Significance
Tumor development is impacted by a set of diverse infiltrating leukocyte populations that can either inhibit or, paradoxically, enhance tumor cell growth. This study characterizes a cellular cross-talk between γδ T lymphocytes and small peritoneal macrophages (SPMs) that is mediated by the proinflammatory cytokine, IL-17, and promotes ovarian cancer growth. IL-17 is preferentially produced by a population of γδ T cells, displaying a distinctive CD27(−) Vγ6(+) phenotype, that strongly proliferate in response to tumor challenge. This associates with the mobilization of SPMs that express protumor and proangiogenic molecular mediators upregulated by IL-17. Critically, these SPMs can directly enhance ovarian cancer cell growth. Our work identifies an IL-17–dependent γδ T cell/SPM axis that promotes tumor development and thus counteracts cancer immunosurveillance.
Keywords: gamma-delta T cells, tumor immunology
Abstract
Cancer-associated inflammation mobilizes a variety of leukocyte populations that can inhibit or enhance tumor cell growth in situ. These subsets include γδ T cells, which can infiltrate tumors and typically provide large amounts of antitumor cytokines, such as IFN-γ. By contrast, we report here that in a well-established transplantable (ID8 cell line) model of peritoneal/ovarian cancer, γδ T cells promote tumor cell growth. γδ T cells accumulated in the peritoneal cavity in response to tumor challenge and could be visualized within solid tumor foci. Functional characterization of tumor-associated γδ T cells revealed preferential production of interleukin-17A (IL-17), rather than IFN-γ. Consistent with this finding, both T cell receptor (TCR)δ-deficient and IL-17–deficient mice displayed reduced ID8 tumor growth compared with wild-type animals. IL-17 production by γδ T cells in the tumor environment was essentially restricted to a highly proliferative CD27(−) subset that expressed Vγ6 instead of the more common Vγ1 and Vγ4 TCR chains. The preferential expansion of IL-17–secreting CD27(−) Vγ6(+) γδ T cells associated with the selective mobilization of unconventional small peritoneal macrophages (SPMs) that, in comparison with large peritoneal macrophages, were enriched for IL-17 receptor A, and for protumor and proangiogenic molecular mediators, which were up-regulated by IL-17. Importantly, SPMs were uniquely and directly capable of promoting ovarian cancer cell proliferation. Collectively, this work identifies an IL-17–dependent lymphoid/myeloid cross-talk involving γδ T cells and SPMs that promotes tumor cell growth and thus counteracts cancer immunosurveillance.
Developing tumors are infiltrated by a variety of leukocyte subsets that can either promote or inhibit inflammation, and thus impact on cancer progression (1). Among such populations are γδ T cells, which are major players in lymphoid stress surveillance likely due to their recognition of stress-inducible molecules independently of MHC-mediated antigen presentation (2). Moreover, abundant IFN-γ secretion and cytotoxic effector functions endow γδ T cells with potent antitumor activity. This has been clearly documented in murine models of spontaneous (3), chemically induced (4), transgenic (5), and transplantable (6, 7) tumors. For example, in the widely used B16 melanoma model, γδ T cells were shown to infiltrate tumors very early and provided a critical source of IFN-γ that significantly delayed tumor growth (6, 7).
Human γδ T cells also possess IFN-γ–secreting potential, which is displayed immediately at birth (8) and display cytotoxicity against tumor lines of diverse origin, including epithelial (9, 10) and hematological (11, 12) tumors. This has prompted the development of cancer clinical trials targeting γδ T cells, which have produced encouraging, albeit highly variable, degrees of therapeutic responses (13–15). There is therefore great interest in maximizing the antitumor functions of γδ T cells for cancer immunotherapy.
Despite these highly promising reports, a clinical study on breast cancer tissue revealed a surprising inverse correlation between infiltrating γδ T cells and overall patient survival (16). In fact, γδ T cells represented the most significant independent prognostic factor for assessing severity of breast cancer (16). Similarly, a recent report on colorectal cancer showed a positive correlation between clinopathological parameters and the infiltration of γδ T cells specifically producing interleukin-17 (IL-17) (17). A tumor-promoting function of γδ T cells was also suggested in murine fibrosarcoma (18) and hepatocellular carcinoma (19) models, in which γδ T cells were the major cellular source of IL-17, which was required for optimal tumor growth in vivo. These data raise the interesting question as to whether distinct functional attributes of γδ T cells, for example differential cytokine production, may associate with markedly different outcomes for tumor growth.
Along these lines, we have pioneered the identification of two distinct functional subsets of murine γδ T cells based on the expression levels of the CD27 coreceptor (20). We showed that robust IFN-γ production is associated with the CD27(+) phenotype, whereas secretion of IL-17 is restricted to CD27(−) γδ T cells. This dichotomy of hard-wired commitment to specific cytokine production is established during thymic development and maintained during the immune response to various infection agents (21, 22). Thus, the overall impact of γδ T cells in a given disease may depend on the balance between distinct proinflammatory effector cell subsets.
Building on these foundations, we have here analyzed the overall and subset-specific contributions of γδ T cells to a well-established murine syngeneic model of ovarian cancer (ID8; transplantable cell line) that has a strong inflammatory component (23–25), akin to that observed in human patients with high-grade serous ovarian cancer (25, 26). In this murine model, we demonstrate that γδ T cells are major sources of IL-17, and both T cell receptor (TCR)δ-deficient and IL-17–deficient mice display reduced ID8 tumor growth. Interestingly, IL-17 production by γδ T cells in the tumor environment is essentially restricted to a CD27(−) subset that does not express the commonly used Vγ1 or Vγ4 TCR chains, but rather Vγ6; these Vγ6(+) cells are highly biased toward IL-17 production, in contrast to their IFN-γ–producing Vγ1(+) and Vγ4(+) counterparts. The ID8 tumor environment gets progressively enriched in the IL-17–promoting factor IL-7, whose receptor is highly expressed on Vγ6(+) cells. This associates with preferential Vγ6(+) cell proliferation and accumulation of IL-17 in the tumor bed, which in turn induces the mobilization of (recently described) small peritoneal macrophages (SPMs) that are enriched in IL-17 receptor A (IL-17RA) and in protumor and proangiogenic molecular mediators. Importantly, in comparison with large peritoneal macrophages (LPMs), SPMs can strongly and directly promote ovarian cancer cell proliferation. In summary, our work identifies an IL-17–dependent γδ T-cell/SPM axis that promotes tumor cell growth and thus opposes the widely accepted antitumor (and IFN-γ mediated) function of γδ T cells.
Results
γδ T Cells Infiltrate ID8 Tumors and Promote Ovarian Cancer Cell Growth in Vivo.
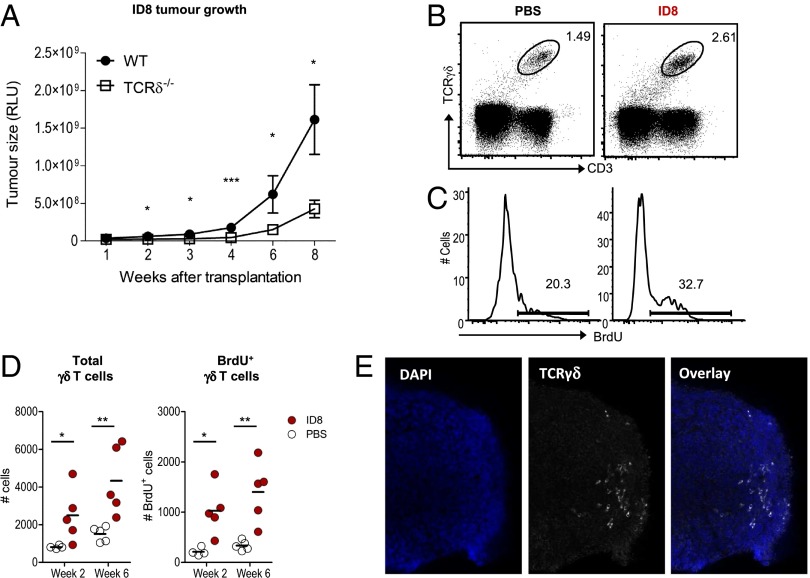
To dissect the role of proinflammatory γδ T cells in tumor progression in vivo, we built on an observation made with the transplantable syngeneic ID8 murine ovarian cancer model. In these experiments, we implanted ID8 tumors into the peritoneal cavities of TCRδ−/− mice and compared them with WT (C57BL/6) controls. We observed significantly decreased tumor load in the absence of γδ T cells along the course of tumor development (Fig. 1A). Flow cytometric analysis of peritoneal exudates from WT animals confirmed that γδ T cells were a sizeable component of the tumor microenvironment (Fig. 1B) and were actively proliferating (5-bromo-2-deoxyuridine, BrdU+) in situ (Fig. 1C), which resulted in an accumulation during tumor progression (Fig. 1D). Moreover, optimized confocal microscopy protocols allowed us to clearly detect γδ T cells infiltrating solid tumor foci (Fig. 1E and Fig. S1). These data suggest that γδ T cells have the capacity to significantly promote ovarian cancer growth in vivo.
Fig. 1.
γδ T cells infiltrate ID8 tumors and enhance ovarian cancer cell growth in vivo. (A) ID8 tumor growth in C57BL/6 WT (n = 6) and TCRδ−/− female mice (n = 8), measured by luciferase bioluminescence at the indicated weeks posttransplantation. (B) Representative FACS plots for γδ T cells in peritoneal exudates of ID8-bearing mice or PBS controls (at week 6 postinoculation). (C and D) Absolute numbers of total and BrdU+ γδ T cells in ID8-bearing mice or PBS controls. BrdU was provided during a period of 2 wk before analysis. Each dot represents one animal. (E) Representative immunofluorescence imaging of γδ T cells in ID8 tumor foci. Data are representative of three independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001.
γδ T Cells Are Major Providers of IL-17, Which Enhances Tumor Growth.
Given that ovarian cancer growth is orchestrated by a dynamic inflammatory cytokine network (23, 26), and that γδ T cells can produce large amounts of IFN-γ and IL-17 (20, 27, 28), we investigated cytokine production by γδ T cells during ID8 ovarian tumor progression. We observed an interesting pattern between earlier (week 2) and later (week 6) stages of tumor development; IFN-γ–producing γδ T cells tended to decrease, whereas IL-17 producers accumulated, both proportionally and in absolute numbers (Fig. 2 A and B). This dynamic behavior of IL-17(+) versus IFN-γ(+) γδ T cells appeared to result from proliferation in situ, as incorporation of BrdU increased in the former (but not in the latter) subset as the tumor progressed (Fig. 2C). This raised the hypothesis that the γδ T-cell–dependent growth of large tumor masses (from week 6 onward; Fig. 1A) could be associated with IL-17 production.
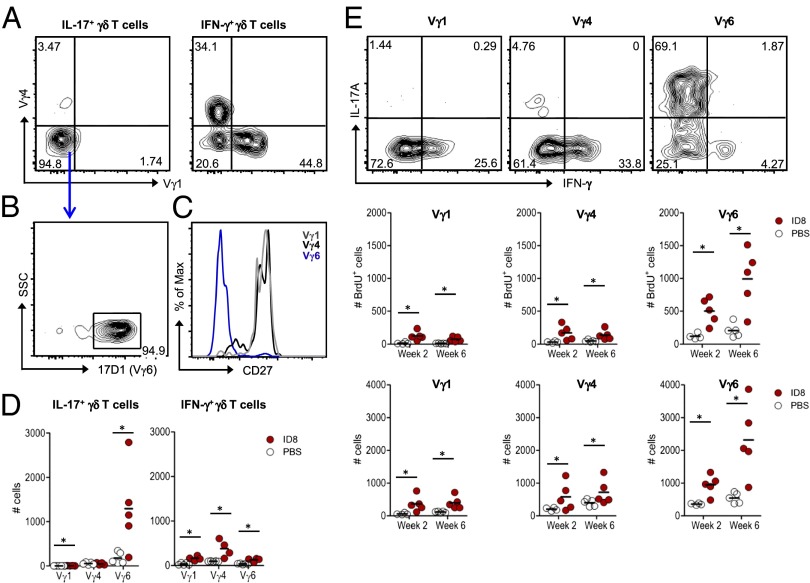
Fig. 2.

γδ T cells are major providers of IL-17A, which promotes ovarian cancer cell growth. (A) Representative FACS plots of intracellular IL-17A and IFN-γ stainings in γδ T cells isolated from the peritoneal cavity at weeks 2 and 6 post-ID8 tumor cell inoculation. (B) Absolute numbers of IL-17(+) or IFN-γ(+) γδ T cells at weeks 2 and 6 after inoculation of PBS or ID8 tumor cells. Each dot represents one animal. (C) Total numbers of BrdU(+) cells within IL-17A(+) or IFN-γ(+) γδ T subsets. BrdU was provided for a period of 2 wk before analysis. (D) Representative plot, absolute numbers, and mean fluorescence intensity (MFI) for IL-17 in total IL-17(+) cells and respective contributions of CD4 and γδ T cells in the peritoneal cavity of tumor-bearing mice (n = 5) at weeks 2 and 6 postinoculation. (E) MFI for IL-17 in total IL-17(+) cells in peritoneal exudates of WT or TCRδ−/− tumor-bearing mice (n = 5) at weeks 2 and 6 postinoculation. (F) ID8 tumor growth in C57BL/6 WT and IL-17A−/− mice, measured by luciferase bioluminescence. Statistical analysis was performed using the Mann–Whitney test. Data are representative of two to four independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001.
Because activated CD4(+) T-helper cells can also be potent producers of IL-17, including in the ID8 model (23), we next compared the relative contribution of these cells and γδ T cells to the pool of IL-17–secreting cells during tumor progression. This revealed comparable numbers of IL-17–secreting CD4(+) and γδ T cells at each of the time points studied (Fig. 2D). However, γδ T cells expressed significantly higher levels of IL-17 on a per cell basis (Fig. 2D). These data suggest that a major functional potential of peritoneal γδ T cells during ID8 tumor development is the production of IL-17. Consistent with this, in the absence of γδ T cells (in TCRδ−/− mice), total IL-17–producing cells displayed reduced IL-17 levels, particularly at 6 wk of tumor development (Fig. 2E). To formally test whether IL-17 is playing a protumor role in this model, we compared tumor growth in IL-17−/− and WT mice. We observed a clear arrest in tumor growth after 6 wk in IL-17−/− hosts, which contrasted the continuous tumor growth in WT (Fig. 2F). Collectively, these data implicate γδ T cells producing IL-17 in the promotion of ovarian cancer cell growth in vivo.
IL-17 Production by γδ T Cells Is Restricted to the CD27(−) Vγ6(+) Subset.
Functional γδ T-cell subsets are commonly defined based on TCRγ variable (Vγ) chain use and/or CD27 expression (27). Thus, we assessed these parameters to further characterize the IL-17–producing γδ T cells associated with ID8 tumor cell growth in vivo. In contrast to their IFN-γ(+) counterparts, the ID8-induced IL-17(+) γδ T cells did not bear the frequently used Vγ1 or Vγ4 chains (Fig. 3A). As these IL-17(+) γδ T cells also did not stain with antibodies against Vγ5 or Vγ7, we used a protocol that combines the GL3 (anti-TCRγδ) and 17D1 (anti-Vγ5Vδ1) monoclonal antibodies to stain for Vγ6(+) γδ T cells (29) (Fig. S2 A and B). This demonstrated that >90% of IL-17(+) γδ T cells expressed Vγ6 (Fig. 3B) and were also characterized by the absence of CD27 expression (Fig. 3C). Thus, IL-17 production in this ovarian cancer model is essentially confined to a distinctive Vγ6(+) γδ T-cell subset that selectively expands in response to tumor challenge, unlike its Vγ1(+) and Vγ4(+) counterparts (Fig. 3D and Fig. S2C).
Fig. 3.
IL-17 production by γδ T cells is essentially restricted to a distinctive CD27(−) Vγ6(+) cell subset. Peritoneal exudates were analyzed at week 2 and week 6 postinoculation of ID8 cells or PBS. (A) Representative FACS plots of Vγ1 and Vγ4 stainings within IL-17A(+) or IFN-γ(+) γδ T cells in tumor-bearing mice (at week 6). (B) FACS staining with GL3 and 17D1 monoclonal antibodies to detect Vγ6(+) γδ T cells. (C) Histogram overlay of CD27 staining in Vγ1, Vγ4, and Vγ6 subsets of γδ T cells in tumor-bearing mice (at week 6). (D) Absolute numbers of Vγ1, Vγ4, and Vγ6 subsets of IL-17A(+) or IFN-γ(+) γδ T cells in ID8 tumor-bearing mice or in PBS-injected controls (at week 6). Each dot represents one animal. (E) Representative intracellular IL-17A and IFN-γ stainings (Top), BrdU incorporation (Middle), and absolute numbers (Bottom) of Vγ1, Vγ4, and Vγ6 subsets of γδ T cells in ID8 tumor-bearing mice or in PBS-injected controls (at weeks 2 and 6). Data are representative of three independent experiments; *P < 0.05.
A further examination of Vγ6(+) γδ T cells showed they were highly biased toward IL-17 production, unlike Vγ1(+) or Vγ4(+) γδ T cells, which essentially made only IFN-γ (Fig. 3E, Top). Moreover, by performing BrdU incorporation experiments, we were able to observe preferential proliferation (Fig. 3E, Middle) and accumulation (Fig. 3E, Bottom) of Vγ6(+) γδ T cells in tumor-bearing mice, in comparison with controls. This raised the question as to which molecular cue was driving the expansion of IL-17–producing Vγ6(+) γδ T cells during tumor progression. To address this question, we quantified various cytokines in peritoneal exudates collected at week 2 and week 6 of tumor development. Within a general tendency for accumulation of type-17–driving cytokines (such as IL-1β or IL-6), IL-7, previously implicated in the selective expansion of IL-17(+) γδ T cells (30), was significantly increased at week 6 compared with the levels observed at week 2 or in PBS controls (Fig. S3A), thus mirroring the proliferation and accumulation of total IL-17(+) γδ T cells (Fig. 2 B and C) and specifically Vγ6(+) γδ T cells (Fig. 3E). Consistent with this finding, Vγ6(+) γδ T cells from the peritoneal cavity expressed higher levels of IL-7Rα than Vγ1(+) cells or Vγ4(+) cells (Fig. S3B), and exogenous administration of recombinant IL-7 showed a tendency to expand Vγ6(+) γδ T cells and enhance ID8 tumor load (Fig. S3 C–E). These data collectively suggest that the tumor microenvironment that progressively becomes enriched for IL-7 and other type-17–driving cytokines, drives the selective expansion of a CD27(−) Vγ6(+) cell subset capable of abundant IL-17 production.
γδ T Cells and IL-17 Promote Angiogenesis and Mobilize SPMs.
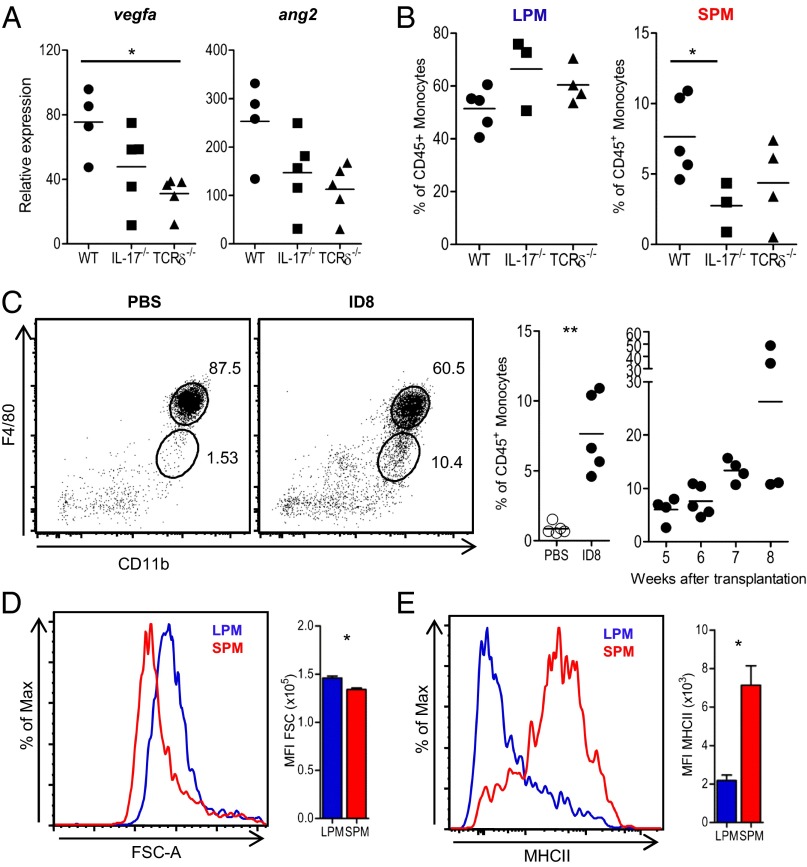
The protumor role of IL-17 has mostly been associated with enhanced angiogenesis (18, 31–33). To assess the significance of IL-17 (and of the γδ T cells responsible for a major fraction of its production) for this process in our ovarian tumor model, we analyzed expression of proangiogenic factors in the cellular peritoneal exudates of WT, TCRδ−/−, and IL-17−/− mice at 6 wk posttumor inoculation. This time point, which coincided with tumor growth arrest in IL-17−/− mice (Fig. 2F), approximately corresponds to the so-called “angiogenic switch” in the ID8 model (25). The angiogenic switch (or initiation of angiogenesis) is a discrete step in tumor development that is required to ensure exponential tumor growth. We have shown this step occurs after leukocyte recruitment to the tumor deposits in the ID8 model (25). Interestingly, we observed a reduction in vascular endothelial growth factor A (VEGFA) and Angiopoietin 2 (Ang-2) expression in both IL-17−/− and TCRδ−/− mice compared with WT controls (Fig. 4A), suggesting that IL-17–producing γδ T cells may indeed promote angiogenesis in this model. Of note, although ID8 cells expressed high levels of IL-17RA, the provision of IL-17 had no direct effect on tumor cell proliferation in vitro (Fig. S4 A and B). Moreover, FACS-sorted Vγ6(+) T cells did not promote ID8 tumor cell proliferation when cocultured together at 1:1 ratio (Fig. S4C).
Fig. 4.
γδ T cells and IL-17 selectively mobilize a population of small peritoneal macrophages. (A) Quantitative PCR expression of vegfa and ang2 in peritoneal exudates of C57BL/6 wild-type (WT), IL-17A−/−, and TCRδ−/− mice, at week 6 of ID8 tumor development. (B) Percentage of F4/80hi (large peritoneal macrophages, LPMs) and F4/80lo (small peritoneal macrophages, SPMs) cells in WT, TCRδ−/−, and IL-17A−/− mice, at week 6 post-ID8 tumor cell inoculation. (C) Representative FACS staining of F4/80hi (LPM) and F4/80lo (SPM) cells in ID8- or PBS-injected mice (Left) and summary graphs for the percentage of SPMs at week 6 (Center) and from weeks 5 to 8 of tumor development (Right). (D and E) Forward scatter (FSC) and MHC class II expression in LPM and SPM cells, and respective MFI (n = 5), at week 6 of tumor development. Data are representative of three independent experiments; *P < 0.05, **P < 0.01.
We next considered that IL-17(+) γδ T cells could promote ID8 ovarian cancer growth by influencing accumulation of one of several tumor-infiltrating leukocyte subsets implicated in cancer biology (1). Compared with WT controls, there was no difference in TCRδ−/− mice in recruitment of immunosuppressive Foxp3(+) regulatory T cells or Gr-1(+) myeloid cells (including neutrophils and myeloid-derived suppressor cells) (Fig. S5). We also observed similar numbers of “tissue-resident” F4/80(hi) macrophages in WT, TCRδ−/−, and IL-17−/− mice (Fig. 4B, Left). By contrast, we found a marked reduction in F4/80(lo) “inflammatory” macrophages in TCRδ−/− and IL-17−/− mice (Fig. 4B, Right), suggesting that γδ T cells and IL-17 secretion were both important for the accumulation of these cells at the site of tumor growth.
F4/80(lo) macrophages have recently been identified as a distinct subset of peritoneal macrophages that derive from blood monocytes that rapidly enter the peritoneal cavity and differentiate upon challenge, such as in response to LPS (34, 35). Interestingly, in our experiments, this population selectively accumulated upon ID8 tumor challenge (Fig. 4C). Given their smaller size [compared with conventional F4/80(hi) macrophages], F4/80(lo) cells were named SPMs (35). We confirmed this characteristic by examining their forward scatter by flow cytometry analysis (Fig. 4D). Furthermore, they also displayed very high surface levels of MHC class II (Fig. 4E), which is another signature feature of SPMs that clearly distinguishes them from F4/80(hi) LPMs (35). This highly selective effect of γδ T cells and IL-17 on SPMs, but not on LPMs (Fig. 4B), or the other eight leukocyte populations analyzed (Fig. S5), suggests a potential protumor role of SPMs in the ID8 ovarian cancer model.
SPMs Express a Proangiogenic Profile and Stimulate Tumor Cell Proliferation.
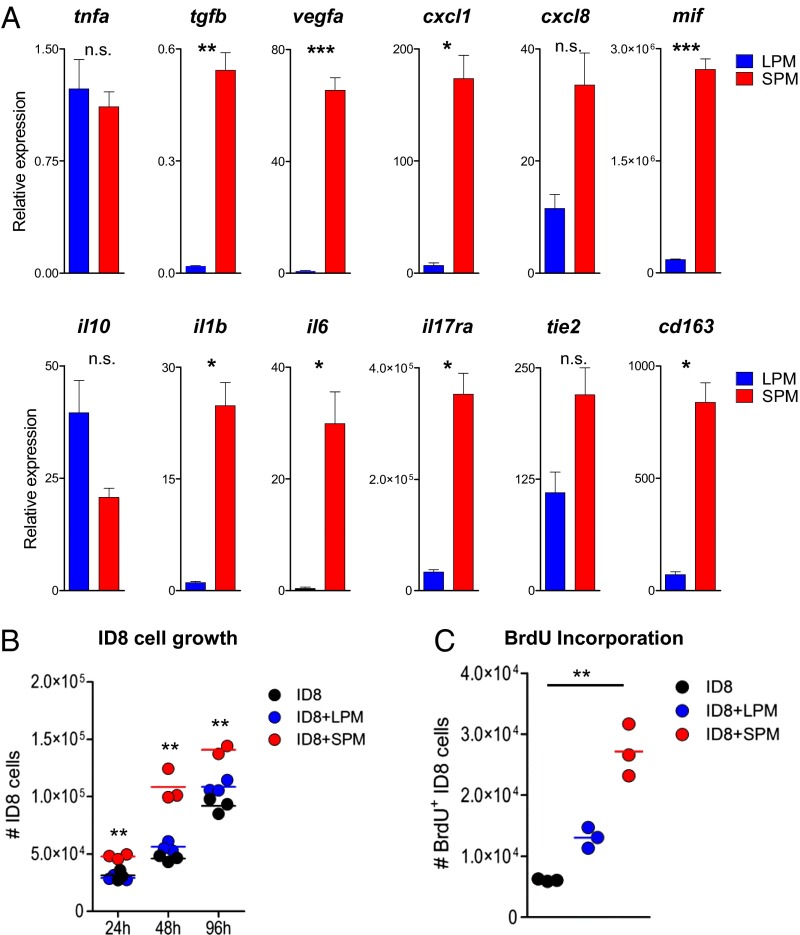
SPMs have been previously characterized in the context of microbial challenge (35), but not in a tumor environment. We isolated SPMs and LPMs after 6 wk of ID8 tumor development and analyzed gene expression by RT-quantitative PCR (RT-qPCR). Whereas both macrophage subsets expressed similar levels of tnfa, SPMs were strikingly enriched in proinflammatory and proangiogenic (Il1b, Il6, vegfa, tgfb, mif, cxcl1, and cxcl8) mediators (Fig. 5A). Consistent with this finding, SPMs expressed high levels of tie2, a well-established hallmark of proangiogenic monocytes and macrophages (36–39). These cells have also been shown to overexpress cd163 (38), transcript for which we found enriched in SPMs (Fig. 5A). These data strongly suggest that SPMs play a protumor role in our ovarian cancer model. Interestingly, the higher levels of Il17ra on SPMs (than LPMs) reinforce their dependence on IL-17 (Fig. 4B). Of note, SPMs presented a heterogeneous phenotype with variable degrees of polarization/differentiation across distinct in vivo tumor development experiments (Fig. S6).
Fig. 5.
Small peritoneal macrophages express a proangiogenic gene profile and directly promote tumor cell proliferation. (A) F4/80hi (LPM) and F4/80lo (SPM) macrophages from the peritoneal exudates of C57BL/6 wild type were FACS sorted after 6 wk of ID8 tumor development and analyzed by RT-qPCR for tnfa, tgfb, vegfa, cxcl1, cxcl8, il10, il1b, il6, il17ra, mif, tie2, and cd163 expression, normalized to the housekeeping gene hprt. Bars represent SD; *P < 0.05, **P < 0.01, ***P < 0.005. (B and C) ID8 tumor cell numbers (at 24, 48, and 96 h) and BrdU incorporation (at 48 h) upon noncontact coculture (at 1:1 ratio) with LPMs or SPMs, sorted from ID8 tumor-bearing mice (at week 6). Data are representative of two to three independent experiments; **P < 0.01.
Next, to test a potential direct effect of SPMs (or LPMs) on ID8 tumor cell growth, we established cocultures of ID8 cells with SPM or LPM populations sorted from ID8-bearing mice (at week 6 postinoculation). Interestingly, we observed a marked increase in total number (Fig. 5B) and in proliferating (Fig. 5C) tumor cells selectively in the presence of SPMs. These data collectively demonstrate that SPMs produce both factors that directly stimulate ovarian cancer cell proliferation and multiple proangiogenic mediators, thus revealing potent protumor SPM functions.
IL-17 Up-Regulates Proangiogenic and Proinflammatory Mediators in SPMs.
Finally, to assess the direct impact of Vγ6(+) T cells and particularly IL-17 on SPM protumor effector genes, we established short-term cultures of purified SPMs to which we added either Vγ6(+) T cells or recombinant IL-17. Upon coculture of SPMs with Vγ6(+) T cells in a Transwell system (thus allowing the retrieval of isolated SPMs at the end of the cultures), we observed a global up-regulation of the proangiogenic and proinflammatory target gene profile, even at a 1 γδ:25 SPM ratio, which resembled the in vivo ratio at week 6 of tumor development (Fig. 6A and Fig. S7A). Interestingly, gata6, a tissue-specific master transcription factor for peritoneal macrophages (40) was also up-regulated in these cocultures (Fig. 6A).
Fig. 6.
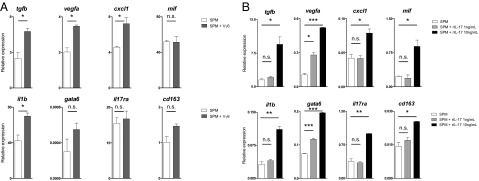
CD27(−) Vγ6(+) γδ T cells and IL-17 up-regulate proangiogenic and proinflammatory mediators in SPMs. FACS-sorted SPMs from peritoneal exudates of week 6 ID8-bearing C57BL/6 wild-type mice were (A) cocultured for 24 h with Vγ6(+) γδ T cells (in a Transwell system) at 1 γδ:25 SPM ratio or (B) incubated for 24 h with recombinant IL-17. Gene expression analysis for tgfb, vegfa, cxcl1, mif, il1b, gata6, il17ra, and cd163 was performed by RT-qPCR and normalized to the housekeeping gene hprt. Bars represent SD; *P < 0.05, **P < 0.01, ***P < 0.005. Additional data from an independent experiment are shown in Fig. S7.
On the other hand, the provision of exogenous IL-17 to purified SPM cultures increased, in a dose-dependent and highly significant manner, the expression of the same proangiogenic and proinflammatory mediators (Fig. 6B and Fig. S7B). Collectively, these data strongly support an IL-17–mediated cross-talk between Vγ6(+) T cells and SPMs in the ID8 ovarian cancer model.
Discussion
Burnet’s “immune surveillance of cancer” theory has been revised to accommodate leukocyte subsets, both of myeloid and lymphoid origin, that enhance cancer cell proliferation (1, 41). The data presented here add to this revised model by identifying and characterizing a population of IL-17–secreting γδ T cells that has the potential to promote ID8 ovarian tumor growth, thus effectively overriding the antitumor function of coexisting IFN-γ–producing γδ T cells. In fact, the latter increased only transiently (at week 2) before returning to baseline (as defined by the PBS-injected control group). By contrast, IL-17(+) γδ T cells accumulated at later stages (week 6) of tumor progression, matching the window where tumor growth was dependent on IL-17.
The preferential expansion of IL-17–producing CD27(−) T cells in our model is likely accounted for by the combined local accumulation of multiple type-17–driving cytokines (IL-1β, IL-6, and IL-7) during ID8 tumor development. Interestingly, CD27(−) γδ T cells in the secondary lymphoid organs have been shown to express higher levels of IL-7R, IL-1R1, and IL-23R than their IFN-γ–secreting counterparts (30, 42, 43), a property that seems to be transcriptionally acquired and epigenetically sustained (42). Among the type-17–driving cytokines, IL-7 was the most consistently up-regulated throughout ID8 growth, and its exogenous administration showed a tendency to increase the tumor load. Nonetheless, the individual and combined contributions of these cytokines to the selective expansion of CD27(−) γδ T cells remains to be formally established.
Interestingly, the tumor microenvironment favored the expansion of a distinct CD27(−) γδ T-cell subset expressing Vγ6 that is similar to the intestinal CD27(−) Vγ6(+) γδ T-cell subset shown to dominate the γδ T-cell response to oral Listeria monocytogenes infection (44). Also in that study, CD27(−) Vγ6(+) γδ T cells were unique among γδ T cells in the extent of IL-17 polarization and in the dynamics of their response in mesenteric lymph nodes (particularly upon secondary challenge) (44). Importantly, the memory-like CD27(−) Vγ6(+) γδ T-cell response reduced bacterial load, showing that CD27(−) Vγ6(+) γδ T cells can play both protective (as in Listeria infection) or detrimental (as for ID8 tumors) roles that merit further investigation in vivo in other models of disease. Interestingly, in a recent study on hepatocellular carcinoma, tumor-promoting IL-17 was mostly provided by Vγ4(+) γδ T cells (19). By contrast, in our model, Vγ4(+) cells contained few IL-17 producers and many more IFN-γ producers. Thus, different tumors may harbor and promote distinct proinflammatory subsets of γδ T cells.
Although the lack of a mouse line expressing Cre recombinase under the control of a γδ T-cell–specific promoter precludes the direct examination of the effect of conditional ablation of IL-17 in γδ T cells, the overlap of phenotypes of TCRδ−/− and IL-17−/− mice, together with the progressive expansion of CD27(−) Vγ6(+) γδ T cells, strongly supports a protumor role for an IL-17–secreting γδ T-cell axis in vivo.
In a previous study on a fibrosarcoma (and colon carcinoma) model(s), γδ T cells were shown to be the major cellular source of IL-17, which supported tumor cell growth (18). However, these experiments were performed on the murine BALB/c background, where lymphoid organs (both in naïve and tumor-bearing mice) were essentially depleted of IFN-γ–producing γδ T cells (18). By contrast, our study was conducted on the murine C57/Bl6 background, where IFN-γ–producing γδ T cells significantly outnumber their IL-17(+) counterparts (20). Thus, our data clearly suggest that even in the backdrop of a dominant IFN-γ–secreting potential, the tumor microenvironment is capable, under certain circumstances, of modulating the balance of γδ T-cell responses from antitumor (IFN-γ based) to protumor (IL-17 mediated) subsets and functions. Moreover, unlike the previous report (18), our study used TCRδ-deficient mice as a critical tool to establish the tumor-promoting role of γδ T cells in vivo. This raises interesting questions regarding γδ T-cell functions in the ID8 ovarian tumor environment in comparison with other models where an overt antitumor role has been demonstrated for γδ T cells (4–7, 45). Along these lines, we have observed that B16 melanoma-infiltrating γδ T cells are enriched for IFN-γ, and essentially devoid of IL-17, expression (7). Thus, the balance between IFN-γ and IL-17 production by γδ T cells may be a critical parameter to assess their overall contribution to tumor surveillance versus protumor progression.
The protumor role of IL-17 has been mostly attributed to promotion of angiogenesis (18, 31–33). Consistent with those reports, we also implicate an impact on the angiogenesis mediators VEGFA and ANG2 by the IL-17–secreting CD27(−) Vγ6(+) γδ T-cell subset in our ovarian cancer model.
Interestingly, we also identified a mechanism in which γδ T cells and IL-17 mobilize SPMs. These cells have recently been identified as a specific subset of peritoneal macrophages characterized by smaller (∼3 μm) size, low F4/80 and CD11b expression, and high MHC class II expression (35). SPMs are mobilized from blood monocytes, whereas LPMs are maintained independently of hematopoiesis (34). Importantly, IL-17 has previously been shown to recruit blood monocytes (46, 47), as well as mature macrophages (48–50) to various tissues; and we have detected high levels of Il17ra selectively on SPMs isolated from ID8-bearing animals. Thus, our work identifies a link between IL-17–secreting γδ T cells and the mobilization/expansion of SPMs in the peritoneal cavity.
SPMs and LPMs also display distinct phagocytic and cytokine secretion patterns (34). Moreover, SPMs (unlike LPMs) fail to produce the antitumor mediator NO when stimulated with LPS in vitro (35). Importantly, our gene expression analysis identified a striking proinflammatory and proangiogenic (il1b, il6, vegfa, tgfb, mif, cxcl1, and cxcl8) gene expression profile for SPMs. Furthermore, SPMs expressed high levels of tie2 and cd163, signature markers of proangiogenic monocytes and macrophages (36–39). Among the protumor mediators produced by SPMs, it is important to note the presence of MIF and IL-6, both known to promote the expression of a large panel of proinflammatory and proangiogenic molecules and to protect tumor cells from apoptosis (51–54). Thus, we propose that the cross-talk with proinflammatory and proangiogenic SPMs, that have further stimulatory effects on tumor cell proliferation, underlies the protumor role of IL-17(+) γδ T cells in the ID8 ovarian cancer model. Interestingly, Ma et al. suggested a distinct mechanism in hepatocellular carcinoma, in which IL-17(+) γδ T cells seemingly act by recruiting myeloid-derived suppressor cells (MDSCs) that inhibit local antitumor CD8(+) T-cell responses (19). An association between IL-17(+) γδ T cells and MDSC accumulation was also recently reported in human colorectal cancer (17). In our model, by contrast, there were no differences in CD11b(+) Gr-1(+) cell recruitment, CD8(+) T-cell infiltration, or IFN-γ production, between γδ T-cell–deficient versus sufficient hosts. This suggests that distinct cellular mechanisms may underlie the protumor functions of IL-17(+) γδ T cells within different tumors.
Notwithstanding the protumor functions that recent experiments disclose for IL-17(+) γδ T cells, it should be noted that these cells have previously been implicated in protective responses in other tumor scenarios. For example, IL-17(+) γδ T cells contributed to the positive (chemo)therapeutic effect of doxorubicin in various transplantable models of epithelial tumors in vivo (55). γδ Tumor-infiltrating lymphocytes were either Vγ4(+) (∼60%) or Vγ6(+) (∼35%), and the therapeutic efficacy of doxorubicin was similarly reduced in TCRδ-deficient and in Vγ4−/−Vγ6−/− hosts. In another study, IL-17(+) γδ T cells were associated with the antitumor effect of bacillus Calmette–Guérin treatment in a bladder cancer model (56). This effect was abolished in both TCRδ-deficient and IL-17–deficient mice, and γδ T cells (whose Vγ use was not determined) were the major intratumor source of IL-17. The discrepancy in IL-17(+) γδ T-cell functions between these studies and the data presented here may be linked to the efficient CD8(+) Tc1 cell priming induced by therapeutic protocols. Zitvogel and coworkers proposed that IL-17 is critical for the afferent phase of the immune response against dying tumor cells (“immunogenic cell death”), namely, CD8(+) T-cell priming for IFN-γ production (55). Therefore, both IL-17 and IFN-γ are likely to be involved in multiple cellular events taking place within the inflammatory tumor microenvironment as well as in draining lymph nodes. Along these lines, it should also be noted that IL-17+ γδ T cells can (under strong inflammatory conditions) differentiate into IL-17/IFN-γ double producers, whose function in the tumor milieu remains to be established (42, 57).
The IL-17–associated protumor/antitumor paradox extends well beyond the γδ T-cell compartment. For example, the adoptive transfer of IL-17–producing CD4(+) (Th17) cells has been shown to promote either tumor development or tumor regression (58–60). And IL-17(R) deficiency/blockade was associated with both enhanced (61) and reduced (23, 62–64) tumor load. The reasons for the highly variable anti- versus protumor activities of IL-17 and IL-17–producing T cells in distinct tumor models remain unclear and deserve further investigation. However, our current data are fully consistent with our previous work in which antibody-mediated IL-17 depletion reduced ID8 cancer cell growth (23). We are therefore convinced that IL-17 is a potent protumor mediator in the ID8 model.
Another area of interest concerns the examination of IL-17(+) γδ T cells in human cancer. In comparison with their murine counterparts, human circulating γδ T lymphocytes are extremely polarized toward IFN-γ production from early stages of life (8, 65). By contrast, IL-17(+) γδ T cells are very rare in the peripheral blood of healthy individuals (65, 66), but accumulate significantly in pathological conditions such as bacterial meningitis (67) or psoriasis (68). With regard to tumors, the data are still limited but suggest that IL-17(+) γδ T cells can be found in some (but not all) types of cancer. Interestingly, the infiltration of IL-17(+) γδ T cells was recently associated with tumor stages and clinopathological features in patients with colorectal cancer (17). We therefore believe that the detailed characterization of tumor-infiltrating γδ lymphocytes, including the balance between major cytokine-producing subsets, may have important prognostic value and may be the key for their successful clinical manipulation.
In conclusion, this study identifies an IL-17–dependent cross-talk between Vγ6(+) γδ T cells and proinflammatory and proangiogenic SPMs that promotes ovarian cancer growth. From a global cancer perspective, our work highlights the importance of tumor-derived factors that drive the selective expansion of IL-17(+) γδ T cells and their tumor-promoting effects via mobilization of particular myeloid subpopulations. Upon investigation of similar mechanisms in human tumors, this may result in the identification of novel targets for cancer immunotherapy.
Materials and Methods
Mice.
C57BL/6J (B6) wild-type mice were purchased from Charles River Laboratories. B6.TCRδ−/− mice were purchased from The Jackson Laboratory. B6.IL-17−/− mice were kindly provided by Fiona Powrie (University of Oxford, Oxford, UK) with permission from Yoichiro Iwakura (Tokyo University of Science, Chiba, Japan). All animals were females, 8–16 wk of age, which were aged matched within 2 wk. Mice were maintained in specific pathogen-free facilities of Queen Mary University of London (QMUL) or Instituto de Medicina Molecular (IMM). All experimental procedures observed the guidelines approved by the ethics committees of QMUL and IMM.
Cell Culture.
The ID8 ovarian cancer cell line was a gift from Kathy Roby (University of Kansas, Kansas City, KS). Cells were maintained in Dulbecco’s modified Eagles’ medium (DMEM) with glutamine, sodium pyruvate, 4% (vol/vol) FCS (Gibco; Life Technologies) and insulin-transferrin-sodium selenite media supplement (Sigma). Lentiviral infection of ID8 cells with luciferase reporter was performed as previously described (69). Proliferation was measured by BrdU incorporation after 30 min incubation with 10 μM BrdU (Sigma).
(Co)cultures of ID8 cells, γδ T cells, and macrophage subsets were performed in Transwell Permeable Support 0.4 μm (Corning), in DMEM supplemented with 0.5% FCS, glutamine, sodium pyruvate, nonessential amino acids, and β-mercaptoethanol (Gibco; Life Technologies). γδ T-cell cocultures were also supplemented with 10 ng/mL IL-2, 50 ng/mL IL-1β (Peprotech), and 50 ng/mL IL-23 (R&D).
In Vivo ID8 Tumor Model.
The 5–10 × 106 tumor cells expressing luciferase (ID8-luc) were injected intraperitoneally or s.c. in a total volume of 100 μL. i.p. Tumor growth was evaluated in situ by bioluminescence imaging as previously described (69). For in vivo administration of IL-7, 5 µg of recombinant mouse IL-7 (Peprotech) (or PBS as control) was injected i.p. every 2/3 d from week 4 to week 6 after tumor injection. For proliferation assays, mice were fed daily with 0.8 mg/mL BrdU (Sigma) in drinking water, during the last 2 wk before analysis.
Flow Cytometry and Cell Sorting.
Peritoneal exudate cells were obtained from the lavage of the peritoneal cavity with 5 mL ice-cold DMEM with 4% (vol/vol) FCS. Erythrocytes were osmotically lysed using RBC Lysis Buffer (Biolegend). For surface staining, cells were Fc blocked with anti-CD16/32 (93; eBioscience) and incubated for 30 min with antibodies in PBS with 2% (vol/vol) FCS and 1 mM EDTA (FACS buffer). The following monoclonal antibodies were purchased from eBioscience: anti-CD3 (17A2), anti-TCRγδ (GL3), anti-CD27 (LG.7F9), anti-CD4 (RM4-5), anti-CD8α (53-6.7), anti–IL-7Rα (A7R34), anti–IL-17RA (PAJ-17R), anti-CD11b (M1/70), anti–Gr-1 (RB6-8C5), anti-F4/80 (BM8), anti-MHC II (M5/114.15.2), and anti-IgM (RM-7B4); from BD Bioscience: anti-TCRγ4 (UC3-10A6); and from Biolegend: anti-CD45 (30-F11), anti-TCRγ1 (2.11), and anti-NK1.1 (PK136). Anti-TCRVγ5Vδ1 (17D1) was kindly provided by Adrian Hayday (King’s College London, London). For TCRγ6 (Vγ6) detection, staining with GL3 and 17D1 monoclonal antibodies was performed as previously described (29). Cells were either analyzed on a FACS Fortessa (BD Bioscience) or sorted on a FACS Aria III (BD Bioscience) for further manipulation. Gating strategies for γδ T cells, SPMs, and LPMs are shown in Fig. S8. Cell numbers were normalized according to the total number of peritoneal exudate cells.
For intracellular cytokine staining, cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma) and 1μg/mL ionomycin (Sigma) for 4 h at 37 °C; 10 μg/mL brefeldin-A (Sigma) and 2 μM monensin (eBioscience) were added during the last 2 h. Cells were stained for surface markers, fixed and permeabilized using the Foxp3/Transcription Factor Staining Buffer set (eBioscience), following the manufacturer’s instructions, and then incubated for 30 min at room temperature with anti-FoxP3 (FJK-16s) and/or anti–IL-17 (17B7) and anti–IFN-γ (XMG1.2) (eBioscience). For BrdU staining, FITC BrdU Flow kit (BD Pharmingen) was used following the manufacturer’s instructions. Data were acquired on a FACS Fortessa (BD Bioscience) and analyzed using FACS Diva or FlowJo software (Tree Star).
RNA Isolation, cDNA Production, and Real-Time PCR.
Total RNA was extracted using the RNeasy Mini kit (Qiagen) according to the manufacturer’s instructions. Concentration and purity were determined using the NanoDrop ND-1000 spectrophotometer (Thermo Scientific). Total RNA was reverse transcribed into cDNA using a Transcriptor High Fidelity cDNA Synthesis kit (Roche). Quantitative real-time PCR was performed on ViiA 7 Real-Time PCR system (Applied Biosystems; Life Technologies). Primers were either designed manually or by using the Universal ProbeLibrary Assay Design Center from Roche (www.roche-applied-science.com). Sequences are available upon request. Analysis of the quantitative PCR results was performed using the ViiA 7 software v1.2 (Applied Biosystems; Life Technologies).
Confocal Microscopy.
The bowel mesentery of ID8 tumor-bearing mice was removed, fixed in ice-cold acetone for 20 min, and allowed to air dry for 1 h. After rehydration in PBS, the intestine was removed and the tumor foci attached to the mesenterium were immunostained. The tissue was blocked and permeabilized in PBS with 1% BSA, 3% (vol/vol) goat serum, 5% (vol/vol) mouse serum, and 1% Triton X-100 (blocking buffer). Tissue was incubated with FITC anti-TCRγδ (Gl3) and/or biotin anti-TCRγδ (UC7) overnight at 4 °C and subsequently with secondary antibodies and Alexa Fluor 546-streptavidin. Tissue was mounted on a slide and embedded in Prolong Gold (Invitrogen; Life Technologies). The tumor foci were imaged under a Zeiss LSM 710 confocal microscope.
Statistical Analysis.
Data are presented as mean ± SE. The tests used were Mann–Whitney or Student t test. Values of P < 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Yoichiro Iwakura (Tokyo University of Science), Dr. Fiona Powrie (University of Oxford), and Dr. Ana Pamplona [Instituto de Medicina Molecular (IMM)] for provision of IL-17–deficient mice; Karine Serre and Sérgio Dias (IMM), and Capucine Grandjean, Anne Monfort, Gemma Everitt, and D. Andrew Leinster [Queen Mary University of London (QMUL)] for advice and technical help; and the staffs of QMUL’s and IMM’s animal, flow cytometry, and microscopy facilities, particularly the expert advice from Joana Marques, Iolanda Moreira, Maria Soares, Ana Vieira, José Rino, and António Temudo (IMM). This work was funded by the Wellcome Trust (D.J.P.) and European Research Council [StG_260352 (to B.S.-S.)]. F.R.B. was funded by the Higher Education Funding Council for England; H.K. was funded by F.R.B.’s Cancer Research UK Programme Grant C587/A10603. M.R. and T.L. received PhD fellowships from Fundação para a Ciência e a Tecnologia.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.L.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403424111/-/DCSupplemental.
References
- 1.Lança T, Silva-Santos B. The split nature of tumor-infiltrating leukocytes: Implications for cancer surveillance and immunotherapy. OncoImmunology. 2012;1(5):717–725. doi: 10.4161/onci.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31(2):184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Street SE, et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med. 2004;199(6):879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girardi M, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294(5542):605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, et al. Protective immunosurveillance and therapeutic antitumor activity of gammadelta T cells demonstrated in a mouse model of prostate cancer. J Immunol. 2008;180(9):6044–6053. doi: 10.4049/jimmunol.180.9.6044. [DOI] [PubMed] [Google Scholar]
- 6.Gao Y, et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198(3):433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lança T, et al. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic γδ T lymphocytes to tumor beds. J Immunol. 2013;190(12):6673–6680. doi: 10.4049/jimmunol.1300434. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons DL, et al. Neonates harbour highly active gammadelta T cells with selective impairments in preterm infants. Eur J Immunol. 2009;39(7):1794–1806. doi: 10.1002/eji.200939222. [DOI] [PubMed] [Google Scholar]
- 9.Corvaisier M, et al. V gamma 9V delta 2 T cell response to colon carcinoma cells. J Immunol. 2005;175(8):5481–5488. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- 10.Wrobel P, et al. Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: Involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol. 2007;66(2-3):320–328. doi: 10.1111/j.1365-3083.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- 11.Gomes AQ, et al. Identification of a panel of ten cell surface protein antigens associated with immunotargeting of leukemias and lymphomas by peripheral blood gammadelta T cells. Haematologica. 2010;95(8):1397–1404. doi: 10.3324/haematol.2009.020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lança T, et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gammadelta T-cell cytotoxicity. Blood. 2010;115(12):2407–2411. doi: 10.1182/blood-2009-08-237123. [DOI] [PubMed] [Google Scholar]
- 13.Fournié JJ, et al. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell Mol Immunol. 2013;10(1):35–41. doi: 10.1038/cmi.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes AQ, Martins DS, Silva-Santos B. Targeting γδ T lymphocytes for cancer immunotherapy: From novel mechanistic insight to clinical application. Cancer Res. 2010;70(24):10024–10027. doi: 10.1158/0008-5472.CAN-10-3236. [DOI] [PubMed] [Google Scholar]
- 15.Hannani D, et al. Harnessing γδ T cells in anticancer immunotherapy. Trends Immunol. 2012;33(5):199–206. doi: 10.1016/j.it.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Ma C, et al. Tumor-infiltrating γδ T lymphocytes predict clinical outcome in human breast cancer. J Immunol. 2012;189(10):5029–5036. doi: 10.4049/jimmunol.1201892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu P, et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40(5):785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakita D, et al. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol. 2010;40(7):1927–1937. doi: 10.1002/eji.200940157. [DOI] [PubMed] [Google Scholar]
- 19.Ma S, et al. IL-17A produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014;74(7):1969–1982. doi: 10.1158/0008-5472.CAN-13-2534. [DOI] [PubMed] [Google Scholar]
- 20.Ribot JC, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10(4):427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribot JC, et al. Cutting edge: adaptive versus innate receptor signals selectively control the pool sizes of murine IFN-γ- or IL-17-producing γδ T cells upon infection. J Immunol. 2010;185(11):6421–6425. doi: 10.4049/jimmunol.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turchinovich G, Pennington DJ. T cell receptor signalling in γδ cell development: Strength isn’t everything. Trends Immunol. 2011;32(12):567–573. doi: 10.1016/j.it.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Charles KA, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119(10):3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73(23):6900–6912. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leinster DA, et al. The peritoneal tumour microenvironment of high-grade serous ovarian cancer. J Pathol. 2012;227(2):136–145. doi: 10.1002/path.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulbe H, et al. Australian Ovarian Cancer Study Group A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72(1):66–75. doi: 10.1158/0008-5472.CAN-11-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prinz I, Silva-Santos B, Pennington DJ. Functional development of γδ T cells. Eur J Immunol. 2013;43(8):1988–1994. doi: 10.1002/eji.201343759. [DOI] [PubMed] [Google Scholar]
- 28.Serre K, Silva-Santos B. Molecular mechanisms of differentiation of murine pro-inflammatory γδ T cell subsets. Front Immunol. 2013;4:431. doi: 10.3389/fimmu.2013.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roark CL, et al. Subset-specific, uniform activation among V gamma 6/V delta 1+ gamma delta T cells elicited by inflammation. J Leukoc Biol. 2004;75(1):68–75. doi: 10.1189/jlb.0703326. [DOI] [PubMed] [Google Scholar]
- 30.Michel ML, et al. Interleukin 7 (IL-7) selectively promotes mouse and human IL-17–producing γδ cells. Proc Natl Acad Sci USA. 2012;109(43):17549–17554. doi: 10.1073/pnas.1204327109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva-Santos B. Promoting angiogenesis within the tumor microenvironment: The secret life of murine lymphoid IL-17-producing gammadelta T cells. Eur J Immunol. 2010;40(7):1873–1876. doi: 10.1002/eji.201040707. [DOI] [PubMed] [Google Scholar]
- 32.Numasaki M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 33.Numasaki M, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175(9):6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 34.Cain DW, et al. Identification of a tissue-specific, C/EBPβ-dependent pathway of differentiation for murine peritoneal macrophages. J Immunol. 2013;191(9):4665–4675. doi: 10.4049/jimmunol.1300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosn EE, et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci USA. 2010;107(6):2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Palma M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8(3):211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Venneri MA, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109(12):5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 38.Pucci F, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114(4):901–914. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 39.Coffelt SB, et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010;70(13):5270–5280. doi: 10.1158/0008-5472.CAN-10-0012. [DOI] [PubMed] [Google Scholar]
- 40.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157(4):832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: Cancer and other tales. Nat Rev Immunol. 2011;11(10):702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 42.Schmolka N, et al. Epigenetic and transcriptional signatures of stable versus plastic differentiation of proinflammatory γδ T cell subsets. Nat Immunol. 2013;14(10):1093–1100. doi: 10.1038/ni.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Sheridan BS, et al. γδ T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 2013;39(1):184–195. doi: 10.1016/j.immuni.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Street SE, et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med. 2004;199(6):879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. IL-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182(6):3884–3891. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shahrara S, et al. IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J Immunol. 2010;184(8):4479–4487. doi: 10.4049/jimmunol.0901942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barin JG, et al. Macrophages participate in IL-17-mediated inflammation. Eur J Immunol. 2012;42(3):726–736. doi: 10.1002/eji.201141737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishida H, et al. IL-23 protection against Plasmodium berghei infection in mice is partially dependent on IL-17 from macrophages. Eur J Immunol. 2013;43(10):2696–2706. doi: 10.1002/eji.201343493. [DOI] [PubMed] [Google Scholar]
- 50.Liu L, et al. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J Thorac Oncol. 2012;7(7):1091–1100. doi: 10.1097/JTO.0b013e3182542752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hudson JD, et al. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190(10):1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calandra T, Roger T. Macrophage migration inhibitory factor: A regulator of innate immunity. Nat Rev Immunol. 2003;3(10):791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagemann T, et al. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol Cancer Ther. 2007;6(7):1993–2002. doi: 10.1158/1535-7163.MCT-07-0118. [DOI] [PubMed] [Google Scholar]
- 54.Kumar J, Ward AC. Role of the interleukin 6 receptor family in epithelial ovarian cancer and its clinical implications. Biochim Biophys Acta. 2014;1845(2):117–125. doi: 10.1016/j.bbcan.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Ma Y, et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med. 2011;208(3):491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeuchi A, et al. IL-17 production by γδ T cells is important for the antitumor effect of Mycobacterium bovis bacillus Calmette-Guérin treatment against bladder cancer. Eur J Immunol. 2011;41(1):246–251. doi: 10.1002/eji.201040773. [DOI] [PubMed] [Google Scholar]
- 57.Hamada S, et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol. 2008;181(5):3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin-Orozco N, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muranski P, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chalmin F, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36(3):362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 61.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114(2):357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L, et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206(7):1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He D, et al. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184(5):2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chae WJ, et al. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci USA. 2010;107(12):5540–5544. doi: 10.1073/pnas.0912675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeBarros A, Chaves-Ferreira M, d’Orey F, Ribot JC, Silva-Santos B. CD70-CD27 interactions provide survival and proliferative signals that regulate T cell receptor-driven activation of human γδ peripheral blood lymphocytes. Eur J Immunol. 2011;41(1):195–201. doi: 10.1002/eji.201040905. [DOI] [PubMed] [Google Scholar]
- 66.Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J Immunol. 2010;184(12):7268–7280. doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caccamo N, et al. Differentiation, phenotype, and function of interleukin-17-producing human Vγ9Vδ2 T cells. Blood. 2011;118(1):129–138. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- 68.Laggner U, et al. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J Immunol. 2011;187(5):2783–2793. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kulbe H, et al. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67(2):585–592. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.