Significance
Despite high energetic demands in the brain, glucose is not always metabolized to produce maximum energy. Aerobic glycolysis, that is, high levels of glucose consumption relative to oxygen use, is connected to cognition and disease, but metabolic plasticity remains challenging to study in vivo owing to the brain’s complexity. We show that decreased oxidative phosphorylation activity, a pattern that resembles aerobic glycolysis, causes increased aggression in honey bees and fruit flies. This effect is specific to neurons and not glia, and the social environment modulates the relationship between metabolism and aggression. The fly–bee system, linking variation in brain metabolism to a natural behavior, could be used to further study the function of brain metabolic plasticity.
Keywords: Warburg effect, behavioral genomics, ND20-like, Ndufs7b
Abstract
Despite ongoing high energetic demands, brains do not always use glucose and oxygen in a ratio that produces maximal ATP through oxidative phosphorylation. In some cases glucose consumption exceeds oxygen use despite adequate oxygen availability, a phenomenon known as aerobic glycolysis. Although metabolic plasticity seems essential for normal cognition, studying its functional significance has been challenging because few experimental systems link brain metabolic patterns to distinct behavioral states. Our recent transcriptomic analysis established a correlation between aggression and decreased whole-brain oxidative phosphorylation activity in the honey bee (Apis mellifera), suggesting that brain metabolic plasticity may modulate this naturally occurring behavior. Here we demonstrate that the relationship between brain metabolism and aggression is causal, conserved over evolutionary time, cell type-specific, and modulated by the social environment. Pharmacologically treating honey bees to inhibit complexes I or V in the oxidative phosphorylation pathway resulted in increased aggression. In addition, transgenic RNAi lines and genetic manipulation to knock down gene expression in complex I in fruit fly (Drosophila melanogaster) neurons resulted in increased aggression, but knockdown in glia had no effect. Finally, honey bee colony-level social manipulations that decrease individual aggression attenuated the effects of oxidative phosphorylation inhibition on aggression, demonstrating a specific effect of the social environment on brain function. Because decreased neuronal oxidative phosphorylation is usually associated with brain disease, these findings provide a powerful context for understanding brain metabolic plasticity and naturally occurring behavioral plasticity.
Metabolic dynamics are critical to brain function in both vertebrate and invertebrate species (1–3). In mammals, cognitive and behavioral tasks result in increased glucose metabolism and minor increases in oxygen consumption (relative to availability), and similar processes have been shown to occur in insects (3, 4). These metabolic changes underlie widely used technologies that measure brain activity (e.g., functional MRI and PET) (5–7). Because the brain is an energetically demanding organ with high ATP requirements (8), temporal and spatial variation in glucose metabolism is generally assumed to fulfill the energetic demands of signaling and recovery (5). Paradoxically, in humans, less than 10% of the glucose that is taken up as a result of brain activity is fully oxidized through oxidative phosphorylation (OX) to produce ATP, despite adequate oxygen availability, a phenomenon known as aerobic glycolysis (6, 9–11). Furthermore total glucose uptake by the adult human brain exceeds oxygen use by 10–12% (12). Thus, increased demand for high levels of ATP is inadequate to explain the function of variation in glucose metabolism in the brain. Understanding the functional significance of metabolic plasticity, which is essential for cognition but also linked to disease, is a critical issue in neuroscience (6, 9, 13).
To study the relationship between metabolic plasticity and brain function, it is necessary to develop in vivo experimental systems that are amenable to precise metabolic manipulations and that link brain metabolic states to specific behavioral states (14). In this study we present a two-species experimental system that meets these requirements.
In our previous study, brain transcriptional profiling in the honey bee (Apis mellifera) revealed a negative correlation between whole-brain OX activity and aggression. This was seen in three different comparisons: in highly aggressive Africanized vs. less aggressive European honey bees; in older, more easily aroused honey bees compared with younger individuals; and in response to alarm pheromone (15). Transcriptomic data and enzyme activity assays showed that effects were most prominent for genes associated with complexes I, IV, and V of the OX pathway (15). We used these results as the basis for experiments that determined the functional implications of plasticity in brain glucose metabolism on aggression.
We manipulated OX in behaviorally relevant ways in honey bees and the fruit fly (Drosophila melanogaster) and tested for a causal relationship between a change in brain OX and aggression. With Drosophila we studied whether the relationship between brain OX and aggression originally discovered in honey bees is evolutionary conserved and also determined whether it is localized to neurons or glia. With honey bees we also assessed how variation in social experience modulates the link between brain OX and aggression.
Results
Experiment 1: OX Inhibition Increased Aggression in Honey Bees.
We treated 7-d-old adult worker bees with two insecticides known to inhibit OX (16–18) and assayed aggression levels 24 h later. Bees were topically treated on the thorax because previous studies showed that such treatments reliably reach the brain (19). We measured aggression levels by testing groups of bees in a laboratory “Intruder Assay.” This assay measured aggressive behaviors toward an intruder bee collected from an unrelated colony (20). We assessed groups of 10 nestmates in which half were treated with a drug and half were treated with an acetone vehicle control. We measured the following aggressive behaviors: antennation (with and without open mandibles), lunging, biting, flexing the abdomen in preparation for stinging, and attempted stinging (21).
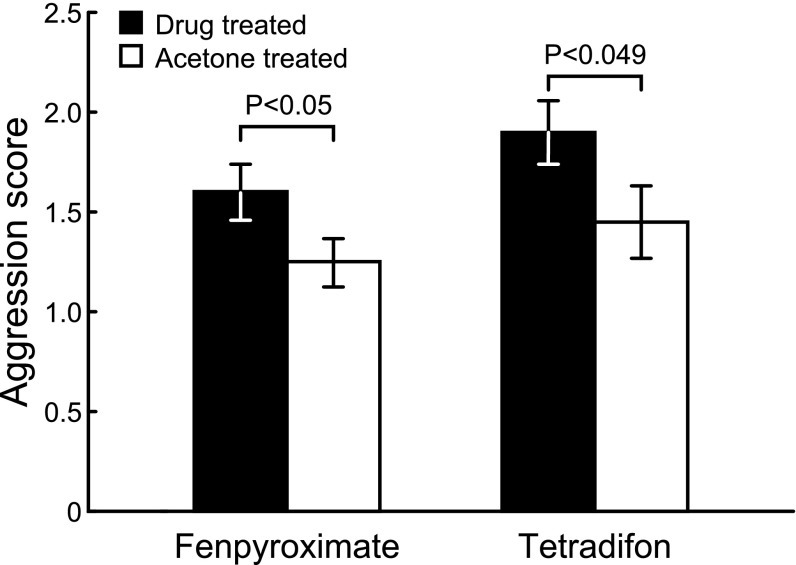
A dose of 0.33 μg/uL fenpyroximate [a complex I inhibitor (16)] or 21.1 μg/uL tetradifon [a complex V inhibitor (18)] resulted in a significant, 28% and 31% increase in aggression, respectively, relative to acetone control (Fig. 1). Dose–response curves showed an inverted U shape for aggression (SI Appendix, Table S1). Treatment at the above-mentioned doses resulted in minimal mortality relative to control, whereas higher doses caused increased mortality in addition to decreased aggression (SI Appendix, Fig. S1 and Table S2).
Fig. 1.
Effect of oxidative phophorylation inhibitors (fenpyroximate and tetradifon) on aggression in honey bees (mean ± SEM). Drug treatment resulted in increased aggression at a dose of 0.33 μg per bee for fenpyroximate (n = 39 groups, two-tailed t test, t38 = 2.03, P < 0.05) and 21.1 μg per bee for tetradifon (n = 20 groups, two-tailed t test, t19 = 2.1, P < 0.049). Aggression was scored following ref. 21; SI Appendix, Table S1 describes aggression at other doses.
Experiment 2: The Relationship Between OX and Aggression Is Conserved in Drosophila and Cell-Type Specific.
We assayed aggression in D. melanogaster elavGAL4 driver lines that knocked down expression of two complex I genes, CG2014 (NADH:ubiquinone oxidoreductase-like, 20kDa subunit, hereafter ND20-like) or CG9140 (NADH:ubiquinone oxidoreductase, 51kDa subunit, hereafter ND51) by ∼50% for each gene in neurons only (SI Appendix, Figs. S2 and S3). We measured aggressive behaviors in 4- to 6-d-old male flies using a previously described laboratory behavioral assay and video analysis method (22). We focused on lunging behavior because it is considered to be an accurate reflection of overall aggression levels in flies (23–25).
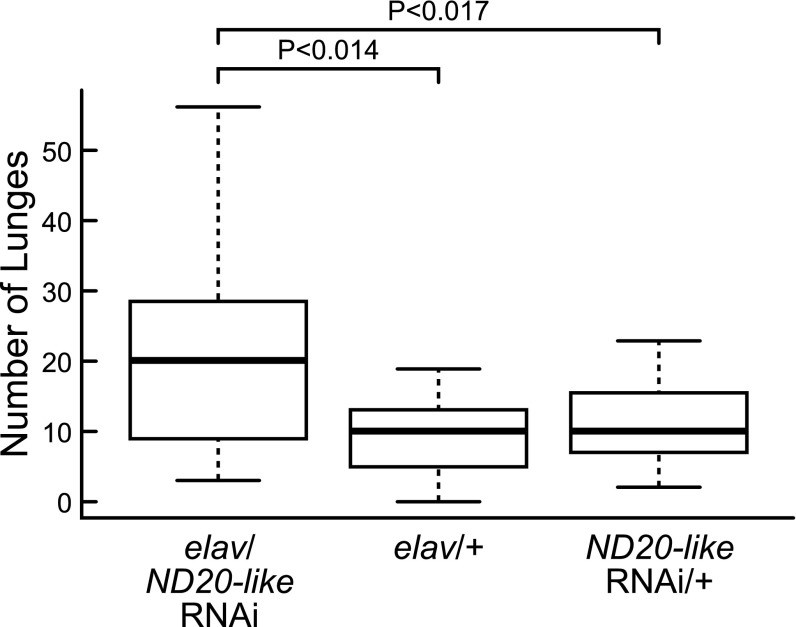
ND20-like knockdown caused increased lunging activity compared with both heterozygous parental controls (Fig. 2). ND51 knockdown caused increased lunging activity compared with one parental control but not the other (SI Appendix, Fig. S4). Unlike for ND20-like, the two ND51 parental control lines showed significant variation in lunging activity (Mann-Whitney U test, Z = −4.08, P < 0.001). Thus, knockdown of ND20-like significantly affected aggressive behavior, whereas the results for ND51 are inconclusive. Additional elav-dicer-GAL4 neuronal knockdown of transcripts in other complexes failed to show behavioral effects or resulted in high mortality (SI Appendix, Table S3). Because our experimental approaches did not target all OX complexes exhaustively, and a change in one complex can affect the stability and function of the others (26, 27), it is difficult to speculate on the reasons for the selective effects.
Fig. 2.
Effect of neuronal RNAi knockdown of metabolic genes on aggression in Drosophila. ND20-like neuronal knockdown flies (elav/ND20-like RNAi, n = 23 pairs) showed significantly more lunges compared with two heterozygous parent controls (ND20-like RNAi/+, n = 20 pairs, elav/+, n = 10 pairs; Kruskal-Wallis test: H2 = 7.08, P < 0.029; post hoc Mann-Whitney U tests: elav/ND20-like RNAi vs. ND20-like RNAi/+, Z = 2.13, P < 0.017, elav/ND20-like RNAi vs. elav/+, Z = 2.21, P < 0.014).
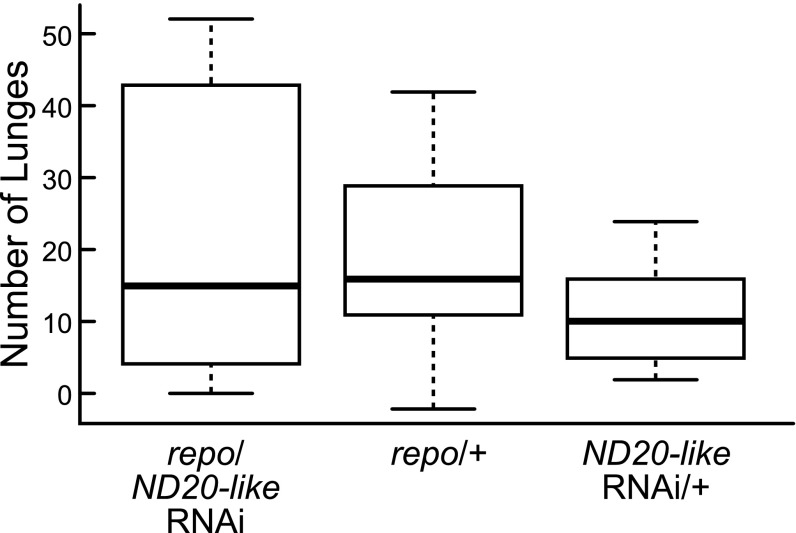
We also assayed aggression in flies from repoGAL4 driver lines that knocked down expression of ND20-like or ND51 by ∼50% for each gene in glia only (SI Appendix, Figs. S2 and S3). This allowed us to determine whether the relationship between brain metabolic state and aggression is cell type-specific, which is not currently possible to do with honey bees. There was no effect of glial knockdowns on aggression (Fig. 3 and SI Appendix, Fig. S5).
Fig. 3.
Effect of glia-specific RNAi knockdown of metabolic genes on aggression in Drosophila. ND20-like glial knockdown flies (repo/ND20-like RNAi, n = 8 pairs) showed no differences in lunging compared with either heterozygous parent controls (ND20-like RNAi/+, n = 8 pairs, repo/+, n = 7 pairs; Kruskal-Wallis test, H2 = 1.13, P < 0.57).
Experiment 3: The Effect of OX Inhibition On Aggression in Honey Bees Is Modulated by the Social Environment.
Honey bee aggression occurs in the context of nest defense, which is a highly integrated collective behavior coordinated among groups of specialized individuals by means of pheromone communication (28). Moreover, individual aggression levels are strongly dependent on past and present threat levels to the colony (15, 29, 30). If the metabolic drugs used in experiment 1 induced naturalistic and socially relevant responses, we would expect to be able to modulate the effects of these drugs by changing the social environment. We tested this hypothesis by using our previously described chronic disturbance method to generate honey bee colonies with reduced aggression levels (29). We exposed bees to these environments for 7 d in the field, collected and treated them with metabolic drugs, and assayed their behavior with the laboratory Intruder Assay. If metabolic effects are independent of social environment, we predicted that all drug-treated bees, regardless of prior social environment, would show increased aggression. By contrast, if the social environment modulates the metabolic effect, we predicted a drug treatment × social environment interaction effect.
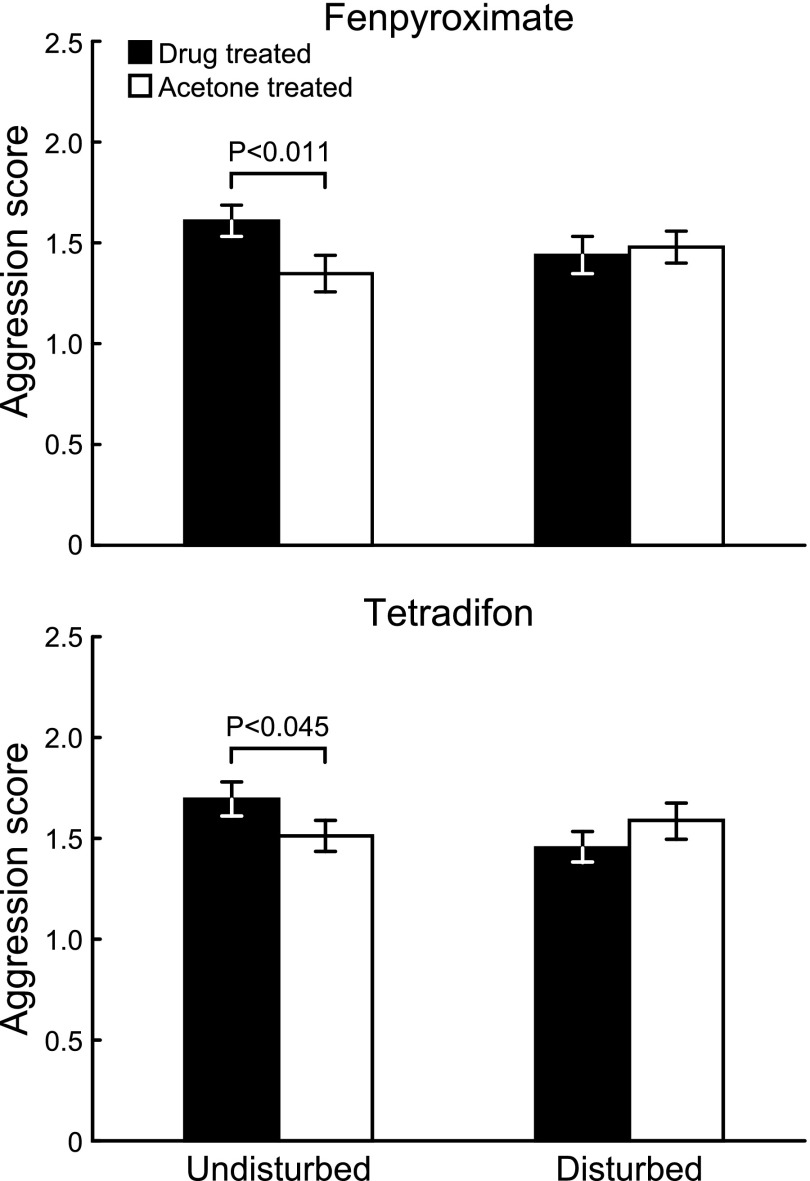
The drug treatment effects in experiment 1 are sensitive to social modulation. Bees collected from control colonies showed the predicted effects of treatment for both drugs (i.e., increased aggression relative to acetone-treated control bees), whereas bees collected from chronically disturbed colonies showed no differences comparing drug-treated bees with acetone-treated bees (Fig. 4). A linear mixed model showed a significant drug × social environment interaction effect for both drugs (fenpyroximate: F96,1 = 4.23, P < 0.043; tetradifon: F91,1 = 5.39, P < 0.023; SI Appendix, Table S4).
Fig. 4.
Effects of metabolic treatments on aggression in honey bees are attenuated by a specific social environment (mean ± SEM). Drug-treated bees collected from undisturbed colonies showed increased aggression relative to acetone-treated control bees (one-tailed t test, fenpyroximate: n = 51 groups, t50 = −2.63, P < 0.011; tetradifon: n = 49 groups, t48 = −1.72, P < 0.045). Drug-treated bees collected from chronically disturbed colonies showed no effects of drug treatment (one-tailed t test, fenpyroximate: n = 51 groups, t50 = 0.40, P = 0.66; tetradifon: n = 48 groups, t47 = −1.4, P = 0.92).
Discussion
Recent studies have shown that the relationship between glucose uptake and OX activity varies with brain region (10) and that plasticity in energy metabolism in the healthy brain is associated with cognition and behavioral plasticity (11). Moreover, changes in relative levels of glucose and oxygen metabolism have been associated with neural degeneration and disorders, including Huntington disease, Parkinson disease, and Alzheimer’s disease (13, 31–33). The broad involvement of metabolic plasticity in both the healthy and diseased brain suggests it is a fundamental characteristic of brain function.
Extending previous correlative findings (15), we demonstrated a causal relationship between OX inhibition and aggression for complexes I and V of OX in the honey bee and complex I in the fruit fly. Correlative studies suggest that the relationship between brain OX and aggression may be conserved in animals beyond insects, including lizards, rodents (34), and humans (35, 36). In addition, abnormalities in complexes I and V have previously been associated with neurological and behavioral disorders, including Leigh syndrome, bipolar disorder, and schizophrenia (26, 27, 37–39).
Variation in glucose uptake and metabolism in the brain (9, 12) could have a variety of functional outcomes. Pathways associated with glucose uptake and OX influence cellular redox balance, neurotransmitter metabolism, and biosynthesis (13, 14, 40, 41). Furthermore, studies in both invertebrate and vertebrate species show that glycolysis and OX are somewhat compartmentalized between the glia and neurons in the brain, and these cell types exchange energetic precursors and metabolites (3, 14, 42, 43). Thus, a brain metabolic state characterized by decreased OX activity could reflect one or several cell type-specific processes (10, 14).
The neural mechanisms by which decreased OX leads to increased aggression are also unknown. One possibility is that OX inhibition increases neural excitability, perhaps by shifting cells to a more excitable reduced redox state (44), or shifting metabolic flux toward increased glycolysis in either the neurons or the metabolically coupled glia (43, 45–48), leading to faster ATP production or glutamate turnover at synapses (10, 13, 49) and increased firing potential (50). Increased neural excitability could allow individuals to respond more quickly to aggressive stimuli, and neural excitability above typical levels has been associated with mood disorders and increased aggression in humans (51).
A second possibility is that OX inhibition decreases neural excitability, a change that is also known to have behavioral consequences (52, 53). Although we do not know whether our manipulations preferentially targeted certain neural cell types with high energetic demands, in mammals, fast-spiking (i.e., energetically demanding) interneurons are typically inhibitory (54). Decreased excitability of inhibitory neurons, due perhaps to decreased ATP levels (55, 56), disruption of membrane ion channels and pumps (1, 57–59), or changes in neurotransmitter release and reuptake (60), could disinhibit neural circuits involved in the aggressive response. In humans, low blood sugar is correlated with increased aggression and impulsiveness, possibly due to a lack of adequate energy substrates to sustain neural and thus behavioral inhibition (61, 62). These effects are likely mediated by insulin signaling (61), which is known to influence cognition in humans (63) and behavioral plasticity in bees (2), suggesting conserved mechanisms could connect glucose availability and metabolism, neural excitability, and behavior across diverse species.
A third possibility is that OX inhibition affects aggression by altering levels of aggression-related neurotransmitters in the brain through changes in cellular redox state (64–68), altered flux through the tricarboxylic acid cycle (69), or increased glucose uptake. If a change in OX influences signaling for specific neurotransmitters, it would suggest that the metabolic pattern in the aggressive brain is localized to particular brain regions.
Aerobic glycolysis is commonly observed in cancer cells, where it is known as the Warburg effect. In this context, high rates of glycolysis relative to OX provide an efficient route to generate amino acids and nucleotides necessary for rapid growth (69). A recent meta-analysis suggests that aerobic glycolysis might serve a similar function in the adult brain (12). Areas of the brain with relatively high levels of aerobic glycolysis tend to show gene expression patterns consistent with synapse formation and growth (12). Similarly, the shift away from oxidative metabolism in the aggressive honey bee brain is accompanied by increased growth-related gene expression, including up-regulation of genes associated with mitosis and protein production (15). Thus, the metabolic pattern in the aggressive brain could be related to biosynthetic processes important for synaptic plasticity and remodeling.
Inhibiting OX in neurons caused a behavioral effect in fruit flies, whereas inhibiting OX in glia did not. It is possible that this reflects a difference in metabolic demand between cell types: for example, in the honey bee retina, glia have very few mitochondria compared with neurons and rely disproportionately on glycolysis during periods of stimulation (3). Neurons may be more susceptible to both changes in ATP availability and redox state, or our treatments may have disproportionately affected mitochondria-rich neurons. In the context of other research on metabolic flux in the brain, decreased OX localized to the neurons seems surprising. Metabolic plasticity in the brain typically manifests as an increase or decrease in glycolysis with little or no change in OX (9, 11). Here we have demonstrated a novel context for brain metabolic plasticity, characterized by a decrease in neuronal OX. To our knowledge this is the first example of such a pattern occurring naturally in a nondisease context (15). Our paradigm can be used in the future to address whether these two types of changes, increased glycolysis alone vs. decreased OX, have similar functional outcomes in the brain.
It is noteworthy that we observed a conserved relationship between OX activity and aggression in honey bees and fruit flies given that they are separated by more than 300 million years of evolution and there are few other clear examples of shared aggression mechanisms between these two species (25, 70). One interpretation of our findings is that variation in OX activity is a broad-acting mechanism that modulates a general aspect of neural function (e.g., excitability), which is a theme that unifies aggression mechanisms, regardless of which neurotransmitters are involved. Modulating OX in the brain may serve a priming function, analogous to modulating whole-organism arousal (71, 72).
Social manipulations of honey bee colonies that decreased individual aggression attenuated the effects of OX inhibition on aggression. Other studies have reported interactions between aggression-inducing mechanisms and the social environment (73, 74), but how these interactions affect specific brain functions is not known. A better understanding of how the social environment is able to modulate the link between brain metabolic plasticity and aggression, in both bees and flies, might provide general insights into the ways in which the social environment exerts lasting effects on individual behavior.
Materials and Methods
Honey Bees.
Experimental animals.
Age affects aggression in honey bees (28), so we controlled for age by collecting 1-d-old adult bees as they emerged from their pupal cells (29). Pupae were obtained from source colonies headed by naturally mated queens, and ∼30 colonies were used as sources over the course of experiments 1 and 3. Because a single queen uses semen from 7 to 17 males (28), there was a broad diversity of naturally occurring outbred genotypes represented in this study. We randomized genotype across treatments by pooling all bees from different source colonies. In experiment 1, bees were kept in 7.0 × 8.0 × 9.0-cm boxes in a 33 °C incubator and supplied honey and water ad libitum. Bees used in experiment 3 were counted and grouped into small field colonies (SI Appendix).
Treatment with metabolic drugs.
We treated bees with fenpyroximate and tetradifon (Sigma-Aldrich), two insecticides that are known OX inhibitors. Powdered drugs were dissolved in 100% acetone (Fisher Scientific). After anesthetizing the bees on ice, we administered a single 1-μL topical application of the appropriate drug to the thorax. Previous studies showed that topical pharmacological treatments to the thorax penetrate the brain (19), although in the present study we did not determine this. Control bees received an equivalent treatment with 1 μL of 100% acetone. We performed the Intruder Assays 24 h after treatment.
Intruder Assay.
The Intruder Assay was modified from ref. 21. To enhance the sensitivity of the assay, we created mixed groups of bees (10 bees per group) in which half of the bees in the group were treated with a drug and half were treated with the acetone vehicle control. We then compared the total aggression level for all drug-treated vs. all acetone-treated bees within each group.
After 60-min acclimation time to a temperature-controlled ventilated room (25–28 °C), we monitored the behavior of the focal bees toward the intruder for 3 min. We scored aggression by scan sampling every 10 s and derived an aggression score by multiplying the number of tallies of each type of aggressive behavior by an index of severity (SI Appendix). We then summed indexed values to give the total aggression score for bees within each group.
Fruit Flies.
Fly-rearing conditions.
SI Appendix provides additional information on fly stocks and crossing schemes. Flies were reared in 25 × 95-mm plastic vials containing a standard fly diet (yeast, corn syrup, and agar) at ∼25 °C and 60% humidity with a 12-h light–dark cycle. Fly cultures were maintained and transferred to new food vials every 2 wk. Newly eclosed males collected for the aggression assay were isolated individually in 10 × 50-mm clear polystyrene tubes containing the standard fly diet.
Aggression assay and analysis.
The fly arena was designed after ref. 22, with minor modifications. Four- to six-day-old adult male flies were used for the behavioral assays. Two individuals of the same genotype were placed into the arena with a food patch containing a 1 × 1-cm square piece of solid food (agarose, sucrose, and apple juice with a small pile of dry yeast). After a 10-min acclimation period, paired flies were video-recorded for 20 min with a Sony HD camcorder (MHS-CM5). One pair of flies from each of the parental control lines and one pair of RNAi knockdown flies were filmed at the same time. After filming, flies were flash-frozen immediately for later validation of gene expression (SI Appendix). The CADABRA software package (Caltech) was used to track and quantify the behavioral activity of the fly pairs (22).
Supplementary Material
Acknowledgments
We thank S. A. Kreher for providing fly lines; D. J. Anderson and L. Wang for help with the fly software and providing ChaGAL4 and UAS-Tra fly lines; O. V. Alekseyenko, Y.-B. Chan, E. A. Kravitz, and M. L. Sokolowski for advice on fly crosses; Y. Ben-Shahar for advice on fly experimental designs; E. McGinnis for fly crosses and filming assistance; C. Coombs and A. Sheriff for assistance with honey bee experiments; and M. E. Raichle, M. L. Sokolowski, and members of the Robinson laboratory for comments that improved the manuscript. This work was supported by an American Fellowship from the American Association of University Women (H.L.-B.); and National Institutes of Health Director’s Pioneer Award 1DP1OD006416 and National Science Foundation Grant IOS-1256705 (to G.E.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1412306111/-/DCSupplemental.
References
- 1.Magistretti PJ, Allaman I. Brain energy metabolism. In: Pfaff DW, editor. Neurosciece in the 21st Century: Basic to Clinical. New York: Springer; 2013. pp. 1591–1619. [Google Scholar]
- 2.Ament SA, Corona M, Pollock HS, Robinson GE. Insulin signaling is involved in the regulation of worker division of labor in honey bee colonies. Proc Natl Acad Sci USA. 2008;105(11):4226–4231. doi: 10.1073/pnas.0800630105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsacopoulos M, Veuthey AL, Saravelos SG, Perrottet P, Tsoupras G. Glial cells transform glucose to alanine, which fuels the neurons in the honeybee retina. J Neurosci. 1994;14(3 Pt 1):1339–1351. doi: 10.1523/JNEUROSCI.14-03-01339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchner E, Buchner S. Mapping stimulus-induced nervous activity in small brains by [3H]2-deoxy-D-glucose. Cell Tissue Res. 1980;211(1):51–64. doi: 10.1007/BF00233722. [DOI] [PubMed] [Google Scholar]
- 5.Sokoloff L, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 6.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 7.Lotze M, Veit R, Anders S, Birbaumer N. Evidence for a different role of the ventral and dorsal medial prefrontal cortex for social reactive aggression: An interactive fMRI study. Neuroimage. 2007;34(1):470–478. doi: 10.1016/j.neuroimage.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Augustine GJ, et al. Neuroscience. 3rd Ed. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- 9.Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- 10.Vaishnavi SN, et al. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci USA. 2010;107(41):17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madsen PL, et al. Persistent resetting of the cerebral oxygen/glucose uptake ratio by brain activation: evidence obtained with the Kety-Schmidt technique. J Cereb Blood Flow Metab. 1995;15(3):485–491. doi: 10.1038/jcbfm.1995.60. [DOI] [PubMed] [Google Scholar]
- 12.Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19(1):49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlassenko AG, et al. Spatial correlation between brain aerobic glycolysis and amyloid-β (Aβ ) deposition. Proc Natl Acad Sci USA. 2010;107(41):17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dienel GA. Brain lactate metabolism: The discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32(7):1107–1138. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alaux C, et al. Honey bee aggression supports a link between gene regulation and behavioral evolution. Proc Natl Acad Sci USA. 2009;106(36):15400–15405. doi: 10.1073/pnas.0907043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motoba K, et al. Species-specific detoxification metabolism of fenpyroximate, a potent acaricide. Pestic Biochem Physiol. 2000;67(2):73–84. [Google Scholar]
- 17.Dahlgren L, Johnson RM, Siegfried BD, Ellis MD. Comparative toxicity of acaricides to honey bee (Hymenoptera: Apidae) workers and queens. J Econ Entomol. 2012;105(6):1895–1902. doi: 10.1603/ec12175. [DOI] [PubMed] [Google Scholar]
- 18.Bustamante E, Pedersen PL. Tetradifon: An oligomycin-like inhibitor of energy-linked activities of rat liver mitochondria. Biochem Biophys Res Commun. 1973;51(2):292–298. doi: 10.1016/0006-291x(73)91255-2. [DOI] [PubMed] [Google Scholar]
- 19.Barron AB, Maleszka J, Vander Meer RK, Robinson GE, Maleszka R. Comparing injection, feeding and topical application methods for treatment of honeybees with octopamine. J Insect Physiol. 2007;53(2):187–194. doi: 10.1016/j.jinsphys.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Breed MD. Nestmate recognition in honey bees. Anim Behav. 1983;31(1):86–91. doi: 10.1006/anbe.1997.0581. [DOI] [PubMed] [Google Scholar]
- 21.Richard FJ, Holt HL, Grozinger CM. Effects of immunostimulation on social behavior, chemical communication and genome-wide gene expression in honey bee workers (Apis mellifera) BMC Genomics. 2012;13:558–575. doi: 10.1186/1471-2164-13-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat Methods. 2009;6(4):297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoyer SC, et al. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18(3):159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 24.Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006;9(12):1469–1471. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: A model system for the study of aggression. Proc Natl Acad Sci USA. 2002;99(8):5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mckenzie M, Ryan MT. Assembly factors of human mitochondrial complex I and their defects in disease. IUBMB Life. 2010;62(7):497–502. doi: 10.1002/iub.335. [DOI] [PubMed] [Google Scholar]
- 27.Kucharczyk R, et al. Mitochondrial ATP synthase disorders: Molecular mechanisms and the quest for curative therapeutic approaches. Biochim Biophys Acta. 2009;1793(1):186–199. doi: 10.1016/j.bbamcr.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Winston ML. The Biology of the Honey Bee. Cambridge, MA: Harvard University Press; 1987. [Google Scholar]
- 29.Rittschof CC, Robinson GE. Manipulation of colony environment modulates honey bee aggression and brain gene expression. Genes Brain Behav. 2013;12(8):802–811. doi: 10.1111/gbb.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alaux C, Robinson GE. Alarm pheromone induces immediate-early gene expression and slow behavioral response in honey bees. J Chem Ecol. 2007;33(7):1346–1350. doi: 10.1007/s10886-007-9301-6. [DOI] [PubMed] [Google Scholar]
- 31.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S26–S36, discussion S36–S38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 32.Varma H, Cheng R, Voisine C, Hart AC, Stockwell BR. Inhibitors of metabolism rescue cell death in Huntington’s disease models. Proc Natl Acad Sci USA. 2007;104(36):14525–14530. doi: 10.1073/pnas.0704482104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powers WJ, et al. Selective defect of in vivo glycolysis in early Huntington’s disease striatum. Proc Natl Acad Sci USA. 2007;104(8):2945–2949. doi: 10.1073/pnas.0609833104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakata JT, Crews D, Gonzalez-Lima F. Behavioral correlates of differences in neural metabolic capacity. Brain Res Brain Res Rev. 2005;48(1):1–15. doi: 10.1016/j.brainresrev.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Aksenov MY, et al. The expression of several mitochondrial and nuclear genes encoding the subunits of electron transport chain enzyme complexes, cytochrome c oxidase, and NADH dehydrogenase, in different brain regions in Alzheimer’s disease. Neurochem Res. 1999;24(6):767–774. doi: 10.1023/a:1020783614031. [DOI] [PubMed] [Google Scholar]
- 36.Choi KH, et al. Expression profiles of mitochondrial genes in the frontal cortex and the caudate nucleus of developing humans and mice selectively bred for high and low fear. PLoS ONE. 2012;7(11):e49183. doi: 10.1371/journal.pone.0049183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schon EA, Santra S, Pallotti F, Girvin ME. Pathogenesis of primary defects in mitochondrial ATP synthesis. Semin Cell Dev Biol. 2001;12(6):441–448. doi: 10.1006/scdb.2001.0281. [DOI] [PubMed] [Google Scholar]
- 38.Ma L, et al. No association between genetic polymorphisms of the NDUFS7 gene and schizophrenia in Han Chinese. Psychiatr Genet. 2013;23(1):29–32. doi: 10.1097/YPG.0b013e32835862c5. [DOI] [PubMed] [Google Scholar]
- 39.Ben-Shachar D, Karry R. Sp1 expression is disrupted in schizophrenia; a possible mechanism for the abnormal expression of mitochondrial complex I genes, NDUFV1 and NDUFV2. PLoS ONE. 2007;2(9):e817. doi: 10.1371/journal.pone.0000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kann O, Kovács R. Mitochondria and neuronal activity. Am J Physiol Cell Physiol. 2007;292(2):C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Brent CS, Fennern E, Amdam GV. Gustatory perception and fat body energy metabolism are jointly affected by vitellogenin and juvenile hormone in honey bees. PLoS Genet. 2012;8(6):e1002779. doi: 10.1371/journal.pgen.1002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Allaman I, Bélanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: For better and for worse. Trends Neurosci. 2011;34(2):76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Wang TA, et al. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science. 2012;337(6096):839–842. doi: 10.1126/science.1222826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Celotto AM, Chiu WK, Van Voorhies W, Palladino MJ. Modes of metabolic compensation during mitochondrial disease using the Drosophila model of ATP6 dysfunction. PLoS ONE. 2011;6(10):e25823. doi: 10.1371/journal.pone.0025823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rera M, Monnier V, Tricoire H. Mitochondrial electron transport chain dysfunction during development does not extend lifespan in Drosophila melanogaster. Mech Ageing Dev. 2010;131(2):156–164. doi: 10.1016/j.mad.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305(5680):99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- 48.Ulanovskaya OA, et al. Synthesis enables identification of the cellular target of leucascandrolide A and neopeltolide. Nat Chem Biol. 2008;4(7):418–424. doi: 10.1038/nchembio.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91(22):10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White RJ, Reynolds IJ. Mitochondria and Na+/Ca2+ exchange buffer glutamate-induced calcium loads in cultured cortical neurons. J Neurosci. 1995;15(2):1318–1328. doi: 10.1523/JNEUROSCI.15-02-01318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keele NB. The role of serotonin in impulsive and aggressive behaviors associated with epilepsy-like neuronal hyperexcitability in the amygdala. Epilepsy Behav. 2005;7(3):325–335. doi: 10.1016/j.yebeh.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 52.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7(5):426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anckarsäter H. Central nervous changes in social dysfunction: Autism, aggression, and psychopathy. Brain Res Bull. 2006;69(3):259–265. doi: 10.1016/j.brainresbull.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Chesselet M-F, Plotkin JL, Wu N, Levine MS. Development of striatal fast-spiking GABAergic interneurons. 2007;160:261–272. doi: 10.1016/S0079-6123(06)60015-0. [DOI] [PubMed] [Google Scholar]
- 55.Davey GP, Peuchen S, Clark JB. Energy thresholds in brain mitochondria. Potential involvement in neurodegeneration. J Biol Chem. 1998;273(21):12753–12757. doi: 10.1074/jbc.273.21.12753. [DOI] [PubMed] [Google Scholar]
- 56.Potter WB, et al. Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS ONE. 2010;5(2):e8996. doi: 10.1371/journal.pone.0008996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hinkle JL, Bowman L. Neuroprotection for ischemic stroke. J Neurosci Nurs. 2003;35(2):114–118. doi: 10.1097/01376517-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34(4-5):325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 59.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 60.Scheller RH. In search of the molecular mechanism of intracellular membrane fusion and neurotransmitter release. Nat Med. 2013;19(10):1232–1235. doi: 10.1038/nm.3339. [DOI] [PubMed] [Google Scholar]
- 61.Gailliot MT, Baumeister RF. The physiology of willpower: Linking blood glucose to self-control. Pers Soc Psychol Rev. 2007;11(4):303–327. doi: 10.1177/1088868307303030. [DOI] [PubMed] [Google Scholar]
- 62.Bushman BJ, Dewall CN, Pond RS, Jr, Hanus MD. Low glucose relates to greater aggression in married couples. Proc Natl Acad Sci USA. 2014;111(17):6254–6257. doi: 10.1073/pnas.1400619111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cholerton B, Baker LD, Craft S. Insulin, cognition, and dementia. Eur J Pharmacol. 2013;719(1-3):170–179. doi: 10.1016/j.ejphar.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yao JK, et al. Associations between purine metabolites and monoamine neurotransmitters in first-episode psychosis. Front Cell Neurosci. 2013;7:90. doi: 10.3389/fncel.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res Rev. 2004;3(4):445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Ramsay RR, Dunford C, Gillman PK. Methylene blue and serotonin toxicity: Inhibition of monoamine oxidase A (MAO A) confirms a theoretical prediction. Br J Pharmacol. 2007;152(6):946–951. doi: 10.1038/sj.bjp.0707430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kravitz EA, Huber R. Aggression in invertebrates. Curr Opin Neurobiol. 2003;13(6):736–743. doi: 10.1016/j.conb.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Nelson RJ, Chiavegatto S. Molecular basis of aggression. Trends Neurosci. 2001;24(12):713–719. doi: 10.1016/s0166-2236(00)01996-2. [DOI] [PubMed] [Google Scholar]
- 69.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hunt GJ. Flight and fight: A comparative view of the neurophysiology and genetics of honey bee defensive behavior. J Insect Physiol. 2007;53(5):399–410. doi: 10.1016/j.jinsphys.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stevenson PA, Dyakonova V, Rillich J, Schildberger K. Octopamine and experience-dependent modulation of aggression in crickets. J Neurosci. 2005;25(6):1431–1441. doi: 10.1523/JNEUROSCI.4258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haller J, Makara GB, Kruk MR. Catecholaminergic involvement in the control of aggression: Hormones, the peripheral sympathetic, and central noradrenergic systems. Neurosci Biobehav Rev. 1998;22(1):85–97. doi: 10.1016/s0149-7634(97)00023-7. [DOI] [PubMed] [Google Scholar]
- 73.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8(7):536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 74.Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008;31(3):120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.