Abstract
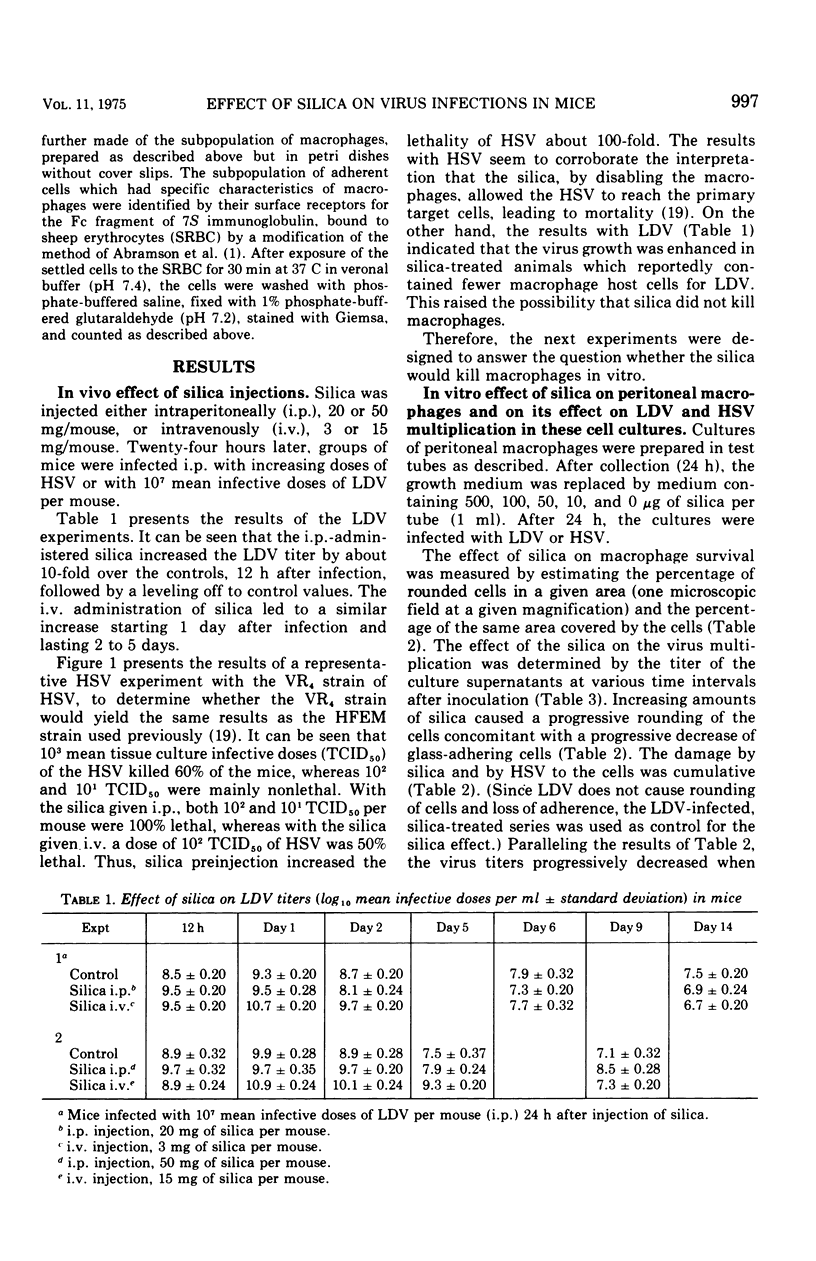
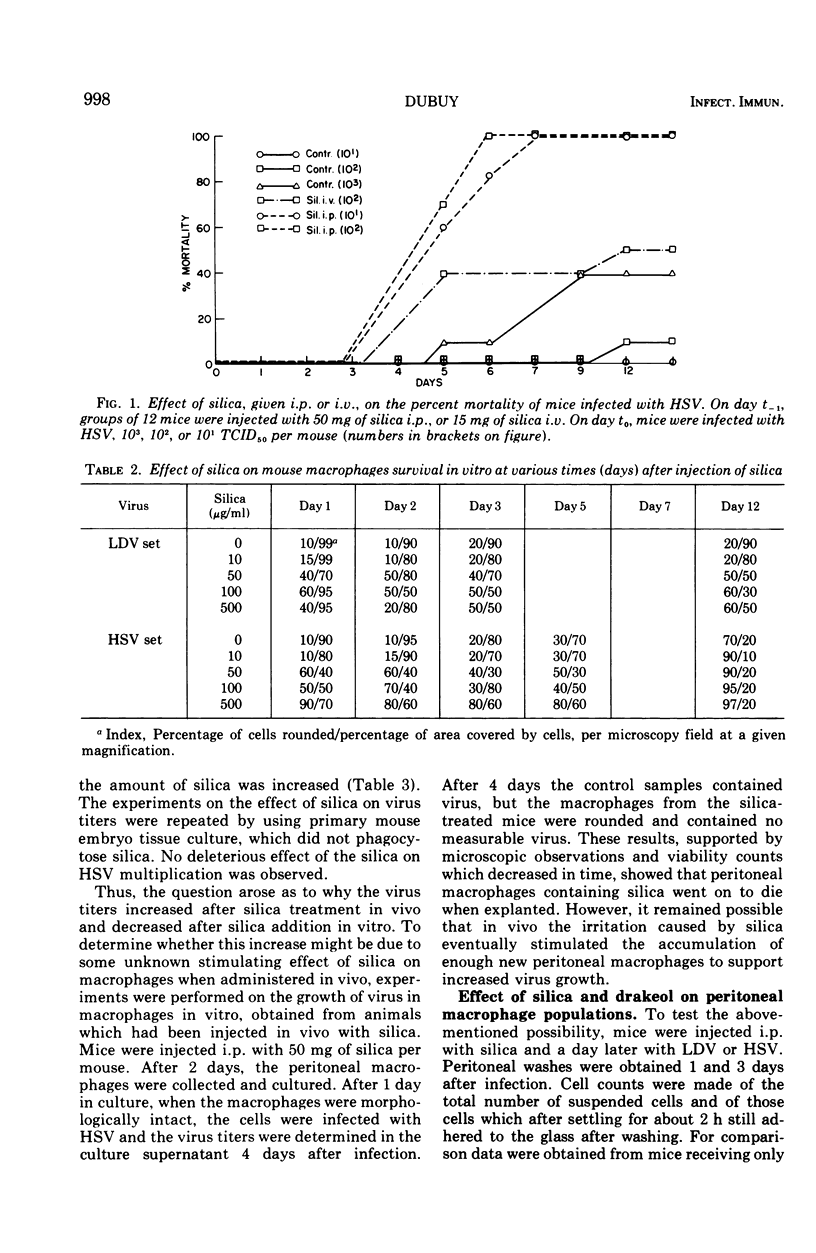
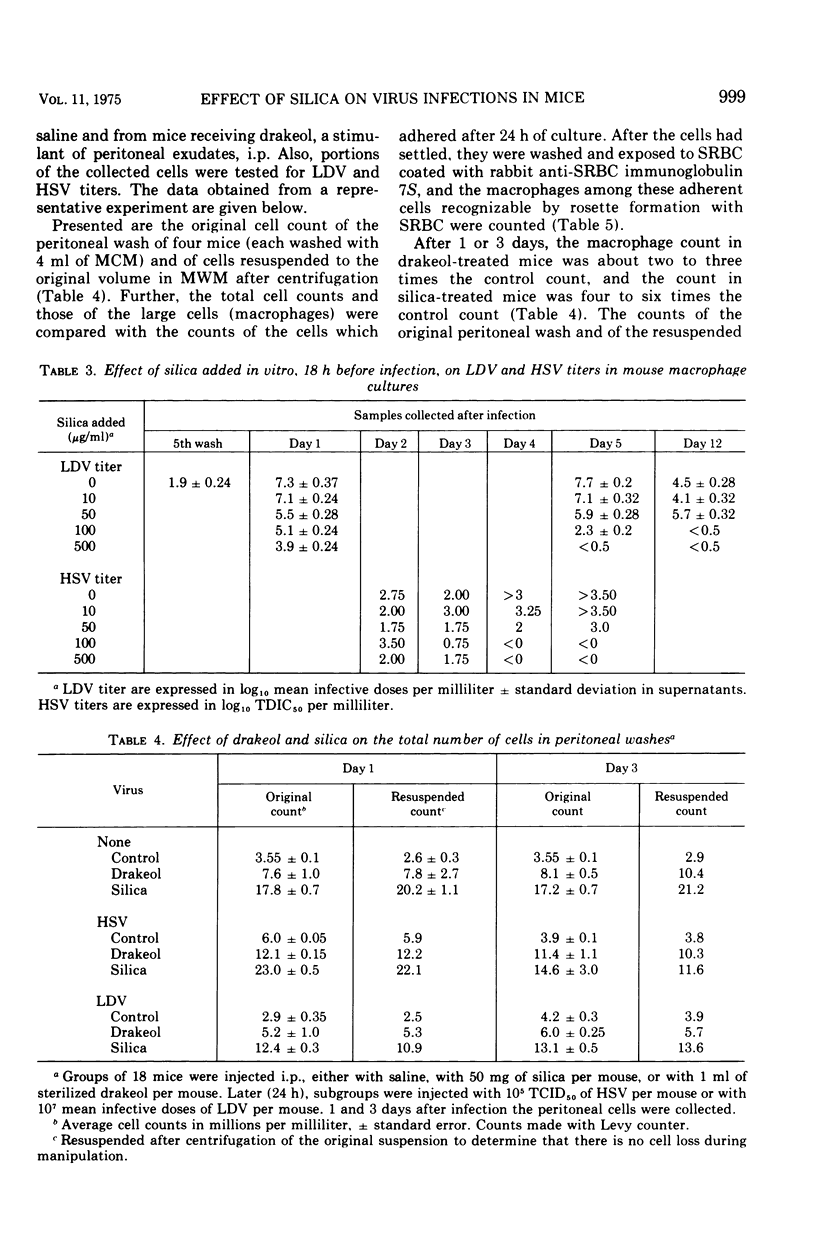
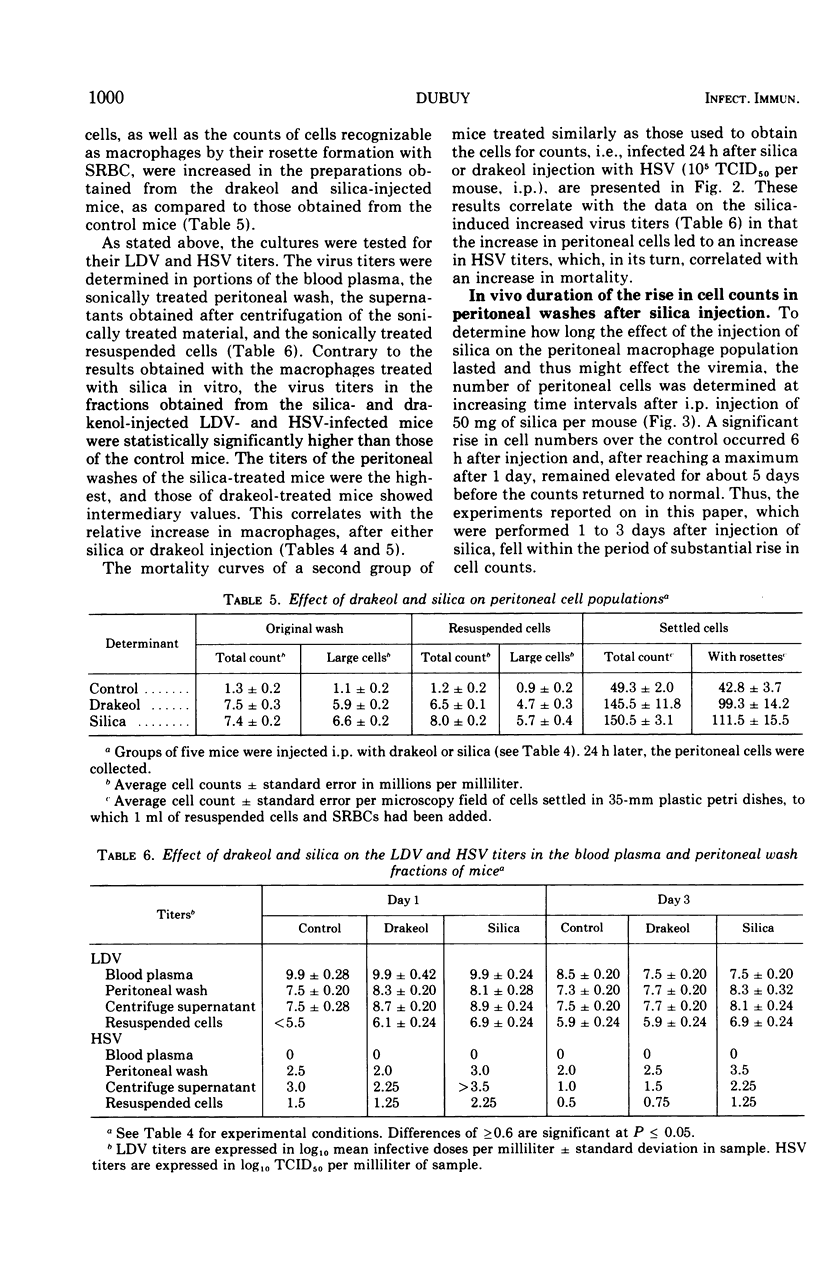
Silica injections of mice have been reported to kill macrophages, thus allowing herpes simplex virus (HSV) to spread rapidly and leading to an increased severity of HSV infection. Thus, silica presumably could be used to eliminate lactic dehydrogenase virus (LDV) (a model for slow viruses), which is known to multiply exclusively in macrophages. Contrary to expectation, it was found that the LDV titers were increased in silica-injected mice as compared to the titers in control mice. Counts of peritoneal cells at different periods after silica injection showed that silica-induced macrophage damage in vivo resulted in proliferation and migration of macrophages, thus providing additional target cells for LDV replication and leading to high LDV titers. In vitro, silica ingestion also damaged the macrophages, but since no replacement of cells could occur by infiltration, decreased LDV titers were found. Similar findings were obtained with HSV. It is suggested that all persistent viruses multiplying in macrophages will show a similar recrudescence under comparable conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson N., Gelfand E. W., Jandl J. H., Rosen F. S. The interaction between human monocytes and red cells. Specificity for IgG subclasses and IgG fragments. J Exp Med. 1970 Dec 1;132(6):1207–1215. doi: 10.1084/jem.132.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Buy H. G., Johnson M. L. Studies on the in vivo and in vitro multiplication of the LDH virus of mice. J Exp Med. 1966 Jun 1;123(6):985–998. doi: 10.1084/jem.123.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuy H., Baron S., Uhlendorf C., Johnson M. L. Role of interferon in murine lactic dehydrogenase virus infection, in vivo and in vitro. Infect Immun. 1973 Dec;8(6):977–984. doi: 10.1128/iai.8.6.977-984.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R., Salaman M. H. Studies on the mechanism of action of Riley virus. 3. Replication of Riley's plasma enzyme-elevating virus in vitro. J Exp Med. 1965 Nov 1;122(5):993–1002. doi: 10.1084/jem.122.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALLUCCI L., ALLISON A. C. LYSOSOMAL ENZYMES IN CELLS INFECTED WITH CYTOPATHIC AND NON-CYTOPATHIC VIRUSES. J Exp Med. 1965 Mar 1;121:477–485. doi: 10.1084/jem.121.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearsall N. N., Weiser R. S. The macrophage in allograft immunity. I. Effects of silica as a specific macrophage toxin. J Reticuloendothel Soc. 1968 Apr;5(2):107–120. [PubMed] [Google Scholar]
- Porter D. D., Porter H. G., Deerhake B. B. Immunofluorescence assay for antigen and antibody in lactic dehydrogenase virus infection of mice. J Immunol. 1969 Feb;102(2):431–436. [PubMed] [Google Scholar]
- Snodgrass M. J., Lowrey D. S., Hanna M. G., Jr Changes induced by lactic dehydrogenase virus in thymus and thymus-dependent areas of lymphatic tissue. J Immunol. 1972 Apr;108(4):877–892. [PubMed] [Google Scholar]
- Stanton M. F., Wrench C. Mechanisms of mesothelioma induction with asbestos and fibrous glass. J Natl Cancer Inst. 1972 Mar;48(3):797–821. [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]



