Significance
Malaria caused by Plasmodium falciparum results in the death of between 500,000 and 800,000 children per year and thus presents a major infectious disease threat to public health. Antibodies directed against the circumsporozoite protein of the infectious form of the parasite can prevent P. falciparum infection. Nevertheless, current candidate malaria vaccines do not elicit consistent, durable protection. Here we demonstrate that an alternative to conventional immunization, antibody gene delivery by a viral vector [vectored immunoprophylaxis (VIP)], can direct sustained expression of protective mAb in vivo and prevent P. falciparum infection in a rodent model. These studies provide a proof of principle for the use of VIP against the deadliest form of human malaria.
Keywords: AAV, vectored gene delivery
Abstract
Malaria caused by Plasmodium falciparum kills nearly one million children each year and imposes crippling economic burdens on families and nations worldwide. No licensed vaccine exists, but infection can be prevented by antibodies against the circumsporozoite protein (CSP), the major surface protein of sporozoites, the form of the parasite injected by mosquitoes. We have used vectored immunoprophylaxis (VIP), an adeno-associated virus-based technology, to introduce preformed antibody genes encoding anti-P. falciparum CSP mAb into mice. VIP vector-transduced mice exhibited long-lived mAb expression at up to 1,200 µg/mL in serum, and up to 70% were protected from both i.v. and mosquito bite challenge with transgenic Plasmodium berghei rodent sporozoites that incorporate the P. falciparum target of the mAb in their CSP. Serum antibody levels and protection from mosquito bite challenge were dependent on the dose of the VIP vector. All individual mice expressing CSP-specific mAb 2A10 at 1 mg/mL or more were completely protected, suggesting that in this model system, exceeding that threshold results in consistent sterile protection. Our results demonstrate the potential of VIP as a path toward the elusive goal of immunization against malaria.
Among infectious diseases, malaria ranks fourth as a cause of death. In Africa in 2012, 500,000–800,000 deaths, mostly among children under 5 y of age, resulted from ∼200 million clinical cases of malaria caused by Plasmodium falciparum, the parasite species responsible for most malaria mortality (1). In addition to its direct effect on health, malaria imposes severe economic burdens. These include crippling treatment costs at the level of individual families and a significant contribution to low national incomes and reduced overall rates of economic growth in nations worldwide (2, 3). The human and economic burdens imposed by malaria make malaria reduction a critical global priority.
Traditional approaches to malaria control such as antimalarial drug treatment, mosquito control by habitat modification and insecticide use, and reduction of exposure to infected mosquitoes have had substantial success in reducing malaria incidence and malaria-specific mortality rates (4). However, these achievements may be difficult to sustain in the face of growing drug resistance among parasites, insecticide resistance in vector populations, and the reduced funding for malaria control anticipated by the World Health Organization for the near future (4). If continued progress against malaria is to be made, it is essential that new approaches for malaria prevention be added to currently available tools.
Sporozoites are the infectious form of the malaria parasite injected by Anopheles mosquitoes. It has been known for decades that immunization of animals or humans with radiation-attenuated sporozoites can elicit sterilizing immunity to malaria, preventing infection, pathogenesis, and transmission (5–8). The predominant antibody response to immunization by irradiated sporozoites is to the circumsporozoite protein (CSP), which coats the sporozoite surface (9, 10). In in vivo and in vitro models of P. falciparum sporozoite infection, infection can be blocked completely by antibody to the immunodominant epitope of CSP, a tetrapeptide [asn-ala-asn-pro (NANP)] found in 30 or more tandemly repeated copies in the central region of the protein (11–13). The NANP repeat is stringently conserved in P. falciparum isolates from diverse geographical locations (14) and thus represents a potentially universal target for P. falciparum immunity. The most advanced malaria infection-blocking vaccine candidate, RTS,S, is comprised of hepatitis B virus-like particles that display a carboxyl-terminal segment of P. falciparum CSP, including part of the central NANP repeat region. Immunization with RTS,S reduces incidence of clinical malaria by 40–70% in children, but levels of protection wane with time (15–18).
Vectored immunoprophylaxis (VIP) provides an alternative to conventional immunization as a route to protective antibody expression (19–21). VIP employs optimized adeno-associated virus (AAV) based vectors to deliver genes encoding mAb with previously characterized specificities to animals. Intramuscular injection of VIP vectors in mice and macaques elicits long-lived antibody or antibody-related immunoadhesin production at levels sufficient to protect against HIV, simian immunodeficiency virus, and influenza A virus infection (19–22). Here we report that in mice, VIP-directed production of sporozoite-neutralizing mAb against the P. falciparum CSP central repeat can confer sterile immunity to infection by a transgenic strain of the rodent parasite Plasmodium berghei whose CSP contains the P. falciparum CSP central repeat [Pb/Pf (12)].
Results
Construction and Characterization of VIP Vectors Expressing mAb Against P. falciparum CSP.
The VIP system (20) permits construction of vectors that express human IgG (hIgG) antibodies that bear the variable region sequences, and thus possess the binding specificities, of previously characterized mAb. Briefly, synthetic DNA segments encoding the variable regions of the mAb are inserted into an optimized hIgG gene in a modular AAV2-derived plasmid backbone that includes a muscle-optimized promoter, a splice donor and splice acceptor pair, a polyadenylation signal, and a posttranscriptional enhancer that together ensure efficient transgene expression. The plasmid also contains AAV2 inverted terminal repeats to permit vector genome encapsidation. AAV particles containing the transgene are produced by transfection of cells in tissue culture with the VIP vector plasmid and helper plasmids that supply required adenovirus products, AAV rep and AAV8 capsid protein. Intramuscular injection of the VIP virions results in production of the hIgG transgene product by transduced muscle cells in vivo. To explore the potential of VIP against P. falciparum, VIP vectors encoding hIgG specific for the P. falciparum CSP central repeat were constructed by inserting the variable regions of mouse mAb 2A10 and 2C11 (23, 24) into the hIgG framework of the VIP expression plasmid (20). The 2A10 and 2C11 mAb each recognize three or more tandem repetitions of the CSP NANP central repeat epitope. Both inhibit hepatic cell invasion by P. falciparum sporozoites in vitro, and passively transferred 2A10 prevents infection of mice by transgenic Pb/Pf parasites. The vectors, 2A10-AAV and 2C11-AAV, expressed hIgG in vitro at levels comparable to those directed by a VIP vector that encodes the HIV mAb b12 and protects humanized mice against HIV infection (b12-AAV; Fig. S1) (20).
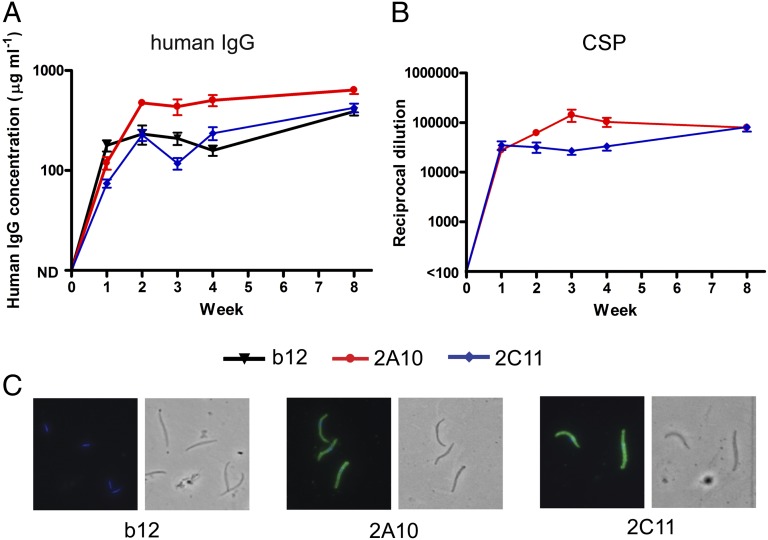
C57BL/6 mice were injected in the cranial thigh muscle with 1 × 1011 genome copies (GC) of 2A10-AAV, 2C11-AAV, b12-AAV, or with buffer. Within 1 wk, AAV-transduced mice expressed hIgG at 50–500 µg/mL in serum (Fig. 1A). Expression increased until ∼4 wk posttransduction, reaching 1,000 µg/mL in two mice, and was sustained through 8 wk when transduced mice were challenged. The 2A10-AAV and 2C11-AAV–transduced mice expressed antibodies that bound recombinant CSP (Fig. 1B) and that recognized whole P. falciparum sporozoites (Fig. 1C). Sera from b12-AAV–transduced mice recognized neither recombinant CSP nor sporozoites.
Fig. 1.
VIP vectors drive expression of human anti-CSP antibodies in mice. Quantification of hIgG (A) and anti-CSP antibodies (B) in serum taken from female C57BL/6 mice at indicated times after intramuscular injection with 1011 GC of vectors 2A10-AAV or 2C11-AAV encoding anti-P. falciparum CSP mAb, b12-AAV encoding anti-HIV glycoprotein120 mAb b12, or vehicle alone (n = 10 per group). Mice transduced with vehicle alone had hIgG below the limit of detection. Means and SEs are shown for the 10 mice in each group. (C) Immunofluorescence micrographs of P. falciparum sporozoites incubated with pooled sera from mice transduced with the indicated vector and stained with anti-human IgG FITC conjugates. The phase contrast images cover the same fields and show the location of sporozoites.
VIP has induced extended mAb production in mice (19, 20). To determine the longevity of the expression of malaria mAb, hIgG in additional 2A10-AAV, 2C11-AAV, and b12-AAV–transduced mice were monitored over several months. Serum antibody concentrations reached a plateau 4–8 wk posttransduction and were maintained at that level for the duration of the study (52 wk; Fig. S2).
i.v. Challenge of AAV-Transduced Mice with Pb/Pf Sporozoites.
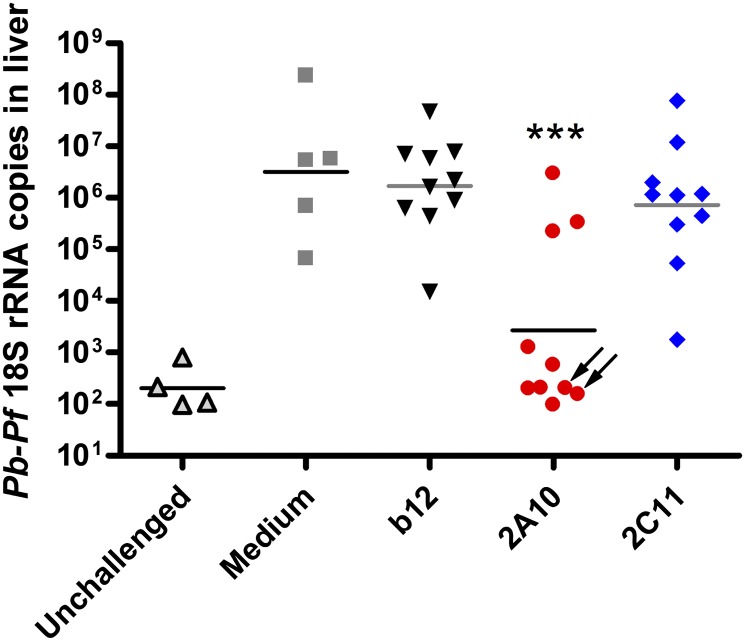
To determine whether VIP-directed mAb production was protective against malaria infection, transduced mice were challenged with Pb/Pf sporozoites either injected i.v. or introduced by the bites of infected mosquitoes. For i.v. challenge, 1 × 104 Pb/Pf sporozoites isolated from infected Anopheles stephensi mosquitoes were injected into the tail vein of mice 8 wk posttransduction. Challenged mice were killed 40–42 h postchallenge, when infectious sporozoites would have successfully invaded and replicated in liver cells. RNA was extracted from whole liver homogenates, and parasites in the liver (liver parasite burden) were quantified by quantitative PCR (qPCR) measurement of P. berghei 18S rRNA (25). The 2A10-AAV–transduced mice had a statistically significant reduction in liver parasite burden compared with b12-AAV mice (Fig. 2), and 7 of 10 2A10-AAV–transduced mice had parasite 18S rRNA levels that were at the background levels seen in unchallenged control mice. Both 2A10 and 2C11-transduced mice displayed a statistically significant decrease in parasite rRNA levels in a second, independent challenge with a different number (2 × 104) of Pb/Pf sporozoites (Fig. S3).
Fig. 2.
VIP-encoded CSP antibodies reduce liver parasite burden. The mice shown in Fig. 1 were challenged i.v. with 1 × 104 Pb/Pf sporozoites isolated from infected A. stephensi mosquitoes 8 wk posttransduction with b12-AAV, 2A10-AAV, 2C11-AAV, or mock transduction. Liver parasite burdens were assessed 40–42 h postchallenge by qRT-PCR measurement of P. berghei 18S rRNA in liver homogenates. Plotted are values for individual mice (n = 5 or 10) and the geometric mean of each group. Unchallenged mice did not receive sporozoites (n = 4). Asterisks indicate mean rRNA levels significantly different (P < 0.001) from b12 control mice by two-tailed t test (n = 10). Arrows indicate mice with a detectable anti-human Fc humoral immune response.
Challenge of VIP-Transduced Mice with Pb/Pf Sporozoites by Infected Mosquito Bite.
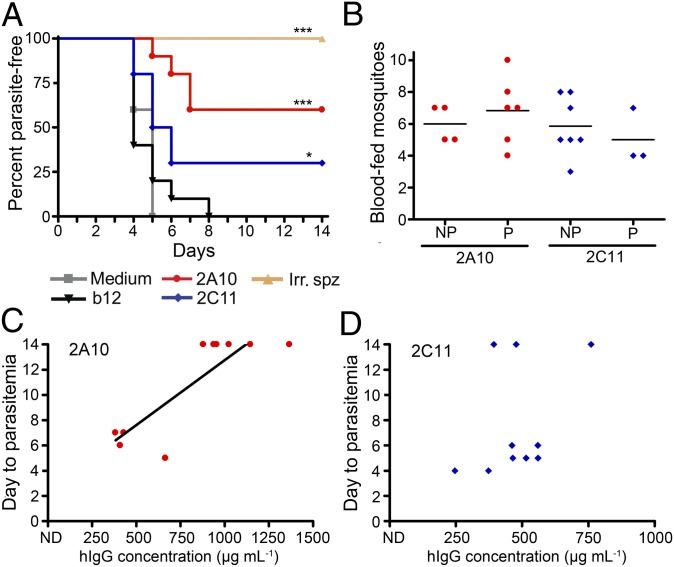
Mosquito bites are the natural route of exposure to malaria. Therefore, groups of transduced mice were challenged by exposure to Pb/Pf-infected mosquitoes. Mice immunized with three i.v. injections of radiation-attenuated Pb/Pf sporozoites were included as a positive protection control. Starting at 4 d postchallenge, mice were checked daily by blood smear to determine when blood-stage parasites (parasitemia) appeared. Parasitemia indicates that sporozoites escaped the anti-CSP mAb and successfully invaded the liver, where they replicate and differentiate to produce blood-stage infection. In a bite challenge, freedom from parasitemia for 14 d indicates complete protection. Sixty percent of 2A10- and 30% of 2C11-AAV–transduced mice did not develop parasitemia by 14 d postexposure, demonstrating sterile protection (Fig. 3A). Both groups of CSP mAb-expressing mice also displayed significant delays in time to parasitemia compared with the b12-AAV control group by Kaplan–Meier survival analysis. All mice vaccinated with irradiated sporozoites were protected from challenge (Fig. 3A), and all mock-transduced mice and those receiving b12-AAV exhibited parasitemia by day 8. To confirm that the sterile protection observed was not a consequence of poor feeding by mosquitoes on transduced mice, the number of mosquitoes that took a blood meal from each mouse was determined. Protected and parasitemic mice fed similar average numbers of mosquitoes (Fig. 3B) and consequently received similar sporozoite inocula.
Fig. 3.
VIP can provide sterile protection in mice challenged by infected mosquito bite. (A) Mice transduced with 1011 GC of 2A10-, 2C11-, or b12-AAV, or mock-transduced (n = 10 per group) were challenged 11 wk posttransduction by mosquito bites from 10 Pb/Pf infected A. stephensi mosquitoes for 5 min. An additional group of mice immunized by three injections with 1 × 105 to 1.5 × 105 irradiated Pb/Pf sporozoites (Irr. spz.) were also challenged (n = 5). Mice were microscopically assessed for blood-stage parasites by blood smear daily, starting at day 4 postchallenge through day 14, when nonparasitemic mice were considered sterilely protected. Asterisks denote a statistically significant difference in survival compared with b12-transduced control mice. *P < 0.05, ***P < 0.005 by Kaplan–Meier logrank test. (B) Number of mosquitoes taking a blood meal from mice that eventually became infected (NP) or from protected mice (P). No significant differences were observed in number of bites per mouse (P = 0.6095 for 2A10; P = 0.3883 for 2C11-AAV by a Mann–Whitney test). The number of mosquitoes that fed on each mouse was determined by the examination of mosquitoes in each challenge cup for the presence of blood in the midgut. (C and D) HIgG levels in individual transduced mice, determined by ELISA immediately before challenge, plotted against time to the appearance of parasitemia in 2A10- (C) and 2C11-AAV–(D) transduced mice. A linear regression line is shown in C for visualization of the correlation of IgG concentration with time to parasitemia for 2A10 (r = 0.78 by a two-tailed Spearman test).
Protection from HIV and influenza infection is correlated with serum antibody levels (19, 20). Therefore, we compared the hIgG levels in 2A10-AAV and 2C11-AAV–transduced mice immediately before challenge with time to parasitemia in bite-challenged groups (Fig. 3 C and D and Fig. S4). A statistically significant correlation was seen between antibody concentration and time to parasitemia for 2A10-AAV mice (P = 0.0105, Fig. 3C). No significant correlation was seen in bite-challenged 2C11-AAV mice (Fig. 3D).
Dose–Response in VIP-Transduced Mice.
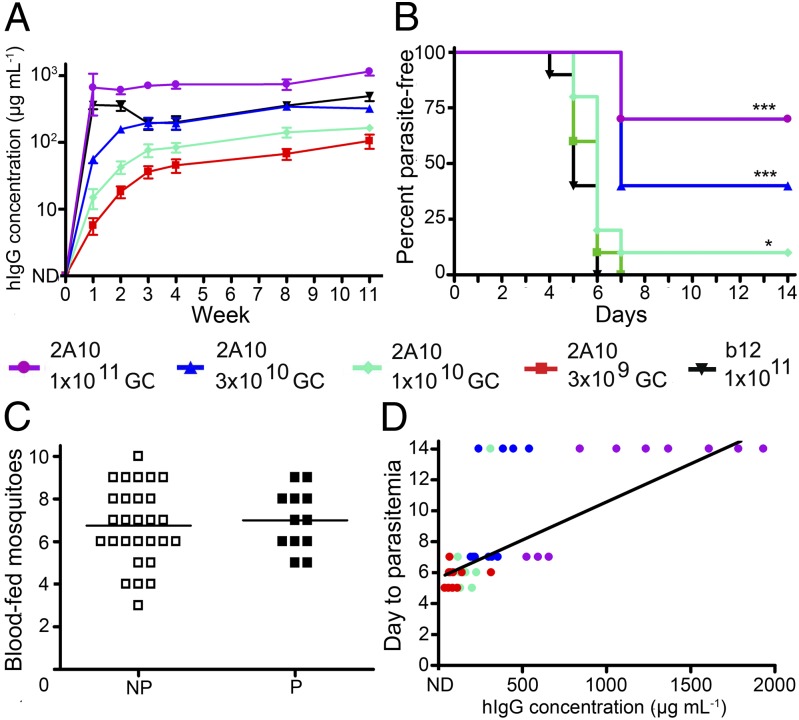
To examine the effects of vector dose on mAb production and the effects of mAb concentration on protection systematically, groups of 10 mice were transduced with doses of 2A10-AAV ranging from 3 × 109 GC to 1011 GC, or with 1011 GC of b12-AAV. hIgG expression showed vector dose dependence at each time point examined (Fig. 4A). Mice were challenged by infected mosquito bite at 11 wk posttransduction and exhibited dose-dependent protection: 70% of mice that received the highest AAV dose (1 × 1011 GC) were protected, as were 40% of mice that received 3 × 1010 GC and 10% of mice that received 1 × 1010 GC (Fig. 4B). All mice transduced with 3 × 109 GC were parasitemic by day 7, and all b12-AAV mice were parasitemic by day 6. The number of mosquitoes taking a blood meal did not impact development of parasitemia (Fig. 4C), but the correlation between 2A10 antibody concentration and day to parasitemia was highly statistically significant (P = 0.0013, Fig. 4D). Notably, all six mice that had greater than 1,000 µg/mL of mAb 2A10 were completely protected from sporozoite challenge. Thus, high mAb concentration contributes to protective efficacy, and in this system, a 2A10 mAb level of 1,000 µg/mL provides reliable protection against sporozoite infection.
Fig. 4.
Antibody expression and protection from sporozoite challenge is dependent upon vector dose. Mice were injected intramuscularly with 1 × 1011, 3 × 1010, 1 × 1010, or 3 × 109 GC of 2A10-AAV or 1 × 1011 GC of b12-AAV. (A) Expression of mAb 2A10 and b12 determined by total hIgG ELISA on serum samples at indicated times. Plot shows the mean and SE for the 10 mice in each group. (B) A Kaplan–Meier survival curve plotting percent of parasite-free mice over time following challenge with bites from 10 Pb/Pf infected A. stephensi mosquitoes for 5 min. *P < 0.05, ***P < 0.005 by Kaplan–Meier logrank test. (C) The number of blood-fed mosquitoes for individual mice that eventually developed blood-stage parasitemia (NP) or that were protected from challenge (P). (D) The day to detection of parasitemia for each mouse as a function of hIgG concentration. Each circle denotes an individual mouse, and a linear regression is shown for visualization of correlation (r = 0.4424; two-tailed Spearman test). The vector dose received by individual mice is indicated in D by the color of the symbol.
Discussion
These data show that in a murine model of human P. falciparum infection, a single intramuscular injection of a VIP vector encoding a mAb that neutralizes malaria sporozoites can confer protection from mosquito-borne malaria infection. In all experiments combined, sterile protection against mosquito bite challenge was achieved in every 2A10-AAV–transduced mouse with a hIgG serum concentration of 1,000 µg/mL or greater (9 out of 9), and in 65% (13 of 20) of mice that received the “standard” vector dose of 1011 GC, regardless of hIgG concentration. For mAb 2C11, serum mAb levels were generally lower than for 2A10, and no mouse achieved a mAb concentration of 1,000 µg/mL Nevertheless, sterile protection was achieved in 3 mice (of 20) inoculated at 1011 GC. The less consistent success of 2C11 in conferring sterile immunity might reflect differences in the fine specificities of the 2A10 and 2C11 mAb (26). The mAb levels apparently required for protection of mice from malaria infection are substantially higher than those required for HIV and influenza (19–21). This probably reflects the limited time sporozoites are exposed to antibodies before liver cell invasion and the capacity of a single unneutralized sporozoite to initiate a full-blown infection (27–30). The efficacy of VIP in protecting against HIV varies among vectors that express different HIV-neutralizing antibodies (20), and surveys of anti-CSP mAb may reveal antibodies more effective in malaria VIP. Targeting other sporozoite antigens, either singly or in combination with CSP, also might provide protection at lower mAb concentrations. It is unknown what levels of VIP-driven mAb production might be achieved in humans, what an effective anti-CSP mAb level might be in humans, or whether mAb other than those examined here might prove effective at a lower serum concentration. These questions must all be considered in looking forward to human trials.
Antigenic diversity presents a major obstacle to vaccine development. Many malaria antigens exhibit antigenic diversity, including CSP (31). However, the conserved CSP central repeat epitope recognized by 2A10 and 2C11 is universally present among P. falciparum isolates (14, 26). Additionally, the NANP epitope is present in multiple tandem copies in each CSP gene, and multiple mutations would be required to ablate reactivity with central repeat mAb. It therefore seems unlikely that CSP antigenic diversity or mutational escape will affect the efficacy of immunoprophylaxis directed against the CSP central repeat. Each Plasmodium species possesses a CSP with a species-specific central repeat region (32), and CSP also provides an attractive target for VIP against other Plasmodium parasites of public health significance.
In macaques, AAV-mediated anti-HIV immunoadhesin production and protection can be long-lived [up to 5 y (22)], suggesting use of VIP for vaccine-like immunization against malaria. In areas where malaria transmission is unstable, VIP might be used to decrease malaria susceptibility to levels where the disease can be locally eliminated (33). The anticipated ability of VIP to confer rapid protection also suggests utility in responding to introduction or reintroduction of the parasite into parasite-free regions and in protecting individuals at risk for infection for shorter periods of time. The roles that VIP might fill in the campaign against malaria will be defined by the properties and efficacy of the technology in humans. However, clinical efficacy of VIP in any of these contexts would be immensely valuable and could provide an exciting new approach to the urgent goal of effective malaria control.
Methods
Mice.
C57BL/6NCr (C57BL/6) female mice 5–8 wk of age were obtained from the National Cancer Institute. Animal experiments were conducted in accordance with the policies and with the approval of the Johns Hopkins University institutional animal care and use committee.
Construction and Production of VIP Vectors.
DNA segments corresponding to the heavy and light chain variable regions of anti-P. falciparum mAb 2A10 and 2C11 (24) were synthesized (Integrated DNA Technologies) and inserted into the hIgG1 constant region framework in the VIP expression vector as previously described (20). VIP vector production, quantitation, and storage were as previously described (20, 34). Briefly, 293T cells were cotransfected with the AAV backbone vector, helper plasmid pHELP (Applied Viromics), and plasmid pAAV2/8 SEED (University of Pennsylvania Vector Core) at a ratio of 0.25:1:2. Culture supernatants were collected at 36, 48, 72, 96, and 120 h after transfection and pooled. Virus was precipitated by a 40% polyethylene glycol solution. Precipitated virus was banded to equilibrium in a 1.36 g mL−1 cesium chloride solution in a Sorvall T1270 rotor at 60,000 rpm for 24 h. Collected fractions exhibiting a refractive index between 1.3755 and 1.3655 were combined and diluted in test formulation buffer 2 (TFB2; 100 mM sodium citrate, 10 mM Tris, pH 8). Virus was concentrated and washed with TFB2 by centrifugation at 500 g at 4 °C using 100 kDa molecular weight cutoff centrifugal filters (Millipore). Virus was aliquoted and stored at −80 °C. Viral genomes were quantified by qPCR.
Vector Administration.
Before intramuscular injection, virus was thawed and diluted to 1 × 1011 GC or the indicated dose with TFB2 in a 50 µL volume. Virus was administered to mice in a single injection of 50 µL into the cranial thigh muscle. At various times following vector administration, mice were bled via the cheek or tail vein.
Quantification of Antibody Production by ELISA.
ELISA plates were coated overnight with 1 µg/well of goat anti-human IgG-Fc antibody (Bethyl) for total hIgG. Plates were blocked with 1% BSA (Sigma) for 1 h. Samples were serially diluted in 1% BSA and incubated in coated wells for 1 h, followed by 1 h incubation with horseradish peroxidase (HRP)-conjugated goat anti-human kappa light chain antibody (Bethyl). Wells were developed using ABTS Peroxidase Substrate 2-Component System (KPL). A standard curve was generated using Human Reference Serum (Bethyl) to quantitate total hIgG.
For detection of CSP antibodies, plates were coated with 0.05 µg per well of recombinant P. falciparum CSP purified from MRA-272 (Malaria Research and Reference Reagent Resource Center) overnight. All subsequent steps are described above, except a standard curve was not used. Purified 2A10 was used as a positive control, and the endpoint ELISA titer was reported as the reciprocal of the highest dilution at which the optical density was two times greater than the background. For detection of CSP antibodies in irradiated sporozoite-immunized mice, an HRP-conjugated anti-mouse IgG (1:5,000 dilution; GE Healthcare) was used as a secondary.
Immunofluorescent Assay.
To determine whether transduced mouse serum recognizes sporozoites, slides (Tekdon, Inc.; Poly-l-Lysine–coated) were spotted with 10 µL of P. falciparum sporozoites at a concentration of 4–6 × 105 sporozoites per milliliter and air-dried. Slides were blocked for 1 h with 10 µL of PBS-1% BSA. Serum samples from each mouse per group were pooled, and 500 ng of hIgG, determined from hIgG ELISA titers, were diluted in PBS-1% BSA and incubated on slides for 1 h at RT. Slides were washed in PBS-1% BSA, and 10 µL of fluorescein isothiocyanate (FITC)-labeled goat anti-monkey IgG (H+L) (KPL) was added for 1 h at RT. Before visualization, slides were washed with PBS-1% BSA, and coverslips were mounted with Prolong Gold antifade reagent with DAPI (Molecular Probes). Fluorescent sporozoites were visualized using an upright fluorescence microscope (Nikon Eclipse 90i).
Irradiated Sporozoite Immunization.
For radiation-attenuated sporozoite immunization, five 5-to-8 wk old C57BL/6 female mice were vaccinated i.v. with 1 × 105 to 1.5 × 105 Pb/Pf sporozoites (see below) previously exposed to 20,000R in a γ irradiator. Mice received a total of three doses of irradiated sporozoites separated by at least 2 wk. Before each dose, mice were bled, and anti-CSP antibody levels were measured.
Sporozoite Challenge.
Pb/Pf-infected A. stephensi mosquitoes provided by the Johns Hopkins Malaria Research Institute Vector and Parasite Cores were dissected in medium for isolation of salivary glands. Glands were spun down at 7,000 rpm for 1 min at 4 °C, the pellet was ground in ∼100 µL of media to break open the salivary glands, and the suspension was spun at 1,000 rpm for 1 min at 4 °C. Supernatant was collected, and sporozoites were counted using a hemocytometer. Mice were challenged by injection into the tail vein with 1.0 × 104 to 2.0 × 104 transgenic Pb/Pf parasites. Approximately 40 h later, mice were killed to assess parasite burden in livers. Whole livers were homogenized in denaturing solution, and RNA was extracted as previously described (35). After cDNA synthesis, parasite burdens were determined by quantitative PCR for P. berghei 18S rRNA (25), and mouse GAPDH was used as an internal control.
For the assessment of sterile protection, A. stephensi mosquitoes infected with Pb/Pf parasites were starved overnight. Mice were anesthetized with 300–350 µL of 2% (wt/vol) Avertin and were individually subjected to feeding from 10 or 15 mosquitoes for 5 min. The number of mosquitoes that fed on each mouse, as indicated by the presence of blood in the midgut, was recorded. Starting on day 4, blood was obtained from the cheek vein daily, and smears were made. Smears were fixed with methanol for 30 s before staining with a 10% Giemsa stain solution (Sigma) for 15 min and observed under a microscope for blood-stage parasites. The day postinfection that blood-stage parasitemia was evident was recorded. After confirmation of parasitemia, mice were killed. Mice were considered to be sterilely protected and were killed if there was no evidence of parasitemia by day 14.
Supplementary Material
Acknowledgments
The authors thank Dr. Christine Zink and Dr. Nathan Pate for performing gross mouse pathology, Dr. Victoria Baxter for invaluable assistance with mice, and Dr. Alan Scott, Dr. Diane Griffin, Dr. Richard Markham, and Dr. David Sullivan for critical comments on the manuscript. This research was supported by a Johns Hopkins Malaria Research Institute pilot grant (to G.K.). C.D. is a recipient of a Bloomberg School of Public Health Sommer Scholarship. A.B.B. was supported by the National Institute of Allergy and Infectious Disease (NIAID) Career Transition Award 1K22AI102769. D.B. was supported by the National Institutes of Health (HHSN266200500035C) through a contract from the NIAID and by the Joint Center for Translational Medicine. F.Z. was supported by National Institutes of Health (NIH) Grant R01AI044375. D.A.E. and C.D. were supported by NIH Grant T32 AI007417.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407362111/-/DCSupplemental.
References
- 1.World Health Organization . World Malaria Report 2013. Geneva: WHO; 2013. [Google Scholar]
- 2.Chuma JM, Thiede M, Molyneux CS. Rethinking the economic costs of malaria at the household level: Evidence from applying a new analytical framework in rural Kenya. Malar J. 2006;5:76–89. doi: 10.1186/1475-2875-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallup JL, Sachs JD. The economic burden of malaria. Am J Trop Med Hyg. 2001;64(1–2 Suppl):85–96. doi: 10.4269/ajtmh.2001.64.85. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . World Malaria Report 2011. Geneva: WHO; 2011. [Google Scholar]
- 5.Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975;24(3):397–401. doi: 10.4269/ajtmh.1975.24.397. [DOI] [PubMed] [Google Scholar]
- 6.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216(5111):160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman SL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185(8):1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 8.Seder RA, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 9.Nardin E, Zavala F, Nussenzweig V, Nussenzweig RS. Pre-erythrocytic malaria vaccine: Mechanisms of protective immunity and human vaccine trials. Parassitologia. 1999;41(1-3):397–402. [PubMed] [Google Scholar]
- 10.Kumar KA, et al. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444(7121):937–940. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 11.Charoenvit Y, et al. Monoclonal, but not polyclonal, antibodies protect against Plasmodium yoelii sporozoites. J Immunol. 1991;146(3):1020–1025. [PubMed] [Google Scholar]
- 12.Persson C, et al. Cutting edge: A new tool to evaluate human pre-erythrocytic malaria vaccines: Rodent parasites bearing a hybrid Plasmodium falciparum circumsporozoite protein. J Immunol. 2002;169(12):6681–6685. doi: 10.4049/jimmunol.169.12.6681. [DOI] [PubMed] [Google Scholar]
- 13.Foquet L, et al. Vaccine-induced monoclonal antibodies targeting circumsporozoite protein prevent Plasmodium falciparum infection. J Clin Invest. 2013;124(1):140–144. doi: 10.1172/JCI70349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zavala F, Masuda A, Graves PM, Nussenzweig V, Nussenzweig RS. Ubiquity of the repetitive epitope of the CS protein in different isolates of human malaria parasites. J Immunol. 1985;135(4):2790–2793. [PubMed] [Google Scholar]
- 15.Stoute JA, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336(2):86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 16.Stoute JA, et al. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J Infect Dis. 1998;178(4):1139–1144. doi: 10.1086/515657. [DOI] [PubMed] [Google Scholar]
- 17.Agnandji ST, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365(20):1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 18.Olotu A, et al. Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. N Engl J Med. 2013;368(12):1111–1120. doi: 10.1056/NEJMoa1207564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balazs AB, Bloom JD, Hong CM, Rao DS, Baltimore D. Broad protection against influenza infection by vectored immunoprophylaxis in mice. Nat Biotechnol. 2013;31(7):647–652. doi: 10.1038/nbt.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balazs AB, et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481(7379):81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balazs AB, et al. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat Med. 2014;20(3):296–300. doi: 10.1038/nm.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson PR, et al. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 2009;15(8):901–906. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nardin EH, et al. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med. 1982;156(1):20–30. doi: 10.1084/jem.156.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anker R, Zavala F, Pollok BA. VH and VL region structure of antibodies that recognize the (NANP)3 dodecapeptide sequence in the circumsporozoite protein of Plasmodium falciparum. Eur J Immunol. 1990;20(12):2757–2761. doi: 10.1002/eji.1830201233. [DOI] [PubMed] [Google Scholar]
- 25.Bruña-Romero O, et al. Detection of malaria liver-stages in mice infected through the bite of a single Anopheles mosquito using a highly sensitive real-time PCR. Int J Parasitol. 2001;31(13):1499–1502. doi: 10.1016/s0020-7519(01)00265-x. [DOI] [PubMed] [Google Scholar]
- 26.Zavala F, et al. Rationale for development of a synthetic vaccine against Plasmodium falciparum malaria. Science. 1985;228(4706):1436–1440. doi: 10.1126/science.2409595. [DOI] [PubMed] [Google Scholar]
- 27.Amino R, et al. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12(2):220–224. doi: 10.1038/nm1350. [DOI] [PubMed] [Google Scholar]
- 28.Yamauchi LM, Coppi A, Snounou G, Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007;9(5):1215–1222. doi: 10.1111/j.1462-5822.2006.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kester KE, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: Safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200(3):337–346. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 30.White MT, et al. The relationship between RTS,S vaccine-induced antibodies, CD4⁺ T cell responses and protection against Plasmodium falciparum infection. PLoS ONE. 2013;8(4):e61395. doi: 10.1371/journal.pone.0061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barry AE, Schultz L, Buckee CO, Reeder JC. Contrasting population structures of the genes encoding ten leading vaccine-candidate antigens of the human malaria parasite, Plasmodium falciparum. PLoS ONE. 2009;4(12):e8497. doi: 10.1371/journal.pone.0008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kappe SH, Buscaglia CA, Nussenzweig V. Plasmodium sporozoite molecular cell biology. Annu Rev Cell Dev Biol. 2004;20:29–59. doi: 10.1146/annurev.cellbio.20.011603.150935. [DOI] [PubMed] [Google Scholar]
- 33.Guerra CA, et al. The limits and intensity of Plasmodium falciparum transmission: Implications for malaria control and elimination worldwide. PLoS Med. 2008;5(2):e38. doi: 10.1371/journal.pmed.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balazs AB, West AP., Jr Antibody gene transfer for HIV immunoprophylaxis. Nat Immunol. 2013;14(1):1–5. doi: 10.1038/ni.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat Protoc. 2006;1(2):581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.