Abstract
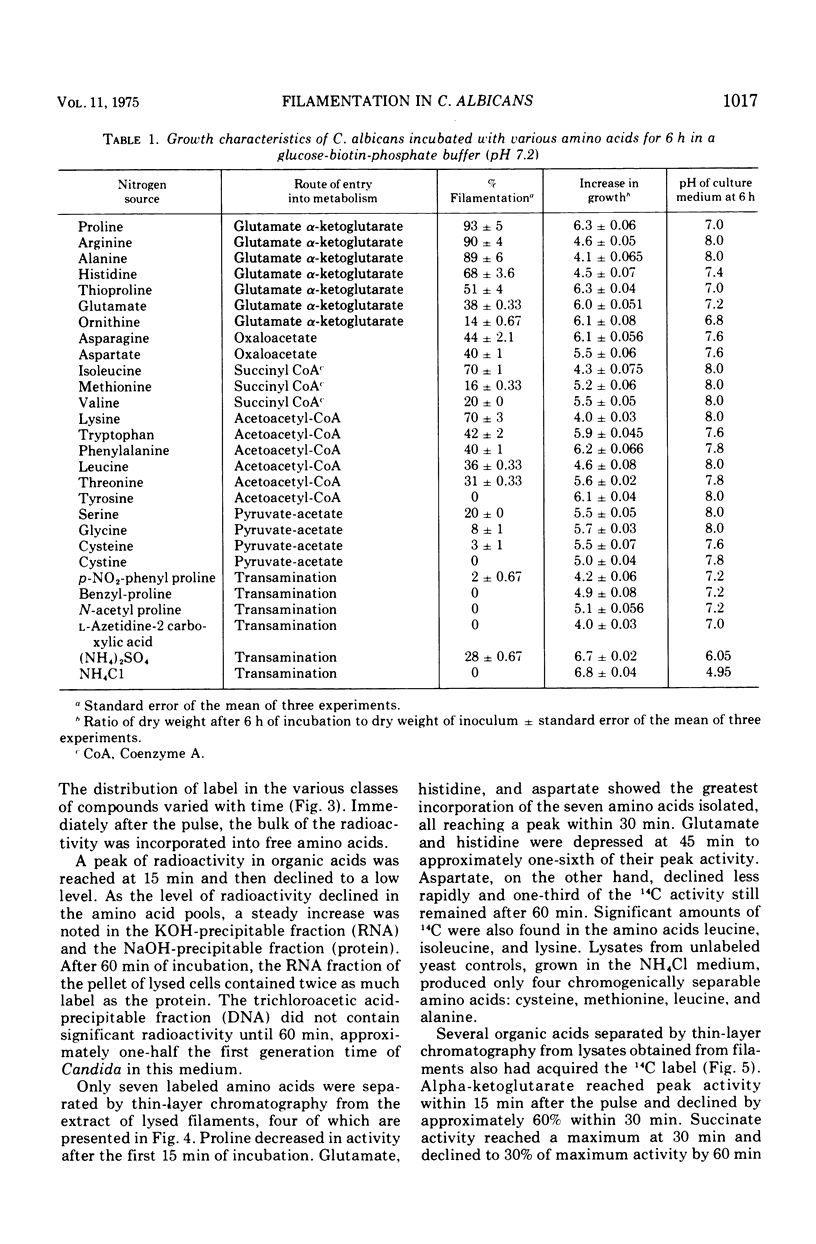
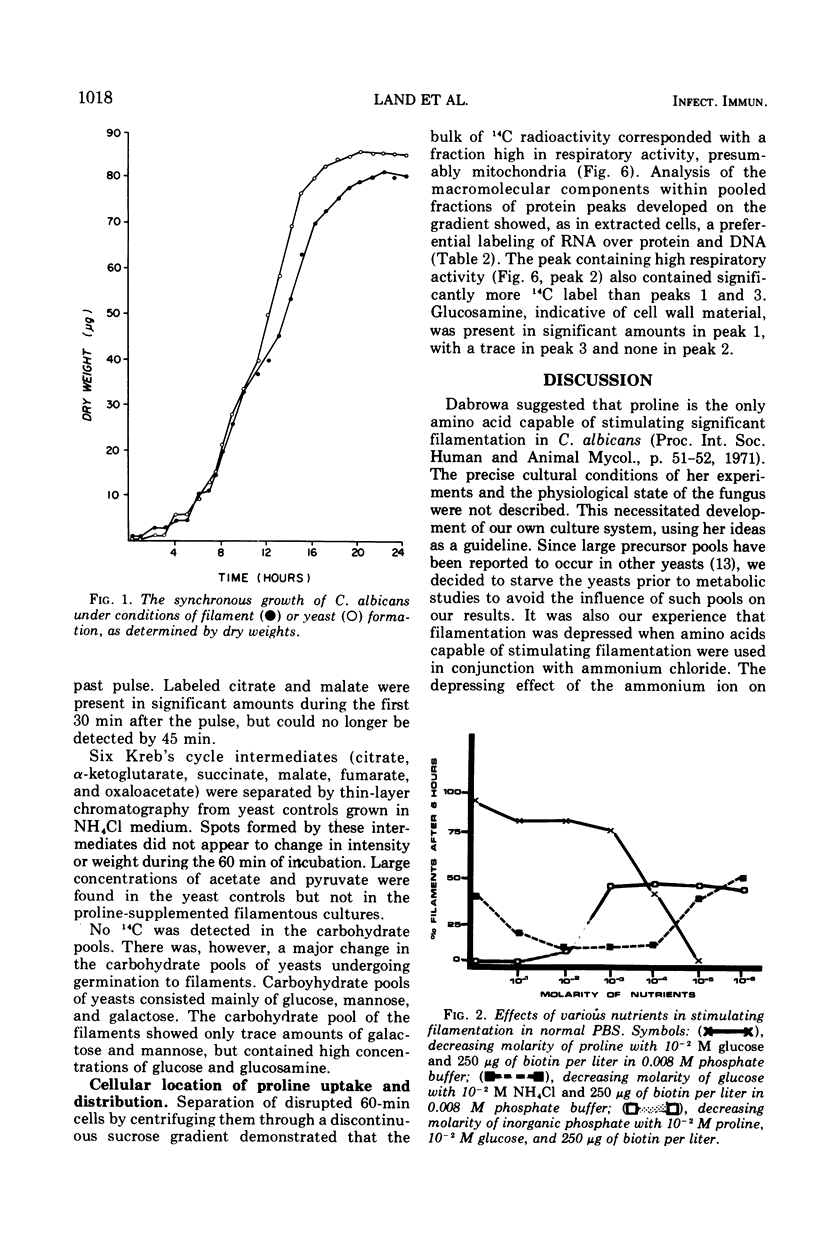
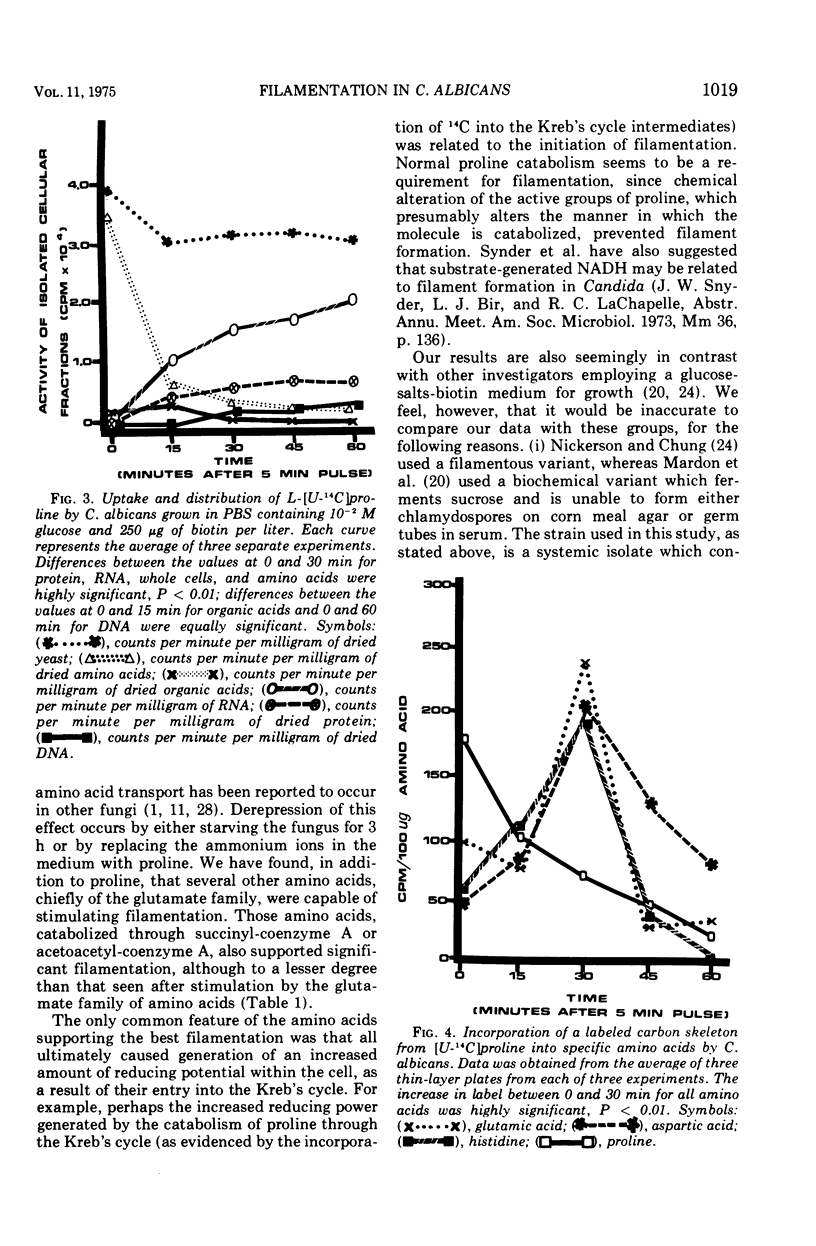
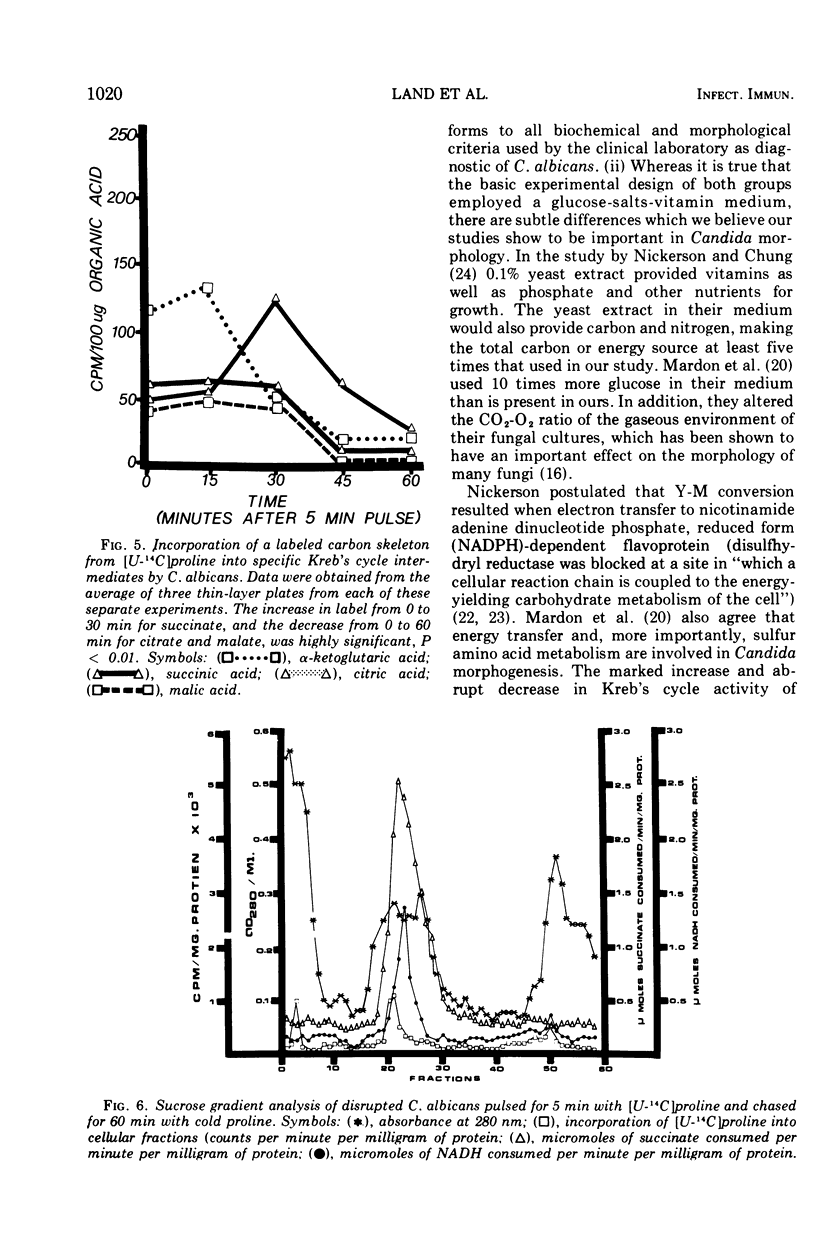
When glucose was present in high concentration, Candida albicans formed filaments in a phosphate-buffered medium, regardless of the nitrogen source. In lower concentrations of glucose, filamentation occurred only when various members of the glutamate, succinyl, or acetoacetyl-coenzyme A families of amino acids were used as sole nitrogen sources. Yeast morphology could be maintained either by replacing the amino acids in the medium with ammonium chloride or by making the medium high in phosphate or biotin. Studies using [U-14C]proline indicated that proline was catabolized in a manner consistent with the generation of increased cellular reducing potential and that the proline label entered into the Kreb's cycle. A reduction in Kreb's cycle activity was evidenced by an initial increase and then a rapid drop of the total organic acid content of the cells as well as in specific Kreb's cycle intermediates. Filamentation under conditions of low phosphate, high glucose, and increased cellular reduction potential, accompanied by a decrease in Kreb's cycle activity, suggests that morphogenesis in C. albicans is correlated with a Crabtree-like effect, i.e., repression of mitochondrial activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arst H. N., Jr, MacDonald D. W. A mutant of Asperigillus nidulans lacking NADP-linked glutamate dehydrogenase. Mol Gen Genet. 1973 May 9;122(3):261–265. doi: 10.1007/BF00278601. [DOI] [PubMed] [Google Scholar]
- Aterman K. Pathology of Candida infection of the umbilical cord. Am J Clin Pathol. 1968 Jun;49(6):798–804. doi: 10.1093/ajcp/49.6.798. [DOI] [PubMed] [Google Scholar]
- Chandra A. K., Banerjee A. B. Oxidation of specifically labelled glucose by Trichophyton rubrum. Acta Microbiol Pol A. 1972;4(4):197–200. [PubMed] [Google Scholar]
- Chattaway F. W., Bishop R., Holmes M. R., Odds F. C., Barlow A. J. Enzyme activities associated with carbohydrate synthesis and breakdown in the yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1973 Mar;75(1):97–109. doi: 10.1099/00221287-75-1-97. [DOI] [PubMed] [Google Scholar]
- Chattaway F. W., Holmes M. R., Barlow A. J. Cell wall composition of the mycelial and blastospore forms of Candida albicans. J Gen Microbiol. 1968 May;51(3):367–376. doi: 10.1099/00221287-51-3-367. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowa N., Howard D. H., Landau J. W., Shechter Y. Synthesis of nueic acids and proteins in the dimorphic forms of Candida albicans. Sabouraudia. 1970 Nov;8(3):163–169. doi: 10.1080/00362177085190831. [DOI] [PubMed] [Google Scholar]
- De Deken R. H. The Crabtree effects and its relation to the petite mutation. J Gen Microbiol. 1966 Aug;44(2):157–165. doi: 10.1099/00221287-44-2-157. [DOI] [PubMed] [Google Scholar]
- Dubois E., Grenson M., Wiame J. M. Release of the "ammonia effect" on three catabolic enzymes by NADP-specific glutamate dehydrogenaseless mutations in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1973 Feb 20;50(4):967–972. doi: 10.1016/0006-291x(73)91500-3. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Concentrations of intermediary metabolites in yeast. Biochimie. 1973;55(2):205–211. doi: 10.1016/s0300-9084(73)80393-1. [DOI] [PubMed] [Google Scholar]
- Gilardi G. L. Nutrition of systemic and subcutaneous pathogenic fungi. Bacteriol Rev. 1965 Sep;29(3):406–424. doi: 10.1128/br.29.3.406-424.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graafmans W. D. The influence of carbon dioxide on morphogenesis in Penicillium isariiforme. Arch Mikrobiol. 1973 Apr 8;91(1):67–76. doi: 10.1007/BF00409539. [DOI] [PubMed] [Google Scholar]
- Hurley R., Stanley V. C. Cytopathic effects of pathogenic and non-pathogenic species of Candida on cultured mouse epithelial cells: relation to the growth rate and morphology of the fungi. J Med Microbiol. 1969 Feb;2(1):63–74. doi: 10.1099/00222615-2-1-63. [DOI] [PubMed] [Google Scholar]
- Koobs D. H. Phosphate mediation of the Crabtree and Pasteur effects. Science. 1972 Oct 13;178(4057):127–133. doi: 10.1126/science.178.4057.127. [DOI] [PubMed] [Google Scholar]
- Kraeger S. J., Hamilton J. G. Quantitative glass-paper chromatography of fungal cell wall acid hydrolysates. J Chromatogr. 1969 Apr 22;41(1):113–115. doi: 10.1016/0021-9673(64)80105-9. [DOI] [PubMed] [Google Scholar]
- Mardon D., Balish E., Phillips A. W. Control of dimorphism in a biochemical variant of Candida albicans. J Bacteriol. 1969 Nov;100(2):701–707. doi: 10.1128/jb.100.2.701-707.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICKERSON W. J. Experimental control of morphogenesis in microorganisms. Ann N Y Acad Sci. 1954 Oct 29;60(1):50–57. doi: 10.1111/j.1749-6632.1954.tb39997.x. [DOI] [PubMed] [Google Scholar]
- NICKERSON W. J. SYMPOSIUM ON BIOCHEMICAL BASES OF MORPHOGENESIS IN FUNGI. IV. MOLECULAR BASES OF FORM IN YEASTS. Bacteriol Rev. 1963 Sep;27:305–324. doi: 10.1128/br.27.3.305-324.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poll van de K. W. Activity of the hexose monophosphate shunt in a mutant of Saccharomyces carlsbergensis lacking NADP dependent glutamate dehydrogenase activity. FEBS Lett. 1973 May 15;32(1):33–34. doi: 10.1016/0014-5793(73)80729-x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwencke J., Magaña-Schwencke N. Derepression of a proline transport system in Saccharomyces chevalieri by nitrogen starvation. Biochim Biophys Acta. 1969 Mar 11;173(2):302–312. doi: 10.1016/0005-2736(69)90113-8. [DOI] [PubMed] [Google Scholar]
- Taschdjian C. L., Kozinn P. J., Toni E. F. Opportunistic yeast infections, with special reference to candidiasis. Ann N Y Acad Sci. 1970 Oct 30;174(2):606–622. doi: 10.1111/j.1749-6632.1970.tb45586.x. [DOI] [PubMed] [Google Scholar]
- WIDRA A. PHOSPHATE DIRECTED Y-M VARIATION IN CANDIDA ALBICANS. Mycopathol Mycol Appl. 1964 Sep 30;23:197–202. doi: 10.1007/BF02068455. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Kanda Y., Iwata K. Biochemical properties of mitochondria from Candida albicans. Sabouraudia. 1971 Nov;9(3):221–230. [PubMed] [Google Scholar]



