Structured Abstract
Objective
To report the impact of microscopic thyroid extension (ETE) on outcome in patients with cT1/cT2 well differentiated thyroid cancer (WDTC), and to determine the effect of extent of surgery and adjuvant RAI on outcome in patients with microscopic ETE.
Patients and Methods
From an institutional database we identified 984 patients (54%) who had surgery for cT1/T2N0 disease. Of these, 869 patients were pT1/T2 and 115 were upstaged to pT3 based on the finding of microscopic ETE.
Disease specific survival (DSS) and recurrence free survival (RFS) were analyzed for each group using the Kaplan-Meier method. In the pT3 group, factors predictive of outcome were analyzed by univariate and multivariate analyses.
Results
There was no significant difference in the 10 year DSS (99% versus 100%, p=0.733) or RFS (98% versus 95%, p=0.188) on comparison of the pT1/pT2 cohort and pT3 cohort. Extent of surgery and administration of post-operative RAI were not significant for recurrence on univariate or multivariate analysis in the pT3 cohort.
Conclusion
Outcomes in patients with cT1T2N0 WDTC are excellent and not significantly affected by microscopic ETE. The extent of resection and administration of post-operative RAI in patients with microscopic ETE does not impact on survival or recurrence.
Introduction
Well differentiated thyroid cancer is a disease with a high cure rate and following appropriate treatment most patients can expect excellent outcomes. Long term follow up is required to detect differences in outcomes which has made prospective randomized controlled trials impractical. Current treatment recommendations are therefore based upon data from retrospective analyses of either institutional experience or national databases. From these studies, the 2 most important factors predictive of outcome have been shown to be age and the presence of gross extra thyroid extension (ETE) (1–6). More recent studies have suggested that microscopic ETE is of less significance than gross ETE in terms of survival, particularly in younger patients(7–11). Despite this recognition, the guideline recommends total thyroidectomy for all patients with any ETE following a biopsy diagnostic for malignancy. Following surgery for intrathyroidal T1 or T2 tumors, in which there is no clinical evidence of ETE, some tumors may be reported by the pathologist as having microscopic ETE. This results in upstaging of tumors to pT3. In young patients less than 45 years of age, this finding has no impact on overall stage with patients remaining at stage I or stage II. However, in patients over 45 years of age, this results in upstaging of patients to stage III, suggesting these patients have a poorer survival. In addition, the ATA in their recent guidelines(12) have recognized ETE as a risk factor for recurrence; patients were classified as low risk if their lesion was intrathyroid, intermediate risk if there was microscopic extension and high risk if there was macroscopic extension. However, there is no data that supports the finding of microscopic ETE as a risk factor for either recurrence or survival. The clinical relevance of this pathological finding is unknown. The objective of our study was therefore to determine the impact of microscopic extrathyroid extension (ETE) on outcome in patients with clinical stage T1 or T2 WDTC. We also wanted to determine whether or not the extent of surgery (lobectomy versus total thyroidectomy) and RAI had any impact on outcome in patients with microscopic ETE.
Patients and Methods
Following approval by the Institutional Review Board, patients who had thyroid surgery for intraglandular clinically T1T2 thyroid cancers were identified from our institutional database of 1810 patients treated for WDTC at Memorial Sloan Kettering Cancer Center between 1985–2005. From this cohort, 869 patients were staged as pathological T1T2N0 (pT1T2N0) and 115 as pT3N0 based on the presence of microscopic ETE. All 984 patients were N0 based upon clinical examination at the time of surgery or if central compartment neck dissection showed no pathological positive nodes. Patients who were pT3 based upon size criteria (ie tumors >4cm in size) or in whom clinical evidence of ETE to fibroadipose tissue or strap muscles was evident at the time of surgery (ie macroscopic ETE) were not included in the analysis. Patients who underwent initial treatment elsewhere prior to referral or those who were considered unresectable at the time of referral were also excluded. Patient demographics, extent of thyroid surgery, details of pathology (tumor histology, size and presence of microscopic ETE) and details of adjuvant RAI were recorded from patient charts.
Patient, tumor and treatment details of all 984 patients are shown in Table 1. The median age of the group was 47 years (range 4–91 years). The male to female ratio was 1:4. Eight hundred and ninety-one patients (91%) had papillary carcinoma, 52 (5%) had follicular carcinoma and 41 (4%) had Hurthle Cell carcinoma. Three hundred and seventy-six patients (38%) had thyroid lobectomy, 574 (58%) had total thyroidectomy, 16 (2%) had isthmusectomy and 18 (2%) had subtotal thyroidectomy. Twenty-two patients who had initial thyroid lobectomy were taken for completion thyroidectomy within weeks due to adverse histopathological findings. These patients were considered as having total thyroidectomy for the purposes of this analysis. Eight hundred and sixty-nine patients (88%) were confirmed as having no extension on histopathological analysis (pT1/pT2), and 115 (12%) were pathologically upstaged to pT3 due to the presence of microscopic ETE. Seventy-six (11%) cT1 and 39 (14%) cT2 lesions were upstaged to pT3. Table 2 shows the rate of microscopic ETE according to tumor size. Patients with microcarcinoma (pT size 1cm or less) had a rate of ETE of only 6% whereas patients with tumors 1–2cms, 2–3cms and 3–4cms had similar rates ETE (16.4%, 15.3% and 11.7% respectively).
Table 1.
Characteristics of whole group
| Variable (n=984) | n (%) |
|---|---|
| Age | |
| <45y | 457 (46%) |
| >45y | 527 (54%) |
| Gender | |
| Male | 208 (21%) |
| Female | 776 (79%) |
| cT Stage | |
| T1 | 707 (72%) |
| T2 | 277 (28%) |
| Surgery | |
| Lobectomy | 376 (38%) |
| Total Thyroidectomy (22 completed) | 574 (58%) |
| Isthmusectomy | 16 (2%) |
| Subtotal Thyroidectomy | 18 (2%) |
| Pathology | |
| Papillary Ca | 891 (91%) |
| Follicular Ca | 52 (5%) |
| Hurthle Cell Ca | 41 (4%) |
| Extension | |
| None | 869 (88%) |
| Microscopic | 115 (12%) |
| pT Stage | |
| T1 | 631 (64%) |
| T2 | 238 (24%) |
| T3 | 115 (12%) |
| RAI | |
| No | 733 (74%) |
| Yes | 251 (26%) |
Table 2.
Percentage of cT1/2 lesions demonstrating microscopic extra-thyroid extension by tumor size
| pT Size | n | ETE (n=115) | % |
|---|---|---|---|
|
| |||
| ≤1 cm | 384 | 23 | 6.0% |
| >1–2 cms | 323 | 53 | 16.4% |
| >2–3 cms | 183 | 28 | 15.3% |
| >3–4 cms | 94 | 11 | 11.7% |
Patient, tumor and treatment characteristics stratified by the presence of ETE are shown in Table 3. Compared to the pT1/pT2 group, the pT3 group had more patients over the age of 45 years (66% versus 52%, p=0.004), were more likely to receive post-operative RAI (57% versus 21%, p<0.001) and were more likely to have total thyroidectomy (75% versus 56%, p=0.002).
Table 3.
Characteristics of group, stratified by microscopic extension
| Variable (n=984) | No Extension (n=869) n (%) pT1/pT2 |
Extension (n=115) n (%) pT3 |
p Value |
|---|---|---|---|
| Age | |||
| <45y | 418 (48%) | 39 (34%) | 0.004 |
| >45y | 451 (52%) | 76 (66%) | |
| Gender | |||
| Male | 184 (21%) | 24 (21%) | 0.940 |
| Female | 685 (79%) | 91 (79%) | |
| cT Stage | |||
| T1 | 631 (73%) | 76 (66%) | 0.144 |
| T2 | 238 (27%) | 39 (34%) | |
| Surgery | |||
| Lobectomy | 350 (40%) | 26 (23%) | 0.002 |
| Total Thyroidectomy | 488 (56%) | 86 (75%) | |
| Isthmusectomy | 15 (2%) | 1 (1%) | |
| Subtotal Thyroidectomy | 16 (2%) | 2 (1%) | |
| Pathology | |||
| Papillary Ca | 785 (90%) | 106 (92%) | 0.654 |
| Follicular Ca | 48 (6%) | 4 (3.5%) | |
| Hurthle Cell Ca | 36 (4%) | 5 (4.5%) | |
| RAI | |||
| No | 683 (79%) | 50 (43%) | <0.001 |
| Yes | 186 (21%) | 65 (57%) |
Survival outcomes for the entire group and for the groups stratified by ETE were determined by the Kaplan Meier method. Outcomes data included overall survival (OS), disease specific survival (DSS), recurrence free survival (RFS), local recurrence free survival (LRFS), regional recurrence free survival (RRFS) and distant recurrence free survival (DRFS). Local recurrence was defined as recurrence in the operative thyroid bed. Regional recurrence was defined as nodal recurrence, either central or regional. The presence of central neck nodes following thyroid surgery was defined as regional recurrence in our institution, not local recurrence. Both local and regional recurrence were based upon clinical examination which was confirmed by cytological or histopathological evidence of disease. Distant disease was determined by imaging studies including radioiodine uptake scans, CT scans, PET scans, or cytological and histopathological evidence where available. During the period of our study from 1986–2005, routine use of ultrasound or serum thyroglobulin were not used to identify local or regional recurrence. We are therefore not able to comment on serum thyroglobulin levels in our cohort of patients. Since 2005, all thyroid cancer patients at our institution now get annual ultrasound and twice yearly serum thyroglobulin measurements. Statistical analysis was performed using JMP statistical package (SAS Institute Inc. SAS Campus Drive, Cary, NC 27513) and SPSS (IBM Company Headquarters, 233 S. Wacker Drive, 11th Floor, Chicago, Illinois 60606). Univariate analysis was carried out by the log rank test and multivariate analysis by Cox proportional hazards method.
Results
Entire Group
With a median follow up of 98 months (range 6–291), the 10 year overall survival (OS) was 92%, disease specific survival (DSS) 99% and recurrence free survival (RFS) 98% respectively. No patient had local recurrence, 8 had regional and 5 had distant recurrence. There were 73 deaths, only one of which was due to disease (a 44year old male, who presented with Hurthle Cell Carcinoma and following total thyroidectomy went on to develop distant recurrence and die 101 months following treatment). The 10 year local recurrence free survival (LRFS), regional recurrence free survival (RRFS) and distant recurrence free survival (DRFS) were 100%, 99% and 99% respectively.
Factors predictive of overall survival by univariate analysis are shown in Table 4. Age over 45 years at the time of thyroid surgery was the only factor predictive of worse outcome. This remained significant on multivariate analysis. Extent of surgery (lobectomy versus total thyroidectomy), pathology (papillary versus follicular or Hurthle cell carcinoma), microscopic ETE or use of postoperative RAI had no impact on overall survival.
Table 4.
Factors Predictive of Overall Free Survival in the Whole Group
| Variable (n=984) | Number (%) | 10y Overall Survival | Univariate Analysis p Value | Multivariate Analysis | |
|---|---|---|---|---|---|
| HR (95% CI) | p Value | ||||
| Age | |||||
| <45y | 457 (46%) | 94% | Referent | ||
| >45y | 527 (54%) | 76% | <0.001 | 3.362 (1.925–5.870) | <0.001 |
| Gender | |||||
| Female | 776 (79%) | 93% | 0.079 | Referent | |
| Male | 208 (21%) | 98% | 1.502 (0.902–2.499) | 0.118 | |
| cT Stage | |||||
| T1 | 707 (72%) | 91% | 0.455 | N/A | |
| T2 | 277 (28%) | 94% | |||
| Surgery | |||||
| Lobectomy | 376 (38%) | 93% | 0.987 | N/A | |
| Total Thyroidectomy | 574 (58%) | 91% | |||
| Isthmusectomy | 16 (2%) | 93% | |||
| Subtotal Thyroidectomy | 18 (2%) | 87% | |||
| Pathology | |||||
| Papillary | 891 (91%) | 92% | 0.988 | N/A | |
| Follicular | 52 (5%) | 92% | |||
| Hurthle Cell | 41 (4%) | 95% | |||
| Extension | |||||
| None | 869 (88%) | 93% | 0.129 | Referent | |
| Microscopic | 115 (12%) | 88% | 1.391 (0.714–2.710) | 0.332 | |
| pT Stage | |||||
| T1 | 631 (64%) | 91% | 0.191 | N/A | |
| T2 | 238 (24%) | 95% | |||
| T3 | 115 (12%) | 88% | |||
| RAI | |||||
| No | 733 (74%) | 93% | 0.384 | Referent | |
| Yes | 251 (26%) | 88% | 0.931 (0.524–1.653) | 0.806 | |
Comparison of pT3 (microscopic extension) and pT1/2 Cohorts
The survival statistics for the 2 cohorts stratified by microscopic extension are shown in Table 5. No significant difference in 10 year OS, DSS or RFS was found between the pT1/pT2 and pT3 groups (OS: 93% versus 88%, p=0.129; DSS:99% versus 100%, p=0.733; RFS: 98% versus 95%, p=0.188 respectively). There was no significant difference in outcome between the pT1/ pT2 and pT3 groups in terms of local or distant recurrence free survival at 10 years (100% versus 100%. P=1 and 99% versus 100%, p=0.389 respectively). A significant difference was found between the pT1/ pT2 and pT3 groups in terms of 10 year regional recurrence free survival (99% versus 95%, p=0.032); 5 of the 869 patients (0.5%) without microscopic evidence of extrathyroid extension recurred in lateral neck nodes (3 pT1 and 2 pT2) compared to 3 of 115 patients with pT3 tumors(3%).
Table 5.
Comparison of outcomes by histopathologicalextrathyroidalextension
| Outcome | Whole Group (n=984) |
No Extension (n=869) |
Extension (n=115) |
p Value |
|---|---|---|---|---|
| Overall Survival 10y | 92% | 93% | 88% | 0.129 |
| Disease Specific Survival 10y | 99% | 99% | 100% | 0.733 |
| Recurrence Free Survival 10y | 99% | 98% | 95% | 0.188 |
| Local Recurrence Free Survival 10y | 100% | 100% | 100% | 1.000 |
| Regional Recurrence Free Survival 10y | 99% | 99% | 95% | 0.032 |
| Distant Recurrence Free Survival 10y | 99% | 99% | 100% | 0.389 |
In older patients, histopathological upstaging to pT3 elevates the overall clinical stage from stage I for T1 and stage II for T2 lesions to stage III. Despite this, we found no difference in overall survival in older patients based on microscopic ETE. The 10 year overall survival of older patients with intra-thyroid lesions versus those with microscopic extension was 88% versus 85% (p=0.675).
The Impact of Extent of Treatment On Patients With Microscopic Extension (pT3)
Patients with microscopic ETE were analyzed to determine factors predictive of outcome within this group. Factors analyzed included age, gender, size of primary tumor, pathology, extent of thyroid surgery, use of RAI. Table 6 shows factors predictive of 10 year recurrence free survival in the pT3 patients. Of the 115 patients, the median age was 53 years (range 26–85) with a male to female ratio of 1:4. Twenty-six patients (23%) had thyroid lobectomy, with one patient (4%) receiving post-operative RAI for ablation of the contra lateral lobe. The remaining 86 patients (77%) had total thyroidectomy, of whom 63 (73%) also received post-operative RAI. There were no disease specific deaths and only 3 recurrences within this group, all of which were in the lateral cervical lymph nodes. These 3 patients had all undergone total thyroidectomy and received post-operative RAI. Univariate analysis of 10 year recurrence free survival identified male gender as a significant predictor of worse outcome (79% versus 99%, p=0.028). However, this significance was lost on multivariate analysis. No other measured variable including type of pathology (papillary versus follicular or Hurthle cell cancer), extent of thyroid surgery (Figure 1) or treatment with adjuvant RAI (Figure 2) were significant predictors of outcome on univariate or multivariable analysis. On univariate analysis of the patients who underwent total thyroidectomy (n=86), the provision of RAI had no impact on RFS in patients who did not receive RAI versus those who did (90% versus 100%, p=0.290) (Figure 2); however it is possible that this difference may be significant if a larger number of patients were used.
Table 6.
Factorspredictive of recurrence in patients with microscopic extrathyroidal extension.
| Variable (n=115) | n (%) | 10 Year Recurrence Free Survival | Univariate p Value | Multivariate p Value |
|---|---|---|---|---|
| Age | ||||
| <45y | 39 (34%) | 100% | 0.252 | N/A |
| >45y | 76 (66%) | 93% | ||
| Gender | ||||
| Female | 91 (79%) | 99% | 0.028 | Referent |
| Male | 24 (21%) | 79% | NS | |
| cT Stage | ||||
| T1 | 76(66%) | 98% | 0.323 | N/A |
| T2 | 39 (34%) | 91% | ||
| RAI | ||||
| No | 50 (43%) | 100% | 0.106 | Referent |
| Yes | 65 (57%) | 90% | NS | |
| Surgery | ||||
| Lobectomy | 26 (23%) | 100% | 0.766 | N/A |
| Total Thyroidectomy | 86 (75%) | 93% | ||
| Isthmusectomy | 1 (1%) | 100% | ||
| Subtotal Thyroidectomy | 2 (1%) | 100% | ||
| Pathology | ||||
| Papillary Ca | 106 (92%) | 95% | 0.907 | N/A |
| Follicular Ca | 4 (4%) | 100% | ||
| Hurthle Cell Ca | 5 (4%) | 100% | ||
| RAI in Total Thyroidectomy (n=86) | ||||
| No | 23 (37%) | 100% | 0.290 | N/A |
| Yes | 63 (73%) | 90% |
Figure 1.
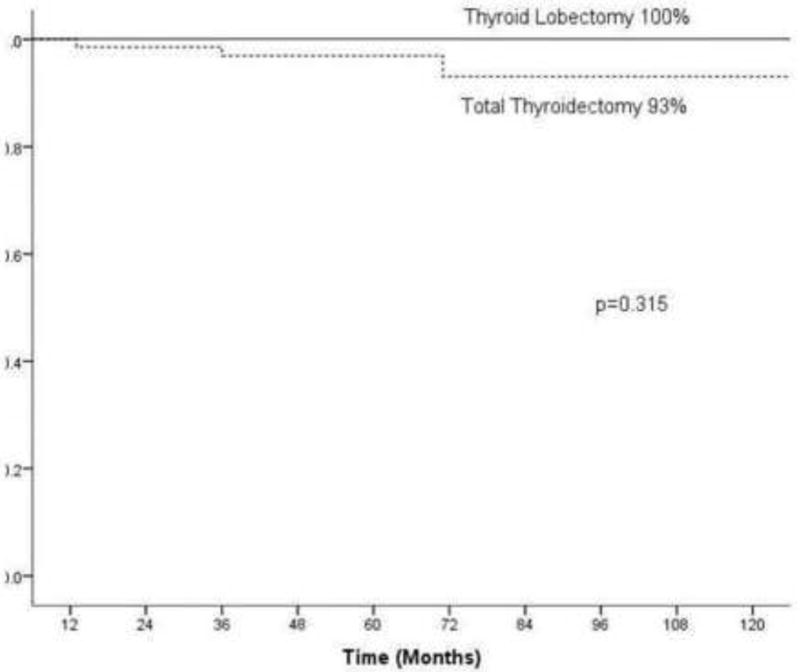
Effect of surgery on 10 year recurrence free survival for pT3 patients (total thyroidectomy versus lobectomy n=115)
Figure 2.
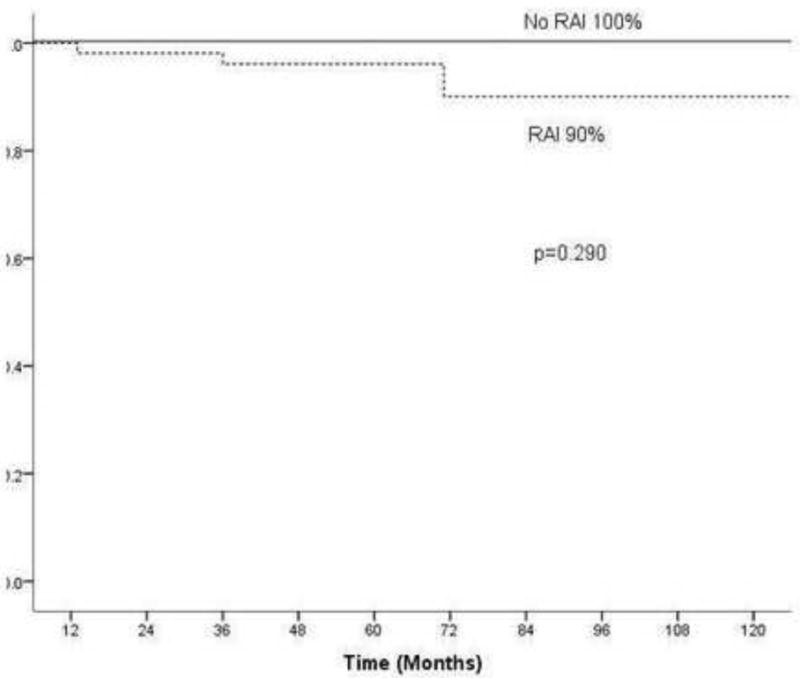
Effect of RAI on 10 year recurrence free survival in pT3 patients having undergone total thyroidectomy (n=86)
Discussion
Well differentiated thyroid cancer is a disease with a high cure rate and following appropriate treatment most patients can expect excellent outcomes (2–6, 8, 13–15). Long term follow up is required to detect differences in outcomes which has made prospective randomized controlled trials impractical(16). Current treatment recommendations are therefore based upon data from retrospective analyses of either institutional experience or national databases. In keeping with previous studies, we report excellent outcomes for patients considered to have well differentiated, clinically intrathyroid lesions (cT1/cT2), with a DSS at 10 years of 99% and LRFS, RRFS and DRFS of 100%, 99% and 99% respectively.
Extra thyroid extension of thyroid cancer has been recognized as a factor predictive of poor outcome by many groups(1–6). More recent analyses have suggested that microscopic ETE is of less significance than gross extension, particularly in younger patients(7–11). This subclassification of ETE has been recognized by the ATA. In their recent guidelines(12), patients were classified as low risk if, amongst other factors, their lesion was intrathyroid, intermediate risk if there was microscopic ETE and high risk if there was macroscopic extension. Despite this recognition, the ATA guidelines recommends total thyroidectomy for all patients with ETE following a biopsy diagnostic for malignancy.
In the current study, 984 (54%) of 1810 patients were identified with cT1T2N0 tumors. Of these 984, 869 were pT1T2N0 and 115 (14%) were upstaged to a pathological pT3 on the basis of microscopic ETE. Although lesions of 1cm or less had a rate of extra thyroid extension of only 6%, those lesions between 1 and 4cms had similar rates of extrathyroid extension independent of size (11.7–16.4%). We found that patients with microscopic ETE were more likely to be older, to receive post-operative RAI and to undergo total thyroidectomy. Age over 45 years was the only independent predictor of overall survival on multivariate analysis. We have shown that for lesions considered to be grossly intraglandular following surgical resection, microscopic ETE had no impact on OS, DSS, LRFS or DRFS. The impact of microscopic ETE on RRFS was questionable. Those patients with microscopic ETE had a regional recurrence rate of 5% at 10 years compared with 1% for those without. Although this difference was significant on univariate analysis (p=0.031), it was non-significant on multivariate analysis (0.052). In addition, although this difference may border on statistical significance, its clinical significance is questionable. As such, a small change in regional recurrence at 10 years which does not translate into a survival advantage is unlikely to change the treatment policies of a disease management team, but rather calls for individualized decision and more careful surveillance during follow up. Clearly, this approach will avoid unnecessarily extended surgery in 95% of patients.
In patients over the age of 45 years, the current AJCC guidelines consider minimal ETE to elevate patients to clinical stage III. Our findings suggest that this upstaging is unwarranted as survival in such patients in unaffected by the microscopic extra-thyroid extension.
Despite the minimal impact that microscopic ETE has on outcome, the current ATA guidelines recommend to perform total thyroidectomy and consider RAI for any lesion which is not confined to the thyroid gland. The clinician therefore may face three problematic postoperative scenarios. The first follows a biopsy suggesting a low risk, well differentiated malignancy which appears to be clinically intrathyroid at surgery. If lobectomy is performed and the histopathological analysis of the specimen reveals microscopic ETE, the surgeon may have to consider advising completion thyroidectomy. The second scenario follows a biopsy which is equivocal for malignancy. Thyroid lobectomy may be performed for diagnostic and treatment purposes. If in this case the histopathological analysis confirms well differentiated thyroid cancer, and also identifies microscopic ETE, again the surgeon may have to consider completion thyroidectomy. The third scenario is after total or completion thyroidectomy and pathology shows microscopic ETE, whether or not RAI should be given. In order to address these clinical problems we analyzed the group with ETE in more detail. There were no disease specific deaths and only 3 recurrences, all in the cervical lymph nodes, in this group of patients. We found that the extent of thyroid surgery did not affect outcome (10 year RFS of 100% versus 93% for thyroid lobectomy and total thyroidectomy respectively, p=0.766). This observation may be more significant given that a higher percentage of those patients who underwent total thyroidectomy also received post-operative RAI in comparison with the thyroid lobectomy group (73% versus 4%). In addition, patients not treated with RAI did not have poorer outcome or increased recurrence compared with the RAI group. The impact of adjuvant RAI in our cohort of pT3 patients is marginal at best.
The current study focuses on a selected surgical group of patients in a major tertiary care referral cancer center, and has the potential bias associated with all retrospective analyses. The patients with ETE were older than those without, which would be expected to result in worse outcomes. They were also more likely to undergo total thyroidectomy and receive post-operative RAI than the group without extension, which may be expected to improve their outcome. There may have been factors which were not recorded that led clinicians to consider the lesions treated with total thyroidectomy as higher risk, resulting in higher rates of distant metastases. The group treated with lobectomy was younger which would be expected to lead to better outcomes, and there was a trend towards total thyroidectomy in the later years, as modern ultrasound identified more contralateral nodularity than clinical examination used earlier in the study period. Despite these limitations, our data suggests that in selected patients, pathological upstaging of a well differentiated, clinically T1 or T2 thyroid cancer without regional or distant metastases has no impact on survival. The vast majority of such patients do well, with only 1 disease specific death recorded in the group as a whole at 101 months. Pathological microscopic ETE had a marginal effect on regional recurrence on univariate analysis, which was not significant on multivariate analysis. Microscopic ETE showed no effect on all other measures of recurrence. We also found that thyroid lobectomy in comparison with total thyroidectomy within this group did not result in worse outcome for patients with microscopic ETE in terms of survival or recurrence, suggesting that a report of microscopic ETE should not automatically prompt completion thyroidectomy. In addition, patients with microscopic ETE who did not have RAI did not have increased rates of recurrence.
In conclusion, outcomes in patients thought to have well differentiated clinically and grossly intrathyroid cancers (cT1/cT2) are excellent and not significantly affected by the discovery of microscopic ETE on histopathological analysis. In addition, the extent of surgical resection or the use of RAI in these low risk lesions does not have an impact on survival or recurrence. Therefore, in select patients, primarily young patients with favorable histology, completion thyroidectomy after lobectomy or the use of postoperative RAI may not be required when a pathological finding of microscopic ETE is found.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Financial Interests and Potential Conflicts of Interest
The authors have no financial interests or conflicts of interest to disclose.
References
- 1.Shaha AR, Shah JP, Loree TR. Patterns of failure in differentiated carcinoma of the thyroid based on risk groups. Head Neck. 1998;20:26–30. doi: 10.1002/(sici)1097-0347(199801)20:1<26::aid-hed5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Shaha AR, Shah JP, Loree TR. Risk group stratification and prognostic factors in papillary carcinoma of thyroid. Ann Surg Oncol. 1996;3:534–8. doi: 10.1007/BF02306085. [DOI] [PubMed] [Google Scholar]
- 3.Sugitani I, Toda K, Yamamoto N, Sakamoto A, Fujimoto Y. Re-Evaluation of Histopathological Factors Affecting Prognosis of Differentiated Thyroid Carcinoma in an Iodine-Sufficient Country. World J Surg. 2009 doi: 10.1007/s00268-009-0305-y. [DOI] [PubMed] [Google Scholar]
- 4.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–53. [PubMed] [Google Scholar]
- 5.McConahey WM, Hay ID, Woolner LB, van Heerden JA, Taylor WF. Papillary thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1986;61:978–96. doi: 10.1016/s0025-6196(12)62641-x. [DOI] [PubMed] [Google Scholar]
- 6.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–7. discussion 7–8. [PubMed] [Google Scholar]
- 7.Andersen PE, Kinsella J, Loree TR, Shaha AR, Shah JP. Differentiated carcinoma of the thyroid with extrathyroidal extension. Am J Surg. 1995;170:467–70. doi: 10.1016/s0002-9610(99)80331-6. [DOI] [PubMed] [Google Scholar]
- 8.Sherman SI, Brierley JD, Sperling M, Ain KB, Bigos ST, Cooper DS, et al. Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012–21. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: massive but not minimal extension affects the relapse-free survival. World J Surg. 2006;30:780–6. doi: 10.1007/s00268-005-0270-z. [DOI] [PubMed] [Google Scholar]
- 10.Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Minimal extrathyroid extension does not affect the relapse-free survival of patients with papillary thyroid carcinoma measuring 4 cm or less over the age of 45 years. Surg Today. 2006;36:12–8. doi: 10.1007/s00595-005-3090-8. [DOI] [PubMed] [Google Scholar]
- 11.Rivera M, Ricarte-Filho J, Tuttle RM, Ganly I, Shaha A, Knauf J, et al. Molecular, morphologic, and outcome analysis of thyroid carcinomas according to degree of extrathyroid extension. Thyroid. 2010;20:1085–93. doi: 10.1089/thy.2010.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 13.Lin HW, Bhattacharyya N. Survival impact of treatment options for papillary microcarcinoma of the thyroid. Laryngoscope. 2009;119:1983–7. doi: 10.1002/lary.20617. [DOI] [PubMed] [Google Scholar]
- 14.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. The American journal of medicine. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 15.Saadi H, Kleidermacher P, Esselstyn C., Jr Conservative management of patients with intrathyroidal well-differentiated follicular thyroid carcinoma. Surgery. 2001;130:30–5. doi: 10.1067/msy.2001.115364. [DOI] [PubMed] [Google Scholar]
- 16.Wong JB, Kaplan MM, Meyer KB, Pauker SG. Ablative radioactive iodine therapy for apparently localized thyroid carcinoma. A decision analytic perspective. Endocrinol Metab Clin North Am. 1990;19:741–60. [PubMed] [Google Scholar]


