Abstract
The early noncry vocalizations of infants are salient social signals. Caregivers spontaneously respond to 30-50% of these sounds, and their responsiveness to infants' prelinguistic noncry vocalizations facilitates the development of phonology and speech. Have infants learned that their vocalizations influence the behavior of social partners? If infants have learned the contingency between their vocalizing and the social responses of others, they should show an extinction burst when the contingency is removed, increasing their rate of noncry vocalizing then decreasing. Thirty-eight 5-month-olds were tested in the still-face paradigm, during which they engaged in a 2-min still-face interaction with an unfamiliar adult. When the adult assumed a still face, infants showed an extinction burst. This pattern of infant vocalizations suggests that 5-month-olds have learned the social efficacy of their vocalizations on caregivers' behavior. Furthermore, the magnitude of 5-month infants' extinction bursts predicted their language comprehension at 13 months.
From early in the first year, infants' prelinguistic noncry vocalizations appear to influence their parents' behavior (e.g., Goldstein & West, 1999; Gros-Louis, West, Goldstein, & King, 2006; Hsu & Fogel, 2003). Contingent responses to prelinguistic vocalizations are a typical characteristic of parent-infant interactions, and parents respond to approximately 30-50% of infants' prelinguistic syllables (Goldstein, King, & West, 2003; Gros-Louis et al., 2006). Reciprocally, infants recognize contingencies in both their parents' and unfamiliar caregivers' responses (e.g., Bigelow, 1998; Bornstein, Tamis-LeMonda, Hahn, & Haynes, 2008). Given the imperfect (less than 100%) feedback that infants receive for vocalizing, have infants learned that their vocalizations change caregivers' behavior?
Learning the relation between their vocalizing and social responding from others is an important developmental achievement for infants, as prelinguistic vocal development is regulated by social partners' contingent responses to vocalizations. Infants begin their first year by producing immature, quasi-resonant vocalizations, which gradually change to fully-resonant vowels and canonical syllables containing consonant-vowel alternations (Oller, 2000). Social interaction influences vocal development, as prelinguistic infants produce more speech-like vocalizations in response to increases in caregivers' contingent nonverbal behavior (Bloom, Russell, & Wassenberg, 1987; Goldstein et al., 2003). When caregivers contingently respond to infants' vocalizations with speech, 9-month-olds structure their own sounds to match the phonological patterns that they hear (Goldstein & Schwade, 2008). Infants who were given vowel sounds as feedback for their vocalizations produced more vowel sounds, whereas infants given words as feedback produced more consonant-vowel syllables. However, identical but non-contingent speech patterns are not learned (Goldstein et al., 2003; Goldstein & Schwade, 2008). These studies demonstrate infants' ability to learn new patterns of vocal production from contingent social feedback.
In addition, infant vocalizations can be operantly conditioned using social reinforcers (e.g., Bloom et al., 1987; Poulson, 1983; Rheingold, Gewirtz, & Ross, 1959). Although these studies show changes in vocalizing in response to high levels of social reinforcement, the 80 – 100% levels typically used are far higher than the 30 – 50% contingencies observed in natural interaction (Goldstein et al., 2003; Goldstein & Schwade, 2008; Gros-Louis et al., 2006).
The present study examines early developmental antecedents of the socially guided vocal learning demonstrated by 9-month-old infants in previous studies (Goldstein et al., 2003; Goldstein & Schwade 2008). In those studies, infants rapidly learned from social responses to their vocalizations, indicating that they had already become aware of the social effects of their babbling. The goal of the present study was to assess younger infants' associations between prelinguistic vocalizing and adult responses that resulted from naturally occurring levels of contingency in previous social interactions. Here, we studied responses to social reinforcers in 5-month-olds because they are capable of socially directed behaviors such as social smiling (Messinger, 2005) and because infants of this age are sensitive to deviations from familiar levels of social contingency from their caregivers (Bigelow, 1998). We also parse the nature of the infants' responses and examine the predictive validity of individual differences among infants for later language comprehension.
In many species, learning an association between a behavior and an outcome under imperfect contingencies leads to longer maintenance of that behavior when the contingencies between the behavior and outcome are removed (see review in Mackintosh, 1974). Such a pattern of behavior change is known as a “partial reinforcement effect” (Amsel, 1958, 1962; Festinger, 1961). For example, when a rat learns a contingency between running down an alley and a food reward under partial reinforcement (e.g., the rat is rewarded for 30-50% of its responses), the learned association resists extinction when the contingency is removed. During extinction, partially reinforced behaviors persist longer than those learned under perfect (100%) contingency (Bacon, 1962). The extinction of an imperfect contingency also temporarily increases the animal's response rate and/or rapidity (e.g., rate of bar-pressing or speed at which it runs down the alley) before the advent of a lasting decrease in responding. This sequence of an increase in behavior followed by a decrease is called the “extinction burst,” and it is characteristic of an extinction effect (Amsel, 1958, 1962; Lerman & Iwata, 1995). The extinction burst is thought to be mediated by a transient increase in frustration or arousal (Tarabulsy, Tessier, & Kappas, 1996).
Human infants learn associations as a result of partial reinforcement. For example, 10- to 11-month-olds learned to touch a cylinder to obtain either food or music rewards when their responses were reinforced on a partial schedule (Lowe, Beasty, & Bentall, 1983), and 4- to 5-month-olds produced arm movements after partial reinforcement with a slide show and music (Sullivan & Lewis, 2003). Infants also increase arousal during extinction (Sullivan & Lewis, 2003). Previous studies of human infant learning, however, have not looked for partial reinforcement effects or extinction bursts. In contrast, partial reinforcement effects are robust in adults and have been demonstrated in a variety of tasks (see review by Halpern & Poon, 1971). For example, when adults learn to move a joystick in one of four directions to receive reinforcement, they persist longer when trained under conditions of partial reinforcement (Pittenger & Pavlik, 1988).
If caregivers are naturally intermittently contingent in their responses to infants' prelinguistic vocalizations, and infants perceive the contingency between their vocalizing and the reactions of adults, then infants might learn the social consequences of their vocalizations. Have young infants already learned that their vocalizations affect others? In the present study, we used the still-face paradigm to assess 5-month-olds' associations between their own vocalizing and obtaining a social response from an unfamiliar adult. The 3-episode still-face paradigm consists, first, of a brief naturalistic face-to face interaction between an adult and infant. Immediately after this interaction, the adult assumes a neutral expression and looks at the infant without speaking or changing expressions (a “still face”). The adult then engages in a second naturalistic interaction episode (Tronick, Als, Adamson, Wise, & Brazelton, 1978).
The still-face paradigm has been used previously to study infant social development and infant expectations about social interactions (e.g., Adamson & Frick, 2003; Moore, Cohn, & Campbell, 2001; Striano, 2004; Tarabulsy et al., 2003). Infants as young as 1.5 months avert gaze and increase fussing when an adult assumes the still face (although newborns do not show similar changes in response to the still face episode; Bertin & Striano, 2006; Striano, 2004). The effects of the still-face interaction on infant gaze, smiling, crying, and fussing have been established for infants of different ages and in different populations (e.g., Nadel et al., 2000). However, only one previous study assessed noncry, non-fuss vocalizations during the still-face episode, as part of a larger set of behavioral measures (Delgado, Messinger, & Yale, 2002). That study manipulated the direction of parents' gaze during the still-face episode (either at the infant's face or above the head). One result was an increase in vocalizing during the still-face when adult gaze was directed at the infant's face. Changes in vocalizing were not examined on a fine time-scale, nor were changes in vocalizing compared to later language development. In addition, Delgado et al. categorized vocalizations as neutral/positive or negative based on coders' impressions. Such a categorization scheme assumes that noncry vocalizations reliably communicate the emotional state of the infant. However, prelinguistic vocalizations are characterized by contextual freedom, in which the same sound form may be used in multiple communicative purposes (Oller, 2000) and thus should be coded based on acoustic criteria.
In the present study, we assessed the effect of extinction of social responsiveness during the still-face episode on infants' rate of vocalizing. In addition, we related changes in amount of vocalizing with later language measures, so as to assess whether young infants' sensitivity to social contingency contributes to their communicative development. Patterns of parental responsiveness to prelinguistic behavior have been linked to long-term developmental outcomes (e.g., Goldstein & Schwade, in press; Tamis-LeMonda & Bornstein, 2002). For example, 9-month-old infants often produce object-directed vocalizations (vocalizing while looking at or holding an object) and parents' contingent responses to those sounds are correlated with vocabulary size at 15 months (Goldstein & Schwade, in press). Thus prelinguistic vocalizations yield opportunities for social learning. Infants' early awareness of the relation between vocalizing and social responses stands at the beginning of a developmental cascade of language acquisition.
To assess the generality or specificity of the extinction effect, we also measured the frequency and duration of infant smiling. We expected smiling to decrease during the still-face episode, as previous studies using the still face paradigm induce negative affect (Adamson & Frick, 2003; Messinger, 2005). Thus, we hypothesized that the extinction burst would be isolated to vocalizing. If, over the first months of life, the imperfect contingencies of adults' responses to prelinguistic vocalizations are sufficient for infant learning, then a lack of adult responsiveness during the still-face should provoke an extinction burst specific to vocalizing -- infants should increase and then decrease their rate of vocalizing within the still-face episode. Thus, we anticipated a dissociation between smiling and vocalizing during the still-face. As smiling decreases, vocalizing should increase and then decrease.
Method
Participants
Infants participated as part of a larger longitudinal study of infant social and cognitive development (Bornstein, Arterberry, & Mash, 2004). Because the present study focused on changes in noncry vocalizations, 10 infants were excluded for crying or excessive fussing. Data from the remaining 38 infants (25 girls; 37 European American, 1 African American) aged 5 months (M = 5.36, SD = .16, range = 5.06 – 5.78) were assessed in the present study. All infants exceeded 2500 g at birth and were healthy at the time of the study. Mothers and fathers had at least graduated from high school, and 84% of mothers and 89% of fathers had a 4-year college degree. Families varied in socioeconomic status (SES; Hollingshead, 1975) with a range of 37 (middle class) to 66 (higher class).
Procedure
Infants were placed in an infant seat on a table at eye level opposite an unfamiliar female experimenter. The still-face procedure consisted of 3 episodes, beginning with a 1-min naturalistic interaction episode, followed by a 2 min still-face episode, and ending with a 1-min naturalistic interaction episode. During the two naturalistic interaction episodes, the experimenter spoke to the infant, but did not touch the infant or engage the infant with any toys. During the still-face episode, the experimenter maintained a neutral expression, without speaking, while looking at the infant. The infant and experimenter were videorecorded during all three episodes. The female experimenter was the same for all infants.
Infant Behavior Coding
The acoustic form of infants' vocalizations during each episode was coded for vowel resonance, presence of consonant-vowel combinations, and the timing of transitions between consonants and vowels (Oller, 2000). To count the number of sounds, each vocalization was divided into syllables containing one vowel per syllable (e.g., /baba/ was counted as 2 syllables). However, if an infant produced a sequence of vowels separated by pauses shorter than 0.2 sec, that sequence was counted as one syllable (i.e., /ai/ was counted as 1 syllable; /a/ <pause>/i/ was counted as 2 syllables). Vocalizations were counted regardless of an infant's focus of attention. Fusses and vegetative sounds (e.g., coughs) were excluded. Vocalizations and pauses were coded by one of 4 coders. To assess reliability, 50% of sessions were double coded. Mean reliability r was .94 (range = .87 – 1.00).
We calculated two ratios of vocalization frequency for each infant. The Learning Ratio (LR) was the number of vocalizations produced per minute during the still-face episode divided by the number of vocalizations produced per minute during the first naturalistic interaction episode:
The LR was large when an infant increased vocalizations during the still-face episode. We interpret a large LR to mean that infants have previously learned that the vocalizations generally elicit adult responding. The Interaction Ratio (IR) was the number of vocalizations produced per minute during the still-face episode divided by the number of vocalizations produced per minute during the second naturalistic interaction episode:
The IR was large when an infant showed a large decline in vocalizations from the still-face to the second naturalistic interaction. We interpret a large IR to indicate that infants recognized normal interaction behavior from the experimenter.
Smile frequency and duration were coded during frame-by-frame inspection of the videorecords when infants raised one or both corners of their mouths by moving the zygomatic muscle (e.g., Jones, Collins, & Hong, 1991). Smiles were categorized by one of six coders. To assess reliability, 50% of sessions were double coded. Mean reliability r was .91 (range = .82- 1.00). (One infant's mouth was obscured by the experimenter for most of the second naturalistic interaction episode; smile data for that infant are excluded from all analyses.)
Language Measures
Parents completed the CDI: Words and Gestures (Fenson et al., 1994) when their infants were 13 months old (M = 13.04, SD = .18, range = 12.85 – 14.03). The questionnaire assesses infants' comprehension of 28 phrases and 396 words.
Results
Vocalizations
Infant vocalizations peaked during the still-face episode (Figure 1). The data were normally distributed, Kolmogorov-Smirnov z = .894, p = .40. A one-way within-subjects ANOVA on the mean number of infant vocalizations per minute across the three episodes (Interact 1, Still Face, Interact 2) revealed a significant main effect of episode, F (2, 74) = 10.88, p < .001, ηp 2 = .23. Tukey's HSD post-hoc tests revealed an increase in the number of noncry vocalizations from the first naturalistic interaction episode to the still-face episode (p < .01) and a decrease in the number of vocalizations from the still-face episode to the second naturalistic interaction episode (p < .05). There was no difference in the number of vocalizations between the two naturalistic interaction episodes. These two patterns of vocal production were typical of the sample. There are 9 possible patterns of increases and decreases in vocalizing that infants could show across the three episodes. For example, infants' vocalizations could increase from the first naturalistic interaction episode to the still-face episode followed by an increase from the still-face episode to the second naturalistic interaction episode, they could increase from the first naturalistic interaction to the still-face episode followed by no change in vocalizing from the still-face episode to the second naturalistic interaction, etc. A binomial test showed that a significant number of infants (22 of 38 or 58%) followed the overall pattern revealed in the ANOVA and depicted in Figure 1,; z = 8.90, p < .00003. In addition, the vast majority of infants' vocalizations (M = 95.9%, SD = 13.4%) were quasi-resonant vowels, which are typical of the vocal repertoires of 5-month-olds.
Figure 1.
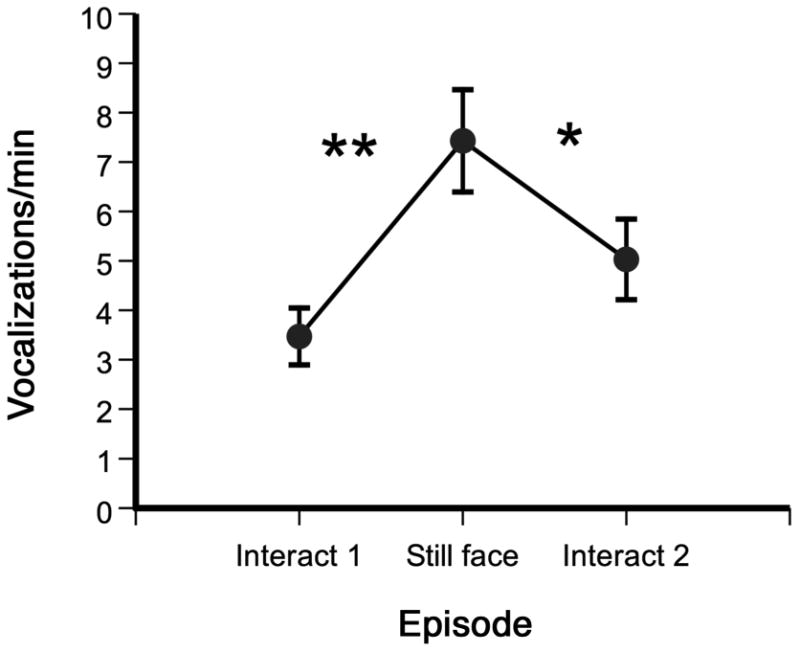
Mean number of noncry vocalizations per min during 1 min of naturalistic interaction (Interact 1), 2 min of still-face interaction, and 1 min of naturalistic interaction (Interact 2) (± 1 SE). * p < .05, ** p < .01.
Relations between Vocalizing and Later Language Comprehension
Infants had a mean LR of 1.92 (SD = 2.03) and a mean IR of 1.68 (SD = 1.35). LR and IR were largely independent (81% of variance between LR and IR was not shared). Language measures were the number of phrases reportedly understood on the CDI at 13 months (M = 14.97, SD = 7.24, range = 2 - 28) and number of words understood (M = 91.86, SD = 73.04, range = 9 - 324). The LR at 5 months was significantly correlated with the number of phrases understood at 13 months; the IR at 5 months was significantly correlated with phrases and words comprehended at 13 months (Table 1).
Table 1. Pearson Correlations between Prelinguistic Vocalizations and CDI Language Measures.
Note
p < .05,
n = 26,
n = 30.
Smiles
Frequency of infants' smiling decreased during the still-face episode (Figure 2). The data were normally distributed, Kolmogorov-Smirnov z = .876, p = .43. A one-way within-subjects ANOVA revealed a significant main effect of episode (Interact 1, Still Face, Interact 2) on frequency of smiles, F (2, 72) = 11.78, p < .001, ηp2 = .25. Tukey's HSD post-hoc tests indicated a decrease in frequency of smiling from the first naturalistic interaction episode to the still-face episode (p < .01) and an increase from the still-face episode to the second naturalistic interaction episode (p < .05). There was no significant difference in smile frequency between the first and second naturalistic interaction episodes.
Figure 2.

Mean number of smiles per min during 1 min of naturalistic interaction (Interact 1), 2 min of still-face interaction, and 1 min of naturalistic interaction (Interact 2) (± 1 SE). * p < .05, ** p < .01.
This pattern of smile frequency across episodes was typical of the sample. Of the 9 possible patterns that infants could show across the three episodes, 21 of the 37 infants (57%) showed this pattern revealed in the ANOVA and depicted in Figure 2. A binomial test showed that a significant number of infants followed the overall pattern, z = 8.58, p < .00003. The same pattern across episodes was found for smile duration, which showed a significant decrease from the first interaction to the still-face, followed by a significant increase from still-face to second interaction.
Changes in Infant Vocalizations and Smiles During the Still-Face Episode
To discern whether infants showed an extinction effect for vocalizations, infant vocalizations during the 2 minute still-face episode were assessed by dividing the still face episode into eight 15-s periods. Infant vocalizations were averaged for each 15-s period because infants tended to vocalize in bursts. During the still-face episode, infants' vocalizations increased to a peak at 75 s and then decreased to the end of the episode (Figure 3). Thirty-seven of the 38 infants (97%) showed a peak in vocalizing (median time of peak vocalizing = 75 s; inter-quartile range = 45 s). To test changes in the number of vocalizations produced across the still-face episode, we compared the mean number of vocalizations in each 15-s period to baseline, defined as the mean number of vocalizations in the last 15 s of the first naturalistic interaction episode (M = .79; Figure 3). We used a Bonferroni correction to adjust the alpha value for multiple tests. The mean number of infant vocalizations was above baseline in the first 15 s, t (37) = 2.92, p <.05; from 30-45 s, t (37) = 3.49, p < .01; from 45-60 s, t (37) = 3.15, p < .05; and from 60-75 s, t (37) = 3.59, p < .01. Mean number of vocalizations was marginally above baseline from 15-30s, t (37) = 2.70, p = .08.
Figure 3.

Mean number of vocalizations for each 15-s period during 1 min of naturalistic interaction (Interact 1), 2 min of still-face interaction, and 1 min of naturalistic interaction (Interact 2) (± 1 SE). The dotted line shows baseline (mean number of vocalizations in the last 15 s of the first naturalistic interaction episode). + Bonferroni-corrected p = .08, * Bonferroni-corrected p < .05, ** Bonferroni-corrected p < .01
In contrast, infants' frequency of smiling decreased from the beginning to the end of the still-face (Figure 4). Thirty-three of the 37 infants (89%) showed a peak in their smiling in Interact 1 followed by steady decreases (median time of peak smiling = 30 s; inter-quartile range = 45 s). To test changes in the number of smiles produced across the still-face episode, we compared the mean number of smiles in each 15-s period to baseline, defined as the mean number of smiles in the last 15 s of the first naturalistic interaction episode (M = 2.26). The mean number of infant smiles was below baseline from 15 s through the end of still face, ts (37) < -4.09, Bonferroni-corrected ps < .001.
Figure 4.

Mean number of smiles for each 15-s period during 1 min of naturalistic interaction (Interact 1), 2 min of still-face interaction, and 1 min of naturalistic interaction (Interact 2) (± 1 SE). The dotted line shows baseline (mean number of smiles in the last 15 s of the first naturalistic interaction episode). ** Bonferroni-corrected p < .01.
Relations between Vocalizing and Smiling
Infants who vocalized during naturalistic interaction episodes tended to vocalize during the still-face, and infants who smiled during naturalistic interaction episodes tended to smile during the still-face (Table 2). However, vocalizing and smiling were independent: frequency of vocalizations was not related to frequency of smiling in any of the three episodes (ps > .42).
Table 2. Pearson Correlations between Vocalization and Smile Frequency across the two Naturalistic Interaction Episodes and the Still-Face Episode.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Vocalizations | Smiles | ||||||
|
|
|||||||
| Interact 1 | Still Face | Interact 2 | Interact 1 | Still Face | Interact 2 | ||
|
|
|||||||
| Vocalizations | Interact 1 | -- | .62*** | .44*** | .04 | .11 | -.09 |
| Still Face | -- | .61*** | .02 | -.02 | -.14 | ||
| Interact 2 | --- | -.02 | -.14 | -.01 | |||
|
| |||||||
| Smiles | Interact 1 | -- | .66*** | .56*** | |||
| Still Face | -- | .66*** | |||||
| Interact 2 | -- | ||||||
p <.001,
Discussion
Five-month infants show a clear and specific extinction effect for vocalizations during the still-face episode. In the still-face paradigm, they significantly increased noncry vocalizations from the first naturalistic interaction episode to the still-face episode and significantly decreased vocalizing from the still-face to the second naturalistic interaction episode. Within the 2-minute still-face episode, infant vocalizations increased to a peak at 75 s, followed by a decrease to the end. The initial increase in vocalizing followed by a decrease is characteristic of a classical extinction burst; the present findings provide the first evidence of an extinction burst in infant vocal behavior. In contrast, infant smiling did not show an extinction burst.
Changes in the number of vocalizations produced across the still-face interaction were also related to later language comprehension. The Learning Ratio (LR) indicated the size of the extinction burst relative to infants' baseline levels of vocalizing. We interpreted the LR as measuring the strength of the association between vocalizing and social responses. The LR was positively correlated with a measure of language comprehension (number of phrases understood) at 13 months. Infants who learned the effects of their vocalizations on adults by 5 months appear to have advantages for later language learning. The differences observed here might be considered a precursor to understanding of social causality in instrumental use of prelinguistic vocalizations. For example, 10-month-olds who are able to search for causes for events also show increased coordination of vocalizations and other socially directed actions compared to infants who do not demonstrate understanding of causality (Harding & Golinkoff, 1979).
The Interaction Ratio (IR) indicated the amount of reduction in vocalizing after the still-face, once social interaction had resumed. We interpreted the IR as measuring infants' resumption of baseline (Naturalistic Interaction 1) rates of vocalizing in recognition of the resumption of normal social interaction behavior from the experimenter. When in face-to-face interactions with responsive social partners, infants typically reduce their amount of vocalizing, making shorter sounds (D'Odorico & Franco, 1991). Infants' IR was positively correlated with measures of their language comprehension (number of words and phrases understood) at 13 months. Infants who recognize a change in the behavior of their social partners may be better attuned to other features of social interaction, such as eye gaze, that predict greater language comprehension (Brooks & Meltzoff, 2005). In addition, by making fewer sounds, these infants change their patterns of vocalizing in ways that create more opportunities to engage in vocal turn-taking, which in turn facilitates language development (Hane, 2003; Locke, 1993). When vocal turn-taking increases as a result of increases in caregivers' contingent responses to the vocalizations of their 9-month-olds, infants' vocalizations become more speech-like and are more likely to incorporate phonological patterns of their caregivers' speech (Goldstein & Schwade, 2008). In contrast, infants who continue to vocalize at high rates during the second naturalistic interaction episode (resulting in a small IR) may be slower to recognize that their social partner is ready to interact. As a result, they have fewer opportunities for interactions that facilitate communicative development.
During the still-face, changes in infant vocalizing and smiling followed different trajectories. Vocalizing showed a rise-fall pattern characteristic of the extinction burst, but smiling fairly consistently decreased. As positive affect decreased, vocalizations peaked and then decreased; thus, vocalization rates were not linked to infants' affective state. The dissociation between smiling and vocalizing also suggests that infants' attempts to re-engage the experimenter were specific to the vocal channel and that infants' association between vocalizing and social responding was acquired during prior learning, and was not due to immediate affective reactions to the experimenter's behavior.
What are the developmental origins of the vocal extinction burst? Although the still-face paradigm increases negative affect in infants as young as 1.5 months (Bertin & Striano, 2006), the effects on noncry vocalizations have not been studied. Infants are capable of contingency learning by 2 months of age (Alessandri, Sullivan, & Lewis, 1990). The onset of social smiling, typically around 2 months (Messinger, 2005) represents the sensitivity of infants to the timing of social interactions. Infants gradually gain control over their production of social smiling from 3 to 6 months (Messinger, 2005). Thus, if the vocal extinction burst is a product of social attunement, then infants who show earlier onset of social smiling might show a larger vocal extinction burst during the still face episode than infants who do not. Current studies in our laboratory are testing this hypothesis.
The strength of the extinction burst, as measured by the LR, might indicate infants' prior history of social interaction. Infants who do not show an extinction burst during the still-face episode may not have previously learned the effects of their vocalizations on others. For example, infants of depressed mothers presumably have fewer opportunities to learn about the effects of their vocalizations on adults. Relative to non-depressed mothers, depressed mothers engage less frequently in contingent interactions with their infants (Murray & Cooper, 1997) and produce less exaggerated infant-dirhoroected speech (Kaplan, Bacwski, Smoski, & Zinser, 2001). We are currently conducting a study on the vocalizations of infants of clinically depressed mothers, and we expect that these infants will show a reduced extinction burst or no extinction burst at all in vocalizing, as they have had fewer opportunities to learn the social efficacy of their sounds.
By 5 months, infants have learned that their prelinguistic vocalizations elicit reactions from others. Vocalizing has acquired instrumental value. Infants expect even unfamiliar social partners to respond to their vocalizations. When that expectancy is violated, infants respond by showing an extinction burst in vocalizing. This pattern may reflect a transitory attempt to elicit social interaction. In light of the relations between the magnitude of the extinction burst (as measured by the LR) and later language, the early social learning demonstrated by the present study may represent the beginning of a developmental cascade of socially guided vocal learning.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NICHD. We thank Maurice Haynes and Diane Putnick for their assistance with data analysis. We thank Fern Baldwin, Casey Berson, Rachel Brandstadter, Jacqueline Briesch, Mi Hae Chung, Melissa Frankel, Angela Narayan, Jessie Northrup, Virtue Sankoh, Rachel Wechsler, Alissa Worly, and Veronika Zeppenfeld for their assistance with coding.
References
- Adamson LB, Frick JE. The still face: A history of a shared experimental paradigm. Infancy. 2003;4:451–473. [Google Scholar]
- Alessandri SM, Sullivan MW, Lewis M. Violation of expectancy and frustration in early infancy. Developmental Psychology. 1990;26:738–744. [Google Scholar]
- Amsel A. The role of frustrative nonreward in noncontinuous reward situations. Psychological Bulletin. 1958;55:102–119. doi: 10.1037/h0043125. [DOI] [PubMed] [Google Scholar]
- Amsel A. Frustrative nonreward in partial reinforcement and discrimination learning: Some recent history and a theoretical extension. Psychological Review. 1962;69:306–328. doi: 10.1037/h0046200. [DOI] [PubMed] [Google Scholar]
- Bacon WE. Partial-reinforcement extinction effect following different amounts of training. Journal of Comparative and Physiological Psychology. 1962;55:998–1003. doi: 10.1037/h0048614. [DOI] [PubMed] [Google Scholar]
- Bertin E, Striano T. The still-face response in newborn, 1.5-, and 3-month-old infants. Infant Behavior and Development. 2006;29:294–297. doi: 10.1016/j.infbeh.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Bigelow AE. Infants' sensitivity to familiar imperfect contingencies in social interaction. Infant Behavior and Development. 1998;21:149–162. [Google Scholar]
- Bloom K, Russell A, Wassenberg K. Turn taking affects the quality of infant vocalizations. Journal of Child Language. 1987;14:211–227. doi: 10.1017/s0305000900012897. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Arterberry ME, Mash C. Long‐term memory for an emotional interpersonal interaction occurring at 5 months of age. Infancy. 2004;6:407–416. [Google Scholar]
- Bornstein MH, Tamis-LeMonda CS, Hahn CS, Haynes OM. Maternal responsiveness to young children at three ages: Longitudinal analysis of a multidimensional, modular, and specific parenting construct. Developmental Psychology. 2008;44:867–874. doi: 10.1037/0012-1649.44.3.867. [DOI] [PubMed] [Google Scholar]
- Brooks R, Meltzoff AN. The development of gaze following and its relation to language. Developmental Science. 2005;8:535–543. doi: 10.1111/j.1467-7687.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado CEF, Messinger DS, Yale ME. Infant responses to the direction of parental gaze: A comparison of two still-face conditions. Infant Behavior and Development. 2002;25:311–318. [Google Scholar]
- D'Odorico L, Franco F. Selective production of vocalization types in different communicative contexts. Journal of Child Language. 1991;18:475–499. doi: 10.1017/s0305000900011211. [DOI] [PubMed] [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Bates E, Thal DJ, Pethick SJ. Variability in early communicative development. 5, Serial No. 242. Vol. 59. Monographs of the Society for Research in Child Development; 1994. pp. 1–173. [PubMed] [Google Scholar]
- Festinger L. The psychological effects of insufficient rewards. American Psychologist. 1961;16:1–11. [Google Scholar]
- Goldstein MH, King AP, West MJ. Social interaction shapes babbling: Testing parallels between birdsong and speech. Proceedings of the National Academy of Sciences. 2003;100:8030–8035. doi: 10.1073/pnas.1332441100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein MH, Schwade JA. Social feedback to infants' babbling facilitates rapid phonological learning. Psychological Science. 2008;19:515–522. doi: 10.1111/j.1467-9280.2008.02117.x. [DOI] [PubMed] [Google Scholar]
- Goldstein MH, Schwade JA. From birds to words: Perception of structure in social interactions guides vocal development and language learning. In: Blumberg MS, Freeman JH, Robinson SR, editors. The Oxford Handbook of Developmental and Comparative Neuroscience. Oxford University Press; in press. [Google Scholar]
- Goldstein MH, West MJ. Consistent responses of mothers to prelinguistic infants: The effect of prelinguistic repertoire size. Journal of Comparative Psychology. 1999;113:52–58. doi: 10.1037/0735-7036.113.1.52. [DOI] [PubMed] [Google Scholar]
- Gros-Louis J, West MJ, Goldstein MH, King AP. Mothers provide differential feedback to infants' prelinguistic sounds. International Journal of Behavioral Development. 2006;30:509–516. [Google Scholar]
- Halpern J, Poon L. Human partial reinforcement extinction effects: An information-processing development from Capaldi's sequential theory. Journal of Experimental Psychology. 1971;89:207–227. [Google Scholar]
- Hane AA. A dyadic approach to the interactional context of language acquisition: The direct and indirect effects of early mother-infant vocal coordination in predicting language outcomes at 24 months Unpublished doctoral dissertation. Unpublished doctoral dissertation, University of Maryland; 2003. [Google Scholar]
- Harding CG, Golinkoff RM. The origins of intentional vocalizations in prelinguistic infants. Child Development. 1979;50:33–40. [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. P.O. Box 1965, Yale Station, New Haven, CT 06520: 1975. [Google Scholar]
- Hsu H, Fogel A. Social regulatory effects of infant nondistress vocalization on maternal behavior. Developmental Psychology. 2003;39:976–991. doi: 10.1037/0012-1649.39.6.976. [DOI] [PubMed] [Google Scholar]
- Jones SS, Collins K, Hong H. An audience effect on smile production in 10-month-old infants. Psychological Science. 1991;2:45–49. [Google Scholar]
- Kaplan PS, Bachorowski JA, Smoski MJ, Zinser MC. Role of clinical diagnosis in effects of maternal depression on infant-directed speech. Infancy. 2001;2:533–544. doi: 10.1207/S15327078IN0204_08. [DOI] [PubMed] [Google Scholar]
- Lerman DC, Iwata BA. Prevalence of the extinction burst and its attenuation during treatment. Journal of Applied Behavior Analysis. 1995;28:93–94. doi: 10.1901/jaba.1995.28-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke JL. The child's path to spoken language. Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- Lowe CF, Beasty A, Bentall RP. The role of verbal behavior in human learning: Infant performance on fixed-interval schedules. Journal of the Experimental Analysis of Behavior. 1983;39:157–164. doi: 10.1901/jeab.1983.39-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. The psychology of animal learning. London: Academic Press; 1974. [Google Scholar]
- Messinger D. Smiling. In: Salkind NJ, editor. The encyclopedia of human development. Vol. 3. Thousand Oaks, CA: Sage; 2005. pp. 1183–1185. [Google Scholar]
- Moore GA, Cohn JF, Campbell SB. Infant affective responses to mother's still face at 6 months differentially predict externalizing and internalizing behaviors at 18 months. Developmental Psychology. 2001;37:706–714. [PubMed] [Google Scholar]
- Murray L, Cooper PJ. Postpartum depression and child development. New York: Guilford Press; 1997. [DOI] [PubMed] [Google Scholar]
- Nadel J, Croue S, Mattlinger MJ, Canet P, Hudelot C, Lecuyer C, Martini M. Do children with autism have expectancies about the social behavior of unfamiliar people? A pilot study using the still face paradigm. Autism. 2000;4:133–146. [Google Scholar]
- Oller DK. The emergence of the speech capacity. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- Pittenger DJ, Pavlik WB. Analysis of the partial reinforcement extinction effect in humans using absolute and relative comparisons of schedules. American Journal of Psychology. 1988;101:1–14. [Google Scholar]
- Poulson C. Differential reinforcement of other-than-vocalization as a control procedure in the conditioning of infant vocalization rate. Journal of Experimental Child Psychology. 1983;36:471–489. doi: 10.1016/0022-0965(83)90047-4. [DOI] [PubMed] [Google Scholar]
- Rheingold HL, Gewirtz JL, Ross HW. Social conditioning of vocalizations in the infant. Journal of Comparative and Physiological Psychology. 1959;52:68–73. doi: 10.1037/h0040067. [DOI] [PubMed] [Google Scholar]
- Striano T. Direction of regard and the still-face effect in the first year: Does intention matter. Child Development. 2004;75:468–479. doi: 10.1111/j.1467-8624.2004.00687.x. [DOI] [PubMed] [Google Scholar]
- Sullivan MW, Lewis M. Contextual determinants of anger and other negative expressions in young infants. Developmental Psychology. 2003;39:693–705. doi: 10.1037/0012-1649.39.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabulsy GM, Provost MA, Deslandes J, St-Laurent D, Moss E, Lemelin J, Bernier A, Dassylva J. Individual differences in infant still-face response at 6 months. Infant Behavior and Development. 2003;26:421–438. [Google Scholar]
- Tarabulsy GM, Tessier R, Kappas A. Contingency detection and the contingent organization of behavior in interactions: Implications for socioemotional development in infancy. Psychological Bulletin. 1996;120:25–41. doi: 10.1037/0033-2909.120.1.25. [DOI] [PubMed] [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, Brazelton TB. The infant's response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child Psychiatry. 1978;17:1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]


