Abstract
With the 2010s declared the Decade of Vaccines, and Millennium Development Goals 4 and 5 focused on reducing diseases that are potentially vaccine preventable, now is an exciting time for vaccines against poverty, that is, vaccines against diseases that disproportionately affect low- and middle-income countries (LMICs). The Global Burden of Disease Study 2010 has helped better understand which vaccines are most needed. In 2012, US$1.3 billion was spent on research and development for new vaccines for neglected infectious diseases. However, the majority of this went to three diseases: HIV/AIDS, malaria, and tuberculosis, and not neglected diseases. Much of it went to basic research rather than development, with an ongoing decline in funding for product development partnerships. Further investment in vaccines against diarrheal diseases, hepatitis C, and group A Streptococcus could lead to a major health impact in LMICs, along with vaccines to prevent sepsis, particularly among mothers and neonates. The Advanced Market Commitment strategy of the Global Alliance for Vaccines and Immunisation (GAVI) Alliance is helping to implement vaccines against rotavirus and pneumococcus in LMICs, and the roll out of the MenAfriVac meningococcal A vaccine in the African Meningitis Belt represents a paradigm shift in vaccines against poverty: the development of a vaccine primarily targeted at LMICs. Global health vaccine institutes and increasing capacity of vaccine manufacturers in emerging economies are helping drive forward new vaccines for LMICs. Above all, partnership is needed between those developing and manufacturing LMIC vaccines and the scientists, health care professionals, and policy makers in LMICs where such vaccines will be implemented.
Vaccination has made a greater impact on global health to date than any other medical intervention (1). As well as alleviating death and suffering, the widespread implementation of vaccines results in improved economic development (2). Much of the global benefit from vaccination has come through the delivery of vaccines to infants in low- and middle-income countries (LMICs) through the Expanded Programme on Immunization (EPI), which was introduced in 1974. The EPI has been key for the delivery of vaccines against diphtheria, tetanus, pertussis, measles, poliomyelitis, and tuberculosis to more than 80% of the world’s children (3) and is being used to roll out vaccines against Haemophilus influenzae b (Hib), rotavirus, and pneumococcus. The success of the EPI in LMICs has been underpinned by support from the Global Alliance for Vaccines and Immunisation (GAVI) Alliance, which was established in 2000 as a public-private partnership with a mission to improve global health through increased access to vaccines in low-income countries (4). Vaccination has stayed at the forefront of global health policy in the new millennium with United Nations Millennium development goals (MDG) 4 and 5, to reduce childhood mortality and improve maternal health (5), very much focused on infectious diseases. With considerable support of the Bill and Melinda Gates Foundation (BMGF), the 2010s were declared the Decade of Vaccines, with new funding pledged for vaccine research and development and for the delivery of vaccines to LIMCs at the 64th World Health Assembly in 2011 (6) and the endorsement of the Global Vaccine Action Plan (GVAP) (7) at the 65th World Health Assembly in 2012.
Need
Many potentially vaccine-preventable diseases in LMICs are being inadequately dealt with because of suboptimal use of existing vaccines. Many other such diseases still have no vaccine, or the vaccines available to protect against them are far from ideal and improved vaccines are required. To better understand which vaccines are most needed and would have the greatest health impact, studies of comprehensive global disease data are invaluable, although inevitably imperfect. Ideally, such studies use the multicause model, which ensures that all causes of death and disability-adjusted life years (DALYs) fit the total number of deaths and DALYs objectively. The Global Burden of Disease, Injuries and Risk Factors Study 2010 (GBD 2010) (8) is the most comprehensive available analysis of causes of death, years of life lost (YLL) from premature mortality, and years lived with disability (YLD). Despite the success of vaccines to date, GBD 2010 confirms that there is much potential for further impact from vaccines. Global burden of disease can be assessed either in terms of mortality (9) or DALYs, which are the sum of years of life lost from premature mortality (YLL) and years lived with disability (YLD) (10). The problem with mortality data is that it is an inevitable fact of life that we will all die from something at some point. With a global aging population, global life expectancy now around 70 y (67. 5 y in men and 73.3 y in women), and 43% of deaths in 2010 occurring in >70 y olds (11), ischemic heart disease and stroke have become the first and third most common causes of global YLL.
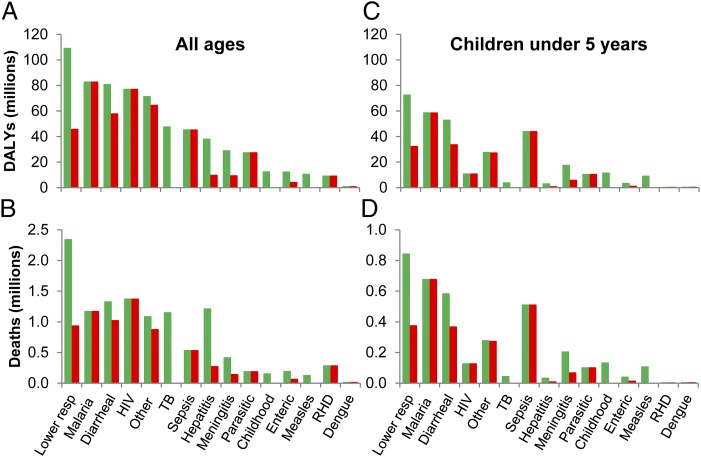
Although this reflects an overall global trend toward increasing mortality from noncommunicable diseases (NCDs), infectious diseases still account for around half of deaths and DALYs in LMICs. In the four poorest regions of the world, all in sub-Saharan Africa, life expectancy is considerably less than 70 y, and the four most common causes of YLL and DALYs are HIV/AIDS, malaria, lower respiratory tract infections (LRTIs), and diarrhea. There is around a 15-fold increased risk of dying from infectious diseases in LMICs compared with high-income countries (HICs), whereas the risk for NCDs is the same. Children less than 5 y of age bear a disproportionate burden of infectious diseases measured both by mortality and DALYs, and children in LMICs are at even higher risk, with ∼34-fold higher death rate than children in HICs. Children less than 5 y of age are at much greater risk of dying from infectious diseases that the total population, even taking into account that many older people with HIV die from infectious diseases. In Fig. 1A, we plotted DALYs for LMICs attributable to vaccine-preventable diseases from GBD 2010 by removing data from regions of HICs: all of Europe, high-income North America, Australasia, and high-income Asia Pacific. For each disease, we indicated the total number of DALYs and estimated those for which no licensed vaccine is currently available, which equates to 68% of total DALYs. The equivalent plot for mortality is remarkably similar (Fig. 1B), and plots restricted to children less than 5 y of age have a much greater proportion of DALYs and deaths due to malaria (Fig. 1 C and D). GBD 2010 includes data on NCDs that can be directly attributed to an infectious cause: liver cirrhosis and carcinoma secondary to hepatitis B and C, and peptic ulcer disease and cervical carcinoma, which are largely caused by Helicobacter pylori and human papilloma virus (HPV), respectively.
Fig. 1.
Global burden of disease from infectious causes. (A) DALYs and (B) deaths for all ages and (C) DALYs and (D) deaths in children aged less than 5 y in LMICs in 2010. Green bars indicate total DALYs and deaths for each disease/disease group. Red bars indicate DALYs and deaths for which no vaccine is available. Data are from GBD 2010 (8). LMIC data were derived by subtracting data from regions of HICs from GBD 2010 data: all of Europe, high-income North America, Australasia, and high-income Asia Pacific. Sepsis, maternal and neonatal sepsis; Childhood, tetanus, diphtheria, whooping cough, and varicella; Enteric, typhoid and paratyphoid fevers; RHD, rheumatic heart disease.
It is little surprise that for LMICs, the diseases most in need of a vaccine are HIV and malaria. Tuberculosis could be added to these in view of the lack of efficacy of bacillus Calmette–Guérin against pulmonary disease. A main target of the sixth strategic objective of the GVAP, relating to research and development, is proof of concept for a vaccine with greater or equal to 75% efficacy for HIV/AIDS, tuberculosis, or malaria (7). Although the design and development of new vaccines against these three diseases are far from straightforward, large amounts of effort and finances are being invested, with good cooperation between public and private partners coordinated, in part, by the Program for Appropriate Technology in Health (PATH) Malaria Vaccine Initiative (MVI) (12), Global HIV Vaccine Enterprise (13), and Aeras tuberculosis initiative (14). The most advanced vaccine against malaria, the sporozoite antigen-based RTS,S/AS01 vaccine, developed by GlaxoSmithKline with support from MVI and funding from BMGF, gave 59% efficacy in a phase 3 multicenter clinical trial in Africa (15). Progress has been slower toward a vaccine against HIV. Nevertheless, the RV41S study, priming with canarypox vector and boosting with glycoprotein 120 (gp120), gave 31% efficacy against infection with HIV (16), providing some hope and indicating the potential importance of broadly neutralizing antibodies against gp120 (17). Unfortunately, in addition to the lack of protection against pulmonary tuberculosis, bacillus Calmette–Guérin provides limited protection in LMICs compared with HICs. Tuberculosis is a particular problem in LMICs, especially in sub-Saharan Africa, due to its strong association with HIV/AIDS. New vaccine efforts have focused on improving bacillus Calmette–Guérin and the immune response that it induces (18).
Following HIV, malaria, and tuberculosis come four disease groups with similar disease burdens not currently preventable by vaccines: LRTI, diarrhea, sepsis, and the neglected tropical diseases (NTDs). The problem with all of these four groups is that none is caused by a single etiological agent. GBD 2010 gives a breakdown of the LRTI, diarrhea, and NTD clusters according to individual disease entities, although sepsis is only subdivided into neonatal and maternal sepsis. Vaccines are available against three of the four principal etiologies of LRTI: pneumococcus, Hib, and influenza, with no vaccine currently against respiratory syncytial virus. Improved implementation in LMICs, particularly of pneumococcal and Hib vaccines, should result in a marked fall in the LRTI, as well as the meningitis disease burden. The diarrheal burden is shared by a larger group of infectious diseases for which only two, rotavirus and cholera, currently have vaccines, with only rotavirus vaccines being widely implemented in LMICs. This leaves diarrhea caused by Cryptosporidium, Campylobacter, enteropathogenic and enterotoxin Escherichia coli (EPEC and ETEC), Shigella, and nontyphoidal Salmonella, which together are responsible for a much larger number of DALYs than those that can be prevented by available diarrhea vaccines.
The NTDs are a group of 17 infections common in impoverished communities that have risen in prominence over recent years largely through the efforts of a group of advocates (19). They comprise 11 parasitic, 4 bacterial, and 2 viral diseases. In 2012, the World Health Organization (WHO) published a roadmap for the control of these diseases (20), which was endorsed in the same year by partners and stakeholders in the London Declaration on Neglected Tropical Diseases (21). By implementing a number of public health strategies, including preventive chemotherapy, good progress has been made toward the goals of eradication of dracunculiasis by 2015 and yaws (endemic treponematoses) by 2020, with elimination (interruption of transmission) of blinding trachoma, leprosy, human African typanosomiasis, and lymphatic filariasis in the same year (22). Hence, the need for new vaccines in this group of diseases may not be as great as for other diseases of LMICs. For one of the NTDs, rabies, a vaccines is already available, whereas for most of the others, vaccines would be far from straightforward to develop.
Given the number of DALYs attributed to maternal and neonatal sepsis, a breakdown by etiology, as for LRTI and diarrhea, would be very helpful in establishing the need for new vaccines. Group B streptococcal disease would be prominent in such a list. The restriction of sepsis in GBD 2010 to mothers and neonates indicates one of the gaps in this report. It has been known for at least 10 y that bacteremia (bacterial infection of the blood), which often manifests as sepsis, is responsible for a large infectious disease burden in LMICs (23). For South Asia, Salmonella enterica Typhi (S. Typhi) is the most common cause of bacteremia (24), and in Africa, this is pneumococcus and invasive nontyphoidal Salmonella (iNTS) (25). Although typhoid comes under the category of enteric fever in GBD 2010 and bacteremia with pneumococcus will often present as pneumonia, the most common presentation of iNTS disease is with fever alone (26). The lack of inclusion of iNTS disease in GBD 2010 obscures its significance as a major cause of morbidity and mortality in LMICs, contributing to the need for a vaccine being overlooked by global health policy makers (26).
Of the remaining LMIC disease burden, there are a number of single etiological agents for which no vaccine is available, where a vaccine could make a major impact on DALYs. Although there is a vaccine against hepatitis B, none is available against hepatitis C. With the sequelae of cirrhosis and carcinoma and problems with coinfection with HIV, a vaccine against hepatitis C would be particularly valuable for LMICs. Similarly, an effective vaccine against group A Streptococcus, the etiological agent of rheumatic fever that causes rheumatic heart disease, could result in a major reduction of disease burden in LMICs and among the aboriginal populations in some HICs such as Australia and New Zealand. A vaccine against Helicobacter pylori could dramatically reduce the burden caused by peptic ulcer disease. For enteric fever, two vaccines have been available for many years against S. Typhi, but both (Vi capsular polysaccharide and Ty21a live attenuated vaccines) have limited efficacy and are not licensed or effective in young children (27). New vaccines are currently in development and becoming available, in particular Vi glycoconjugate vaccines (27, 28), that can be given to young children, but vaccines are also needed for S. Paratyphi A, the other main cause of enteric fever. Among the grouping of sexual transmitted diseases, syphilis has by far the highest disease burden but can be effectively treated with antibiotics.
Challenge
A major challenge to developing new vaccines for LMICs is the economic one. Without the commercial incentive present for HIC vaccines, there is limited attraction for the vaccine industry to spend the large sums of money [estimated in 2003 at US$802 million for each new licensed vaccine (29)] and time (a minimum of 10 y) to develop vaccines where it will be challenging to recoup the investment. The time required for vaccine development is not helped by what is often a slow pathway to regulatory approval (30). The cost of developing a vaccine is compounded by the need to maintain minimal pricing on any LMIC vaccine.
The case is different for diseases where there is a HIC and LMIC market, such as HIV, Hib, and pneumococcus, but timely implementation of such vaccines in LMICs has been a challenge. Tragically, much of the DALYs for LMICs is caused by diseases for which vaccines are available, yet have not yet been implemented globally. This is well recognized by the GVAP, which has equitable extension of vaccine coverage to all people as its third strategic objective (7). The reasons for poor vaccine coverage in LMICs include cost, lack of appreciation of the relevant burden of disease, reduced effectiveness in LMICs, and differences in strain coverage between HICs and LMICs. Reduced effectiveness of HIC vaccines in phase 3 clinical trials in LMICs can occur for several reasons including differences in strain coverage of the vaccine in HICs and LMICs, a problem for the 7-valent pneumococcal conjugate vaccine in Africa (31). Reduced effectiveness of live oral vaccines, including those against poliomyelitis, rotavirus, and cholera, could be caused by malnutrition, maternal antibodies, host genetic factors, and chronic environmental enteropathy (32). Hence, a one-size-fits-all policy toward vaccine development for HICs and LMICs is not necessarily the most effective strategy. Vaccine development for LMICs needs to be timely. For every extra year that a new vaccine takes to reach licensure in HICs, tens to hundreds of deaths may occur that could otherwise be averted, whereas for a LMIC vaccine, this could be thousands to tens of thousands. Once a new vaccine for LMICs has been developed and licensed, it needs to be used, and therefore, the presence of a demand for the vaccine within LMICs is important.
Global investment into research and development for new products for neglected diseases has been tracked for the last 6 y by the Global Funding of Innovation for Neglected Diseases (G-FINDER) survey, which is produced annually by Policy Cures with support from BMGF (33). A broad definition of “neglected diseases” is used to encompass diseases “disproportionately affecting people in developing countries for which there is a need for new products (i.e., there is no existing product or improved or additional products are needed) and market failure (i.e., insufficient commercial market to attract research and development (R&D) by private industry).” In 2012, US$1.3 billion was invested in new vaccines against neglected diseases. Most was from the public sector, predominantly HIC governments, with the remainder from philanthropic organizations (mainly BMGF and the Wellcome Trust) and the private sector, mostly multinational vaccine companies.
Most neglected disease vaccine research and development (R&D) funding (US$933 million, 71%) went to the three “top tier” diseases, HIV/AIDS, malaria, and tuberculosis, whereas the remaining US$374 million was spent primarily on the “second tier diseases”: dengue, diarrheal disease, kinetoplastids, bacterial pneumonia and meningitis, helminths, and Salmonella infections. This left less than 1% (around US$10 million) of spending for all other neglected disease (the third tier), including group A Streptococcus. In relation to how this funding was spent, the vast majority went to basic research, with only 12% going to product development partnerships (PDPs) in 2012. With the available finances (US$374 million) and cost of developing a vaccine (US$802 million), at best, one new vaccine could be developed against a G-FINDER tier 2 or tier 3 disease every 2 y, whereas the amount spent on HIV/AIDS, malaria, and tuberculosis vaccines could fund the development of one tier 2 or 3 vaccine every year. This skewing of R&D funding to the top tier is not addressed by the GVAP. With the absence of any other named vaccine in its research and development objective, other than a vaccine against a top tier disease or a universal influenza vaccine (7), the plan is unfortunately likely to support the status quo with regard to the distribution of R&D funding.
The second biggest challenge is prioritizing which vaccines to work on. This primarily needs a proper understanding of the burden of disease each potential vaccine could prevent. Although GBD 2010 provides the clearest picture to date of which infectious diseases affecting LMICs require vaccines, the study should be considered as a general guide, particularly in relation to data from LMICs, where hospital infrastructure and diagnostic capacity are limited compared with HICs. Much data concerning cause of death in the poorest countries, particularly in sub-Saharan Africa, is recorded through “verbal autopsy,” which has a high rate of misclassification (34, 35). The Child Health Epidemiology Reference Group of the WHO and UNICEF (CHERG) found that only 2.7% of deaths in children less than 5 y of age in 2012 were reported using medically certified vital registration data (36).
For some disease entities, particularly diarrhea, the presence of more than one potential pathogen in diagnostic samples can cause difficulties in attributing burden of disease. Such scenarios require robust and ongoing surveillance data, with testing for multiple pathogens in cases and controls across multiple sites in LMICs, as was carried out in the recently published Global Enteric Multicenter Study (GEMS) (37). With other disease entities, such as LRTI, the absence of an obvious etiology is a problem. To address this, another BMGF-supported study is underway, the Pneumonia Etiology Research for Child Health (PERCH) project, which will use a range of diagnostic techniques for children with severe pneumonia across seven LMICs (38). This study will also help to assess the impact of implementation of Hib and pneumococcal vaccines in these countries. Similar studies are required to address the etiologies of maternal and neonatal sepsis.
As well as understanding disease burden targeted by each vaccine, a careful assessment is required of the probability of success for a new vaccine. Vaccines that target conserved antigens on the pathogen and elicit protection through antibody-mediated mechanisms will be quicker and more cost-effective to develop than those targeting antigenically diverse molecules and relying on T-cell effector mechanisms (39). A high level of antigenic diversity has been a major obstacle for the development of vaccines against malaria and HIV/AIDS, along with the belief that T-cell immunity is required for protection against these diseases. In contrast, many of the bacteria responsible for high burdens of disease in LMICs have conserved antigenic targets that are amenable to protection through antibody-mediated mechanisms. Vaccines have more likelihood of success if they target acute rather than chronic infections.
Vaccines for LMICs need to be affordable, as well as safe and effective. Some currently available HIC vaccines have not been implemented in LMICs owing to their cost. For example, two multivalent conjugate vaccines are available that could protect against meningococcal serogroup W (as well as A, C, and Y), which is currently causing outbreaks of meningococcal meningitis in the African Meningitis Belt, but is not used due to cost. An affordable vaccine needs to have affordability at three levels: it needs to be affordable to develop, affordable to manufacture, and affordable to deliver. This approach will favor the use of relatively simple technologies to produce vaccines with low cost of goods that can ideally be delivered as part of the EPI. Complex multicomponent vaccines are less attractive for LMIC use. This hurdle can be partly offset by the use of tier marketing whereby vaccines are priced much lower in LMICs than in HICs.
Realization
Despite the challenges above, there is much to be hopeful about regarding the development of vaccines for LMICs. Concerning implementation of existing vaccines, measles and tetanus were ranked the 12th and 20th causes of global YLL in 1990. In GBD 2010, these ranks had fallen to 38th and 52nd (9). As a result, both diseases are on track to achieve MDG 4 objectives regarding reduction in childhood mortality. Hib vaccination is now being widely implemented in LMICs. There was a delay of 10–20 y between use of this vaccine in HICs and LMICs (40). More recently, GlaxoSmithKline’s Rotarix vaccine against rotavirus was released simultaneously in Latin America and HICs (41). Part of the issue with timely implementation of new vaccines into LMICs has been securing the necessary financing, as well as understanding their potential public health benefit. The Advanced Market Commitments strategy of financing vaccines through the GAVI Alliance (42) is being used to bring about the timely deployment of new rotavirus and pneumococcal conjugate vaccines in LMICs.
A paradigm shift in the development of vaccines for LMICs was achieved with the licensing and rolling out of MenAfriVac, a monovalent conjugate vaccine against meningococcal serogroup A (43), across the African Meningitis Belt, beginning in 2010. Meningococcal group A was the main cause of both endemic meningitis in the Belt and the periodic epidemics that would often paralyze fragile health care services in affected countries. This vaccine was the product of the Meningitis Vaccine Project, a partnership between PATH and WHO with support from BMGF. The vaccine was developed solely for use in LMICs with a price of less than US$1 per dose. The necessary technology for its production was transferred to the Serum Institute of India Ltd., the largest vaccine manufacturer in India, with the capacity to produce large quantities of quality low-cost vaccines. Although financing for LMIC vaccines is less than that available for vaccines aimed at HICs, which can afford to pay a higher price, the funding situation for such vaccines has greatly improved in the last 20 y, through support from HIC governments, both directly and indirectly through the GAVI Alliance, philanthropic institutions, and industry corporate and social responsibility initiatives.
Over the same period, the number of vaccine manufacturers in emerging economies, such as the Serum Institute of India and Biological E in India and the Lanzhou Institute in China, has been increasing. These have the ability to produce high quantities of low-cost vaccines for LMICs using a low profit margin business model. The companies have high levels of industrial expertise allowing the necessary transfer of technology to them from vaccine institutes and potentially directly from academia in order to manufacture new LMIC vaccines. These manufacturers have formed an international alliance known as the Developing Countries Vaccine Manufacturers Network (DCVMN), which now comprises 37 manufacturers from 14 countries (44). Between them, the manufacturers have contributed more than 30 WHO prequalified vaccines for global immunization programs, with many other products in their vaccine pipelines.
In addition, a number of vaccines institutes have emerged with missions aimed at the development of vaccines for LMICs (45). The first was the Sabin Vaccine Institute in Washington, DC. Founded in 1993, its focus has been on vaccines against hookworm, schistosomiasis, and malaria. In 1997, the International Vaccine Institute was established in Seoul, South Korea, with a focus on infectious diseases in Asia and the development of vaccines against Japanese encephalitis, Shigella, cholera, and typhoid fever. Ten years later, the opening of the Novartis Vaccines Institute for Global Health in Siena, Italy, brought a new dimension to vaccine institutes by having direct access to a major commercial vaccine manufacturer. In 2013, the institute entered into an agreement with Biological E, a major Indian manufacturer, for the commercial development of its first two vaccines: a Vi-CRM197 glycoconjugate vaccine against S. Typhi (28) and Vi-CRM197/O:2-CRM197 bivalent vaccine against enteric fever (caused by S. Typhi and S. Paratyphi A). Other vaccines are being developed against shigellosis, iNTS disease, and meningococcal meningitis. Following this industry-linked model, partnership between Merck and the Wellcome Trust led to the foundation of the Hilleman Laboratories in India.
The MenAfriVac meningococcal vaccine and Vi-CRM197 vaccine represent vaccines that use conjugation technology to transform earlier unconjugated polysaccharide vaccines, which elicit only T-independent antibody responses, to vaccines that can induce T-dependent antibody responses. This has significant advantages in relation to being immunogenic in infants and inducing memory responses and enhanced antibody levels (46). Other relatively simple vaccine technologies that are suitable for LMIC vaccines are live attenuated vaccines and bacterial inactivated vaccines. The latter have traditionally had problems with reactogenicity, and this is being overcome by a new low-cost vaccine strategy known as generalized modules for membrane antigens (GMMA). GMMA take advantage of the potential to up-regulate the release of outer membrane blebs from Gram-negative bacteria, such as Shigella, Salmonella, and meningococcus, by introducing mutations into proteins that link the inner and outer membranes (47, 48). Finally, the application of new adjuvants, such as MF59, AS01, and toll-like receptor (TLR) agonists, to LMIC vaccines, has the potential to make such vaccines more immunogenic and more affordable by dose-sparing and reducing the number of doses required to induce long-lived protective immunity. The onus will constantly be on those seeking to develop LMIC vaccines to find innovative ways to produce effective and affordable vaccines in a timely manner with the resources available.
Improved surveillance of the disease targets for new LMIC vaccines is key to both the development of vaccines with appropriate strain coverage and monitoring their effectiveness after implementation. The Wellcome Trust-supported MenAfriCar study has monitored the effect on meningococcal carriage and changes in patterns of disease around the implementation of the MenAfriVac meningococcal A vaccine and has been key to following its success in reducing meningococcal A disease and also for monitoring meningitis caused by other meningococcal serogroups, particularly W and X. The BMGF-supported Typhoid Surveillance in Africa Program (TSAP) has been valuable for identifying the burden of disease caused by Salmonella in Africa, including disease caused by nontyphoidal serovars of S. enterica, as well as the emergence of typhoid fever caused by S. Typhi. Such surveillance programs have the added advantage of collecting disease-causing isolates from LMICs. Analysis of the genotypes of these isolates, through the use of high-throughput whole genome sequencing, can help ensure that new vaccines are designed with appropriate disease coverage (45).
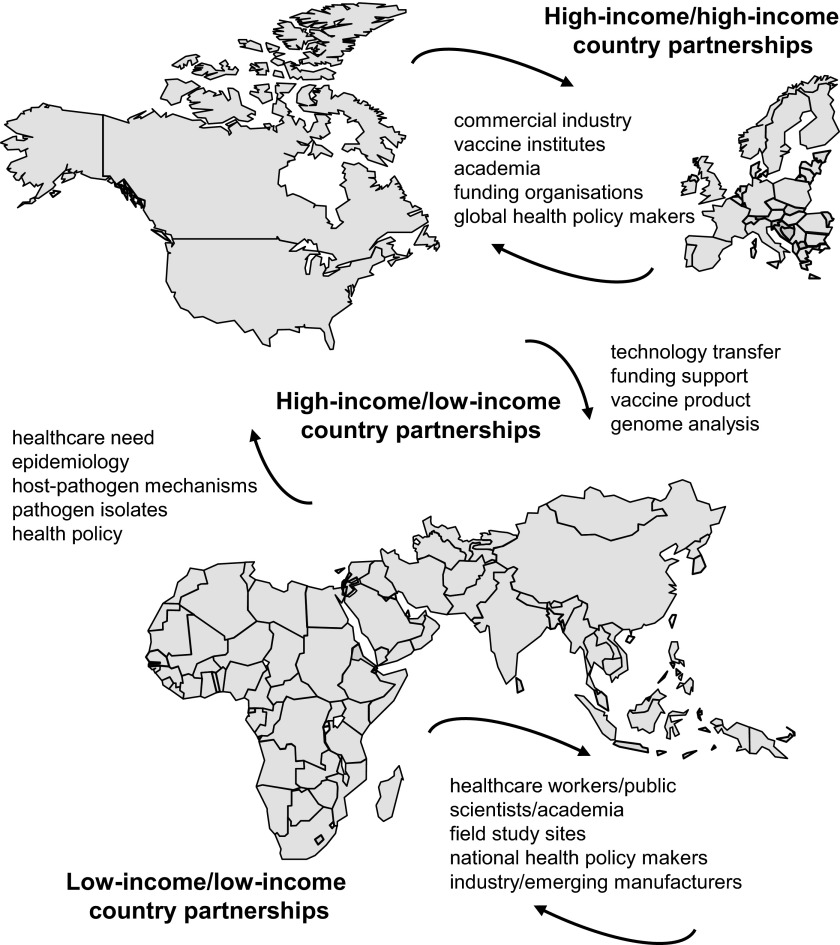
A theme throughout this Perspective is the need for partnership so that new vaccines for LMICs become a reality and effectively reduce the burden of disease in these countries (Fig. 2). The most important partnership is between those seeking to develop new vaccines and the countries that the vaccines are being developed for. The burden of disease that could be prevented by a new vaccine needs to be clearly established, and the public health benefit of such a vaccine needs to be recognized by the affected LMICs, so that a clear need for the vaccine is established and recognized. This involves interaction with scientists, health care professionals, and policy makers in LMICs, and needs to begin early in the vaccine development process. Partnership and interaction with scientists and health care professionals who work on the disease first hand are important for understanding the clinical presentation and groups affected, qualities of the pathogen responsible for the disease, and mechanisms of protective immunity that the vaccine needs to elicit. Increasingly, such vaccines will be manufactured by companies in LMICs, and therefore, partnership at the industrial level is also important. Finally, partnership between academia, vaccine institutes, and industry in the developed world is also important for accelerating the development of these vaccines and optimizing the limited resources available for this truly globally significant work.
Fig. 2.
Global partnerships in development of vaccines for LMICs. Successful development of new vaccines for LMICs will rely on partnerships between HICs and LMICs and among both HICs and LMICs. These partnerships need to involve industry, academia, health care professionals and health policy makers in both groups of countries.
In conclusion, current spending on R&D for new vaccines for LMICs, other than HIV, malaria, and tuberculosis, could support the development of one new vaccine every 2 y. Therefore, the decision concerning which vaccines to work on is critical and needs to consider burden of disease, probability of success of each vaccine, and economic issues, including cost of development and production. The potential to develop new LMIC vaccines has been enhanced by research leading to improved understanding of the pathogens and mechanisms of protection against the diseases they cause in LMICs, the establishment of vaccine institutes that have the technical understanding to move vaccine ideas through to clinical proofs of concept, and increasing numbers of manufacturers, particularly in the DCVMN, that can produce large quantities of high quality vaccines for the global market at low cost. The full realization of this potential requires partnership and cooperation, combined with an increase in funding for LMIC vaccines and/or more effective ways of developing these vaccines, including an improvement in the efficiency of the regulatory approval process.
Supplementary Material
Footnotes
Conflict of interest statement: C.A.M. and A.S. are employees of the Novartis Vaccines Institute for Global Health. C.A.M. is the recipient of a clinical research fellowship from GlaxoSmithKline.
This article is a PNAS Direct Submission.
This article is part of the special series of PNAS 100th Anniversary articles to commemorate exceptional research published in PNAS over the last century.
References
- 1.Plotkin SA. Vaccines: The fourth century. Clin Vaccine Immunol. 2009;16(12):1709–1719. doi: 10.1128/CVI.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom DE, Canning D, Weston M. The value of vaccination. World Econ. 2005;6(3):15–39. [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Global routine vaccination coverage, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(42):1367–1371. [PubMed] [Google Scholar]
- 4. GAVI Alliance (2014) Saving children's lives and protecting people's health by increasing access to immunisation in poor countries. Available at http://www.gavialliance.org/. Accessed February 12, 2014.
- 5. United Nations Development Programme (2014) The Millenium development goals. Available at http://www.undp.org/content/undp/en/home/mdgoverview/. Accessed February 12, 2014.
- 6. World Health Organization (2011) Mr Bill Gates, Co-chair of the Bill & Melinda Gates Foundation Speech to the sixty-fourth World Health Assembly. Available at http://www.who.int/mediacentre/events/2011/wha64/bill_gates_speech_20110517/en/index.html. Accessed February 12, 2014.
- 7. World Health Organization (2013) Global vaccine action plan 2011-2020. Available at http://www.dovcollaboration.org/wp-content/uploads/2013/03/GlobalVaccineActionPlan_interactive.pdf. Accessed March 29, 2014.
- 8. Institute for Health Metrics and Evaluation (2010) Global burden of diseases, injuries, and risk factors study 2010. Available at http://www.healthmetricsandevaluation.org/gbd/research/project/global-burden-diseases-injuries-and-risk-factors-study-2010. Accessed February 12, 2014.
- 9.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray CJ, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, et al. Age-specific and sex-specific mortality in 187 countries, 1970-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2071–2094. doi: 10.1016/S0140-6736(12)61719-X. [DOI] [PubMed] [Google Scholar]
- 12. PATH (2014) Malaria vaccine initiative. Available at http://www.malariavaccine.org/. Accessed February 12, 2014.
- 13. Global HIV Vaccine Enterprise (2014) Global HIV Vaccine Enterprise. Available at http://www.vaccineenterprise.org/. Accessed February 12, 2014.
- 14. AERAS. (2014) AERAS advancing tuberculosis vaccines for the world. Available at http://www.aeras.org. Accessed February 12, 2014.
- 15.Agnandji ST, et al. RTS,S Clinical Trials Partnership First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365(20):1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 16.Rerks-Ngarm S, et al. MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 17.Scheid JF, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458(7238):636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 18.McShane H. Tuberculosis vaccines: Beyond bacille Calmette-Guerin. Philos Trans R Soc Lond B Biol Sci. 2011;366(1579):2782–2789. doi: 10.1098/rstb.2011.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotez PJ, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357(10):1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization (2012) Accelerating work to overcome the global impact of neglected tropical diseases: A roadmap for implementation. Available at http://www.who.int/neglected_diseases/NTD_RoadMap_2012_Fullversion.pdf. Accessed February 12, 2014.
- 21. Uniting to Combat NTDs (2012) The London declaration on neglected tropical diseases. Available at http://www.unitingtocombatntds.org/downloads/press/london_declaration_on_ntds.pdf. Accessed February 12, 2014.
- 22. World Health Organization (2013) Sustaining the drive to overcome the global impact of neglected tropical diseases. Available at www.who.int/iris/bitstream/10665/77950/1/9789241564540_eng.pdf. Accessed February 12, 2014.
- 23.Berkley JA, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352(1):39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 24.Deen J, et al. Community-acquired bacterial bloodstream infections in developing countries in south and southeast Asia: A systematic review. Lancet Infect Dis. 2012;12(6):480–487. doi: 10.1016/S1473-3099(12)70028-2. [DOI] [PubMed] [Google Scholar]
- 25.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: A systematic review and meta-analysis. Lancet Infect Dis. 2010;10(6):417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLennan CA, Levine MM. Invasive nontyphoidal Salmonella disease in Africa: current status. Expert Rev Anti Infect Ther. 2013;11(5):443–446. doi: 10.1586/eri.13.27. [DOI] [PubMed] [Google Scholar]
- 27.Martin LB. Vaccines for typhoid fever and other salmonelloses. Curr Opin Infect Dis. 2012;25(5):489–499. doi: 10.1097/QCO.0b013e328356ffeb. [DOI] [PubMed] [Google Scholar]
- 28.Bhutta ZA, et al. Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and southeast Asia: Results from two randomised, observer-blind, age de-escalation, phase 2 trials. Lancet Infect Dis. 2014;14(2):119–129. doi: 10.1016/S1473-3099(13)70241-X. [DOI] [PubMed] [Google Scholar]
- 29.Douglas RG, Sadoff J, Samant V. The vaccine industry. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Philadelphia: Saunders Elsevier; 2008. pp. 37–44. [Google Scholar]
- 30.Moxon ER, et al. A call to action for the new decade of vaccines. Lancet. 2011;378(9788):298–302. doi: 10.1016/S0140-6736(11)60766-6. [DOI] [PubMed] [Google Scholar]
- 31.Gordon SB, et al. Poor potential coverage for 7-valent pneumococcal conjugate vaccine, Malawi. Emerg Infect Dis. 2003;9(6):747–749. doi: 10.3201/eid0906.030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmgren J, Svennerholm AM. Vaccines against mucosal infections. Curr Opin Immunol. 2012;24(3):343–353. doi: 10.1016/j.coi.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 33. Policy Cures G-FINDER (2013) Neglected disease research and development: The public divide. Available at http://www.policycures.org/downloads/GF_report13_all_web.pdf. Accessed February 12, 2014.
- 34.Anker M. The effect of misclassification error on reported cause-specific mortality fractions from verbal autopsy. Int J Epidemiol. 1997;26(5):1090–1096. doi: 10.1093/ije/26.5.1090. [DOI] [PubMed] [Google Scholar]
- 35.Lozano R, et al. Population Health Metrics Research Consortium (PHMRC) Performance of physician-certified verbal autopsies: Multisite validation study using clinical diagnostic gold standards. Popul Health Metr. 2011;9:32. doi: 10.1186/1478-7954-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, et al. Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 37.Kotloff KL, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet. 2013;382(9888):209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 38.Levine OS, et al. The Pneumonia Etiology Research for Child Health Project: A 21st century childhood pneumonia etiology study. Clin Infect Dis. 2012;54(Suppl 2):S93–101. doi: 10.1093/cid/cir1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rappuoli R. Bridging the knowledge gaps in vaccine design. Nat Biotechnol. 2007;25(12):1361–1366. doi: 10.1038/nbt1207-1361. [DOI] [PubMed] [Google Scholar]
- 40.Levine OS, et al. The future of immunisation policy, implementation, and financing. Lancet. 2011;378(9789):439–448. doi: 10.1016/S0140-6736(11)60406-6. [DOI] [PubMed] [Google Scholar]
- 41.Plosker GL. Rotavirus vaccine RIX4414 (Rotarix™): A pharmacoeconomic review of its use in the prevention of rotavirus gastroenteritis in developed countries. Pharmacoeconomics. 2011;29(5):439–454. doi: 10.2165/11207130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Hargreaves JR, et al. Making new vaccines affordable: A comparison of financing processes used to develop and deploy new meningococcal and pneumococcal conjugate vaccines. Lancet. 2011;378(9806):1885–1893. doi: 10.1016/S0140-6736(11)60687-9. [DOI] [PubMed] [Google Scholar]
- 43.Sow SO, et al. Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N Engl J Med. 2011;364(24):2293–2304. doi: 10.1056/NEJMoa1003812. [DOI] [PubMed] [Google Scholar]
- 44.Pagliusi S, et al. Developing countries vaccine manufacturers network: Doing good by making high-quality vaccines affordable for all. Vaccine. 2013;31(Suppl 2):B176–B183. doi: 10.1016/j.vaccine.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 45.MacLennan CA. Vaccines for low-income countries. Semin Immunol. 2013;25(2):114–123. doi: 10.1016/j.smim.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9(3):213–220. doi: 10.1038/nri2494. [DOI] [PubMed] [Google Scholar]
- 47.Berlanda Scorza F, et al. High yield production process for Shigella outer membrane particles. PLoS ONE. 2012;7(6):e35616. doi: 10.1371/journal.pone.0035616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koeberling O, et al. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on generalized modules for membrane antigens (GMMA) Vaccine. 2014;32(23):2688–2695. doi: 10.1016/j.vaccine.2014.03.068. [DOI] [PubMed] [Google Scholar]