Significance
The cellular mechanisms by which nicotinic and muscarinic cholinergic systems facilitate learning and memory largely remain to be elucidated. This study identified a common signaling pathway stimulated by cognitive-enhancing drugs targeted to nicotinic and m1 muscarinic receptors and acetylcholinesterase. Stimulation of this signaling pathway induces significant increases in glutamate receptor, ionotropic, N-methyl D-aspartate 2B (GluN2B)-containing NMDA receptor (NMDAR)-mediated responses at Schaffer collateral synapses in the hippocampal CA1 region, the most widely studied synapses for a cellular correlate of hippocampus-dependent memory. Because the GluN2B-containing NMDAR is a critical component for long-term potentiation and learning and memory, the identified signaling pathway is most likely involved in the cholinergic control of learning and memory. Thus, targeting components of the pathway may be effective treatment for memory impairment.
Keywords: m1 muscarinic receptor, donepezil, hippocampus
Abstract
Nicotinic and muscarinic ACh receptor agonists and acetylcholinesterase inhibitors (AChEIs) can enhance cognitive function. However, it is unknown whether a common signaling pathway is involved in the effect. Here, we show that in vivo administration of nicotine, AChEIs, and an m1 muscarinic (m1) agonist increase glutamate receptor, ionotropic, N-methyl D-aspartate 2B (GluN2B)-containing NMDA receptor (NR2B-NMDAR) responses, a necessary component in memory formation, in hippocampal CA1 pyramidal cells, and that coadministration of the m1 antagonist pirenzepine prevents the effect of cholinergic drugs. These observations suggest that the effect of nicotine is secondary to increased release of ACh via the activation of nicotinic ACh receptors (nAChRs) and involves m1 receptor activation through ACh. In vitro activation of m1 receptors causes the selective enhancement of NR2B-NMDAR responses in CA1 pyramidal cells, and in vivo exposure to cholinergic drugs occludes the in vitro effect. Furthermore, in vivo exposure to cholinergic drugs suppresses the potentiating effect of Src on NMDAR responses in vitro. These results suggest that exposure to cholinergic drugs maximally stimulates the m1/guanine nucleotide-binding protein subunit alpha q/PKC/proline-rich tyrosine kinase 2/Src signaling pathway for the potentiation of NMDAR responses in vivo, occluding the in vitro effects of m1 activation and Src. Thus, our results indicate not only that nAChRs, ACh, and m1 receptors are on the same pathway involving Src signaling but also that NR2B-NMDARs are a point of convergence of cholinergic and glutamatergic pathways involved in learning and memory.
Nicotinic and muscarinic agonists can produce cognitive enhancement (1, 2). Acetylcholinesterase inhibitors (AChEIs) also cause cognitive enhancement by increasing ACh levels (3, 4). However, it is largely unknown whether the effect of ACh is mediated by nicotinic ACh receptors (nAChRs), muscarinic receptors, or both. Studies involving cholinergic lesions and local administration of cholinergic antagonists indicate that both nAChRs and muscarinic receptors located in the hippocampus are of particular importance for learning and memory processes (5–8). However, the mechanisms by which these receptors mediate cognitive enhancement largely remain to be elucidated.
Synaptic plasticity is thought to be a critical component underlying learning and memory (9, 10), and the NMDA receptor (NMDAR) is a key component of synaptic plasticity (9, 11). Thus, studies of the modulation of NMDAR responses and long-term potentiation (LTP) induction by cholinergic drugs (12–20) help elucidate the mechanisms of cholinergic facilitation of learning and memory. In vitro acute nicotine can potentiate NMDAR-mediated responses in CA1 pyramidal cells in hippocampal slices via at least two different mechanisms (16, 18). One of these mechanisms is absent after a selective cholinergic lesion (21) and is paradoxically blocked by the muscarinic antagonist atropine (18), suggesting not only a critical role of nicotine-induced ACh release but also the involvement of muscarinic receptor activation in the effect of nicotine. This pathway appears to be stimulated by systemic nicotine administration in rats and most likely involves Src signaling (18, 19), which is known to be initiated via acute activation of m1 muscarinic (m1) receptors in CA1 pyramidal cells (22). An implication of these observations is that there is a common signaling pathway stimulated by cognitive-enhancing cholinergic drugs, leading to the enhancement of NMDAR-mediated responses in CA1 pyramidal cells. Thus, in this study, we investigated the link between nicotine and NMDARs in rats by administrating drugs that target different cholinergic proteins.
Results
In the following experiments, we gave rats repeated injections of the indicated drugs and then examined hippocampal slices after the drugs had cleared. In this way, we target long-lasting, but not acute, effects of the drugs. To assess the effect of treatment on the NMDAR responses, we measured NMDAR/AMPA receptor (AMPAR) ratios. This readout is independent of the number of synapses activated, and therefore allows for comparison of synaptic currents recorded in different slices.
Activation of Muscarinic Receptors Is Involved in Nicotine-Induced Enhancement of NMDAR Responses in Vivo.
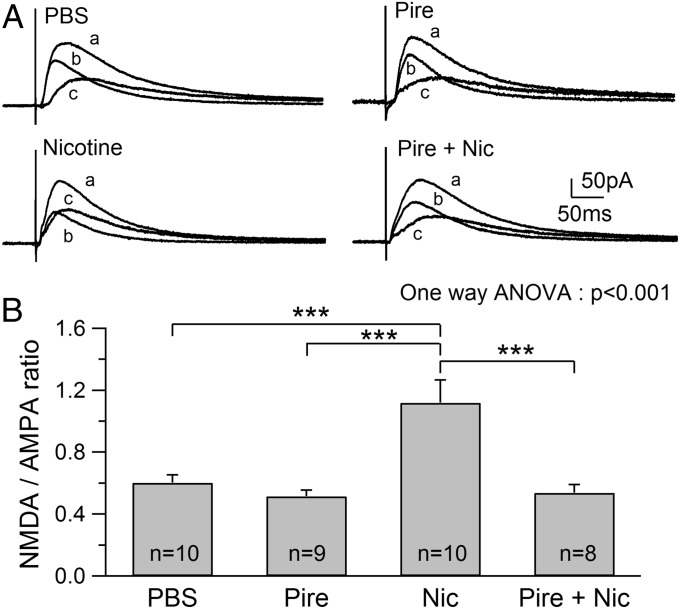
The hippocampus receives extensive cholinergic input from the medial septum/diagonal band, and it has been shown that both systemic and local administration of nicotine increases the release of ACh in the hippocampus in vivo (23). To examine whether this effect of nicotine leads to the activation of m1 receptors that trigger signaling for the enhancement of NMDAR responses (22), nicotine was coadministered with pirenzepine, a relatively selective m1 antagonist. NMDAR/AMPAR ratios obtained from rats treated with pirenzepine alone (0.52 ± 0.04, n = 9) were similar to those obtained from PBS-treated rats (0.60 ± 0.05, n = 10), suggesting that administration of pirenzepine alone had no significant effect on NMDAR responses (Fig. 1 A and B). Administration of nicotine caused a robust increase in NMDAR/AMPAR ratios (1.12 ± 0.15, n = 10) as reported previously (17), which were significantly higher than those obtained from both PBS-treated (ANOVA, P < 0.001) and pirenzepine-treated (ANOVA, P < 0.001) rats (Fig. 1 A and B). Coadministration of pirenzepine with nicotine prevented the effect of nicotine (0.54 ± 0.05, n = 8; ANOVA, P < 0.001; Fig. 1 A and B), suggesting that eliciting nicotine’s effect requires the activation of m1 receptors. Increased NMDAR/AMPAR ratios could be due to the enhancement of NMDAR excitatory postsynaptic currents (EPSCs) or suppression of AMPAR EPSCs. However, our previous study (17) has shown that this increase is due to the enhancement of NMDAR responses.
Fig. 1.
Pirenzepine (Pire) blocks the nicotine-induced increase in NMDAR/AMPAR ratios. (A) Traces show examples of NMDAR/AMPAR ratios obtained from animals treated with the indicated substance. Total EPSCs comprising both AMPAR- and NMDAR-mediated EPSCs were first recorded (a), and 2-amino-5-phosphopentanoate (40 μM) was then applied to obtain the AMPA EPSC (b). (c) NMDA EPSC was obtained by digital subtraction of the AMPA EPSC from the initial EPSC. (B) Summary of NMDAR/AMPAR ratios obtained from animals administered PBS, Pire, nicotine (Nic), or Pire + Nic. ***P < 0.001.
In Vivo Exposure to AChEIs Increases NMDAR/AMPAR Ratios via Muscarinic Receptor Activation.
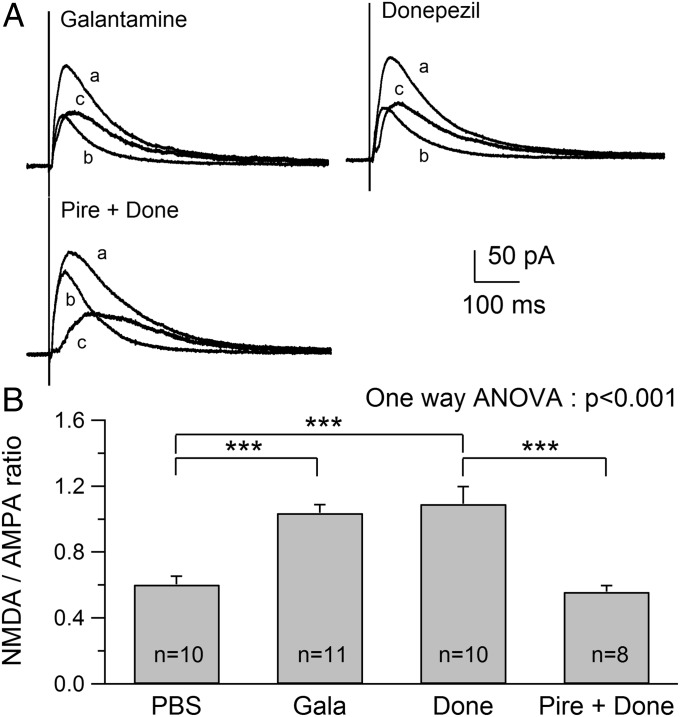
The requirement of m1 receptor activation for nicotine-induced enhancement of NMDAR responses suggests a critical role of nicotine-induced ACh release. To confirm the role of ACh, we elevated the synaptic levels of available ACh by administering AChEIs and determined whether this treatment mimicked the effect of nicotine on NMDAR responses. We used two AChEIs, donepezil and galantamine, currently used for treatment of mild to moderate Alzheimer’s disease (AD) and found a robust increase in NMDAR/AMPAR ratios from rats treated with galantamine (1.03 ± 0.05, n = 11; ANOVA, P < 0.001) and donepezil (1.09 ± 0.10, n = 10; ANOVA, P < 0.001) compared with PBS-treated rats (Fig. 2 A and B). The observed increases in the ratios were similar to that caused by nicotine exposure (1.12 ± 0.15, n = 10), suggesting a common role of increased ACh levels in the effect. Because blocking m1 receptors prevented the nicotine-induced enhancement of NMDAR responses, we examined whether blocking m1 receptors also prevents AChEI-induced increases in NMDAR/AMPAR ratios. Thus, pirenzepine was coadministered with donepezil to rats. We found that the ratio (0.56 ± 0.04, n = 8; Fig. 2 A and B) was significantly lower than that obtained in rats exposed to donepezil alone (ANOVA, P < 0.001) and was similar to that found in PBS- or pirenzepine-treated rats (Fig. 1). These observations suggest that the increased level of ACh causes the activation of m1 receptors that, in turn, enhances NMDAR responses, and that ACh and m1 receptors are downstream of nAChRs in the pathway.
Fig. 2.
Administration of galantamine (Gala) or donepezil (Done) induces an increase in NMDAR/AMPAR ratio. (A) Traces show examples of NMDAR/AMPAR ratios obtained from Gala-, Done-, and Pire + Done-treated animals. (B) Summary of NMDAR/AMPAR ratios obtained from animals administered PBS, Gala, Done, or Pire + Done. ***P < 0.001.
In Vivo Exposure to a Muscarinic Agonist Increases NMDAR/AMPAR Ratios.
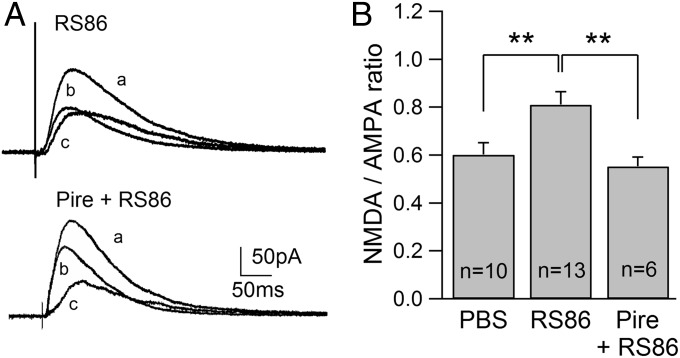
The m1 receptor is quite densely expressed in CA1 pyramidal cells, and acute activation of this subtype is known to cause short-lasting enhancement of NMDAR responses (19, 22, 24). Thus, this subtype is a reasonable target of ACh released from cholinergic terminals during in vivo nicotine and AChEI treatment. Furthermore, our results above suggest that m1 receptors are downstream of nAChRs and ACh. The implication of this is that direct activation of m1 receptors should mimic the effects of nicotine and AChEI on NMDARs. Thus, we used RS86, an m1 receptor agonist (25, 26), and found that in vivo exposure significantly increased the NMDAR/AMPAR ratio (0.81 ± 0.06, n = 13; P < 0.01) compared with PBS-exposed rats (Fig. 3 A and B). Furthermore, when pirenzepine was coadministered with RS86, the effect of RS86 was prevented (0.55 ± 0.04, n = 6; P < 0.01). These observations demonstrate that direct activation of m1 receptors causes the sustained enhancement of NMDAR responses in vivo, supporting our prediction that m1 receptors are downstream of nAChRs and ACh.
Fig. 3.
RS86 enhances NMDAR/AMPAR ratios. (A) Traces show examples of NMDAR/AMPAR ratios obtained from RS86- and Pire + RS86-treated animals. (B) Summary of NMDAR/AMPAR ratios obtained from animals administered PBS, RS86, or Pire + RS86. **P < 0.01.
In Vivo Exposure to Donepezil and RS86 Affect Muscarinic Modulation of Glutamate Receptor, Ionotropic, N-Methyl D-Aspartate 2B-NMDAR Responses in Vitro.
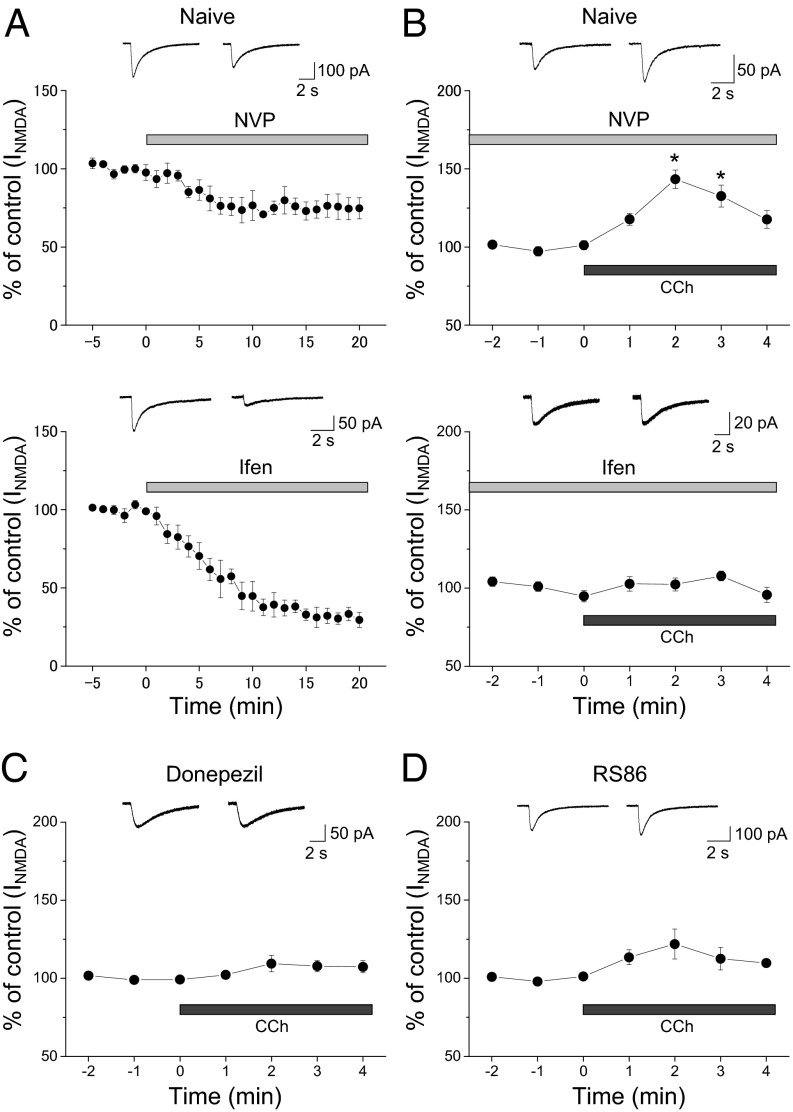
Bath application of the cholinergic agonist carbachol potentiates NMDAR responses evoked by NMDA puff onto CA1 pyramidal cells, and its effect is prevented in the presence of the selective m1 antagonist m1-toxin (24). The predominant NMDAR subtypes in CA1 pyramidal cells are those containing glutamate receptor, ionotropic, N-methyl D-aspartate 2A (GluN2A) (NR2A) and GluN2B (NR2B) subunits. It remains unknown whether muscarinic modulation is preferentially targeted to a particular NMDAR subtype. Thus, we pharmacologically isolated NR2A- and NR2B-containing NMDAR-mediated responses using the NR2B-selective antagonist ifenprodil (3 μM) and the NR2A-selective antagonist NVP-AAM077 (50 nM), respectively, and examined the effect of carbachol on the remaining responses. Application of ifenprodil reduced NMDA responses by ∼68%, whereas NVP-AAM077 decreased responses by about 25% (Fig. 4A), indicating that the response contains more NR2B than NR2A component. Because NR2B-containing NMDARs are common in the extrasynaptic site in addition to the synaptic site (27, 28), our observations suggest that NMDA puffs result in activation of both extrasynaptic and synaptic NMDARs. We then found that carbachol potentiated NMDA responses in the presence of NVP-AAM077 (143.4 ± 5.9%, n = 3; P < 0.05) but not in the presence of ifenprodil (107.2 ± 3.1%, n = 3), suggesting that this muscarinic modulation is selectively targeted to NR2B-containing NMDARs (Fig. 4B).
Fig. 4.
Repeated donepezil and RS86 treatment prevent the muscarinic receptor-mediated potentiation of NR2B-containing NMDAR responses. NMDAR responses were evoked by ejecting NMDA (1 mM) through a pipette to the patched cells at 1-min intervals and were recorded in CA1 pyramidal cells of naive (A and B), donepezil-exposed (C), and RS86-treated (D) rats. The time course of the effects of indicated drugs on the amplitude of NMDA responses is shown as percentage changes (mean ± SEM) in the peak amplitude of NMDAR responses. Representative traces of NMDA responses recorded before and 20 min (A) or 2 min (B–D) after drug application are shown. (A) Effects of the NR2A-selective antagonist NVP-AAM077 (50 nM, Upper) or the NR2B-selective antagonist ifenprodil (Ifen, 3 μM, Lower) on NMDA responses. (B) Effect of bath application of carbachol (CCh; 10 μM) on NR2B-containing NMDAR-mediated responses (recorded in the presence of NVP-AAM077, Upper) and NR2A-containing NMDAR-mediated responses (recorded in the presence of Ifen, Lower). (C) Effect of bath application of CCh (10 μM) on NMDAR-mediated responses in pyramidal cells of donepezil-exposed rats. (D) Effect of bath application of CCh (10 μM) on NMDAR-mediated responses in pyramidal cells of RS86-treated rats. *P < 0.05.
We previously confirmed that bath application of carbachol (10 μM) enhances NMDAR responses and this effect is blocked by pirenzepine (75 nM) in the naive hippocampus (19). However, this muscarinic modulation is not found after in vivo exposure to nicotine, suggesting that nicotine exposure alters m1 receptor-mediated signaling (19). Thus, we next examined whether in vivo exposure to donepezil and RS86 affect m1 signaling. We found that in donepezil-exposed pyramidal cells, as in the case of nicotine-exposed cells, m1 modulation of NMDAR responses was almost absent (donzepil: 109.4 ± 5.2%, n = 4; nicotine: 98.3 ± 12.4% of control, n = 4; Fig. 4C). However, in RS86-treated pyramidal cells, application of carbachol slightly potentiated NMDAR responses (121.9 ± 9.6%, n = 5; Fig. 4D), but this muscarinic modulation was significantly less than that in the naive pyramidal cells (168.5 ± 15.9% of control, n = 7; P < 0.01). These findings suggest that the m1 receptor signaling mediating the enhancement of NR2B-containing NMDAR responses is affected by in vivo exposure to these cholinergic drugs.
In Vivo Exposure to Nicotine, Donepezil, and RS86 Enhance NR2B-NMDAR–Mediated EPSCs.
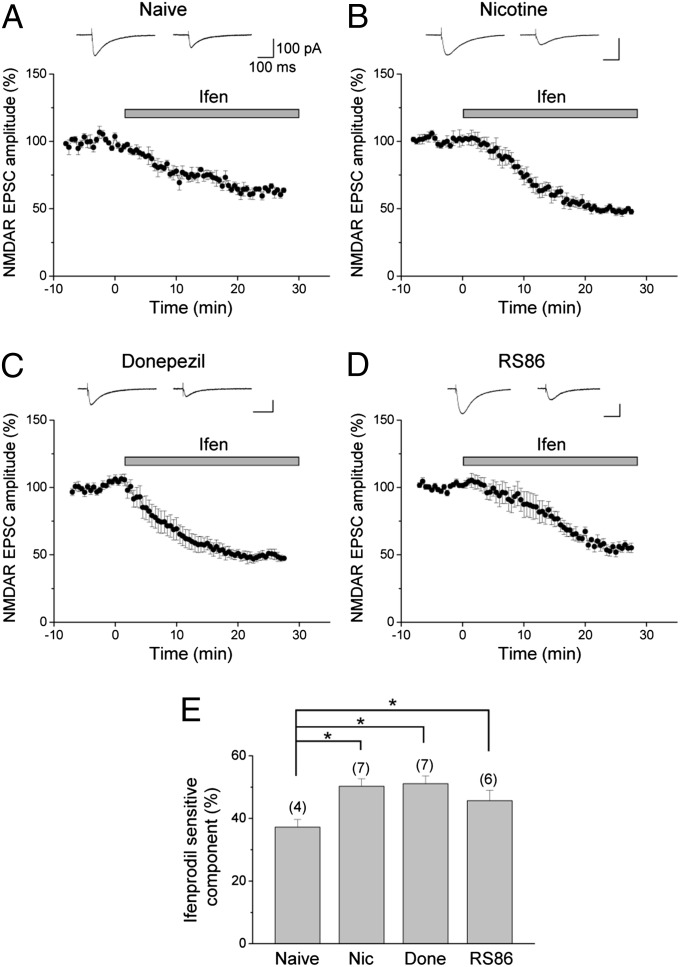
We further examined the effects of in vivo exposure to cholinergic drugs on synaptic NR2B-NMDAR–mediated responses. In control CA1 pyramidal cells, application of NVP-AAM077 caused a 50.7 ± 4.9% reduction in the amplitude of NMDAR-mediated EPSCs, whereas ifenprodil reduced the response by 37.2 ± 2.5% (Fig. 5A). This supports previous studies (27, 28) showing that CA1 synapses contain more NR2A-containing than NR2B-containing NMDARs. To assess the effects on synaptic NR2B-containing NMDAR responses, we then measured the changes in the ifenprodil-sensitive (NR2B) component of NMDAR-mediated EPSCs. We found that it was enhanced following in vivo nicotine exposure [naive (37.2 ± 2.5%) vs. nicotine (50.3 ± 2.4%); P < 0.05; Fig. 5 B and E]. Furthermore, as in the case of nicotine exposure, in vivo donepezil and RS86 exposure also caused significant increases in the NR2B component (51.1 ± 2.5% for donepezil, P < 0.05; 45.7 ± 3.3% for RS86, P < 0.05; Fig. 5 C–E). These findings demonstrate that in vivo exposure of cholinergic drugs acting on different cholinergic targets causes the enhancement of NR2B-NMDAR–mediated synaptic responses in hippocampal CA1 pyramidal cells.
Fig. 5.
Repeated nicotine, donepezil, and RS86 treatment enhances the NR2B component of NMDAR-mediated EPSCs. (A–D) NMDAR-mediated EPSCs were recorded in the presence of 6,7-dinitroquinoxalline-2,3-dione to block AMPAR-mediated EPSCs. To assess the effects of nicotine, donepezil, and RS86 exposure on the NR2B component of NMDAR-mediated EPSCs, the Ifen sensitivity of NMDAR EPSCs was monitored. The time course of the effect of Ifen on the amplitude of NMDAR EPSCs is shown as percentage changes (mean ± SEM) in the peak amplitude of the responses. Representative traces above the graph were taken before and 25 min after bath application of Ifen (3 μM). Effects of bath application of Ifen on NMDAR EPSCs in naive (A), nicotine-exposed (B), donepezil-exposed (C), and RS86-exposed (D) rats are shown. (E) Summary of the ifenprodil-sensitive component of NMDAR-mediated EPSCs obtained from naive, Nic-, Done-, and RS86-treated rats. *P < 0.05.
In Vivo Exposure to Nicotine, Donepezil, and RS86 Affect Src Signaling.
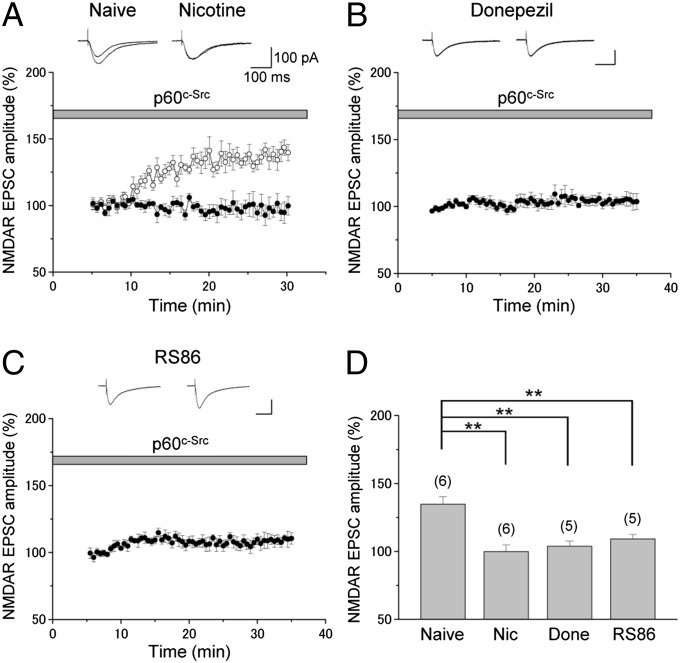
We next investigated the link between m1 receptors and NMDARs. Acute activation of m1 receptors can stimulate the guanine nucleotide-binding protein subunit alpha q (Gq)/PKC/proline-rich tyrosine kinase 2 (Pyk2)/Src signaling pathway for the enhancement of NMDAR responses in CA1 pyramidal cells (22). Thus, Src appears to act as the intermediate signaling molecule between m1 receptors and NMDARs. Because in vivo nicotine exposure appears to stimulate the Gq/PKC/Pyk2/Src signaling pathway maximally and, as a consequence, occludes any further potentiation of NMDAR responses by intracellular application of Src (19), we examined the effects of exogenous Src on NMDAR responses after in vivo donepezil and RS86 exposure. We found that infusion of Src into donepezil-exposed cells resulted in almost no effect on the amplitude of NMDAR-mediated EPSCs (103.8 ± 3.7% of control responses, n = 5; Fig. 6 B and D), although exogenous Src caused a significant increase in the amplitude of NMDAR-mediated EPSCs (134.8 ± 5.5% of control responses, n = 5; Fig. 6 A and D) in naive rats as reported previously (19). The effect of exogenous Src on NMDAR responses found in slices from donepezil-exposed rats (naive vs. donepezil: P < 0.01) is very similar to that observed in in vivo nicotine-exposed cells, in which exogenous Src had no significant effect on NMDAR EPSCs (99.9 ± 5.0% of control responses, n = 6; naive vs. nicotine: P ≤ 0.01; Fig. 6 A and D). When we applied Src directly into RS86-exposed pyramidal cells, the enhancement (109.2 ± 3.3% of control responses, n = 5) was significantly less than that observed in the cells of naive rats (134.8 ± 5.5% of control responses, n = 5) (naive vs. RS86: P < 0.01; Fig. 6 C and D). These results suggest that both donepezil and RS86 mimic the action of nicotine in vivo. As in the case of nicotine exposure (19), the significantly reduced Src effects in donepezil- and RS86-exposed pyramidal cells are most likely due to nearly saturated stimulation of the same pathway during in vivo donepezil and RS86 exposure, preventing any further potentiation of NMDAR responses by exogenous Src in vitro. These observations suggest that in vivo exposure to these cholinergic drugs enhances NR2B-NMDAR EPSCs via a common signaling cascade involving Src.
Fig. 6.
Repeated donepezil and RS86 exposure prevent the Src-mediated potentiation of NMDAR-mediated responses. (A–C) Time course of the effect of exogenous Src (p60c-Src) on NMDAR EPSCs is shown as percentage change (mean ± SEM) in the peak amplitude of NMDAR responses. NMDAR EPSCs were recorded in CA1 pyramidal cells from naive (A, ○), nicotine-treated (A, ●), donepezil-treated (B), and RS86-treated (C) rats. Src (30 U/mL) was applied directly into the cells by diffusional exchange from the patch pipette while simultaneously monitoring NMDAR EPSCs. (A–C, Insets) Representative traces from the first 5-min period and the period from 25 to 30 min are displayed. Each trace is the average of four NMDAR EPSCs. (D) Changes in the mean amplitudes of NMDA EPSCs by Src in naive, Nic-, Done- and RS86-treated rats. The peak amplitudes of NMDA EPSCs during the first 5–10 min (after whole-cell configuration was established) and the period from 25 to 30 min were used to calculate the percentage changes. **P < 0.01.
Discussion
The current study demonstrates that administration of cholinergic cognitive-enhancing drugs that act on nAChRs, acetylcholinesterase, or m1 receptors induces significant increases in NR2B-containing NMDAR-mediated responses in CA1 pyramidal cells of rat hippocampus, a critical component for enhancing LTP and learning and memory (29).
Role of nAChRs in the Nicotine-Induced Potentiation of NR2B-NMDAR Responses.
Cholinergic neurons originating from the medial septum/diagonal band project to the hippocampus, and these neurons contain α7, non-α7 (possibly α4β2), and α7β2 nAChR subtypes (30, 31). Activation of any of these nAChRs localized at somatodendritic or presynaptic sites by nicotine potentially increases ACh release in the hippocampus (23). α7β2 nAChR subtype is highly sensitive to functional inhibition by pathologically relevant concentrations of amyloid peptides (31). If this subtype is involved in nicotine-induced ACh release to trigger the cholinergic signaling, it would provide a possible mechanism for deficits in cholinergic signaling contributing to cognitive function in early AD. In hippocampal slices, bath application of nicotine enhances NMDAR responses in CA1 pyramidal cells (18). This effect of nicotine is paradoxically blocked by atropine and is absent after a selective lesion of cholinergic neurons by 192-IgG-saporin (18, 21). These observations suggest that the effect of nicotine is most likely secondary to increased release of ACh via activation of presynaptic nAChRs at cholinergic terminals in hippocampal slices and involves postsynaptic muscarinic receptors. In vivo exposure to nicotine increases ACh release (23) and enhances NMDAR responses in CA1 pyramidal cells (17). The current study demonstrates that this enhancement is prevented by coadministration of pirenzepine with nicotine, suggesting the involvement of muscarinic receptor activation in the effect. Thus, in vivo and in vitro nicotine exposure appears to stimulate the same signaling pathway, and repeated in vivo exposure to nicotine maintains the effect.
Role of m1 Receptors in Cholinergic Drug-Induced Potentiation of NR2B-NMDAR Responses.
Although RS86 has been used as an m1 receptor agonist (25, 26), its selectivity under the conditions used remains unknown. Nevertheless, the fact that the effect of RS86 is blocked by coadministration of pirenzepine suggests that the effect of RS86 is due to direct stimulation of the m1 receptor-mediated signaling cascade. However, pirenzepine also has a relatively high affinity for the m4 subtype of muscarinic AChRs and is proposed as an inverse agonist of the m2 subtype (32, 33). Thus, it is not clear whether the doses of pirenzepine used in our experiments achieved selectivity for the m1 subtype. Nevertheless, our observations that pirenzepine blocked the effects of nicotine, AChEIs, and RS86 on NR2B-NMDAR responses suggest the involvement of muscarinic receptor activation in the effects. Pirenzepine has been shown to stimulate ACh release (34), although the mechanism is unclear. The m2 receptors are located on presynaptic cholinergic terminals projecting to the hippocampus (35). Activation of this muscarinic subtype inhibits the release of ACh, whereas blockade of this subtype leads to increased levels of ACh (2, 36, 37). Thus, pirenzepine might act as an inverse agonist at the m2 subtype and increase levels of ACh. This effect of pirenzepine should mimic the effects of nicotine and AChEIs. However, our current study demonstrated that administration of pirenzepine had no effect on NMDAR/AMPAR ratios. Pirenzepine might enhance ACh levels via the m2 receptor, but pirenzepine acting through m1 receptors on postsynaptic CA1 pyramidal cells prevented its effect on NR2B-NMDAR responses. The m1, m3, and m5 receptors can couple to the Gq family, Gq and G11 (38), and are expressed in pyramidal cells (39). However, muscarinic receptor-stimulated Gq/11-specific 35SGTPγ (GTP labeled on the gamma phosphate with 35S) binding to G proteins, which has been used as a sensitive assay of G protein-coupled receptor activation, was virtually abolished in the hippocampus of m1 receptor KO mice (38). This suggests a role for the m1 receptor subtype as the primary Gq/11-coupled muscarinic receptor in the hippocampus. Thus, it is mostly likely that m1 is the muscarinic receptor subtype involved in the nicotine-, AChEI-, and RS86-induced enhancement of NMDAR responses, and that the effects of pirenzepine are due to blocking this muscarinic receptor subtype.
Possible Role of NMDAR Activation in Cholinergic Drug-Induced Potentiation of NR2B-NMDAR Responses.
Tyrosine phosphorylation of NMDARs returns to basal levels when stimulation of the Src signaling cascade is terminated, and protein tyrosine phosphatases remove phosphate groups. Thus, modulation of NMDARs by tyrosine phosphorylation is likely to be short lived. However, our data demonstrate that enhancement of NMDAR function in cholinergic drug-exposed pyramidal cells is sustained, suggesting the involvement of an additional mechanism for the sustained increase in NMDAR responses. We expect that each administration of cholinergic drugs activates m1 receptors to potentiate NMDAR responses via a Gq/PKC/Pyk2/Src signaling cascade (22), thereby increasing Ca2+ entry through NMDARs. In addition to PKC, Pyk2 is activated by Ca2+, and therefore enhanced Ca2+ entry through NMDARs may set up a feedforward cycle that maintains activation of Src, and thereby sustained up-regulation of NR2B-NMDAR function. This may explain why exogenous Src had no significant effect on NMDAR-mediated responses in nicotine-, donepezil-, and RS86-exposed pyramidal cells.
Role of Cholinergic Drug-Induced Potentiation of NR2B-NMDAR Responses in LTP.
It is known that activation of m1 receptors in CA1 pyramidal cells enhances NMDAR-mediated responses (22, 24). However, to our knowledge, the present study is the first to demonstrate that this effect is due to selective enhancement of NR2B-NMDAR responses, providing a link between m1 receptors and NR2B-NMDARs. This link is most likely involved in m1 receptor-mediated facilitation of LTP induction in the hippocampal CA1 region (15). Because transgenic mice overexpressing NR2B in the forebrain show enhanced LTP and learning and memory (29), the pathway found in the present study may be critically involved in cholinergic drug-induced cognitive enhancement. Repeated in vivo nicotine exposure results in the facilitation of LTP induction and long-lasting LTP that is NR2B-containing NMDAR-dependent and protein synthesis-independent (12, 17). Furthermore, repeated in vivo treatment of animals with AChEIs also causes long-lasting LTP in the hippocampal CA1 region (40). The long-lasting nature of LTP might imply an important role of the cholinergic NR2B-NMDAR pathway revealed in the current study in long-term memory formation.
AChEIs cause cognitive enhancement via their ability to increase ACh levels, and they are the primary treatment for the cognitive impairments of AD. In addition to AChEIs, clinical studies using nicotine skin patches show the efficacy of nicotine in treating cognitive deficits associated with AD (1), and RS86 also shows positive clinical changes in cognitive function in a minority of patients with AD (41). Our current study indicates that AChEIs, nicotine, and RS86 all potentiate NR2B-containing NMDAR responses via an m1/Gq/PKC/Pyk2/Src signaling pathway. Global KOs of m1, Gq, and Src are known to affect NMDAR-dependent LTP induction in mice (15, 42, 43). Furthermore, cholinergic lesions impair the signaling pathway (18, 21, 44), and the overproduction of β-amyloid causes uncoupling of m1 receptor/G protein interaction (45). Thus, deficits in the signaling pathway most likely contribute to the loss of cognitive function in AD. Indeed, impaired coupling of m1 receptors to G proteins is associated with the severity of dementia in AD (46). Thus, the identified cholinergic-glutamatergic signaling appears to be a critical pathway involved in cognitive function that is affected in AD.
Materials and Methods
Methods are described in detail in SI Materials and Methods. All animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (47) and with protocols approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Chronic Drug Treatment.
Sprague–Dawley rats (∼3 wk old) were injected with drugs as described in SI Materials and Methods.
Electrophysiological Recording.
EPSCs and NMDAR-mediated responses were recorded using the whole-cell patch clamp technique as described previously (17). NMDAR/AMPAR ratios were calculated by measuring the average peak EPSC at +40 mV (EPSCs recorded over 5 min) before and after application of the NMDAR antagonist 2-amino-5-phosphopentanoate (40 μM). The NMDAR EPSC amplitude was obtained by digital subtraction of AMPAR EPSC amplitude from the initial EPSC. Src (30 U/mL; Upstate) was directly applied into pyramidal cells by diffusional exchange through patch pipettes as described previously (17).
Drugs.
Most chemicals were obtained from Sigma Chemical Company. Donepezil hydrochloride (Aricept), and RS86 and NVP-AAM077 were generous gifts from Eisai Company and Novartis Pharma AG, respectively.
Statistical Analysis.
Data were analyzed offline using Origin (OriginLab) and pCLAMP 7 (Axon Instruments). Data were expressed as means ± SEM. Sample size n refers to the total number of neurons analyzed in electrophysiological recordings; each cell was recorded from a separate hippocampal slice, obtained from three to six rats. For statistical analysis, differences between means were compared using a two-sample, two-tailed Student t test. For multiple comparisons of the means, one-way ANOVA followed by a post hoc Fisher’s protected least significant difference test was used. A comparison was considered statistically significant if P < 0.05.
Supplementary Material
Acknowledgments
This research was supported by the National Institute on Drug Abuse (Grants DA14542, DA025676, and DA026458).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408805111/-/DCSupplemental.
References
- 1.Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49(3):258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- 2.Fisher A. Cholinergic treatments with emphasis on m1 muscarinic agonists as potential disease-modifying agents for Alzheimer’s disease. Neurotherapeutics. 2008;5(3):433–442. doi: 10.1016/j.nurt.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winblad B, et al. Donepezil Nordic Study Group A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57(3):489–495. doi: 10.1212/wnl.57.3.489. [DOI] [PubMed] [Google Scholar]
- 4.Mintzer JE, Kershaw P. The efficacy of galantamine in the treatment of Alzheimer’s disease: Comparison of patients previously treated with acetylcholinesterase inhibitors to patients with no prior exposure. Int J Geriatr Psychiatry. 2003;18(4):292–297. doi: 10.1002/gps.826. [DOI] [PubMed] [Google Scholar]
- 5.McGurk SR, Levin ED, Butcher LL. Impairment of radial-arm maze performance in rats following lesions involving the cholinergic medial pathway: Reversal by arecoline and differential effects of muscarinic and nicotinic antagonists. Neuroscience. 1991;44(1):137–147. doi: 10.1016/0306-4522(91)90256-n. [DOI] [PubMed] [Google Scholar]
- 6.Decker MW, Majchrzak MJ, Anderson DJ. Effects of nicotine on spatial memory deficits in rats with septal lesions. Brain Res. 1992;572(1-2):281–285. doi: 10.1016/0006-8993(92)90485-r. [DOI] [PubMed] [Google Scholar]
- 7.Iversen SD. Behavioural evaluation of cholinergic drugs. Life Sci. 1997;60(13-14):1145–1152. doi: 10.1016/s0024-3205(97)00059-3. [DOI] [PubMed] [Google Scholar]
- 8.Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008;38(1):101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 10.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 11.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Fujii S, Ji Z, Morita N, Sumikawa K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res. 1999;846(1):137–143. doi: 10.1016/s0006-8993(99)01982-4. [DOI] [PubMed] [Google Scholar]
- 13.Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31(1):131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 14.Ovsepian SV, Anwyl R, Rowan MJ. Endogenous acetylcholine lowers the threshold for long-term potentiation induction in the CA1 area through muscarinic receptor activation: In vivo study. Eur J Neurosci. 2004;20(5):1267–1275. doi: 10.1111/j.1460-9568.2004.03582.x. [DOI] [PubMed] [Google Scholar]
- 15.Shinoe T, Matsui M, Taketo MM, Manabe T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci. 2005;25(48):11194–11200. doi: 10.1523/JNEUROSCI.2338-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki Y, Jia Y, Hamaue N, Sumikawa K. Nicotine-induced switch in the nicotinic cholinergic mechanisms of facilitation of long-term potentiation induction. Eur J Neurosci. 2005;22(4):845–860. doi: 10.1111/j.1460-9568.2005.04259.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki Y, Jia Y, Niu R, Sumikawa K. Nicotine exposure in vivo induces long-lasting enhancement of NMDA receptor-mediated currents in the hippocampus. Eur J Neurosci. 2006;23(7):1819–1828. doi: 10.1111/j.1460-9568.2006.04714.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki Y, Fujii S, Jia Y, Sumikawa K. Nicotine withdrawal suppresses nicotinic modulation of long-term potentiation induction in the hippocampal CA1 region. Eur J Neurosci. 2006;24(10):2903–2916. doi: 10.1111/j.1460-9568.2006.05160.x. [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki Y, Jia Y, Wong JK, Sumikawa K. Chronic nicotine-induced switch in Src-family kinase signaling for long-term potentiation induction in hippocampal CA1 pyramidal cells. Eur J Neurosci. 2006;24(11):3271–3284. doi: 10.1111/j.1460-9568.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 20.Nakauchi S, Brennan RJ, Boulter J, Sumikawa K. Nicotine gates long-term potentiation in the hippocampal CA1 region via the activation of alpha2* nicotinic ACh receptors. Eur J Neurosci. 2007;25(9):2666–2681. doi: 10.1111/j.1460-9568.2007.05513.x. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki Y, Hamaue N, Sumikawa K. Nicotine compensates for the loss of cholinergic function to enhance long-term potentiation induction. Brain Res. 2002;946(1):148–152. doi: 10.1016/s0006-8993(02)02935-9. [DOI] [PubMed] [Google Scholar]
- 22.Salter MW, Kalia LV. Src kinases: A hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5(4):317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 23.Reid RT, Lloyd GK, Rao TS. Pharmacological characterization of nicotine-induced acetylcholine release in the rat hippocampus in vivo: Evidence for a permissive dopamine synapse. Br J Pharmacol. 1999;127(6):1486–1494. doi: 10.1038/sj.bjp.0702683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ. Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-D-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1998;95(19):11465–11470. doi: 10.1073/pnas.95.19.11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gower AJ. Effects of acetylcholine agonists and antagonists on yawning and analgesia in the rat. Eur J Pharmacol. 1987;139(1):79–89. doi: 10.1016/0014-2999(87)90500-0. [DOI] [PubMed] [Google Scholar]
- 26.Seo H, Ferree AW, Isacson O. Cortico-hippocampal APP and NGF levels are dynamically altered by cholinergic muscarinic antagonist or M1 agonist treatment in normal mice. Eur J Neurosci. 2002;15(3):498–506. doi: 10.1046/j.0953-816x.2001.01884.x. [DOI] [PubMed] [Google Scholar]
- 27.Rauner C, Köhr G. Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem. 2011;286(9):7558–7566. doi: 10.1074/jbc.M110.182600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petralia RS. Distribution of extrasynaptic NMDA receptors on neurons. ScientificWorldJournal. 2012;2012:267120. doi: 10.1100/2012/267120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang YP, et al. Genetic enhancement of learning and memory in mice. Nature. 1999;401(6748):63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 30.Thinschmidt JS, Frazier CJ, King MA, Meyer EM, Papke RL. Medial septal/diagonal band cells express multiple functional nicotinic receptor subtypes that are correlated with firing frequency. Neurosci Lett. 2005;389(3):163–168. doi: 10.1016/j.neulet.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 31.Liu Q, et al. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J Neurosci. 2009;29(4):918–929. doi: 10.1523/JNEUROSCI.3952-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billard W, Binch H, 3rd, Crosby G, McQuade RD. Identification of the primary muscarinic autoreceptor subtype in rat striatum as m2 through a correlation of in vivo microdialysis and in vitro receptor binding data. J Pharmacol Exp Ther. 1995;273(1):273–279. [PubMed] [Google Scholar]
- 33.Daeffler L, Schmidlin F, Gies JP, Landry Y. Inverse agonist activity of pirenzepine at M2 muscarinic acetylcholine receptors. Br J Pharmacol. 1999;126(5):1246–1252. doi: 10.1038/sj.bjp.0702407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imperato A, Scrocco MG, Ghirardi O, Ramacci MT, Angelucci L. In vivo probing of the brain cholinergic system in the aged rat. Effects of long-term treatment with acetyl-L-carnitine. Ann N Y Acad Sci. 1991;621:90–97. doi: 10.1111/j.1749-6632.1991.tb16971.x. [DOI] [PubMed] [Google Scholar]
- 35.Wall SJ, Wolfe BB, Kromer LF. Cholinergic deafferentation of dorsal hippocampus by fimbria-fornix lesioning differentially regulates subtypes (m1-m5) of muscarinic receptors. J Neurochem. 1994;62(4):1345–1351. doi: 10.1046/j.1471-4159.1994.62041345.x. [DOI] [PubMed] [Google Scholar]
- 36.Pohorecki R, Head R, Domino EF. Effects of selected muscarinic cholinergic antagonists on [3H]acetylcholine release from rat hippocampal slices. J Pharmacol Exp Ther. 1988;244(1):213–217. [PubMed] [Google Scholar]
- 37.Quirion R, et al. Facilitation of acetylcholine release and cognitive performance by an M(2)-muscarinic receptor antagonist in aged memory-impaired. J Neurosci. 1995;15(2):1455–1462. doi: 10.1523/JNEUROSCI.15-02-01455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porter AC, et al. M1 muscarinic receptor signaling in mouse hippocampus and cortex. Brain Res. 2002;944(1-2):82–89. doi: 10.1016/s0006-8993(02)02721-x. [DOI] [PubMed] [Google Scholar]
- 39.Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ. Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci. 1995;15(5 Pt 2):4077–4092. doi: 10.1523/JNEUROSCI.15-05-04077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnes CA, et al. Chronic treatment of old rats with donepezil or galantamine: Effects on memory, hippocampal plasticity and nicotinic receptors. Neuroscience. 2000;99(1):17–23. doi: 10.1016/s0306-4522(00)00180-9. [DOI] [PubMed] [Google Scholar]
- 41.Wettstein A, Spiegel R. Clinical trials with the cholinergic drug RS 86 in Alzheimer’s disease (AD) and senile dementia of the Alzheimer type (SDAT) Psychopharmacology (Berl) 1984;84(4):572–573. doi: 10.1007/BF00431470. [DOI] [PubMed] [Google Scholar]
- 42.Miura M, Watanabe M, Offermanns S, Simon MI, Kano M. Group I metabotropic glutamate receptor signaling via Galpha q/Galpha 11 secures the induction of long-term potentiation in the hippocampal area CA1. J Neurosci. 2002;22(19):8379–8390. doi: 10.1523/JNEUROSCI.22-19-08379.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitcher GM, et al. Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat Med. 2011;17(4):470–478. doi: 10.1038/nm.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potter PE, Gaughan C, Assouline Y. Lesion of septal-hippocampal neurons with 192 IgG-saporin alters function of M1 muscarinic receptors. Neuropharmacology. 1999;38(4):579–586. doi: 10.1016/s0028-3908(98)00207-x. [DOI] [PubMed] [Google Scholar]
- 45.Janíčková H, et al. Uncoupling of M1 muscarinic receptor/G-protein interaction by amyloid β(1-42) Neuropharmacology. 2013;67:272–283. doi: 10.1016/j.neuropharm.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Tsang SW, et al. Impaired coupling of muscarinic M1 receptors to G-proteins in the neocortex is associated with severity of dementia in Alzheimer’s disease. Neurobiol Aging. 2006;27(9):1216–1223. doi: 10.1016/j.neurobiolaging.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Committee on Care and Use of Laboratory Animals 1985. Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.