Abstract
Vaccination is the most effective medical intervention ever introduced and, together with clean water and sanitation, it has eliminated a large part of the infectious diseases that once killed millions of people. A recent study concluded that since 1924 in the United States alone, vaccines have prevented 40 million cases of diphtheria, 35 million cases of measles, and a total of 103 million cases of childhood diseases. A report from the World Health Organization states that today vaccines prevent 2.5 million deaths per year: Every minute five lives are saved by vaccines worldwide. Overall, vaccines have done and continue to do an excellent job in eliminating or reducing the impact of childhood diseases. Furthermore, thanks to new technologies, vaccines now have the potential to make an enormous contribution to the health of modern society by preventing and treating not only communicable diseases in all ages, but also noncommunicable diseases such as cancer and neurodegenerative disorders. The achievement of these results requires the development of novel technologies and health economic models able to capture not only the mere cost–benefit of vaccination, but also the value of health per se.
Keywords: reverse vaccinology, adjuvants, cost-effectiveness, immunotherapy, life expectancy
In 1900 life expectancy in the United States was 47.3 y (1). Communicable diseases such as pneumonia, influenza, tuberculosis, diphtheria, smallpox, pertussis, measles, and typhoid fever were the leading causes of mortality. Children and young adults were mostly affected. Vaccination, together with improved hygiene practices and antibiotics, have played an essential role during the last century in eliminating most of the mortality from infectious diseases. A recent study collected the data of the reported infectious diseases in United States from 1888 and concluded that since 1924 vaccines prevented 40 million cases of diphtheria, 35 million cases measles, and a total of 103 million cases of childhood diseases (2). Today life expectancy is 78.7 y, and noncommunicable diseases such as ischemic heart disease, stroke, and cancer are the leading cause of death (3). Other noncommunicable diseases such as diabetes, Alzheimer, and other neurodegenerative diseases are becoming leading causes of morbidity.
Globally data are available only for more recent times. However, it is not difficult to imagine the impact of vaccination if we just think that in the 20th century smallpox alone killed 300 million people, and that no one dies from it today because the virus has been eradicated thanks to vaccination in 1978 (4). Worldwide life expectancy also increased moving from 58.5 to 70 y from 1970 through 2010 (5). The highest decrease in mortality was measured in children and young adults up to 20 y of age. During this period, the Expanded Program on Immunization and more recently the establishment of the Global Alliance for Vaccines and Immunization (GAVI) increased complete infant immunization from less than 5% to more than 90% (6). A study from the World Health Organization (WHO), reports that today vaccines save more than 2.5 million deaths annually (7). The impact of communicable diseases worldwide decreased from 33% of the total deaths in 1990 to 25% in 2010 and noncommunicable diseases became the first cause of global mortality and morbidity (8).
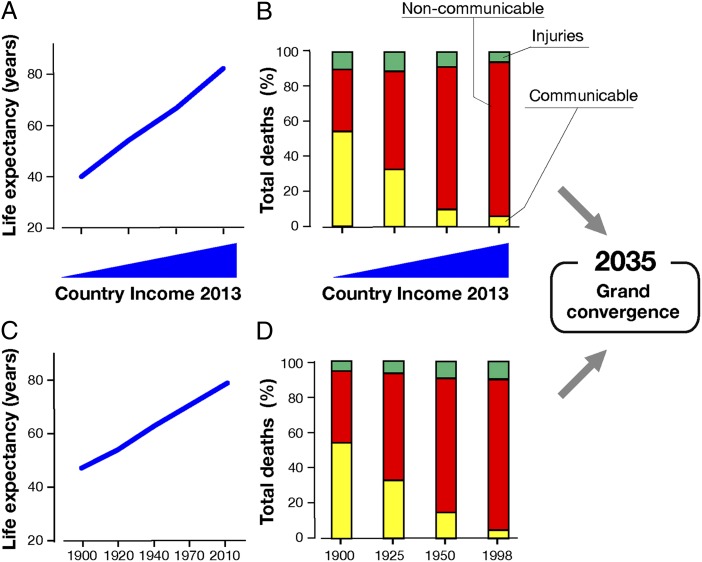
Fig. 1 shows the life expectancy (Fig. 1A) and the relative contribution to mortality of communicable versus noncommunicable diseases in very low-, low-, middle-, and high-income countries calculated in 2013 (Fig. 1B) (9). Remarkably, a similar pattern is observed if we analyze the life expectancy and the evolution of communicable diseases during the last century in a high-income country such as the United States (Fig. 1 C and D, respectively). With the appropriate investment in health, it may be possible to reduce infectiousness and reduce child and maternal mortality rates to universally low levels by 2035 and close the health gap between the countries, a phenomenon that has been termed “grand convergence” (9, 10). Existing vaccines will continue to improve global health as shown in Fig. 1. The question we will address here is whether new vaccines will have an impact on the diseases that are now becoming the most prominent cause of mortality. In doing so, we will briefly review the history of vaccines, the great progress already achieved by vaccine technologies, and importantly, the potential that vaccines may have to prevent and cure the diseases of modern society across all age groups and all countries if the vaccines succeed in unleashing the full power of the immune system.
Fig. 1.
Worldwide life expectancy (A) and total deaths from communicable and noncommunicable diseases (B) in countries with very low, low, middle, or high income. Fig. 1B is modified from ref. 9. Life expectancy (C) and total deaths from communicable and noncommunicable diseases in the United States during the last century (D).
Brief History of Vaccines
The beginning of vaccination stems from the observation reported first by Thucydides in 430 BC that people that survived deadly contagious diseases did not contract the disease twice (11). Already in the Middle Ages in China people practiced variolation, a procedure where healthy people were exposed to air-dried pustules of smallpox. The procedure, though effective, was very dangerous and medicine made great progress when Jenner discovered it was sufficient to expose individuals to an agent that mimicked the disease, such as the pustulae derived from cowpox, for inoculation rather than exposing people to the disease directly (12, 13). Once the microbial origin of infectious diseases was discovered a century later, Pasteur pioneered vaccine development by exposing people to dead or attenuated microorganisms that mimicked the infectious agent, but did not cause disease (14, 15). In the 1940s the discovery that viruses could be grown in in vitro cultures on animal cells allowed the development of many vaccines against poliomyelitis, measles, mumps, rubella, varicella, hepatitis A and, more recently, rotavirus and influenza.
More recently, sophisticated technologies have improved the way we mimic microorganisms and induce protective immunity. Modern vaccines are made of components purified from the pathogen, recombinant antigens produced in yeast, Escherichia coli or baculovirus, as well as antigens modified with structure-based design or synthesized in the laboratory (16, 17).
Evolution of Vaccine Technologies
For more than a century vaccines have been developed following the principles of Pasteur by isolating, inactivating, and injecting the microorganism causing the disease or a portion of it (14, 15). During the last 30 y several waves of new and unconventional technologies allowed for the development of vaccines that conquered several diseases. The advent of recombinant DNA in the late 1970s made it possible to safely make large quantities of hepatitis B vaccine by producing it in yeast viral-like particles (VLPs) identical to those released in the plasma by the hepatitis B virus (18). More recently VLP technology has been used to produce the human papillomavirus (HPV) vaccines in yeast or baculovirus (19) and many other experimental vaccines such as respiratory syncytial virus (RSV), influenza, norovirus, and parvovirus B19. Recombinant DNA technologies were also used to engineer the Bordetella pertussis genome to produce a nontoxic mutant of the pertussis toxin that induced protective immunity superior to the same toxin detoxified by conventional chemicophysical methods (20, 21). Another revolutionary technology was the conjugation of bacterial capsular polysaccharides to carrier proteins to make polysaccharide antigens T-cell dependent and therefore immunogenic in infants. This technology has already allowed the development and licensure of vaccines against Haemophilus influenza type B; meningococcus C; meningococcus A, C, W, and Y; and pneumococcus. Additional candidates, such as vaccines against group B Streptococcus (GBS) and typhoid fever, are in a late stage of development (22). More recently, reverse vaccinology used the entire genomic sequence of pathogens to discover antigens that could not be discovered by conventional approaches. Reverse vaccinology technology allowed the development and licensure of the vaccine against meningococcus B (MenB) and advanced preclinical and clinical vaccine studies against several bacteria, including those resistant to antibiotics such as Staphylococcus aureus and E. coli (23, 24). Although reverse vaccinology has an untapped potential for bacteria and parasites, the next technology that is likely to profoundly change the way we make vaccines against viral infections is structural vaccinology. Information derived from the 3D structure of viral envelope proteins has been used to design antigens with improved immunogenicity and protection. An extraordinary example of this technology has been the ability to lock the F protein of RSV in the prefusion conformation making the development of a vaccine that people have been expecting for decades possible (25, 26). Structure-based antigen design recently also hit another milestone, where, for the first time, a peptide epitope from RSV has been shown to induce functional neutralizing antibodies in nonhuman primates, provided that it is inserted into a scaffold that keeps the peptide in a conformation that is thermally stable and recognized with picomolar affinity by antibodies to the native protein (27).
Synthetic biology has recently emerged as a new and potent way to make vaccines. Gene synthesis in the laboratory with less than 1 error in 10,000 bases has been recently used to make a vaccine seed for a potentially pandemic influenza virus in a matter of a few days instead of the typical 2–3 mo needed with conventional technologies (28). The same approach was used to make a completely synthetic RNA vaccine able to induce protective antibody titers in preclinical models in less than 40 d after the discovery of the pandemic H7N9 virus (29). It is worth mentioning that not all new and exciting technologies emerging during this period were successful. For instance, some initially promising technologies, such as vaccines based on antiidiotype antibodies (30), synthetic B- and T-cell epitopes, and DNA vaccines did not lead to vaccine licensure (31).
Finally, a new platform that is going to help all vaccines is the development of novel adjuvants. After a century when the only adjuvants licensed for human use were hydroxide and phosphate salts of aluminium (alum), the licensure of the new adjuvant MF59 led to the improved effectiveness of seasonal influenza vaccines in the elderly (32) and the creation of vaccines against pandemic influenza strains such as H5N1 or H7N9, that without adjuvants, are not effective. Additionally, recent clinical data have demonstrated that MF59 can improve the efficacy of seasonal influenza vaccines also in children from 43% to 86% (33). Other adjuvants recently licensed for human use are ASO3 and ASO4 for influenza and HPV, respectively (34). The potential of developing novel adjuvants has increased exponentially with the discovery of innate immune receptors such as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLR) as reviewed Philpott and coworkers (35). Several TLR agonists have already been tested in many clinical trials; however, the potential of this field resides in the use of medicinal chemistry and formulation strategies to rationally design and optimize TLR adjuvants inducing the appropriate immune response in the absence of systemic effects. Different adjuvants can synergize if combined in the same formulation. For example, AS01 is a mix of liposome, saponin, and monophosphoryl lipid A, and was used to enhance the efficacy in the RTS,S malaria vaccine. In a phase II study RTS,S/AS01 showed 53% efficacy against first malaria episodes in children (36).
Emerging Technologies to Unleash the Power of the Immune System for the Prevention and Therapy of Tumors and Chronic Infections
Vaccines that prevent infections can improve the quality of life of special target populations suffering from several diseases. For example, people with chronic infections, cancer, and autoimmune and neurodegenerative diseases need special attention and require more frequent vaccination with antiinfective vaccines, such as influenza and pneumococcus. In the future, these special target populations may benefit from vaccination against additional pathogens such as CMV, Staphylococcus, and tuberculosis. Similarly, patients suffering from sickle-cell anemia may benefit from a vaccine against parvovirus B19, which is particularly dangerous to this population. However, the sizeable challenge the field of vaccination will face is whether vaccination can cure or stave off these underlying diseases. Recently, it has been shown that the immune system can indeed cure HIV and cancer, two results that were beyond imagination just few years ago (37–39). The first observation is that passive administration of potent and broadly neutralizing monoclonal antibodies against the HIV envelope can rapidly reduce plasma viremia to undetectable levels and keep it at bay for months, as shown in rhesus monkeys chronically infected with pathogenic simian-human immunodeficiency virus SHIV-SF162P3 (37). This finding suggests that if we are able to induce such antibodies by vaccination, we would be able not only to prevent HIV infection, but also to cure chronically infected people.
The second observation is that infusion with autologous T cells engineered in vitro to express high-affinity chimeric antigen receptors (CARs) targeting the CD19 antigen present in B cells can proliferate and efficiently eliminate aggressive, treatment-refractory leukemia cells from patients with chronic lymphocytic leukemia and acute lymphoblastic leukemia, resulting in complete tumor remission (38, 39). These results demonstrate that appropriately harmed T cells have the power to specifically eliminate large amounts of established tumors.
The third observation is that antibodies blocking the negative regulators of T-cell activation such as cytotoxic T-cell antigen 4 (CTLA4) and programmed cell death 1 (PD-1) alone, but especially in combination, can induce rapid tumor regression in a large proportion of melanoma patients (40). This shows that by removing the brakes that cancer and chronic infectious diseases impose on the immune system, we can empower natural immunity to fight these diseases.
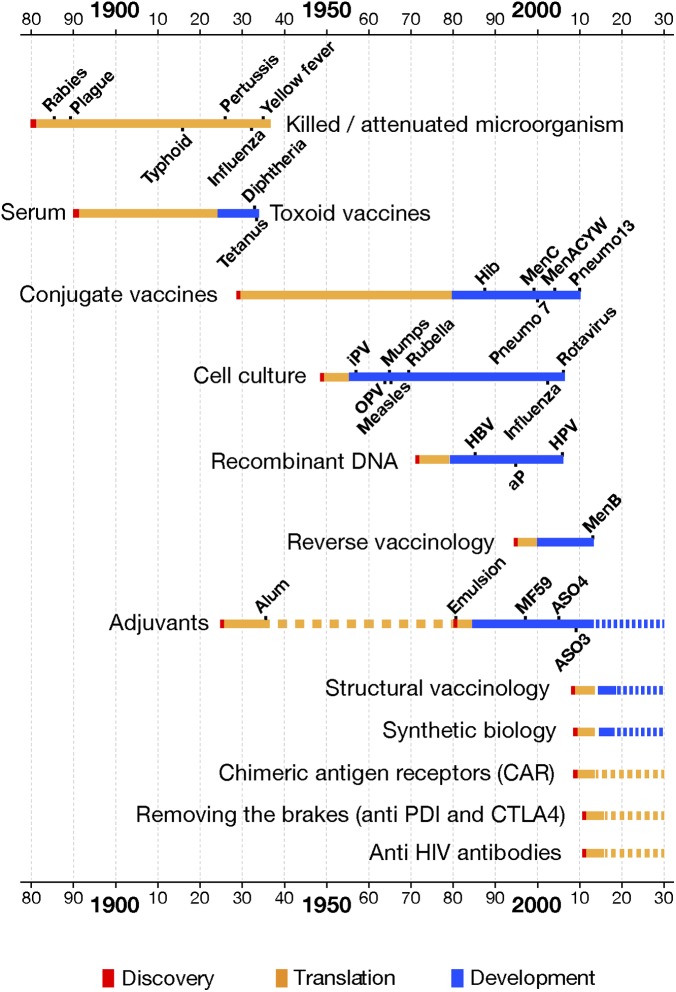
The three examples above show that the immune system has the power to control and eliminate chronic infections and tumors. The question is whether we can use vaccination alone or in combination with immunotherapy to elicit a protective immune response. Somehow in these areas we are in a situation similar to that in which Behring and Kitasato found themselves in 1890 (41, 42). They had shown that passive administration of antibodies contained in a serum protected against diphtheria and tetanus, proving the principle that the immune system could prevent and control these diseases. However, considerable time passed before they could find a safe way to educate the immune system into doing so naturally by vaccination. The great challenge for the vaccine field is whether vaccines will be able to unleash the full power of the immune system and succeed in prevention and cure of these diseases in the near future. How long will it take to translate these new findings into routine vaccination practices? Fig. 2 shows that, after scientific discovery, in most cases (shown in red in Fig. 2) a long period has been historically necessary to translate the discovery into safe and scalable vaccine technology (shown in orange in Fig. 2). Finally, an additional time is required to develop, license, and make one or more vaccines deriving from that technology (blue bars in Fig. 2) available to the public. What is striking in Fig. 2 is the observation that the time from ideation to development and finally to registration did not progressively shorten with any progress in science and technologies. New progress in immunology and in systems biology is paving the way for toolkits, which could enable prediction of whether a subject will respond well or poorly to a vaccination and whether a given formulation will have the appropriate tolerability needed to guarantee safe progression toward full-blown development (43). This translational and mechanistic approach may accelerate the development of new, high-demand vaccines.
Fig. 2.
Historical view of the time needed to translate a scientific discovery (shown in red) into a safe and scalable vaccine technology (orange bars). The blue bars represent the time needed to develop and implement one or more vaccines derived from the same technology. IPV, inactivated polio virus; OPV, oral polio virus.
Vaccines for All Ages
During the last century vaccines have been developed mostly against infectious diseases that were a major cause of mortality in children and infants. Thanks to their success, today people live longer and there are only a few major infectious agents still causing life-threatening diseases in infants living in developed countries. Among them there are MenB, GBS, and RSV. However, these diseases will also be conquered soon: A MenB vaccine was recently licensed in Europe, Australia, and Canada; a maternal vaccine preventing GBS (see below) is in late-stage development; and a recent breakthrough in stabilization of the prefusion form of the RSV vaccine F protein suggests that even for RSV a vaccine will become available during the next 10 to 15 y (26). Once these vaccines become widely available, all major diseases in infants and children will be eliminated. Today, we can extend the benefits of vaccination beyond children and infants to all age groups, including the elderly, the population that consumes most of today’s healthcare budgets (44).
The first new population that is already benefiting from vaccines is newborns before they can build their own immunity by vaccination. During this period, usually lasting until 4 to 6 mo of age, infants are exposed to GBS, influenza, RSV, pertussis, meningococcus, and tetanus, among others. Recently it has been found that vaccination of pregnant women during the third trimester against tetanus, pertussis, and influenza induces protective immunity in the mother and in the newborn baby through the transplacental transfer of the antibodies. Pertussis and influenza vaccination of pregnant women is presently recommended by the Center for Disease Control for use in the United States and more recently also by the WHO (45), whereas tetanus vaccination is recommended by the WHO for low-income countries with a high risk of neonatal tetanus. A vaccine against GBS is presently in late-stage development to prevent early and late onset of meningitis and decrease the risk of premature birth and stillbirth (46).
The second population with a high need of vaccines is adolescents. In this age group, a booster is required to increase the protective levels of antibodies against pertussis, meningococcus, diphtheria, tetanus, and influenza. In addition, adolescents are vaccinated to prevent infection with HPV, which could lead to cervix cancer later in life. In the future adolescents could be vaccinated against human herpes virus, CMV, and parvovirus B19.
Adults should also get their annual influenza vaccine and periodic boosts of diphtheria, tetanus, pertussis, meningococcus, hepatitis B, and RSV.
The population that most needs the development of new and better vaccines is the elderly. Aging immune systems expose the elderly to many diseases that occur in the young, such as influenza, RSV, diphtheria, tetanus, pneumococcus, meningococcus, RSV, and Varicella zooster. In some cases a boost with a normal vaccine may be sufficient, as is the case with diphtheria and tetanus. However, in most cases an aged immune system needs more potent vaccines. For instance, in influenza, adjuvanted vaccines or high-dose vaccines have been shown to increase the efficacy in the elderly. Similarly, in zoster, a high dose of varicella vaccine is required.
In addition, the elderly are often exposed to antibiotic-resistant bacteria, especially during hospitalization. Vaccines against Pseudomonas, E. coli, Klebsiella, and S. aureus are within reach of modern technologies and may be developed soon (44). Finally, progress in modern medicine has rendered creation of vaccines to prevent cancer and neurodegenerative and metabolic diseases feasible. The hope is that in the future vaccines may improve the health status of a population that is constantly aging.
Vaccines Against Emerging Infections
Emerging and reemerging infectious diseases have marked human history with devastating events and lead to massive population mortality, such as the Plague of Athens, the Black Death (that killed one-third of the European population in the 14th century), the importation of smallpox in 1520 and the endogenous hemorrhagic fever known as cocoliztli (that killed more than 25 million people in Mexico in the 16th century), and the 1918 influenza pandemic (that killed at least 50 million people) (47, 48). During the last 30 y we experienced the emergence of HIV (which has claimed more than 35 million people), Creutzfeldt–Jakob disease spongiform encephalopathy, severe acute respiratory syndrome, the H1N1 influenza pandemic, and multiple-drug-resistant S. aureus (49). The emergence of new communicable diseases will continue despite modern advances in science and technology because they are mostly derived from the natural evolution of microbes occurring in their natural hosts (animals) while they adapt to their new hosts, humans. Increasing global population, global travel, and climate change, are some of the factors that favor the evolution of new pathogens, and these factors are unlikely to change. One recent example is the emergence of the Middle East respiratory syndrome. Vaccines are, and will continue to be, one of the most important tools for the prevention and control of emerging infections. New technologies such as synthetic biology, adjuvants, and structural vaccinology will help by providing a rapid response from efficacious vaccines against most of these diseases.
Vaccines for the Prevention of and Therapy in Cancer and Neurodegenerative Diseases
Today we know of two types of cancer. There are those induced by chronic infections, such as those of the stomach, caused by Helicobacter pylori; the liver, caused by human hepatitis B (HBV) and C (HCV) viruses; and the cervix, caused by human papillomavirus (HPV), as well as Burkitt lymphoma and nasopharyngeal caricinoma, caused by the Epstein–Barr virus. These can be eliminated just with vaccination against the infectious agent. HBV and HPV vaccines are already in use and are already preventing cancer (50). For cancers that are not associated with infectious agents—such as prostate, breast, colon, etc.—experiments in animal models suggest that early vaccination with properly delivered and adjuvanted self-antigens may induce immune responses able to kill the tumorigenic cells before they grow into large tumors. It is therefore possible to think that in the near future we may vaccinate people when they are in their 50s with vaccines that may delay several cancers for 10 to 20 y or even forever. Cancer therapy can also be improved by vaccination (51, 52). The licensure of the first therapeutic vaccine against prostate cancer is a milestone in the vaccinology field (53). The power of immunotherapy as seen in CAR technology (38, 39) by releasing the brakes of the immune system by anti–PD-1 and anti-CTLA4 (40), combined with the progress in vaccine platforms and adjuvants, shows that curing cancer, most likely in combination with other therapies, is within the capacity of vaccinology. In the case of neurodegenerative diseases, passive and active immunizations can reduce the levels of the molecules associated with dementia, such as amyloid-β and tau (54). Although clinical trials have not yet produced encouraging results, conceivably this could change in the near future as we learn more about how to measure benefits and improve trial design (55).
Vaccines for Impoverished Populations
The global convergence by 2035 shown in Fig. 1 requires vaccination to be available globally against most communicable diseases, especially those present only in low-income countries. Although thanks to GAVI, the Vaccine Fund, and the Bill and Melinda Gates Foundation we have made enormous progress in providing already existing vaccines, we are still struggling to develop those vaccines because there is no market to justify the investments in vaccine development. In many cases we have the technologies but not the resources to develop these vaccines for the same reason. Several institutions dedicated to the development of vaccines against diseases present only in low-income countries emerged during the last few years. These include the International Vaccine Institute in Korea, the Novartis Vaccines Institute for Global Health in Italy, the Hillemann Institute in India, the Sabin Vaccine Institute, and the Infectious Disease Research Institute in the United States (44). However, solving this problem will require a much more significant global effort than what we have now. A paper fully dedicated to this problem is published in PNAS (56).
The Value of Vaccines
Up to very recently decisions about vaccine implementation were simple to make. When three of five children were dying before the age of 25 (57), having succumbed to smallpox, polio, tetanus, diphtheria, or other infectious diseases, it did not take sophisticated health economics calculations to decide whether vaccines to save their lives should be used. Thanks to the success of vaccines and hygiene practices, today parents, doctors, and healthcare officials no longer have first-hand experience with these devastating diseases, and often they question whether vaccines are still necessary or whether they are cost-effective. The recent economic crisis has challenged healthcare budgets and a lack of leadership in most decision-making bodies has pressured decision makers to use health economics analysis to decide on vaccine implementation. Unfortunately, there are no appropriate tools to calculate vaccine values, and tools imported from other disciplines have been used, especially therapy, to calculate the cost-effectiveness of vaccines (58). These primitive analyses restrict the calculation to cost/quality-adjusted life-year (QALY) and do not consider the real value of vaccination. As a consequence, the calculated value of vaccines is probably one or more orders of magnitude lower than the effective benefits that vaccines provide to society. Consequently these tools lead to incorrect decisions because vaccines are prioritized purely on the basis of economic considerations without calculating the price we would assign to a human life (59). To overcome this dilemma, the Institute of Medicine has developed a new platform termed “Strategic Multi-Attribute Ranking Tool for Vaccines,” or SMART Vaccines, that tries to capture many of the tangible and intangible attributes of vaccination and improve decision making (60–62). Capturing the value of better health in itself is essential to make better decisions, considering that people are willing to trade off income, pleasure, and convenience for an increase in the quality of life expectancy. A further example of how to improve the calculation of vaccine value is provided by Bloom and coworkers in this issue of PNAS (63).
Conclusions
Vaccination has done an incommensurable work for our society and has contributed to the decrease of communicable diseases that used to kill people before the age of 20. Today vaccines have the potential to make a similar contribution to an aging society, by decreasing communicable diseases for all age groups, especially the elderly, in both high- and low-income countries. In addition, thanks to the great technological progress made, vaccines may help keep people healthy by also preventing and curing noncommunicable diseases, including cancer and neurodegenerative diseases. For this to happen, appropriate health economics models able to capture the value of an improved quality of a healthy life are necessary.
Supplementary Material
Acknowledgments
This project has received funding from the European Union’s Seventh Programme for research, technological development, and demonstration under Grant Agreement 280873 [Advanced Immunization Technologies (ADITEC)] and from Innovative Medicines Initiative 115308 [Biomarkers for Enhanced Vaccine Safety (BIOVACSAFE)].
Footnotes
Conflict of interest statement: All authors are full-time employees of Novartis Vaccines.
This article is part of the special series of PNAS 100th Anniversary articles to commemorate exceptional research published in PNAS over the last century.
This article is a PNAS Direct Submission.
References
- 1.Arias E. United States life tables, 2008. Natl Vital Stat Rep. 2012;61(3):1–64. [PubMed] [Google Scholar]
- 2.van Panhuis WG, et al. Contagious diseases in the United States from 1888 to the present. N Engl J Med. 2013;369(22):2152–2158. doi: 10.1056/NEJMms1215400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy SL, et al. Deaths. Final data for 2010. Natl Vital Stat Rep. 2013;61(4):1–118. [PubMed] [Google Scholar]
- 4.Oldstone MBA. Viruses, Plagues, and History. Oxford: Oxford Univ Press; 1978. [Google Scholar]
- 5.Wang H, et al. Age-specific and sex-specific mortality in 187 countries, 1970-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2071–2094. doi: 10.1016/S0140-6736(12)61719-X. [DOI] [PubMed] [Google Scholar]
- 6.Keja K, Chan C, Hayden G, Henderson RH. 1988. Expanded programme on immunization. World Health Statistics Quarterly. Rapport rimestriel de statistiques sanitaires mondiales 41(2):59–63. [PubMed]
- 7. World Health Organization (2013) Global Vaccine Action Plan 2011 - 2020. Available at www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/index.html. Accessed June 1, 2014.
- 8.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamison DT, et al. Global health 2035: A world converging within a generation. Lancet. 2013;382(9908):1898–1955. doi: 10.1016/S0140-6736(13)62105-4. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous Grand convergence: A future sustainable development goal? Lancet. 2014;383(9913):187. doi: 10.1016/S0140-6736(14)60051-9. [DOI] [PubMed] [Google Scholar]
- 11.Thucydides . History of the Peloponnesian War. Chicago: Univ of Chicago Press; 1989. [Google Scholar]
- 12.Jenner E. An Inquiry into the Causes and Effects of the Variolae Vaccinae. 1798;(Sampson Low, London) [Google Scholar]
- 13.Tognotti E. The eradication of smallpox, a success story for modern medicine and public health: what lessons for the future? J Infect Dev Ctries. 2010;4(5):264–266. doi: 10.3855/jidc.1204. [DOI] [PubMed] [Google Scholar]
- 14.Pasteur L. De l'attenuation du virus du cholera des poules. C R Acad Sci Paris. 1880;91:673–680. French. [Google Scholar]
- 15.Pasteur L. Méthode pour prévenir la rage après morsure. C R Acad Sci Paris. 1885;101:765–772. French. [Google Scholar]
- 16.Plotkin SA, Plotkin SL. The development of vaccines: How the past led to the future. Nat Rev Microbiol. 2011;9(12):889–893. doi: 10.1038/nrmicro2668. [DOI] [PubMed] [Google Scholar]
- 17. Plotkin S (2014) History of vaccination. Proc Natl Acad Sci USA 111:12283–12287. [DOI] [PMC free article] [PubMed]
- 18.Valenzuela P, Medina A, Rutter WJ, Ammerer G, Hall BD. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982;298(5872):347–350. doi: 10.1038/298347a0. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology. 1991;185(1):251–257. doi: 10.1016/0042-6822(91)90772-4. [DOI] [PubMed] [Google Scholar]
- 20.Pizza M, et al. Mutants of pertussis toxin suitable for vaccine development. Science. 1989;246(4929):497–500. doi: 10.1126/science.2683073. [DOI] [PubMed] [Google Scholar]
- 21.Rappuoli R. Rational design of vaccines. Nat Med. 1997;3(4):374–376. doi: 10.1038/nm0497-374. [DOI] [PubMed] [Google Scholar]
- 22.Schneerson R, et al. Haemophilus influenzae type B polysaccharide-protein conjugates: Model for a new generation of capsular polysaccharide vaccines. Prog Clin Biol Res. 1980;47:77–94. [PubMed] [Google Scholar]
- 23.Pizza M, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287(5459):1816–1820. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 24.Sette A, Rappuoli R. Reverse vaccinology: Developing vaccines in the era of genomics. Immunity. 2010;33(4):530–541. doi: 10.1016/j.immuni.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dormitzer PR, Ulmer JB, Rappuoli R. Structure-based antigen design: A strategy for next generation vaccines. Trends Biotechnol. 2008;26(12):659–667. doi: 10.1016/j.tibtech.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLellan JS, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342(6158):592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correia BE, et al. Proof of principle for epitope-focused vaccine design. Nature. 2014;507(7491):201–206. doi: 10.1038/nature12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dormitzer PR, et al. Synthetic generation of influenza vaccine viruses for rapid response to pandemics. Sci Trans Med. 2013;5(185):185ra168. doi: 10.1126/scitranslmed.3006368. [DOI] [PubMed] [Google Scholar]
- 29.Hekele A. Rapidly produced SAM® vaccine against H7N9 influenza is immunogenic in mice. Emerg Microb Infect. 2013;2:e52. doi: 10.1038/emi.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ladjemi MZ. Anti-idiotypic antibodies as cancer vaccines: Achievements and future improvements. Front Oncol. 2012;2:158. doi: 10.3389/fonc.2012.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulmer JB, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259(5102):1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 32.Mannino S, et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am J Epidemiol. 2012;176(6):527–533. doi: 10.1093/aje/kws313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vesikari T, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011;365(15):1406–1416. doi: 10.1056/NEJMoa1010331. [DOI] [PubMed] [Google Scholar]
- 34.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22(3):411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 35. Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E (2014) Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc Natl Acad Sci USA 111:12294–12299. [DOI] [PMC free article] [PubMed]
- 36.Bejon P, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359(24):2521–2532. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barouch DH, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503(7475):224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Behring E, et al. Uber das Zustandekommen der Diphtherie-Immunitat und der Tetanus-Immunitat bie Tieren. Dtsch Med Wochenschr. 1890;16:1113–1114. German. [PubMed] [Google Scholar]
- 42.von Behring E. Uber das Zustandekommen der Diphtherie-Immunitat und der Tetanus-Immunitat bie Tieren. Dtsch Med Wochenschr. 1890;16:1145–1148. German. [PubMed] [Google Scholar]
- 43.Rappuoli R, Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473(7348):463–469. doi: 10.1038/nature10124. [DOI] [PubMed] [Google Scholar]
- 44.Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11(12):865–872. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization Meeting of the Strategic Advisory Group of Experts on immunization, April 2012—conclusions and recommendations. Wkly Epidemiol Rec. 2012;87(21):201–216. [PubMed] [Google Scholar]
- 46.Rappuoli R, Black S. Introduction: Addressing the challenge of group B streptococcal disease. Vaccine. 2013;31(Suppl 4):D1–D2. doi: 10.1016/j.vaccine.2013.06.072. [DOI] [PubMed] [Google Scholar]
- 47.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430(6996):242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Acuna-Soto R, Stahle DW, Cleaveland MK, Therrell MD. Megadrought and megadeath in 16th century Mexico. Emerg Infect Dis. 2002;8(4):360–362. doi: 10.3201/eid0804.010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morens DM, Fauci AS. Emerging infectious diseases: Threats to human health and global stability. PLoS Pathog. 2013;9(7):e1003467. doi: 10.1371/journal.ppat.1003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kane MA. Preventing cancer with vaccines: Progress in the global control of cancer. Cancer Prev Res (Phila) 2012;5(1):24–29. doi: 10.1158/1940-6207.CAPR-11-0533. [DOI] [PubMed] [Google Scholar]
- 51.Lollini PL, Cavallo F, Nanni P, Forni G. Vaccines for tumour prevention. Nat Rev Cancer. 2006;6(3):204–216. doi: 10.1038/nrc1815. [DOI] [PubMed] [Google Scholar]
- 52.Naz RK, Shiley B. Prophylactic vaccines for prevention of prostate cancer. Front Biosci (Schol Ed) 2012;4:932–940. doi: 10.2741/s309. [DOI] [PubMed] [Google Scholar]
- 53.Michael A, Relph K, Annels N, Pandha H. Prostate cancer vaccines. Expert Rev Vaccines. 2013;12(3):253–262. doi: 10.1586/erv.13.27. [DOI] [PubMed] [Google Scholar]
- 54.Panza F, et al. Immunotherapy for Alzheimer’s disease: From anti-β-amyloid to tau-based immunization strategies. Immunotherapy. 2012;4(2):213–238. doi: 10.2217/imt.11.170. [DOI] [PubMed] [Google Scholar]
- 55.Lambracht-Washington D, Rosenberg RN. Advances in the development of vaccines for Alzheimer’s disease. Discov Med. 2013;15(84):319–326. [PMC free article] [PubMed] [Google Scholar]
- 56. MacLennan S (2014) Vaccines against poverty. Proc Natl Acad Sci USA 111:12307–12312. [DOI] [PMC free article] [PubMed]
- 57.Blower S, Bernoulli D. An attempt at a new analysis of the mortality caused by smallpox and of the advantages of inoculation to prevent it. 1766. Rev Med Virol. 2004;14(5):275–288. doi: 10.1002/rmv.443. [DOI] [PubMed] [Google Scholar]
- 58.Black S. The role of health economic analyses in vaccine decision making. Vaccine. 2013;31(51):6046–6049. doi: 10.1016/j.vaccine.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 59.Mekalanos JJ. Vaccine economics: What price has human life? Sci Transl Med. 2013;5(204):204ed16. doi: 10.1126/scitranslmed.3007588. [DOI] [PubMed] [Google Scholar]
- 60. Institute of Medicine of the National Academies (2012) Ranking Vaccines: A Prioritization Framework - Phase I: Demonstration of Concept and a Software Blueprint. Available at www.nap.edu/smartvaccines. Accessed June 1, 2014.
- 61. Institute of Medicine of the National Academies (2013) Ranking Vaccines: A Prioritization Software Tool - Phase II: Prototype of a Decision-Support System. Available at www.nap.edu/smartvaccines. Accessed June 1, 2014.
- 62.Phelps C, et al. A priority-setting aid for new vaccine candidates. Proc Natl Acad Sci USA. 2014;111(9):3199–3200. doi: 10.1073/pnas.1400945111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bärnighausen T, Bloom DE, Cafiero-Fonseca ET, O'Brien JC (2014) Valuing vaccination. Proc Natl Acad Sci USA 111:12313–12319. [DOI] [PMC free article] [PubMed]