Abstract
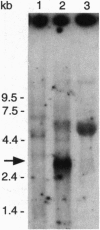
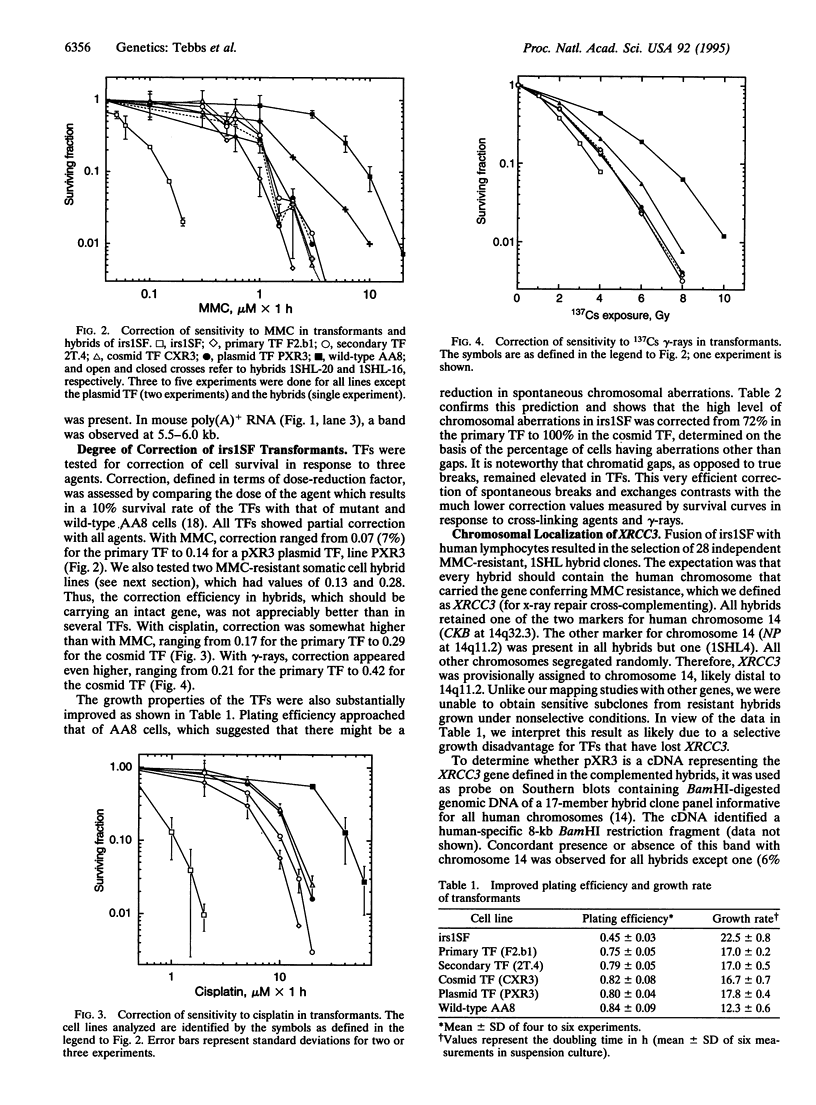
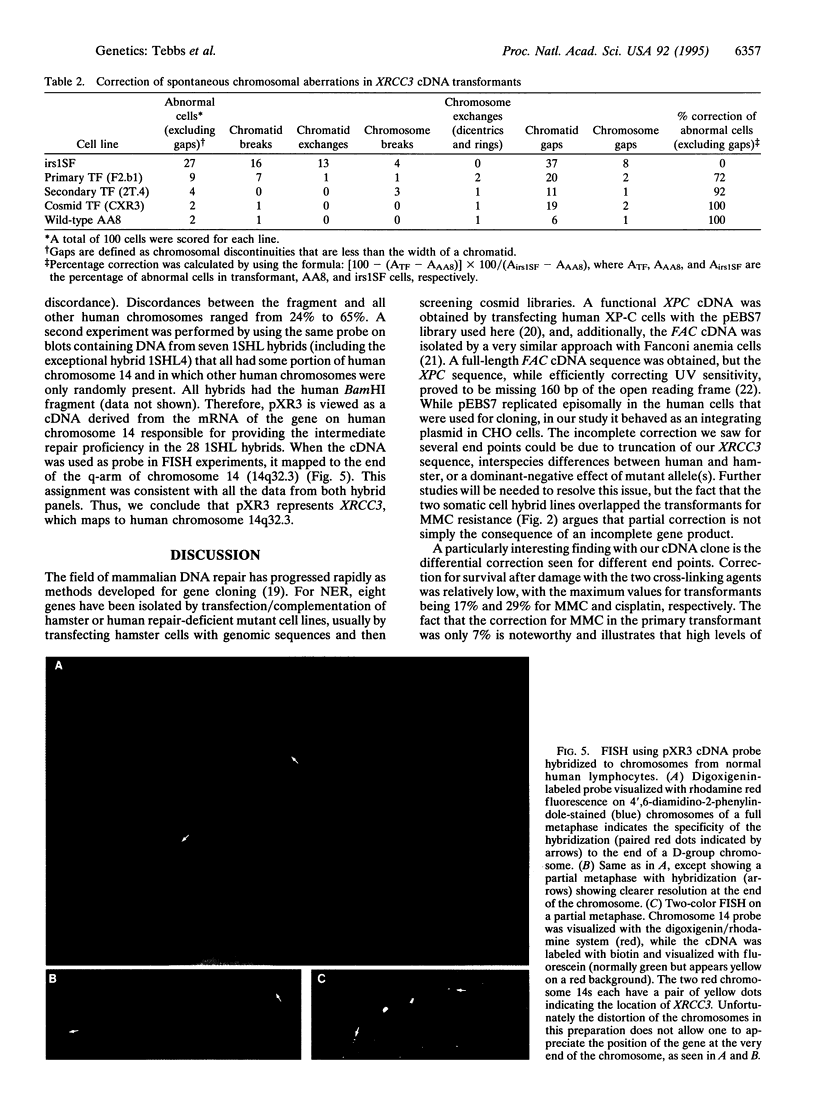
The mutagen-sensitive CHO line irs1SF was previously isolated on the basis of hypersensitivity to ionizing radiation and was found to be chromosomally unstable as well as cross-sensitive to diverse kinds of DNA-damaging agents. The analysis of somatic cell hybrids formed between irs1SF and human lymphocytes implicated a human gene (defined as XRCC3; x-ray repair cross-complementing), which partially restored mitomycin C resistance to the mutant. A functional cDNA that confers mitomycin C resistance was transferred to irs1SF cells by transforming them with an expression cDNA library and obtaining primary and secondary transformants. Functional cDNA clones were recovered from a cosmid library prepared from a secondary transformant. Transformants also showed partial correction of sensitivity to cisplatin and gamma-rays, efficient correction of chromosomal instability, and substantially improved plating efficiency and growth rate. The XRCC3 cDNA insert is approximately 2.5 kb and detects an approximately 3.0-kb mRNA on Northern blots. The cDNA was mapped by fluorescence in situ hybridization to human chromosome 14q32.3, which was consistent with the chromosome concordance data of two independent hybrid clone panels.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldecott K., Jeggo P. Cross-sensitivity of gamma-ray-sensitive hamster mutants to cross-linking agents. Mutat Res. 1991 Sep;255(2):111–121. doi: 10.1016/0921-8777(91)90046-r. [DOI] [PubMed] [Google Scholar]
- Chen D. J., Marrone B. L., Nguyen T., Stackhouse M., Zhao Y., Siciliano M. J. Regional assignment of a human DNA repair gene (XRCC5) to 2q35 by X-ray hybrid mapping. Genomics. 1994 May 15;21(2):423–427. doi: 10.1006/geno.1994.1287. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Do we know the cause of xeroderma pigmentosum? Carcinogenesis. 1990 Jun;11(6):875–882. doi: 10.1093/carcin/11.6.875. [DOI] [PubMed] [Google Scholar]
- Collins A. R. Mutant rodent cell lines sensitive to ultraviolet light, ionizing radiation and cross-linking agents: a comprehensive survey of genetic and biochemical characteristics. Mutat Res. 1993 Jan;293(2):99–118. doi: 10.1016/0921-8777(93)90062-l. [DOI] [PubMed] [Google Scholar]
- Dianov G., Price A., Lindahl T. Generation of single-nucleotide repair patches following excision of uracil residues from DNA. Mol Cell Biol. 1992 Apr;12(4):1605–1612. doi: 10.1128/mcb.12.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller L. F., Painter R. B. A Chinese hamster ovary cell line hypersensitive to ionizing radiation and deficient in repair replication. Mutat Res. 1988 Mar;193(2):109–121. doi: 10.1016/0167-8817(88)90041-7. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H. Nucleotide excision repair I: from E. coli to yeast. Trends Genet. 1993 May;9(5):173–177. doi: 10.1016/0168-9525(93)90164-d. [DOI] [PubMed] [Google Scholar]
- Hoy C. A., Thompson L. H., Mooney C. L., Salazar E. P. Defective DNA cross-link removal in Chinese hamster cell mutants hypersensitive to bifunctional alkylating agents. Cancer Res. 1985 Apr;45(4):1737–1743. [PubMed] [Google Scholar]
- Huang J. C., Svoboda D. L., Reardon J. T., Sancar A. Human nucleotide excision nuclease removes thymine dimers from DNA by incising the 22nd phosphodiester bond 5' and the 6th phosphodiester bond 3' to the photodimer. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3664–3668. doi: 10.1073/pnas.89.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. J. Genetic analysis of mitomycin C-hypersensitive Chinese hamster cell mutants. Mutagenesis. 1994 Sep;9(5):477–482. doi: 10.1093/mutage/9.5.477. [DOI] [PubMed] [Google Scholar]
- Jones N. J., Stewart S. A., Thompson L. H. Biochemical and genetic analysis of the Chinese hamster mutants irs1 and irs2 and their comparison to cultured ataxia telangiectasia cells. Mutagenesis. 1990 Jan;5(1):15–23. doi: 10.1093/mutage/5.1.15. [DOI] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R., Peterson C. Expression cloning of a human DNA repair gene involved in xeroderma pigmentosum group C. Nature. 1992 Sep 3;359(6390):70–73. doi: 10.1038/359070a0. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Satoh M. S., Dianov G. Enzymes acting at strand interruptions in DNA. Philos Trans R Soc Lond B Biol Sci. 1995 Jan 30;347(1319):57–62. doi: 10.1098/rstb.1995.0009. [DOI] [PubMed] [Google Scholar]
- Liu P., Siciliano J., Seong D., Craig J., Zhao Y., de Jong P. J., Siciliano M. J. Dual Alu polymerase chain reaction primers and conditions for isolation of human chromosome painting probes from hybrid cells. Cancer Genet Cytogenet. 1993 Feb;65(2):93–99. doi: 10.1016/0165-4608(93)90213-6. [DOI] [PubMed] [Google Scholar]
- Mares A., Jr, Ledbetter S. A., Ledbetter D. H., Roberts R., Hejtmancik J. F. Isolation of a human chromosome 14-only somatic cell hybrid: analysis using Alu and LINE-based PCR. Genomics. 1991 Sep;11(1):215–218. doi: 10.1016/0888-7543(91)90122-u. [DOI] [PubMed] [Google Scholar]
- Marlton P., Claxton D. F., Liu P., Estey E. H., Beran M., LeBeau M., Testa J. R., Collins F. S., Rowley J. D., Siciliano M. J. Molecular characterization of 16p deletions associated with inversion 16 defines the critical fusion for leukemogenesis. Blood. 1995 Feb 1;85(3):772–779. [PubMed] [Google Scholar]
- Masutani C., Sugasawa K., Yanagisawa J., Sonoyama T., Ui M., Enomoto T., Takio K., Tanaka K., van der Spek P. J., Bootsma D. Purification and cloning of a nucleotide excision repair complex involving the xeroderma pigmentosum group C protein and a human homologue of yeast RAD23. EMBO J. 1994 Apr 15;13(8):1831–1843. doi: 10.1002/j.1460-2075.1994.tb06452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overkamp W. J., Rooimans M. A., Neuteboom I., Telleman P., Arwert F., Zdzienicka M. Z. Genetic diversity of mitomycin C-hypersensitive Chinese hamster cell mutants: a new complementation group with chromosomal instability. Somat Cell Mol Genet. 1993 Sep;19(5):431–437. doi: 10.1007/BF01233248. [DOI] [PubMed] [Google Scholar]
- Peterson C., Legerski R. High-frequency transformation of human repair-deficient cell lines by an Epstein-Barr virus-based cDNA expression vector. Gene. 1991 Nov 15;107(2):279–284. doi: 10.1016/0378-1119(91)90328-9. [DOI] [PubMed] [Google Scholar]
- Sancar A. Mechanisms of DNA excision repair. Science. 1994 Dec 23;266(5193):1954–1956. doi: 10.1126/science.7801120. [DOI] [PubMed] [Google Scholar]
- Stallings R. L., Olson E., Strauss A. W., Thompson L. H., Bachinski L. L., Siciliano M. J. Human creatine kinase genes on chromosomes 15 and 19, and proximity of the gene for the muscle form to the genes for apolipoprotein C2 and excision repair. Am J Hum Genet. 1988 Aug;43(2):144–151. [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Duncan A. M., Buchwald M. Evidence for at least four Fanconi anaemia genes including FACC on chromosome 9. Nat Genet. 1992 Jun;1(3):196–198. doi: 10.1038/ng0692-196. [DOI] [PubMed] [Google Scholar]
- Strathdee C. A., Gavish H., Shannon W. R., Buchwald M. Cloning of cDNAs for Fanconi's anaemia by functional complementation. Nature. 1992 Apr 30;356(6372):763–767. doi: 10.1038/356763a0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Jones N. J., Allen S. A., Carrano A. V. Molecular cloning of the human XRCC1 gene, which corrects defective DNA strand break repair and sister chromatid exchange. Mol Cell Biol. 1990 Dec;10(12):6160–6171. doi: 10.1128/mcb.10.12.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Weber C. A., Salazar E. P., Reardon J. T., Sancar A., Deng Z., Siciliano M. J. Molecular cloning of the human nucleotide-excision-repair gene ERCC4. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6855–6859. doi: 10.1073/pnas.91.15.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Mooney C. L., Burkhart-Schultz K., Carrano A. V., Siciliano M. J. Correction of a nucleotide-excision-repair mutation by human chromosome 19 in hamster-human hybrid cells. Somat Cell Mol Genet. 1985 Jan;11(1):87–92. doi: 10.1007/BF01534738. [DOI] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. Molecular cloning and biological characterization of a human gene, ERCC2, that corrects the nucleotide excision repair defect in CHO UV5 cells. Mol Cell Biol. 1988 Mar;8(3):1137–1146. doi: 10.1128/mcb.8.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin M., de Wit J., Odijk H., Westerveld A., Yasui A., Koken M. H., Hoeijmakers J. H., Bootsma D. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986 Mar 28;44(6):913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]