Abstract
Aims
To correlate the immunohistochemical detection of WWOX with histological measures and disease progression within the whole spectrum of urothelial bladder neoplasms.
Methods and results
One hundred and one patients with primary bladder tumours were retrospectively analysed. Immunohistochemically, a polyclonal antibody was utilized and the level of WWOX protein expression was analysed by using a combined score system based on intensity of the reaction and percentage of immunoreactive tumour cells. WWOX protein expression was consistently expressed in nonneoplastic urothelium, whereas a progressive loss of immunoreactivity was observed as tumour grade and stage increased (P < 0.05). Principal component analysis showed that reduced WWOX immunoexpression was significantly associated with high histological grades (P = 0.001), advanced stage (P = 0.002), tumour size (P = 0.04) and cancer progression (P = 0.028). Invasive urothelial carcinomas of the bladder with squamous metaplasia presented the lowest levels of WWOX protein. Kaplan–Meier analyses demonstrated a significant correlation between loss of WWOX expression and a shorter progression-free survival (P = 0.042), whereas the prediction of overall survival achieved borderline significance (P = 0.053).
Conclusion
Loss of WWOX immunoexpression strongly correlates with classical clinicopathological factors and appears to be a potential predictive marker of progressive disease.
Keywords: bladder neoplasms, immunohistochemistry, prognosis, WWOX protein
Introduction
It is well known that human chromosomes display non-random regions of fragility prone to breakage.1 These unstable loci are referred to as common or constitutive chromosomal fragile sites, and this initial observation has led many authors to hypothesize that mutations affecting these fragile-related genes may contribute to cancer development.2–4 Two of the most commonly altered fragile sites within the human genome are located within the short arm of chromosome 3 (3p) and the long arm of chromosome 16 (16q), where the FHIT and the WWOX genes, respectively, have been mapped and are considered to be the most representative cancer-related fragile site genes.5–7 The WWOX gene was cloned by Bednarek et al. in 2000 as a gene spanning a huge genomic region of >1 mbp located in a genomic area showing a high incidence of loss of heterozygosity and allelic imbalances in the preinvasive stages of breast cancer development.7 Recent studies have confirmed that this gene behaves as a putative tumour suppressor gene and is strongly related to the neoplastic process in hormonally dependent tumour types such as breast, ovarian and prostatic carcinomas.8–11 However, significant down-regulation of WWOX gene and protein expression has also been observed in non-hormonally regulated human neoplasias.12–18
In the current study, we characterized, for the first time, the level of immunohistochemical expression of WWOX protein in a large series of patients (n = 101) with primary urinary bladder tumours in order to assess the potential predictive value of alterations in WWOX protein expression in comparison with classical clinicopathological prognostic factors such as histological grade, tumour stage, tumour size and multiplicity.
Materials and methods
CLINICOPATHOLOGICAL DATA
The study group encompassed 101 patients with nonconsecutive primary bladder tumours [81 transurethral resection (TUR) and 20 radical cystectomy specimens]. Clinical and pathological data were obtained from cystoscopic, operative and histopathological reports. Informed consent was required for each patient to enter the study and all research carried out was in compliance with the Helsinki Declaration. First, a representative histological section of the tumour was selected by an expert uropathologist so as to assess the histological grade and tumour stage. Subsequently, assessment of grade and stage was carried out by two independent uropathologists and consensus was reached in cases of discrepancy. According to the 2004 World Health Organization (WHO) Consensus Classification for Histological Grading of Bladder Neoplasms, 19 the study included patients with papillary urothelial neoplasms of low malignant potential (PUNLMP), low-grade papillary urothelial carcinomas (LGPUC), high-grade (papillary and non-papillary) urothelial carcinomas (HGUC) and urothelial carcinomas with squamous metaplasia (UCSC). Secondary urothelial carcinoma in situ was present in 13 cases (12.87%) accompanying high-grade urothelial carcinomas. All tumours were staged according to the 2004 WHO Consensus Staging System as non-invasive (pTa) tumours, tumours with invasion of the lamina propria (pT1) and tumours with invasion of the muscularis propria or beyond (pT2–4) [19]. Likewise, normal bladder urothelium and squamous metaplasia were obtained from a control group of non-cancer patients.
Follow-up data were obtained by retrospective review of out-patient records. Patients treated with TUR of the lesion were monitored for follow-up purposes with voided urinary cytology, cystoscopies, and cystoscopies with second TUR or random biopsies, whereas patients treated with radical cystectomy were monitored by means of radiological examinations for tumour relapses and spread of the disease to regional lymph nodes or distant organ metastases. Tumour recurrences were defined as a histologically confirmed tumour relapse during patient follow-up provided that this new tumour presented the same histological grade and stage as the primary lesion, although they were not located at the same anatomical location in the bladder wall. Otherwise, tumour progression was defined as an increase in histological grade or tumour stage during follow-up of recurrent disease or the development of lymph node metastases (N+) or systemic organ metastasis (M+). Three patients were N+ at the time of diagnosis. The period of time from primary diagnosis to the assessment of a tumour recurrence [relapse-free survival (RFS)] or to the assessment of tumour progression [progression-free survival (PFS)] was also taken into account. Finally, overall survival (OS) was considered for patients who died as a result of their disease during follow-up.
IMMUNOHISTOCHEMISTRY
The immunohistochemical study was carried out on 4-μm-thick formalin-fixed paraffin-embedded tissue sections following the streptavidin–biotin complex method (LSAB-2). Primary polyclonal antibody was a rabbit anti-WWOX (140 mg / ml) with an optimal dilution of 1:400. All samples were treated for antigen retrieval heating in an autoclave at 147.1 kPa for 3 min (pH 8.0 ethylenediamine tetraacetricacid). The Dako Techmate Kit (Dako, Glostrup, Denmark) was used as the secondary antibody and the revelation method was based on diaminobenzidine chromogen. Specificity and sensitivity of the WWOX antibody employed and raised against the WW domains of this protein have been previously demonstrated in various publications.7,9,10 The immunoreaction was subjectively evaluated by two experienced uropathologists by using a combined score system. Normal bladder urothelium and squamous metaplasia of the bladder from non-tumour samples were used as the external control of the reaction. In both normal urothelium and squamous metaplasia, WWOX immunoexpression consistently showed strong and diffusely granular cytoplasmic immunoreactivity without any nuclear reactivity. Lack of primary antibody was used as the negative control of the reaction. In tumour samples, the immunoreaction was quantified by means of two parameters: intensity of reactivity, which was scored as absent (0), weak (1), moderate (2) or intense (3); and percentage of reactive tumour cells, which was scored as none (0), 0–25% of stained tumour cells (1), 26–50% (2), 51–75% (3) and >75% (4). Afterwards, a combined score was obtained by multiplying the intensity of the reaction and the percentage of stained tumour cells, which resulted in a final value ranging between 0 and 12 arbitrary units. To simplify these data, scores ranging from 0 to 4 were considered a low level of WWOX protein expression, between 5 and 7 was considered moderate, and >7 was considered high.
STATISTICS
Multivariate analysis was performed by principal component analysis in order to simplify the dataset into a lower dimension analysis in three axes. Variables were codified and then compared by means of Kendall’s τ-b correlation coefficient. In order to enable visualization of the factorial analysis, we employed a 3D representation of each component plotted in rotated space. anova tests were utilized to compare and confirm differences among the variables. Kaplan–Meier estimates were generated to calculate the specific correlation of WWOX protein expression level with survival parameters (RFS, PFS and OS). For this purpose, the log rank test was utilized to contrast differences between the variables. The basic significance level was fixed at P < 0.05 and all data were analysed using SPSS® commercially available statistical software (SPSS Inc., Chicago, IL, USA).
Results
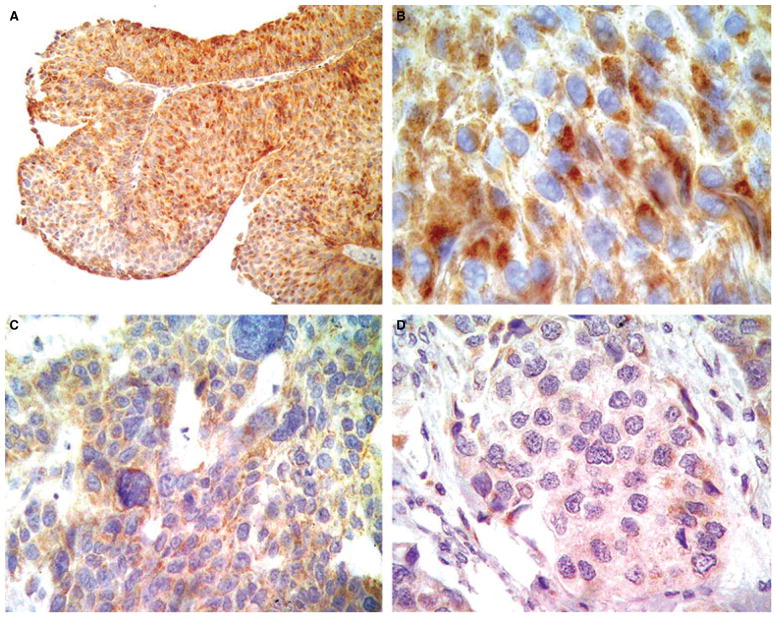
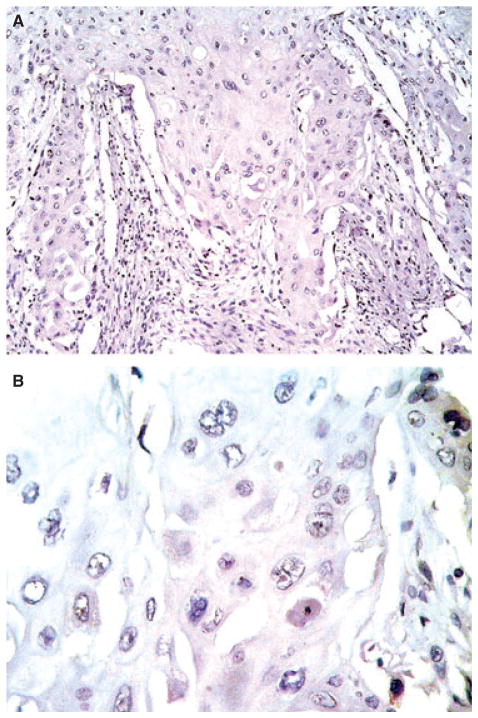
The mean patient age was 68.1 years (range 42–91 years); 86 were male (85.1%) and 15 female (14.9%), which meant a male-to-female ratio of 5.7:1. Complete clinicopathological data are shown in Table 1. Strong and diffuse WWOX immunoreactivity was observed in 10 patients with normal bladder urothelium as well as in five with squamous metaplasia, all of them without tumour. Subsequently, this pattern of reactivity was utilized as an external positive control for the evaluation of WWOX protein expression in bladder cancer patients. WWOX immunoreactivity was stratified in tumour cases into three different categories according to the level of WWOX protein expression: low level (26 cases, 25.7%), moderate (25 cases, 24.8%) and high (50 cases, 49.5%) (Table 2; Figures 1A,B and 2A–D). In addition, a total absence of WWOX protein expression could be observed in four cases (4%), all of which were urothelial carcinomas with extensive squamous metaplasia (Figure 3A,B). A reduced level of WWOX expression was also identified in areas of secondary urothelial carcinoma in situ accompanying high-grade urothelial carcinoma in 13 cases (13%), which was similar to that observed in the remaining HGUC. Within the specific subgroup of HGUC (pure urothelial plus urothelial carcinomas with extensive squamous metaplasia), only 23 patients (33.3%) showed a reduced level of WWOX protein expression, whereas 15 (21.7%) and 31 (44.9%) showed moderate or intense immunoreactivity, respectively. However, up to 13 out of 31 pT2 carcinomas (41.9%) presented a low level of WWOX protein expression as well as 20 out of 54 (37%) of all invasive carcinomas (pT1 plus pT2). In contrast, seven out of eight (87.5%) PUNLMP and 12 out of 24 (50%) LGPUC presented intense WWOX immunoexpression. Similarly, higher levels of WWOX protein immunoreactivity were observed in 28 out of 47 (59.6%) non-invasive neoplasms (pTa tumours) when compared with 11 out of 23 (47.8%) pT1 carcinomas in which the level of WWOX reactivity was significantly lower (P = 0.002).
Table 1.
Clinicopathological patient data and treatment strategies
| Mean patient age | 68.1 years (range 38–91) |
|
| |
| Gender (male / female) | 86 (85.2%)/15 (14.9%) |
|
| |
| Secondary carcinoma in situ | 13 (12.9%) |
|
| |
| Mean follow-up | 42.2 months (range 15–60) |
|
| |
| Papillary / sessile or ulcerated | 77 (76.2%)/24 (23.8%) |
|
| |
| Treatment strategies | |
| TUR | 39 (38.6%) |
|
| |
| TUR + MMC | 22 (21.8%) |
|
| |
| TUR + BCG | 11 (10.9%) |
|
| |
| TUR + CT | 9 (8.9%) |
|
| |
| Radical cystectomy | 15 (14.9%) |
|
| |
| Radical cystectomy + CT | 5 (5%) |
TUR, transurethral resection; MMC, mytomycin; BCG, bacillus Calmette–Guérin; CT, chemotherapy.
Table 2.
Correlation of WWOX protein expression with classical prognostic factors such as histological grade, tumour stage, tumour size, multiplicity, and with predictive factors related to recurrent and progressive disease
| WWOX protein expression level
|
Total | P-value | |||
|---|---|---|---|---|---|
| Low | Moderate | High | |||
|
| |||||
| Grade | |||||
| PUNLMP | 0 | 1 (12.5%) | 7 (87.5%) | 8 (100%) | |
|
| |||||
| LGPUC | 3 (12.5%) | 9 (37.5%) | 12 (50%) | 24 (100%) | |
|
| |||||
| HGPUC | 19 (29.7%) | 14 (21.9%) | 31 (48.4%) | 64 (100%) | 0.012* |
|
| |||||
| UCSM | 4 (80%) | 1 (20%) | 0 (0%) | 5 (100%) | 0.001 |
|
| |||||
| Stage | |||||
| pTa | 6 (12.8%) | 13 (27.7%) | 28 (59.6%) | 47 (100%) | |
|
| |||||
| pT1 | 7 (30.4%) | 5 (21.7%) | 11 (47.8%) | 23 (100%) | 0.002 |
|
| |||||
| pT2–4 | 13 (42%) | 7 (22.6%) | 11 (35.5%) | 31 (100%) | |
|
| |||||
| Size, mm | |||||
| <10 | 2 (25%) | 0 (0%) | 6 (75%) | 8 (100%) | |
|
| |||||
| 10–30 | 8 (17.4%) | 14 (30.4%) | 24 (52.2%) | 46 (100%) | |
|
| |||||
| >30 | 16 (34%) | 11 (23.4%) | 20 (42.6%) | 47 (100%) | 0.04 |
|
| |||||
| Multiplicity | |||||
| No | 13 (24.5%) | 10 (18.9%) | 31 (57.4%) | 54 (100%) | |
|
| |||||
| Yes | 13 (27.1%) | 15 (31.6%) | 19 (40.4%) | 47 (100%) | 0.11 |
|
| |||||
| Recurrence | |||||
| No | 17 (25%) | 17 (25%) | 34 (50%) | 68 (100%) | |
|
| |||||
| Yes | 9 (27.3%) | 8 (24.2%) | 16 (48.5%) | 33 (100%) | 0.416 |
|
| |||||
| Progression | |||||
| No | 15 (19.7%) | 21 (27.6%) | 40 (52.6%) | 76 (100%) | |
|
| |||||
| Yes | 11 (44%) | 4 (16%) | 10 (40%) | 25 (100%) | 0.028 |
|
| |||||
| N+ | |||||
| No | 16 (22.2%) | 18 (25%) | 39 (53.4%) | 73 (100%) | |
|
| |||||
| Yes | 10 (35.7%) | 7 (25%) | 11 (39.3%) | 28 (100%) | 0.067 |
|
| |||||
| M+ | |||||
| No | 23 (24.5%) | 25 (26.6%) | 46 (48.9%) | 94 (100%) | |
|
| |||||
| Yes | 3 (42.9%) | 0 (0%) | 4 (57.1%) | 7 (100%) | 0.379 |
|
| |||||
| Treatment | |||||
| TUR alone | 7 (18%) | 11 (28.2%) | 21 (53.8%) | 39 (100%) | |
|
| |||||
| TUR + AT | 8 (19%) | 11 (26.2%) | 23 (54.8%) | 42 (100%) | |
|
| |||||
| Cystectomy | 11 (55%) | 3 (15%) | 6 (30%) | 20 (100%) | 0.024 |
|
| |||||
| Total | 18 (17.8%) | 33 (32.7%) | 50 (45.5%) | 101 (100%) | |
PUNLMP, Papillary urothelial neoplasms of low malignant potential; LGPUC, low-grade papillary urothelial carcinomas; HGPUC, high-grade papillary urothelial carcinomas; UCSM, urothelial carcinomas with squamous metaplasia; L+, lymph node metastases; M+, systemic metastases; P-value, PCA and Kendall’s correlation coefficient; AT, adjuvant therapy.
When HGPUC plus UCSM are grouped into the same category of high-grade urothelial carcinomas, then the P-value is 0.012 when compared with PUNLMP, although no significant differences with LGPUC were observed.
Figure 1.
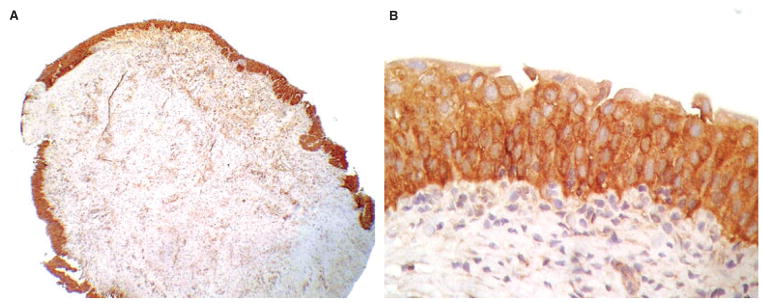
Non-neoplastic urothelium showing intense WWOX protein expression.
Figure 2.
Note the intense WWOX immunoreactivity in low-grade papillary urothelial neoplasms (A, B) in comparison with the weak immunoexpression observed in invasive high-grade urothelial carcinomas (C, D).
Figure 3.
Urothelial carcinomas with extensive squamous metaplasia displayed a complete absence of WWOX protein expression.
The mean follow-up was 42.2 months (range 15–60 months) and during this period 33 patients (32.7%) presented recurrent disease, 25 (24.8%) showed tumour progression in grade and / or stage and 19 (18.8%) died of their disease. Lymph node and distant metastases were observed in 28 (27.7%) and seven patients (6.9%), respectively.
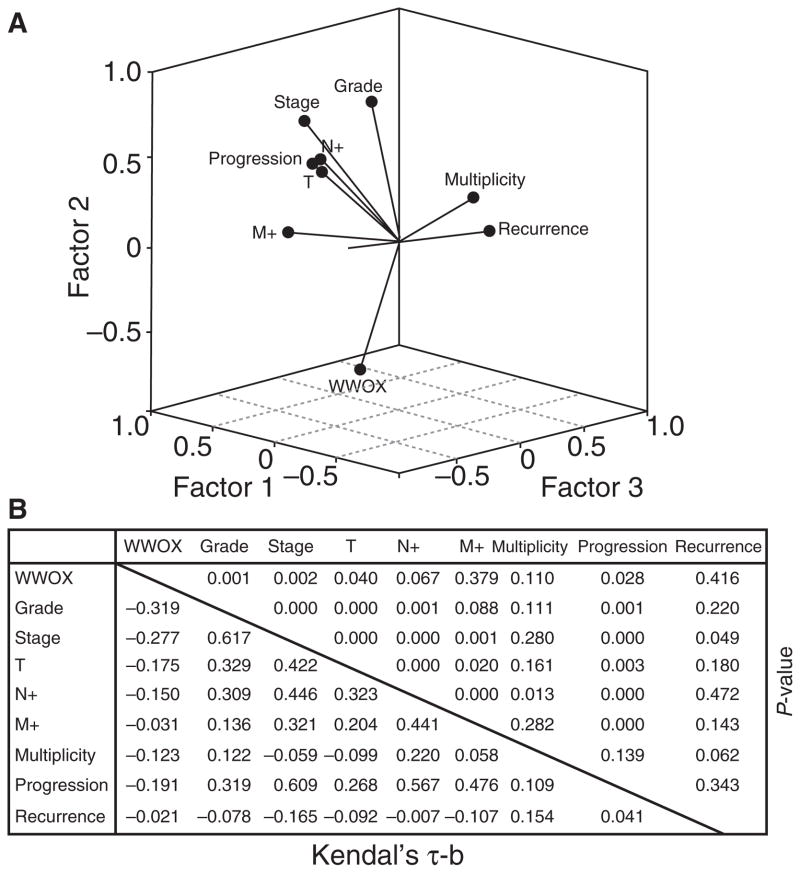
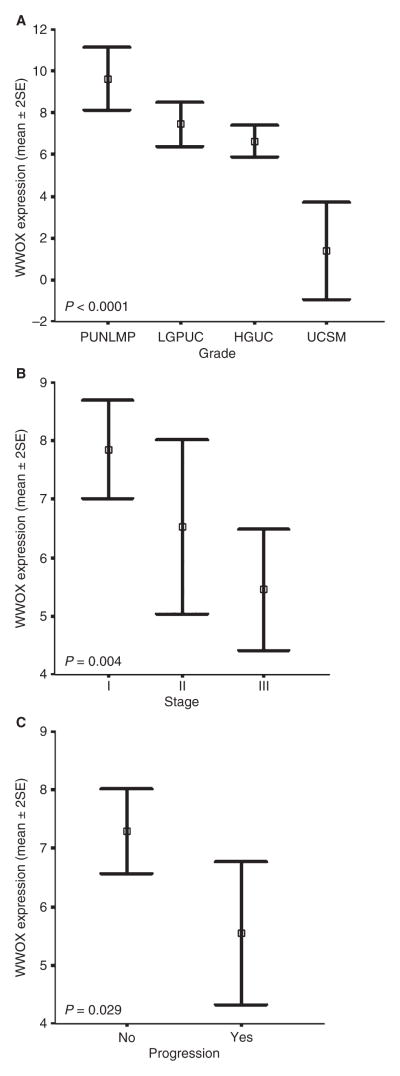
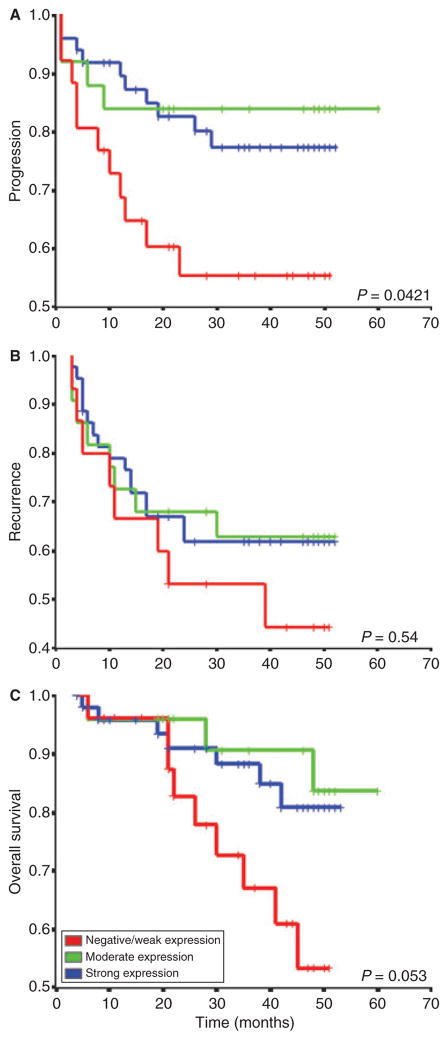
Multivariate analysis demonstrated that many variables were statistically associated, and in the case of WWOX protein expression an inverse correlation with histological grade (P = 0.001), tumour stage (P = 0.002), tumour size (P = 0.040) and tumour progression (P = 0.028) could be observed (Figure 4A,B). In univariate statistical studies, the level of WWOX protein expression was significantly different between PUNLMP and HGUC (P = 0.012), and especially between UCSC and all the remaining pure urothelial tumours (P = 0.001). As regards tumour stage, significant differences between non-invasive tumours and muscle-invading carcinomas were also observed (P = 0.003) (Figure 5A,B). Similarly, significant differences in terms of tumour progression were also observed between those cases showing the lowest levels of WWOX protein expression and those with high levels of WWOX immunoexpression (P = 0.029) (Figure 5C). In contrast, WWOX protein levels did not correlate with tumour multiplicity (P = 0.11) or metastatic disease (N orMstatus) (P = 0.067 and P = 0.379, respectively). In survival studies, Kaplan–Meier estimates corroborated a significant correlation between the loss of WWOX protein immunoreactivity and a shorter PFS (Figure 6A). However, no statistical association could be demonstrated regarding recurrence-free survival and OS (Figure 6B). Moreover, loss of WWOX protein immunoexpression was of borderline significance for the prediction of OS (P = 0.053) (Figure 6C).
Figure 4.
Principal component analysis (PCA) of 101 bladder tumours analysed. Note the inverse correlation of WWOX protein expression in comparison with the remaining variables. Component matrix showing correlation (Kendal’s τ-b) and statistical significances (P-values) of PCA study. All P-values are included.
Figure 5.
anova graphs show the statistical correlation of grade (A) stage (B) and tumour size (C) with respect to WWOX expression levels in univariate studies.
Figure 6.
Kaplan–Meier plot analysis showing the prognostic value of WWOX protein expression levels in predicting tumour progression (A), recurrence (B) and overall survival (C). Log rank P-values are indicated.
Discussion
The WWOX gene encodes a 414-amino acid protein which displays two main peptide domains: a region at its NH2 terminus containing two WW domains and a centrally located oxidoreductase (OX) domain. By analogy with other WW domain-containing proteins, WWOX is considered to play an important role in protein–protein interactions.6 The high expression of the WWOX gene and protein, particularly in hormonally regulated tissues such as testis, ovary, prostate and breast, has led Bednarek et al. to speculate on the possibility that this gene could play an important role in steroid-receptor cell signalling.6 In this regard, inhibition of tumorigenicity of MDA-MB-435 breast cancer cells in vivo has been revealed with WWOX gene restoration.8 Similar findings have been recently described by Qin et al. in prostate cells and tissues and by Fabbri et al. in lung cancer cell lines.11,20 In agreement with some of the above-mentioned studies, immunohistochemistry of WWOX protein in our study displayed intense granular cytoplasmic reactivity with a perinuclear enhancement of the reaction probably localized to the Golgi apparatus. Likewise, as previously described, no immunoreactivity could be detected in cell nuclei of normal or tumour cells.8,21 Intriguingly, loss of WWOX protein expression has been shown to be associated with loss of oestrogen, progesterone and androgen receptors in breast, ovarian and prostatic carcinomas.8,10,11 In particular, in prostatic cancer series up to 84% of the cases presented a remarkable reduction in WWOX immunoexpression.11 In ovarian tumours, loss of WWOX protein expression correlated with histological tumour type and tumour stage; and presented, furthermore, a strong statistical correlation with shorter RFS of patients.11 Furthermore, several in vitro and in vivo studies have provided additional evidence supporting the putative role of WWOX as a tumour suppressor gene in non-hormonally regulated tumour such as liver, gastric, pancreatic, oesophageal, lung, bladder and oral cancers,3,12,16,17,20,22 where a remarkable loss of WWOX protein expression has been demonstrated by means of Western blot, Southern blot, quantitative reverse transcriptase-PCR and immunohistochemistry, although by immunohistochemical methods it could be demonstrated only in a rather small series of patients. In our study, the level of WWOX protein immunoexpression was found to be highly heterogeneous in primary urinary bladder neoplasms.
It is noteworthy that bladder tumours are not known to be greatly influenced by hormonal receptor status. In fact, only a few studies on bladder neoplasms have demonstrated prognostic correlation regarding alterations in androgen or oestrogen receptor status.22–24 Importantly, loss of WWOX protein expression was strongly associated in our series with tumour grade. In addition, correlation was also seen with classical clinicopathological prognostic markers such as stage, which further suggests that WWOX could play a significant role in both bladder cancer progression and tumour differentiation. In this regard, it is well known that, in general, histological grading is not mainly based on molecular expression profiles. However, a strong inverse statistical association between tumour differentiation (grade) and WWOX protein immunoexpression is noteworthy. The possibility exists, however, that alterations affecting the WWOX gene and protein expression are consequential to other genetic alterations rather than being a primary causative factor in bladder carcinogenesis.25
In addition to the significant loss of WWOX protein expression in high-grade and muscle-invasive bladder carcinomas, complete loss of WWOX immunoexpression could be specifically observed in a subset of high-grade urothelial carcinomas with extensive squamous metaplasia. In agreement with this observation, a dramatic decrease in WWOX protein and mRNA expression has been previously reported in some series of pure squamous cell carcinomas of the lung, oral mucosa and oesophagus.3,15,18 Unfortunately, the number of urothelial carcinomas with extensive squamous metaplasia was not high enough for a more robust statistical analysis. Similarly, WWOX expression was not statistically linked to nodal disease, although the number of patients was not high enough for robust statistical analysis.
In summary, WWOX protein immunoexpression is strongly associated with classical clinicopathological factors such as histological grade, tumour stage and tumour size. Furthermore, progressive loss of WWOX protein expression appears to be useful in predicting progressive disease in urothelial bladder carcinoma. Nevertheless, future studies with larger series of patients undergoing similar treatments are necessary to determine the true value of WWOX protein assessment as a useful prognostic biomarker in bladder cancer patients.
Acknowledgments
This work was partially supported by the European Urology Program Grant from the European Association of Urology (2003) and by the “Fundación para la Investigación en Urología” from the Spanish Urological Association. The technical assistance of Laura Martínez and Alejo Sempere is gratefully acknowledged.
Abbreviations
- HGUC
high-grade urothelial carcinoma
- LGPUC
low-grade papillary urothelial carcinoma
- OS
overall survival
- PFS
progression-free survival
- PUNLMP
papillary urothelial neoplasms of low malignant potential
- RFS
relapse-free survival
- TUR
transurethral resection
- UCSC
urothelial carcinoma with squamous metaplasia
- WHO
World Health Organization
References
- 1.Hetch F, Glover TW. Cancer chromosome breakpoints and common fragile sites induced by aphidicolin. Cancer Genet Cytogenet. 1984;13:185–188. doi: 10.1016/0165-4608(84)90060-8. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Darling J, Zhang JS, et al. Frequent homozygous deletions in the FRA3B region in tumor cell lines still leave the FHIT exons intact. Oncogene. 1998;16:635–642. doi: 10.1038/sj.onc.1201576. [DOI] [PubMed] [Google Scholar]
- 3.Iliopoulos D, Guler G, Han SY, et al. Fragile genes as biomarkers: epigenetic control of WWOX and FHIT in lung, breast and bladder cancer. Oncogene. 2005;24:1625–1633. doi: 10.1038/sj.onc.1208398. [DOI] [PubMed] [Google Scholar]
- 4.Huebner K, Croce CM. FRA3B and other common fragile sites: the weakest links. Nat Rev Cancer. 2001;1:214–221. doi: 10.1038/35106058. [DOI] [PubMed] [Google Scholar]
- 5.Inoue H, Ishii H, Alder H, et al. Sequence of the FRA3B common fragile region: implications for the mechanism of FHIT deletion. Proc Natl Acad Sci USA. 1997;94:14584–14589. doi: 10.1073/pnas.94.26.14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3–24.1, a region frequently affected in breast cancer. Cancer Res. 2000;60:2140–2145. [PubMed] [Google Scholar]
- 7.Ludes-Meyers JH, Bednarek AK, Popesku NC, Bedford M, Aldaz CM. WWOX, the common chromosomal fragile site, FRA 16D, cancer gene. Cytogenet Genome Res. 2003;100:101–110. doi: 10.1159/000072844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bednarek AK, Keck-Waggoner CL, Daniel RL, et al. WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res. 2001;61:8068–8073. [PubMed] [Google Scholar]
- 9.Nunez MI, Ludes-Meyers J, Abba MC, et al. Frequent loss of WWOX expression in breast cancer: correlation with estrogen receptor status. Breast Cancer Res Treat. 2005;89:99–105. doi: 10.1007/s10549-004-1474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunez MI, Rosen DG, Ludes-Meyers J, et al. WWOX protein expression varies among ovarian carcinoma histotypes and correlates with less favourable outcome. BMC Cancer. 2005;5:64–74. doi: 10.1186/1471-2407-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin HR, Iliopoulos D, Semba S, et al. A role for the WWOX gene in prostate cancer. Cancer Res. 2006;66:6477–6481. doi: 10.1158/0008-5472.CAN-06-0956. [DOI] [PubMed] [Google Scholar]
- 12.Aqeilan RI, Kuroki T, Pekarsky Y, et al. Loss of WWOX expression in gastric carcinoma. Clin Cancer Res. 2004;10:3053–3058. doi: 10.1158/1078-0432.ccr-03-0594. [DOI] [PubMed] [Google Scholar]
- 13.Kuroki T, Trapasso F, Shiraishi T, et al. Genetic alterations of the tumor suppressor gene WWOX in esophageal squamous cell carcinoma. Cancer Res. 2002;62:2258–2260. [PubMed] [Google Scholar]
- 14.Kuroki T, Yendamuri S, Trapasso F, et al. The tumor suppressor gene WWOX at FRA16D is involved in pancreatic carcinogenesis. Clin Cancer Res. 2004;10:2459–2465. doi: 10.1158/1078-0432.ccr-03-0096. [DOI] [PubMed] [Google Scholar]
- 15.Kuroki T, Tajima Y, Furui J, Kanematsu T. Common fragile genes and digestive tract cancers. Surg Today. 2006;36:1–5. doi: 10.1007/s00595-005-3094-4. [DOI] [PubMed] [Google Scholar]
- 16.Paige AJW, Taylor KJ, Taylor C, et al. WWOX: A candidate tumor suppressor gene involved in multiple tumor types. Proc Natl Acad Sci USA. 2001;98:11417–11422. doi: 10.1073/pnas.191175898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SW, Ludes-Meyers J, Zimonjic DB, Durkin ME, Popescu NC, Aldaz CM. Frequent down-regulation and loss of WWOX gene expression in human hepatocellular carcinoma. Br J Cancer. 2004;91:753–759. doi: 10.1038/sj.bjc.6602023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pimenta FJ, Gomes DA, Perdigao PF, et al. Characterization of the tumor suppressor gene WWOX in primary human oral squamous cell carcinomas. Int J Cancer. 2006;118:1154–1158. doi: 10.1002/ijc.21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and genetics of tumours of the urinary system and male genital organs. Geneva: WHO Press; 2004. WHO classification of tumours. [Google Scholar]
- 20.Fabbri M, Iliopoulos D, Trapasso F, et al. WWOX gene restoration prevents lung cancer growth in vitro and in vivo. Proc Natl Acad Sci USA. 2005;102:15611–15616. doi: 10.1073/pnas.0505485102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludes-Meyers JH, Kil H, Bednarek AK, Drake J, Bedford MT, Aldaz CM. WWOX binds the specific proline-rich ligand PPXY: identification of candidate interacting proteins. Oncogene. 2004;23:5049–5055. doi: 10.1038/sj.onc.1207680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen SS, Smith CL, Hsieh JT, et al. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer. 2006;15:2610–2616. doi: 10.1002/cncr.21945. [DOI] [PubMed] [Google Scholar]
- 23.Boorjian S, Ugras S, Mongan NP, et al. Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology. 2004;64:383–388. doi: 10.1016/j.urology.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Croft PR, Lathrop SL, Feddersen RM, Joste NE. Estrogen receptor expression in papillary urothelial carcinoma of the bladder and ovarian transitional cell carcinoma. Arch Pathol Lab Med. 2005;129:194–199. doi: 10.5858/2005-129-194-EREIPU. [DOI] [PubMed] [Google Scholar]
- 25.Pluciennik E, Kusinska R, Potemski P, Kubiak R, Kordek R, Bednarek AK. WWOX, the FRA16D cancer gene: expression correlation with breast cancer progression and prognosis. Eur J Surg Oncol. 2006;32:153–157. doi: 10.1016/j.ejso.2005.11.002. [DOI] [PubMed] [Google Scholar]