preterm delivery in Hong Kong Chinese women. Ultrasound Obstet Gynecol. 2005;26:713–717. doi: 10.1002/uog.2617. [DOI] [PubMed] [Google Scholar]
- 70.Tongsong T, Kamprapanth P, Srisomboon J, Wanapirak C, Piyamongkol W, Sirichotiyakul S. Single transvaginal sonographic measurement of cervical length early in the third trimester as a predictor of preterm delivery. Obstet Gynecol. 1995;86:184–187. doi: 10.1016/0029-7844(95)00152-h. [DOI] [PubMed] [Google Scholar]
- 71.Heath VC, Souka AP, Erasmus I, Gibb DM, Nicolaides KH. Cervical length at 23 weeks of gestation: the value of Shirodkar suture for the short cervix. Ultrasound Obstet Gynecol. 1998;12:318–322. doi: 10.1046/j.1469-0705.1998.12050318.x. [DOI] [PubMed] [Google Scholar]
- 72.Heath VC, Southall TR, Souka AP, Novakov A, Nicolaides KH. Cervical length at 23 weeks of gestation: relation to demographic characteristics and previous obstetric history. Ultrasound Obstet Gynecol. 1998;12:304–311. doi: 10.1046/j.1469-0705.1998.12050304.x. [DOI] [PubMed] [Google Scholar]
- 73.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gray DJ, Robinson HB, Malone J, Thomson RB., Jr Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn. 1992;12:111–117. doi: 10.1002/pd.1970120206. [DOI] [PubMed] [Google Scholar]
- 75.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 76.Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends in endocrinology and metabolism: TEM. 2010;21:353–361. doi: 10.1016/j.tem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahendroo M. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction. 2012;143:429–438. doi: 10.1530/REP-11-0466. [DOI] [PubMed] [Google Scholar]
- 78.Mazor M, Hershkovitz R, Chaim W, Levy J, Sharony Y, Leiberman JR, Glezerman M. Human preterm birth is associated with systemic and local changes in progesterone/17 beta-estradiol ratios. Am J Obstet Gynecol. 1994;171:231–236. doi: 10.1016/0002-9378(94)90474-x. [DOI] [PubMed] [Google Scholar]
- 79.Mazor M, Hershkowitz R, Ghezzi F, Cohen J, Silber A, Levy J, Leiberman JR, Glezerman M. Maternal plasma and amniotic fluid 17 beta-estradiol, progesterone and cortisol concentrations in women with successfully and unsuccessfully treated preterm labor. Arch Gynecol Obstet. 1996;258:89–96. doi: 10.1007/BF00626029. [DOI] [PubMed] [Google Scholar]
- 80.Romero R, Scoccia B, Mazor M, Wu YK, Benveniste R. Evidence for a local change in the progesterone/estrogen ratio in human parturition at term. Am J Obstet Gynecol. 1988;159:657–660. doi: 10.1016/s0002-9378(88)80029-2. [DOI] [PubMed] [Google Scholar]
- 81.Chwalisz K, Shi Shao O, Neff G, Elger J. The effect of antigestagen ZK 98, 199 on the uterine cervix. Acta Endocrinol. 1987;283:113. [Google Scholar]
- 82.Norman J. Antiprogesterones. Br J Hosp Med. 1991;45:372–375. [PubMed] [Google Scholar]
- 83.Elliott CL, Brennand JE, Calder AA. The effects of mifepristone on cervical ripening and labor induction in primigravidae. Obstet Gynecol. 1998;92:804–809. doi: 10.1016/s0029-7844(98)00284-1. [DOI] [PubMed] [Google Scholar]
- 84.Stenlund PM, Ekman G, Aedo AR, Bygdeman M. Induction of labor with mifepristone--a randomized, double-blind study versus placebo. Acta Obstet Gynecol Scand. 1999;78:793–798. [PubMed] [Google Scholar]
- 85.Giacalone PL, Daures JP, Faure JM, Boulot P, Hedon B, Laffargue F. The effects of mifepristone on uterine sensitivity to oxytocin and on fetal heart rate patterns. Eur J Obstet Gynecol Reprod Biol. 2001;97:30–34. doi: 10.1016/s0301-2115(00)00506-6. [DOI] [PubMed] [Google Scholar]
- 86.Hegele-Hartung C, Chwalisz K, Beier HM, Elger W. Ripening of the uterine cervix of the guinea-pig after treatment with the progesterone antagonist onapristone (ZK 98.299): an electron microscopic study. Hum Reprod. 1989;4:369–377. doi: 10.1093/oxfordjournals.humrep.a136909. [DOI] [PubMed] [Google Scholar]
- 87.Chwalisz K. The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery. Hum Reprod. 1994;9(Suppl 1):131–161. doi: 10.1093/humrep/9.suppl_1.131. [DOI] [PubMed] [Google Scholar]
- 88.Wolf JP, Sinosich M, Anderson TL, Ulmann A, Baulieu EE, Hodgen GD. Progesterone antagonist (RU 486) for cervical dilation, labor induction, and delivery in monkeys: effectiveness in combination with oxytocin. Am J Obstet Gynecol. 1989;160:45–47. doi: 10.1016/0002-9378(89)90084-7. [DOI] [PubMed] [Google Scholar]
- 89.Stys SJ, Clewell WH, Meschia G. Changes in cervical compliance at parturition independent of uterine activity. Am J Obstet Gynecol. 1978;130:414–418. doi: 10.1016/0002-9378(78)90282-x. [DOI] [PubMed] [Google Scholar]
- 90.Levine RU, Berkowitz KM. Conservative management and pregnancy outcome in diethylstilbestrol-exposed women with and without gross genital tract abnormalities. Am J Obstet Gynecol. 1993;169:1125–1129. doi: 10.1016/0002-9378(93)90267-m. [DOI] [PubMed] [Google Scholar]
- 91.Craig CJ. Congenital abnormalities of the uterus and foetal wastage. S Afr Med J. 1973;47:2000–2005. [PubMed] [Google Scholar]
- 92.Mangan CE, Borow L, Burtnett-Rubin MM, Egan V, Giuntoli RL, Mikuta JJ. Pregnancy outcome in 98 women exposed to diethylstilbestrol in utero, their mothers, and unexposed siblings. Obstet Gynecol. 1982;59:315–319. [PubMed] [Google Scholar]
- 93.Ludmir J, Landon MB, Gabbe SG, Samuels P, Mennuti MT. Management of the diethylstilbestrol-exposed pregnant patient: a prospective study. Am J Obstet Gynecol. 1987;157:665–669. doi: 10.1016/s0002-9378(87)80025-x. [DOI] [PubMed] [Google Scholar]
- 94.Goldstein DP. Incompetent cervix in offspring exposed to diethylstilbestrol in utero. Obstet Gynecol. 1978;52:73S–75S. [PubMed] [Google Scholar]
- 95.Goldberg JM, Falcone T. Effect of diethylstilbestrol on reproductive function. Fertility and sterility. 1999;72:1–7. doi: 10.1016/s0015-0282(99)00153-3. [DOI] [PubMed] [Google Scholar]
- 96.Salle B, Sergeant P, Awada A, Bied-Damon V, Gaucherand P, Boisson C, Guibaud S, Benchaib M, Rudigoz RC. Transvaginal ultrasound studies of vascular and morphological changes in uteri exposed to diethylstilbestrol in utero. Hum Reprod. 1996;11:2531–2536. doi: 10.1093/oxfordjournals.humrep.a019153. [DOI] [PubMed] [Google Scholar]
- 97.Raynal P, Le Meaux JP, Epelboin S, Tournaire M. [Early prophylactic cervical cerclage for hypoplastic cervix following exposure to DES in utero] J Gynecol Obstet Biol Reprod (Paris) 2005;34:572–580. doi: 10.1016/s0368-2315(05)82882-0. [DOI] [PubMed] [Google Scholar]
- 98.Cukier J, Batzofin JH, Conner JS, Franklin RR. Genital tract reconstruction in a patient with congenital absence of the vagina and hypoplasia of the cervix. Obstet Gynecol. 1986;68:32S–36S. [PubMed] [Google Scholar]
- 99.Kriplani A, Kachhawa G, Awasthi D, Kulshrestha V. Laparoscopic-Assisted Uterovaginal Anastomosis in Congenital Atresia of Uterine Cervix: Follow-up Study. Journal of minimally invasive gynecology. 2012;19:477–484. doi: 10.1016/j.jmig.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 100.Carlomagno G, Di Blasi A, Monica MD. Congenital scoliosis associated with agenesis of the uterine cervix. Case report. BMC women's health. 2004;4:4. doi: 10.1186/1472-6874-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee CL, Wang CJ, Swei LD, Yen CF, Soong YK. Laparoscopic hemi-hysterectomy in treatment of a didelphic uterus with a hypoplastic cervix and obstructed hemivagina. Hum Reprod. 1999;14:1741–1743. doi: 10.1093/humrep/14.7.1741. [DOI] [PubMed] [Google Scholar]
- 102.Moinian M, Andersch B. Does cervix conization increase the risk of complications in subsequent pregnancies? Acta Obstet Gynecol Scand. 1982;61:101–103. doi: 10.3109/00016348209156537. [DOI] [PubMed] [Google Scholar]
- 103.Kristensen J, Langhoff-Roos J, Wittrup M, Bock JE. Cervical conization and preterm delivery/low birth weight. A systematic review of the literature. Acta Obstet Gynecol Scand. 1993;72:640–644. doi: 10.3109/00016349309021157. [DOI] [PubMed] [Google Scholar]
- 104.Romero R. Prenatal Medicine: the child is the father of the man. Prenatal and Neonatal Medicine. 1996;1:8–11. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 105.Raio L, Ghezzi F, Di NE, Gomez R, Luscher KP. Duration of pregnancy after carbon dioxide laser conization of the cervix: influence of cone height. Obstet Gynecol. 1997;90:978–982. doi: 10.1016/s0029-7844(97)00489-4. [DOI] [PubMed] [Google Scholar]
- 106.Berghella V, Pereira L, Gariepy A, Simonazzi G. Prior cone biopsy: prediction of preterm birth by cervical ultrasound. Am J Obstet Gynecol. 2004;191:1393–1397. doi: 10.1016/j.ajog.2004.06.087. [DOI] [PubMed] [Google Scholar]
- 107.Bruinsma FJ, Quinn MA. The risk of preterm birth following treatment for precancerous changes in the cervix: a systematic review and meta-analysis. Bjog. 2011;118:1031–1041. doi: 10.1111/j.1471-0528.2011.02944.x. [DOI] [PubMed] [Google Scholar]
- 108.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, Nien JK, Berry SM, Bujold E, Camacho N, Sorokin Y. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol. 2009;200:374. doi: 10.1016/j.ajog.2009.01.047. e1–5. [DOI] [PubMed] [Google Scholar]
- 110.Keeler SM, Kiefer DG, Rust OA, Vintzileos A, Atlas RO, Bornstein E, Hanna N. Comprehensive amniotic fluid cytokine profile evaluation in women with a short cervix: which cytokine(s) correlates best with outcome? Am J Obstet Gynecol. 2009;201:276. doi: 10.1016/j.ajog.2009.05.045. e1–6. [DOI] [PubMed] [Google Scholar]
- 111.Vaisbuch E, Hassan SS, Mazaki-Tovi S, Nhan-Chang CL, Kusanovic JP, Chaiworapongsa T, Dong Z, Yeo L, Mittal P, Yoon BH, Romero R. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433. doi: 10.1016/j.ajog.2010.02.007. e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633. doi: 10.1016/j.ajog.2007.11.047. e1–8. [DOI] [PubMed] [Google Scholar]
- 113.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, Mazor M, Treadwell MC, Cotton DB. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167:1086–1091. doi: 10.1016/s0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 114.Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95:652–655. doi: 10.1016/s0029-7844(99)00633-x. [DOI] [PubMed] [Google Scholar]
- 115.ACOG Practice Bulletin. Cervical insufficiency. Obstet Gynecol. 2003;102:1091–1099. doi: 10.1016/j.obstetgynecol.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 116.Grant A, Chalmers I, Enkin M, Keirse MJNC. Effective care in pregnancy and childbirth. New York, NY: Oxford University Press; 1989. Cervical cerclage to prolong pregnancy; pp. 633–646. [Google Scholar]
- 117.Guzman ER, Mellon R, Vintzileos AM, Ananth CV, Walters C, Gipson K. Relationship between endocervical canal length between 15–24 weeks gestation and obstetric history. J Matern Fetal Med. 1998;7:269–272. doi: 10.1002/(SICI)1520-6661(199811/12)7:6<269::AID-MFM3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 118.Warren JE, Silver RM, Dalton J, Nelson LT, Branch DW, Porter TF. Collagen 1Alpha1 and transforming growth factor-beta polymorphisms in women with cervical insufficiency. Obstet Gynecol. 2007;110:619–624. doi: 10.1097/01.AOG.0000277261.92756.1a. [DOI] [PubMed] [Google Scholar]
- 119.Botsis D, Papagianni V, Vitoratos N, Makrakis E, Aravantinos L, Creatsas G. Prediction of preterm delivery by sonographic estimation of cervical length. Biol Neonate. 2005;88:42–45. doi: 10.1159/000084457. [DOI] [PubMed] [Google Scholar]
- 120.Anum EA, Hill LD, Pandya A, Strauss JF., 3rd Connective tissue and related disorders and preterm birth: clues to genes contributing to prematurity. Placenta. 2009;30:207–215. doi: 10.1016/j.placenta.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Andersen HF. Transvaginal and transabdominal ultrasounography of the uterine cervix during pregnancy. J Clin Ultrasound. 1991;19:77–83. doi: 10.1002/jcu.1870190204. [DOI] [PubMed] [Google Scholar]
- 122.To MS, Skentou C, Cicero S, Nicolaides KH. Cervical assessment at the routine 23-weeks' scan: problems with transabdominal sonography. Ultrasound Obstet Gynecol. 2000;15:292–296. doi: 10.1046/j.1469-0705.2000.00094.x. [DOI] [PubMed] [Google Scholar]
- 123.Mason GC, Maresh MJ. Alterations in bladder volume and the ultrasound appearance of the cervix. Br J Obstet Gynaecol. 1990;97:457–458. doi: 10.1111/j.1471-0528.1990.tb01839.x. [DOI] [PubMed] [Google Scholar]
- 124.Hernandez-Andrade E, Romero R, Ahn H, Hussein Y, Yeo L, Korzeniewski SJ, Chaiworapongsa T, Hassan SS. Transabdominal evaluation of uterine cervical length during pregnancy fails to identify a substantial number of women with a short cervix. J Matern Fetal Neona. 2012 doi: 10.3109/14767058.2012.657278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jeanty P, d'Alton M, Romero R, Hobbins JC. Perineal scanning. Am J Perinatol. 1986;3:289–295. doi: 10.1055/s-2007-999882. [DOI] [PubMed] [Google Scholar]
- 126.Hertzberg BS, Livingston E, DeLong DM, McNally PJ, Fazekas CK, Kliewer MA. Ultrasonographic evaluation of the cervix: transperineal versus endovaginal imaging. J Ultrasound Med. 2001;20:1071–1078. doi: 10.7863/jum.2001.20.10.1071. [DOI] [PubMed] [Google Scholar]
- 127.Braithwaite JM, Economides DL. Acceptability by patients of transvaginal sonography in the elective assessment of the first-trimester fetus. Ultrasound Obstet Gynecol. 1997;9:91–93. doi: 10.1046/j.1469-0705.1997.09020091.x. [DOI] [PubMed] [Google Scholar]
- 128.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 129.Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, Vijayaraghavan J, Trivedi Y, Soma-Pillay P, Sambarey P, Dayal A, Potapov V, O'Brien J, Astakhov V, Yuzko O, Kinzler W, Dattel B, Sehdev H, Mazheika L, Manchulenko D, Gervasi MT, Sullivan L, Conde-Agudelo A, Phillips JA, Creasy GW. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Higgins JPT, Altman DG, Sterne JAC. Higgins JPT, Green S, editors. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 510 (updated March 2011) The Cochrane Collaboration 2011. 2011 [Google Scholar]
- 131.Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet. 1993;341:418–422. doi: 10.1016/0140-6736(93)93004-k. [DOI] [PubMed] [Google Scholar]
- 132.Thompson SG, Higgins JP. Treating individuals 4: can meta-analysis help target interventions at individuals most likely to benefit? Lancet. 2005;365:341–346. doi: 10.1016/S0140-6736(05)17790-3. [DOI] [PubMed] [Google Scholar]
- 133.Sutton AJ, Kendrick D, Coupland CA. Meta-analysis of individual- and aggregate-level data. Stat Med. 2008;27:651–669. doi: 10.1002/sim.2916. [DOI] [PubMed] [Google Scholar]
- 134.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 135.Romero R, Nicolaides K, Conde-Agudelo A, Tabor A, O'Brien JM, Cetingoz E, Da Fonseca E, Creasy GW, Klein K, Rode L, Soma-Pillay P, Fusey S, Cam C, Alfirevic Z, Hassan SS. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124. doi: 10.1016/j.ajog.2011.12.003. e1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.O'Brien JM, Adair CD, Lewis DF, Hall DR, Defranco EA, Fusey S, Soma-Pillay P, Porter K, How H, Schackis R, Eller D, Trivedi Y, Vanburen G, Khandelwal M, Trofatter K, Vidyadhari D, Vijayaraghavan J, Weeks J, Dattel B, Newton E, Chazotte C, Valenzuela G, Calda P, Bsharat M, Creasy GW. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:687–696. doi: 10.1002/uog.5158. [DOI] [PubMed] [Google Scholar]
- 137.Rode L, Klein K, Nicolaides K, Krampl-Bettelheim E, Tabor A. Prevention of preterm delivery in twin gestations (PREDICT): A multicentre randomised placebo-controlled trial on the effect of vaginal micronised progesterone. Ultrasound Obstet Gynecol. 2011;38:272–280. doi: 10.1002/uog.9093. [DOI] [PubMed] [Google Scholar]
- 138.Cetingoz E, Cam C, Sakalli M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high-risk pregnancies: a randomized placebo-controlled trial. Arch Gynecol Obstet. 2011;283:423–429. doi: 10.1007/s00404-009-1351-2. [DOI] [PubMed] [Google Scholar]
- 139.Werner EF, Han CS, Pettker CM, Buhimschi CS, Copel JA, Funai EF, Thung SF. Universal cervical-length screening to prevent preterm birth: a cost-effectiveness analysis. Ultrasound Obstet Gynecol. 2011;38:32–37. doi: 10.1002/uog.8911. [DOI] [PubMed] [Google Scholar]
- 140.Norman JE, Mackenzie F, Owen P, Mactier H, Hanretty K, Cooper S, Calder A, Mires G, Danielian P, Sturgiss S, MacLennan G, Tydeman G, Thornton S, Martin B, Thornton JG, Neilson JP, Norrie J. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373:2034–2040. doi: 10.1016/S0140-6736(09)60947-8. [DOI] [PubMed] [Google Scholar]
- 141.Wood S, Ross S, Tang S, Miller L, Sauve R, Brant R. Vaginal progesterone to prevent preterm birth in multiple pregnancy: a randomized controlled trial. J Perinat Med. 2012 doi: 10.1515/jpm-2012-0057. [DOI] [PubMed] [Google Scholar]
- 142.Serra V, Perales A, Meseguer J, Parrilla J, Lara C, Bellver J, Grifol R, Alcover I, Sala M, Martinez-Escoriza J, Pellicer A. Increased doses of vaginal progesterone for the prevention of preterm birth in twin pregnancies: a randomised controlled double-blind multicentre trial. Bjog. 2012 doi: 10.1111/j.1471-0528.2012.03448.x. [DOI] [PubMed] [Google Scholar]
- 143.Romero R. Progesterone to prevent preterm birth in twin gestations: what is the next step forward? Bjog. 2012 doi: 10.1111/1471-0528.12019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Conde-Agudelo A, Romero R, Hassan SS, Yeo L. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:128. doi: 10.1016/j.ajog.2010.02.064. e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Grobman W. Randomized Controlled Trial of Progesterone Treatment for Preterm Birth Prevention in Nulliparous Women with Cervical Length Less Than 30mm. Am J Obstet Gynecol. 2012 [Google Scholar]
- 146.Romero R. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol. 2007;30:675–686. doi: 10.1002/uog.5174. [DOI] [PubMed] [Google Scholar]
- 147.Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW, Leveno KJ, Caritis SN, Iams JD, Wapner RJ, Conway D, O'Sullivan MJ, Carpenter M, Mercer B, Ramin SM, Thorp JM, Peaceman AM, Gabbe S. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 148.Keirse MJ. Progesterone and preterm: seventy years of "deja vu" or "still to be seen"? Birth. 2004;31:230–235. doi: 10.1111/j.0730-7659.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 149.Iams JD, Newman RB, Thom EA, Goldenberg RL, Mueller-Heubach E, Moawad A, Sibai BM, Caritis SN, Miodovnik M, Paul RH, Dombrowski MP, Thurnau G, McNellis D. Frequency of uterine contractions and the risk of spontaneous preterm delivery. N Engl J Med. 2002;346:250–255. doi: 10.1056/NEJMoa002868. [DOI] [PubMed] [Google Scholar]
- 150.Combs CA, Garite T, Maurel K, Das A, Porto M. Failure of 17-hydroxyprogesterone to reduce neonatal morbidity or prolong triplet pregnancy: a double-blind, randomized clinical trial. Am J Obstet Gynecol. 2010;203:248. doi: 10.1016/j.ajog.2010.06.016. e1–9. [DOI] [PubMed] [Google Scholar]
- 151.Shirodkar VN, et al. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic. 1955;52:299–300. [Google Scholar]
- 152.McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynaecol Br Emp. 1957;64:346–350. doi: 10.1111/j.1471-0528.1957.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 153.Althuisius SM, Dekker GA, Hummel P, van Geijn HP. Cervical incompetence prevention randomized cerclage trial: emergency cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2003;189:907–910. doi: 10.1067/s0002-9378(03)00718-x. [DOI] [PubMed] [Google Scholar]
- 154.To MS, Alfirevic Z, Heath VC, Cicero S, Cacho AM, Williamson PR, Nicolaides KH. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363:1849–1853. doi: 10.1016/S0140-6736(04)16351-4. [DOI] [PubMed] [Google Scholar]
- 155.Althuisius S, Dekker G, Hummel P, Bekedam D, Kuik D, van Geijn H. Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): effect of therapeutic cerclage with bed rest vs. bed rest only on cervical length. Ultrasound Obstet Gynecol. 2002;20:163–167. doi: 10.1046/j.1469-0705.2002.00770.x. [DOI] [PubMed] [Google Scholar]
- 156.Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185:1106–1112. doi: 10.1067/mob.2001.118655. [DOI] [PubMed] [Google Scholar]
- 157.Final report of the Medical Research Council/Royal College of Obstetricians and Gynaecologists multicentre randomised trial of cervical cerclage. MRC/RCOG Working Party on Cervical Cerclage. Br J Obstet Gynaecol. 1993;100:516–523. doi: 10.1111/j.1471-0528.1993.tb15300.x. [DOI] [PubMed] [Google Scholar]
- 158.Berghella V, Rafael TJ, Szychowski JM, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta-analysis. Obstet Gynecol. 2011;117:663–671. doi: 10.1097/AOG.0b013e31820ca847. [DOI] [PubMed] [Google Scholar]
- 159.Rust OA, Atlas RO, Reed J, van Gaalen J, Balducci J. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: why cerclage therapy may not help. Am J Obstet Gynecol. 2001;185:1098–1105. doi: 10.1067/mob.2001.118163. [DOI] [PubMed] [Google Scholar]
- 160.Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191:1311–1317. doi: 10.1016/j.ajog.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 161.Owen J, Hankins G, Iams JD, Berghella V, Sheffield JS, Perez-Delboy A, Egerman RS, Wing DA, Tomlinson M, Silver R, Ramin SM, Guzman ER, Gordon M, How HY, Knudtson EJ, Szychowski JM, Cliver S, Hauth JC. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201:375. doi: 10.1016/j.ajog.2009.08.015. e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Berghella V, Mackeen AD. Cervical length screening with ultrasound-indicated cerclage compared with history-indicated cerclage for prevention of preterm birth: a meta-analysis. Obstet Gynecol. 2011;118:148–155. doi: 10.1097/AOG.0b013e31821fd5b0. [DOI] [PubMed] [Google Scholar]
- 163.Conde-Agudelo A, Romero R, Nicolaides KH, Chaiworapongsa T, O'Brien J, Cetingoz E, da Fonseca E, Creasy GW, Soma-Pillay P, Fusey S, Cam C, Alfirevic Z, Hassan SS. Vaginal progesterone vs cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol. 2012;208 doi: 10.1016/j.ajog.2012.10.877. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Arabin B, Halbesma JR, Vork F, Hubener M, van Eyck J. Is treatment with vaginal pessaries an option in patients with a sonographically detected short cervix? J Perinat Med. 2003;31:122–133. doi: 10.1515/JPM.2003.017. [DOI] [PubMed] [Google Scholar]
- 165.Goya M, Pratcorona L, Merced C, Rodo C, Valle L, Romero A, Juan M, Rodriguez A, Munoz B, Santacruz B, Bello-Munoz JC, Llurba E, Higueras T, Cabero L, Carreras E. Cervical pessary in pregnant women with a short cervix (PECEP): an open-label randomised controlled trial. Lancet. 2012;379:1800–1806. doi: 10.1016/S0140-6736(12)60030-0. [DOI] [PubMed] [Google Scholar]
- 166.Lee J, Romero R, Dong Z, Xu Y, Qureshi F, Jacques S, Yoo W, Chaiworapongsa T, Mittal P, Hassan SS, Kim CJ. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology. 2011;59:928–938. doi: 10.1111/j.1365-2559.2011.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu Y, Tarquini F, Romero R, Kim CJ, Tarca AL, Bhatti G, Lee J, Sundell IB, Mittal P, Kusanovic JP, Hassan SS, Kim JS. Peripheral CD300a+CD8+ T lymphocytes with a distinct cytotoxic molecular signature increase in pregnant women with chronic chorioamnionitis. Am J Reprod Immunol. 2012;67:184–197. doi: 10.1111/j.1600-0897.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lee J, Romero R, Xu Y, Kim JS, Park JY, Kusanovic JP, Chaiworapongsa T, Hassan SS, Kim CJ. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011;66:510–526. doi: 10.1111/j.1600-0897.2011.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee J, Romero R, Xu Y, Kim JS, Topping V, Yoo W, Kusanovic JP, Chaiworapongsa T, Hassan SS, Yoon BH, Kim CJ. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PloS one. 2011;6:e16806. doi: 10.1371/journal.pone.0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ogge G, Romero R, Lee DC, Gotsch F, Than NG, Lee J, Chaiworapongsa T, Dong Z, Mittal P, Hassan SS, Kim CJ. Chronic chorioamnionitis displays distinct alterations of the amniotic fluid proteome. The Journal of pathology. 2011;223:553–565. doi: 10.1002/path.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, Gotsch F, Yoon BH, Chi JG, Kim JS. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2010;23:1000–1011. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Espinoza J, Erez O, Romero R. Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol. 2006;194:630–637. doi: 10.1016/j.ajog.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 173.Romero R, Sibai B, Caritis S, Paul R, Depp R, Rosen M, Klebanoff M, Sabo V, Evans J, Thom E, et al. Antibiotic treatment of preterm labor with intact membranes: a multicenter, randomized, double-blinded, placebo-controlled trial. Am J Obstet Gynecol. 1993;169:764–774. doi: 10.1016/0002-9378(93)90003-2. [DOI] [PubMed] [Google Scholar]
- 174.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 175.Mazor M, Chaim W, Maymon E, Hershkowitz R, Romero R. The role of antibiotic therapy in the prevention of prematurity. Clin Perinatol. 1998;25:659–685. x. [PubMed] [Google Scholar]
- 176.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 177.Romero R, Hagay Z, Nores J, Sepulveda W, Mazor M. Eradication of Ureaplasma urealyticum from the amniotic fluid with transplacental antibiotic treatment. Am J Obstet Gynecol. 1992;166:618–620. doi: 10.1016/0002-9378(92)91686-5. [DOI] [PubMed] [Google Scholar]
- 178.Romero R, Ghidini A, Mazor M, Behnke E. Microbial invasion of the amniotic cavity in premature rupture of membranes. Clin Obstet Gynecol. 1991;34:769–778. doi: 10.1097/00003081-199112000-00013. [DOI] [PubMed] [Google Scholar]
- 179.Romero R, Scioscia AL, Edberg SC, Hobbins JC. Use of parenteral antibiotic therapy to eradicate bacterial colonization of amniotic fluid in premature rupture of membranes. Obstet Gynecol. 1986;67:15S–17S. doi: 10.1097/00006250-198603001-00005. [DOI] [PubMed] [Google Scholar]