Significance
Ammonia-oxidizing archaea (AOA) influence the form and availability of nitrogen in marine environments and are a major contributor to N2O release and plausible indirect source of methane in the upper ocean. Thus, their sensitivity to ocean acidification and other physicochemical changes associated with climate change has global significance. Here, we report on the physiological response of marine AOA isolates to key environmental variables. Although reported as highly sensitive to reduction in ocean pH, we now show that some coastal marine AOA can remain active with increasing acidification of the oceans. All AOA isolates assimilate fixed carbon and two are obligate mixotrophs, suggesting this globally significant assemblage serves a significant function in coupling chemolithotrophy with organic matter assimilation in marine food webs.
Keywords: marine ammonia-oxidizing archaea, ecophysiology, urea utilization
Abstract
Ammonia-oxidizing archaea (AOA) are now implicated in exerting significant control over the form and availability of reactive nitrogen species in marine environments. Detailed studies of specific metabolic traits and physicochemical factors controlling their activities and distribution have not been well constrained in part due to the scarcity of isolated AOA strains. Here, we report the isolation of two new coastal marine AOA, strains PS0 and HCA1. Comparison of the new strains to Nitrosopumilus maritimus strain SCM1, the only marine AOA in pure culture thus far, demonstrated distinct adaptations to pH, salinity, organic carbon, temperature, and light. Strain PS0 sustained nearly 80% of ammonia oxidation activity at a pH as low as 5.9, indicating that coastal strains may be less sensitive to the ongoing reduction in ocean pH. Notably, the two novel isolates are obligate mixotrophs that rely on uptake and assimilation of organic carbon compounds, suggesting a direct coupling between chemolithotrophy and organic matter assimilation in marine food webs. All three isolates showed only minor photoinhibition at 15 µE⋅m−2⋅s−1 and rapid recovery of ammonia oxidation in the dark, consistent with an AOA contribution to the primary nitrite maximum and the plausibility of a diurnal cycle of archaeal ammonia oxidation activity in the euphotic zone. Together, these findings highlight an unexpected adaptive capacity within closely related marine group I Archaea and provide new understanding of the physiological basis of the remarkable ecological success reflected by their generally high abundance in marine environments.
The discovery of ammonia-oxidizing archaea (AOA), sometimes constituting up to nearly 40% of marine microbial plankton, challenged the traditional view of microbial controls of nitrogen speciation in the ocean (1–5). The AOA are now generally recognized as the major drivers of nitrification in marine environments (5–8). Their activities are of importance to trophic interactions that influence primary production and export of carbon to the deep ocean, they are a known source of atmospheric greenhouse gases (nitrous oxide and indirectly methane), and their high demand for copper may also alter food web dynamics (9–14).
Although enrichments of AOA strains belonging to groups I.1a and I.1b from a variety of marine and terrestrial environments have been reported (15–22), no additional pure cultures of marine representatives have been described since the isolation of Nitrosopumilus maritimus strain SCM1 (henceforth referred to as SCM1) (23). However, many complex biological traits of significance to marine biogeochemistry and marine food webs cannot be unambiguously established using metagenomic, metatranscriptomic studies and enrichment cultures. Initial understanding of the physiological basis for high AOA abundance in the marine water column came from the demonstration that SCM1 is an extreme oligotroph, having one of the highest affinities (low Ks) for ammonia (here defined as combined ammonia/ammonium) yet observed in pure culture (24). The discovery of a previously unidentified pathway for methylphosphonate synthesis, a plausible source of methane in the upper ocean, in the AOA and other abundant marine plankton (including Pelagibacter and Prochlorococcus) was made possible by genomic and biochemical characterization of SCM1 (13, 14). More recent physiological studies examining the copper requirements of SCM1 provided a framework to evaluate the significance of copper in controlling the environmental distribution and activity of marine AOA (12).
The capture of a greater representation of AOA environmental diversity in pure culture should therefore serve to expand understanding of traits influencing their activity patterns in coastal and open marine systems. For example, a capacity to use fixed carbon and urea as alternative carbon and energy sources, respectively, has been suggested by tracer, metagenomic, and metatranscriptomic studies (25–33). The contribution of AOA to endogenously generated nitrate in marine surface waters, of fundamental relevance to the source of nitrogen sustaining primary production in the photic zone, was suggested by relating nitrification activity and the archaeal amoA (the gene coding for the α-subunit of the ammonia monooxygenase) distribution patterns (7, 8, 34–36). A reported sensitivity of marine populations to small reductions in pH, important to understanding the possible impact of ongoing ocean acidification on the marine nitrogen cycle, was inferred from a pH perturbation study (37). Thus, as was shown for studies of SCM1, the availability of additional AOA isolates will both inform inferences made in the field as well as provide a physiological basis to direct future field research.
We now report the isolation of two novel coastal marine AOA strains from the Puget Sound estuary system in Washington, significantly expanding the ecotypic diversity of marine Thaumarchaeota available in pure culture. Despite relatively close phylogenetic relationships, the three marine isolates comprise physiologically distinct ecotypes of AOA, varying in their capacity to use different carbon and energy sources, and in their tolerance to changes in pH, salinity, and light. These distinctive differences are directly relevant to their possible contribution to nitrification in different marine environments—including lower salinity coastal regions, the photic zone of the upper water column, and the increasing acidification of ocean waters associated with climate change.
Results
Enrichment and Isolation of Marine AOA.
Enrichment cultures were initiated from Puget Sound main basin near surface (47.55 N, 122.28 W) and 50 m water from the Puget Sound Regional Synthesis Model (PRISM) Station P10 (47.91 N, 122.62 W) in Hood Canal. Our previous molecular surveys showed these coastal waters to be dominated by AOA, relative to ammonia-oxidizing bacteria (AOB) (8, 38, 39). Predominance of AOA was consistent with generally submicromolar concentrations of ammonia at these stations, sufficient to support AOA such as SCM1 but not known AOB (24). Enrichment conditions were designed to simulate the low substrate availability of these marine systems. The growth medium was supplemented with 2 µM NH4Cl, and cultures were incubated at 15 °C (SI Materials and Methods).
Once the enrichments showed stable activity, they were transferred to artificial seawater medium supplemented with 2 µM NH4Cl and 100 µM α-ketoglutaric acid. Earlier studies of strain SCM1 had shown that only a few central metabolites, including α-ketoglutaric acid, stimulated growth (SI Materials and Methods). Highly enriched AOA cultures were obtained following ∼2 y of consecutive transfer of 10% (vol/vol) late–exponential-phase subcultures into the same medium. Comparable growth kinetics was observed at ammonia concentrations between 2 and 500 µM ammonia, but concentrations above 1 mM significantly impaired growth. Thus, 500 µM ammonia was subsequently used to isolate the two new AOA strains from enrichment culture. Pure cultures were ultimately obtained by filtration of the enrichment cultures through a 0.22-µm Millex-GP syringe filter, and diluting the filtrate to extinction (SI Materials and Methods). The two new oligotrophic AOA strains were designated HCA1 (Hood Canal station P10) and PS0 (Puget Sound main basin).
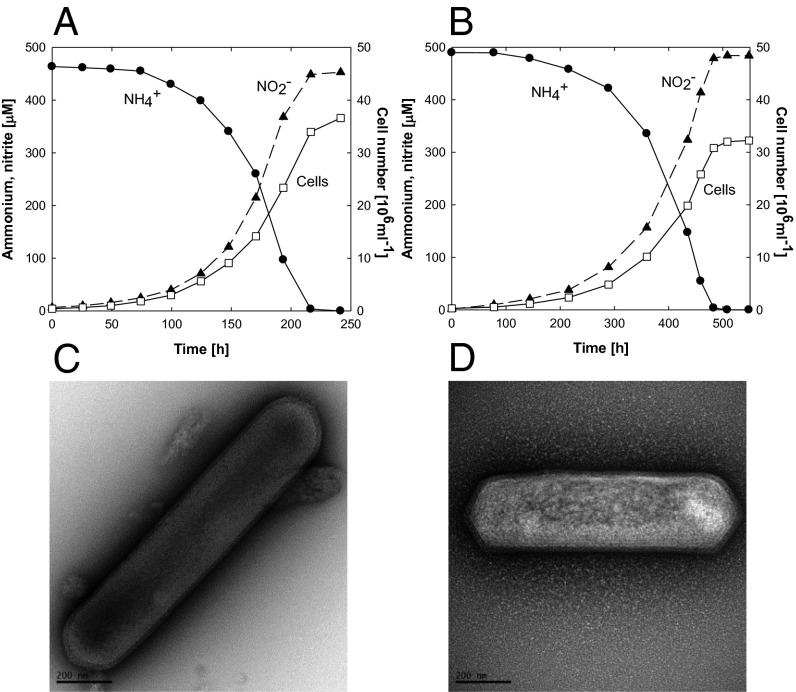
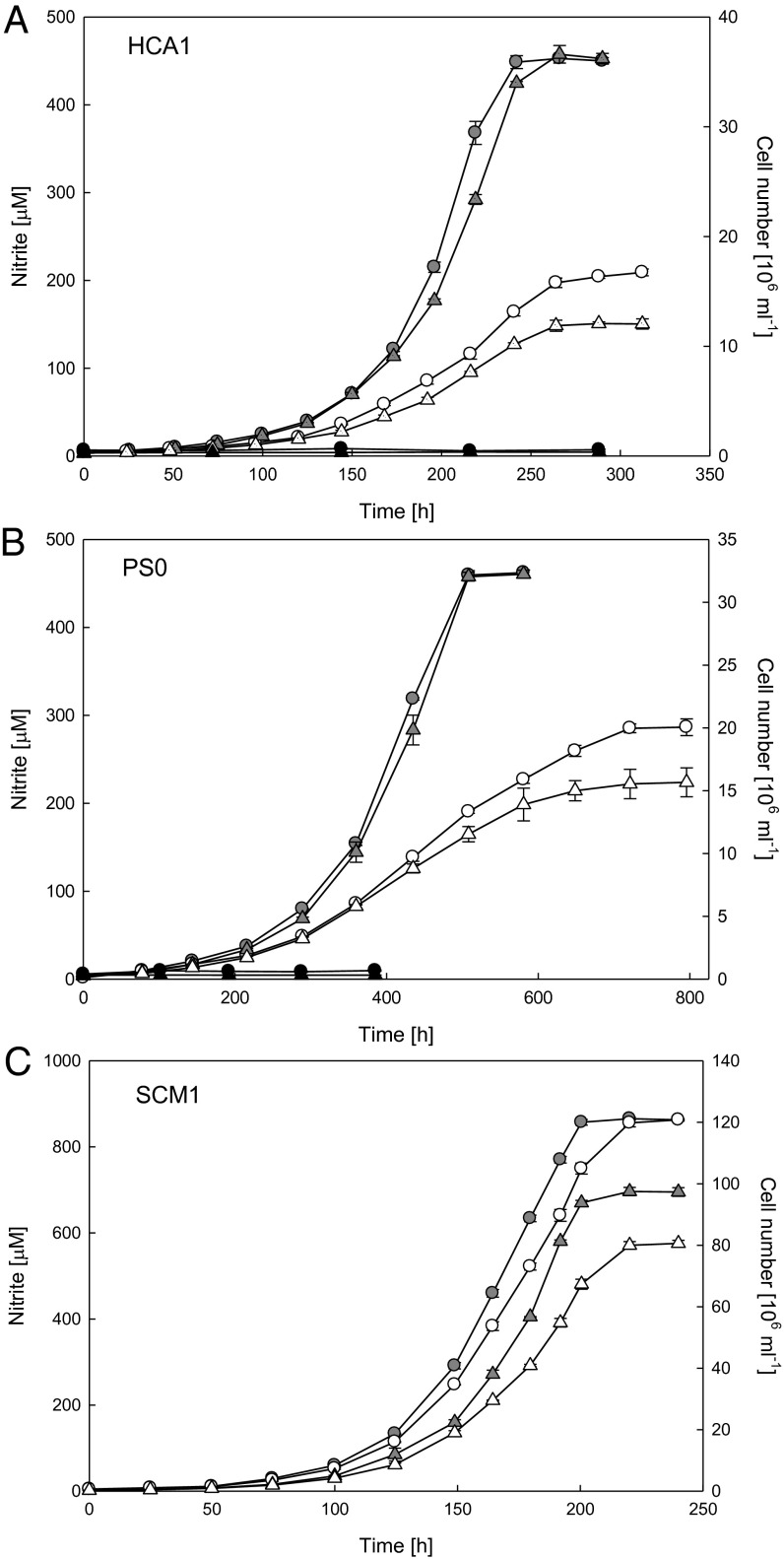
Exponential growth of both isolates in synthetic seawater medium containing 500 µM ammonia and 100 µM α-ketoglutaric acid was supported by the near stoichiometric oxidation of ammonia to nitrite (Fig. 1 A and B). No growth was observed in medium containing α-ketoglutaric acid and no ammonia, supplemented with nitrite or nitrate as possible nitrogen sources. The maximum specific growth rates for strains HCA1 and PS0 were 0.55 d−1 and 0.23 d−1, respectively. The growth rate of HCA1 was comparable to SCM1 (0.65 d−1) and the one described thermophilic AOA, “Candidatus Nitrosocaldus yellowstonii” (0.8 d−1) (22, 23). The lower growth rate of PS0 was similar to the previously described pelagic AOA enrichments, CN25 and CN75 (0.17 d−1) and to the estimated in situ growth rates of AOA in winter polar waters (0.21 d−1) (18, 28). Because both strains were of similar shape and size to SCM1 (Fig. 1 C and D; see description below), calculations of maximum ammonia oxidation activity were based on the reported cellular biomass of SCM1, using a factor of 10.2 fg of protein per cell (24). The values for HCA1 (24.5 µmol of NH4+ per mg of protein per h) and PS0 (12.2 µmol of NH4+ per mg of protein per h) (Table S1) were both lower than SCM1 (51.9 µmol of NH4+ per mg of protein per h) and characterized AOB (30–80 µmol of NH4+ per mg of protein per h) (40, 41), but within the range estimated for in situ cell-specific rates of a natural marine community (0.2–15 fmol of NH4+ per cell per d) (6).
Fig. 1.
Growth and morphology of strains HCA1 and PS0. Correlation between ammonia oxidation and growth of HCA1 (A) and PS0 (B) in an artificial seawater medium containing 500 µM NH4+ and 100 µM α-ketoglutaric acid. Transmission electron micrographs of negative-stained cells of HCA1 (C) and PS0 (D). (Scale bars: 200 nm.)
Morphology and Phylogeny.
The morphologies of strains HCA1 and PS0, as characterized by transmission electron microscopy (Fig. 1 C and D), are very similar to SCM1 (23). Both are small rods, with a diameter of 0.15–0.26 µm and a length of 0.65–1.59 µm. Unlike “Candidatus Nitrosoarchaeum limnia” (16), no flagella were observed. Cells reproducing by typical binary fission were also observed in images of mid–exponential-phase cultures.
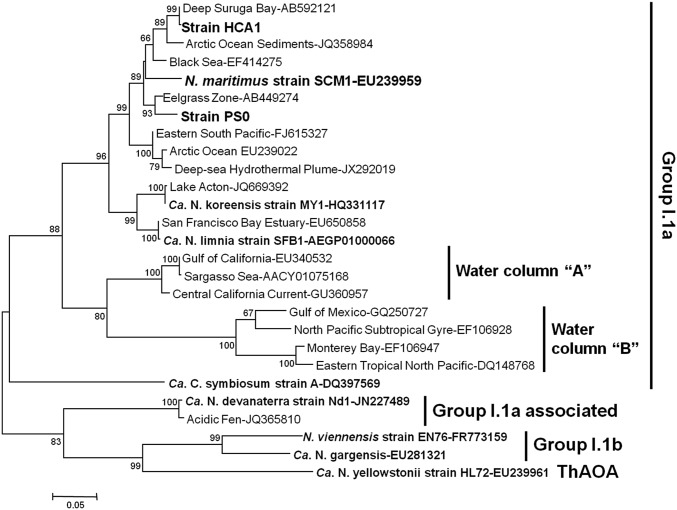
We earlier reported on the high similarity and synteny of the SCM1 genome to the metagenome of globally distributed marine group I Archaea (11). A close phylogenetic relationship between strain SCM1 and the two new isolates was here established by 16S rRNA and amoA gene sequencing. All are affiliated with Thaumarchaeota group I.1a, together forming a monophyletic clade sharing >95% amoA and >99% 16S rRNA gene sequence identity (Fig. 2 and Fig. S1). The amoA genes are more than 11% divergent from environmental sequences previously termed marine water column clusters “A” and “B,” and more than 16% divergent from the symbiotic archaeon “Candidatus Cenarchaeum symbiosum” (2, 42). “Candidatus Nitrosoarchaeum limnia,” previously described in an enrichment from a low-salinity estuarine system in California, differed from the new isolates by greater than 4% and 7% sequence divergence of the 16S rRNA and amoA genes, respectively (43). All share less than 90% and 80% identity with the 16S rRNA and amoA genes, respectively, of the cultured soil representatives “Candidatus Nitrosotalea devanaterra” and Nitrososphaera viennensis (15, 17).
Fig. 2.
Phylogenetic relationships among amoA gene sequences of strains HCA1, PS0, and SCM1 and described AOA representatives, as well as relevant environmental clone sequences. The tree was constructed using the maximum-likelihood method with Kimura two-parameter correction. Confidence values were based on 1,000 bootstrap replications. (Scale bar represents 0.05 nucleotide changes per position.)
Relationships Among Growth Rate, Temperature, Salinity, and pH.
The temperature-dependent growth kinetics of the new isolates differed significantly from strain SCM1. The highest growth rates for strains HCA1 and PS0 were observed at 25 °C and 26 °C, respectively, in contrast to a maximum at 32 °C for strain SCM1 (Fig. S2 A–C). No growth (nitrite production) could be detected for SCM1 at 10 °C, whereas the two new isolates continued to grow at 10 °C. An Arrhenius analysis revealed a good linear relationship between the natural logarithm of the ammonia oxidation rate and the inverse of the absolute temperature (Fig. S2D). The inferred activation energy (78.25 kJ⋅mol−1) and Q10 value (2.89) of SCM1 were somewhat greater than strain HCA1 (Ea = 67.67 kJ⋅mol−1, Q10 = 2.62) and strain PS0 (Ea = 64.20 kJ⋅mol−1, Q10 = 2.49). These values are comparable to those estimated for representatives of estuarine nitrifying bacteria (Ea = 67.4–82.5 kJ⋅mol−1, Q10 = 2.7–3.3) (44) (Table S2).
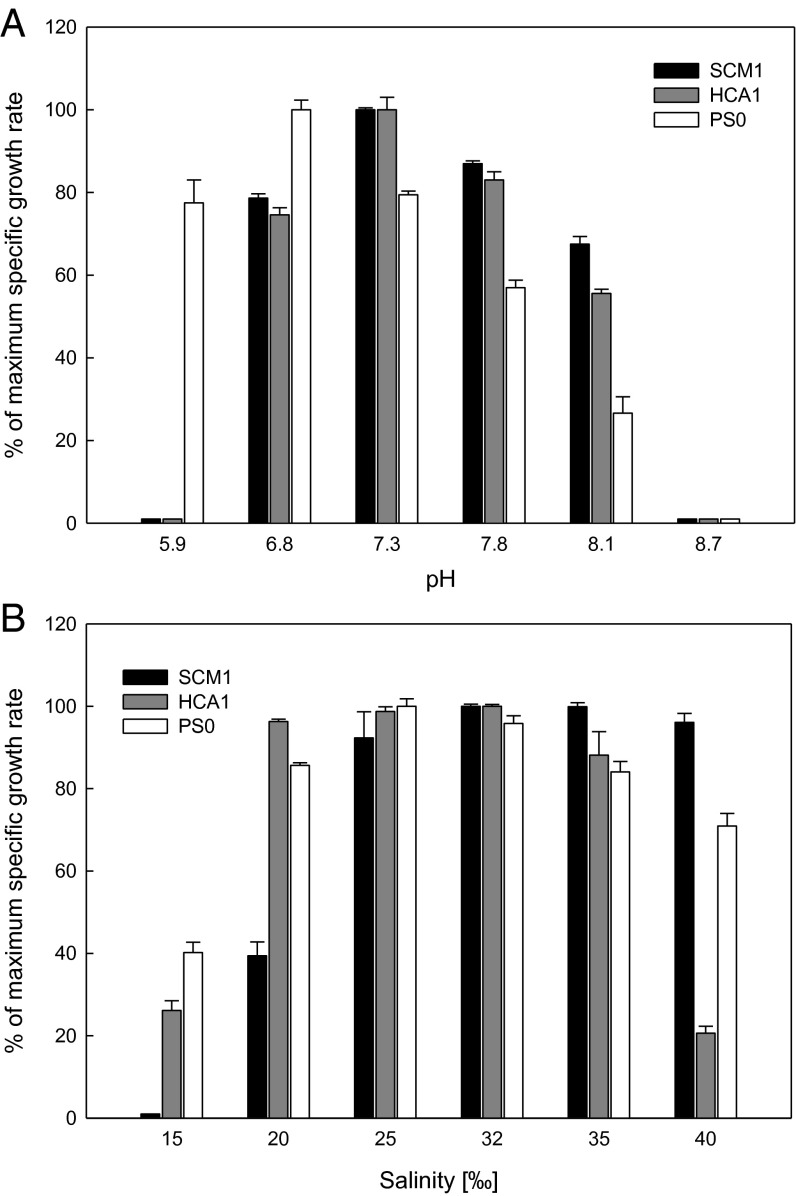
Growth of strains SCM1 and HCA1 was restricted to pH values between 6.8 and 8.1, with the highest ammonia oxidation rates observed at pH 7.3 (Fig. 3A). In contrast, strain PS0 grew well at significantly lower pH values, having a maximum growth rate at pH 6.8 and maintaining nearly 80% of its maximum growth rate at pH values as low as 5.9. At pH values closer to that of open ocean surface waters (∼8.1), the growth of PS0 was depressed relative to the other two isolates. Thus, strain PS0 appears well adapted to the lower pH waters of the Puget Sound main basin from where it was isolated, having a pH range of 7.71–8.05 near the surface and of 7.70–7.83 at depths greater than 100 m (45). No ammonia oxidation was observed at pH 8.7 for any of the strains, which is consistent with observations of other neutrophilic AOA (15, 19, 46).
Fig. 3.
Influence of pH (A) and salinity (B) on growth. Values represent percentage of specific growth rates of cultures grown under different pH and salinity values relative to those at pH and salinity optima. Error bars represent the SD of triplicate cultures.
Salinity is also a significant environmental variable, particularly in coastal regions influenced by varying inputs of terrestrial freshwater. The Hood Canal fjord is influenced by seasonally varying riverine and surface runoff sources of freshwater, and by the intrusion of low pH and high-salinity water from seasonal coastal upwelling of deep ocean water (47). All strains grew best at midsalinity (25–32‰), but differed markedly in response to low- and high-salinity conditions (Fig. 3B). Strain SCM1 grew well at high salinities (35–40‰), was significantly inhibited at 20‰, and ceased to grow at 15‰. Strains HCA1 and PS0 were less inhibited at 20‰ and still remained active at 15‰. However, unlike SCM1, both of these strains were significantly inhibited at 40‰. Because 40‰ is much higher than the normal oceanic environment, these differences likely reflect strain specific adaptations to the varying salinities intrinsic to this coastal system.
Photoinhibition.
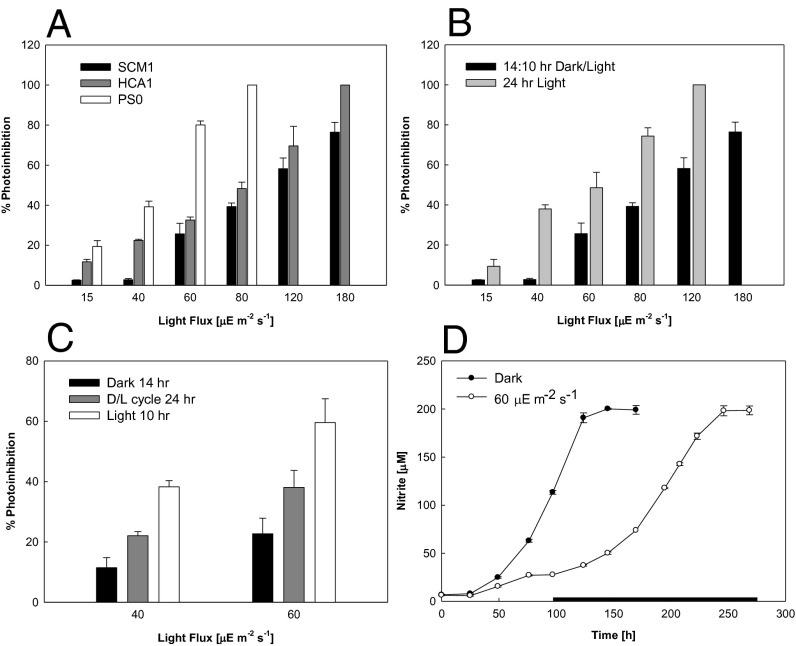
The influence of light on growth kinetics was examined by controlled exposure of the isolates to polychromatic light from a cool, white fluorescent lamp. Growth rate was measured at different intensities of illumination and under three different illumination regimes: continuous dark, continuous illumination, and a 14-h dark/10-h light cycle. Differential growth during light and dark periods of the imposed diurnal cycle was also examined. All isolates were differentially inhibited. Strain SCM1 was significantly less photosensitive (Fig. 4A) than the other two isolates when exposed to a diurnal light cycle, showing no apparent inhibition (relative to cultures incubated in the dark) at low light fluxes (15 and 40 µE⋅m−2⋅s−1) and retaining about 20% of its maximum growth rate at the highest light intensity examined (180 µE⋅m−2⋅s−1). However, under continuous illumination, this strain was completely inhibited at 120 µE⋅m−2⋅s−1 (Fig. 4B). Although exhibiting somewhat greater light sensitivity, the response of HCA1 was similar to SCM1, showing reduced specific growth rates of 11% and 22% at 15 and 40 µE⋅m−2⋅s−1, respectively, and complete inhibition at 180 µE⋅m−2⋅s−1 (Fig. 4A). Strain PS0 was the most light sensitive of the three AOA isolates, being partially inhibited at low light fluxes (19% and 39% inhibition at 15 and 40 µE⋅m−2⋅s−1, respectively), 80% inhibited at 60 µE⋅m−2⋅s−1, and completely inhibited at 80 µE⋅m−2⋅s−1.
Fig. 4.
Photoinhibition and recovery of ammonia oxidation of the three AOA strains. Values represent percentage inhibition of cultures grown under continuous illumination or dark/light cycles relative to cultures grown in the dark. (A) The inhibitory effects of light on all three isolates under a 14:10-h dark/light cycle at different intensities from 15 to 180 µE⋅m−2⋅s−1. (B) Photoinhibition of strain SCM1 under continuous light (15–120 µE⋅m−2⋅s−1) and dark/light cycles (15–180 µE⋅m−2⋅s−1). (C) The reduction in specific growth rate of strain HCA1 with cycling between periods of 14 h of dark and 10 h of light at 40 and 60 µE⋅m−2⋅s−1. (D) The recovery of light-inhibited SCM1 cultures (open circles) following transfer to the dark after 100 h of continuous illumination (60 µE⋅m−2⋅s−1) compared with continuous growth in the dark (dark circles). Error bars represent the SD of triplicate incubations.
The greater inhibition observed with continuous illumination relative to an imposed dark/light cycle suggested that a recovery of activity was possible during the dark period. Dark recovery of strain HCA1 was shown by an increased growth rate during the dark period (Fig. 4C). To further evaluate dark recovery of completely light-inhibited cultures, strain SCM1 cultures were first incubated under continuous illumination at 60 µE⋅m−2⋅s−1 until growth ceased (∼100 h) and then transferred to the dark. These cultures began to recover within 24 h and completely converted all ammonia to nitrite, but at a significantly reduced growth rate compared with dark-incubated controls (Fig. 4D). Thus, although the data confirmed sensitivity of AOA to light, they also suggest that significant ammonia oxidation is possible in the upper water column.
Assimilation of Organic Carbon.
Isolation of HCA1 and PS0 required the addition of a low concentration of α-ketoglutaric acid to the inorganic basal medium. Both strains oxidized approximately one-half of the added ammonia to nitrite following initial transfer from α-ketoglutaric acid supplemented into organic carbon-free media (1% inoculum), and failed to grow following a second transfer into organic carbon-free media (Fig. 5 A and B). The oxidation of one-half of the ammonia following the first transfer likely reflected either use of endogenous cellular reserves or the carryover of a low amount of α-ketoglutaric acid in the inoculum. As previously reported, strain SCM1 was capable of chemolithoautotrophic growth, oxidizing all added ammonia in the absence of an organic carbon supplement. However, SCM1 growth rate and cell yield were greater in cultures supplemented with α-ketoglutaric acid (Fig. 5C and Table S3). Because cell yield per mole of ammonia oxidized was greater for all strains in organic carbon supplemented media (from 93.4 to 112.6 × 1012 cells per mol of NH4+ for SCM1, no growth to 80.8 × 1012 cells per mol of NH4+ for HCA1, and no growth to 70.4 × 1012 cells per mol of NH4+ for PS0) (Fig. S3), these results suggested that mixotrophic growth may be the preferred lifestyle of marine AOA.
Fig. 5.
Nitrite production (circles) and growth curves (triangles) of strains HCA1 (A), PS0 (B), and SCM1 (C) with 100 µM α-ketoglutaric acid (gray) relative to controls without organic carbon supplements (first transfer, white; second transfer, black). No second transfer is included in C, because SCM1 has the demonstrated capacity to sustain growth under strictly autotrophic conditions (23, 24). Error bars represent the SD of triplicate cultures (please note that, in most cases, the error bars are too small to be visible in the figure).
Growth of Strain PS0 on Urea.
The genetic potential for using urea to fuel nitrification has been reported for the marine group I Thaumarchaeota (28, 30). However, direct physiological support has to date been lacking. We therefore examined the capacity of each strain to grow in an ammonia-free medium supplemented with ∼100 µM urea. As predicted from the SCM1 genome sequence, previously reported to lack genes annotated for urea transport and degradation, no growth or nitrite production was observed for SCM1 cultures following more than 3 mo of incubation. Likewise, no growth of strain HCA1 was observed in the urea medium. In contrast, strain PS0 grew by near-stoichiometric conversion of urea (∼90 µM) to nitrite (∼180 µM) in 17 d (Fig. S4) at a specific growth rate (0.18 d−1) comparable to its growth on ammonia (0.23 d−1).
Discussion
Since the successful isolation of the first ammonia-oxidizing archaeon (SCM1) from a seawater aquarium (23) using low ammonia concentrations for selective enrichment, the same general approach has been widely used to enrich additional AOA from soil, fresh water, marine, and geothermal habitats (15–22, 46). However, despite significant efforts to obtain isolates from enrichment cultures, other than SCM1 only Nitrososphaera viennensis strain EN76 from garden soil has been reported in pure culture (15). In contrast to chemolithoautotrophic growth of SCM1, N. viennensis and the new marine isolates require organic carbon, a requirement that may account for the virtual absence of publications describing additional isolates. Presumably the coexisting bacteria provide essential nutrients, and without appropriate nutrient supplementation, the AOA cannot be maintained (15, 19, 21). For example, the isolation of the soil AOA N. viennensis required the addition of pyruvate (15). However, pyruvate addition has not been sufficient for the isolation of AOA from other enrichment cultures (21), suggesting that different ecotypes vary in their carbon assimilation capabilities. Here, we isolated two N. maritimus-related strains from coastal water using α-ketoglutaric acid as an organic nutrient source, demonstrating the significance of another organic compound to the growth of an AOA lineage in marine environments.
The three strains (SCM1, HCA1, and PS0) described here are relatively closely related members of a marine AOA lineage that differ markedly in basic physiological features. Although strain SCM1 was isolated from a tropical marine aquarium, it shares high genomic similarity with marine metagenomic sequences and has an apparent half-saturation constant (Ks) for ammonia oxidation (133 nM) comparable to Ks values determined directly in open ocean waters (65–112 nM) (8, 11, 23, 24, 48). The optimum growth temperature near 30–33 °C, and a preference for salinities (32–40‰) higher than the newly described coastal isolates, also suggests that strain SCM1 was originally native to tropical ocean waters.
All isolates vary significantly in sensitivity to light and pH. These features relate directly to abiotic factors controlling their environmental distribution and response to the ongoing reduction in ocean pH (49). The sensitivity of marine AOA to ocean acidification has been inferred from a limited number of short-term studies of experimentally acidified ocean water (37, 50, 51). For example, a recent study by Beman et al. (37) reported significant inhibition of ammonia oxidation (8–38% reduction in rates) following relatively small pH reductions of both coastal and open ocean waters in which AOA were the dominant ammonia-oxidizing population. This effect was associated with the reported requirement for the use of ammonia (NH3), not ammonium (NH4+), as a substrate for ammonia-oxidizing bacteria such as the marine AOB Nitrosococcus oceani (40). The concentration of this form is significantly reduced by small reductions in pH. However, as yet there is no evidence that AOA have the same substrate requirement as AOB. In fact, the description of “Candidatus Nitrosotalea devanaterra,” an acidophilic terrestrial AOA growing optimally at pH values near 4, suggests that the NH4+ ion is more likely the substrate for this organism (17). Our observation that marine AOA strain PS0 grows well at pH 5.9 suggests that at least some AOA populations have the capacity to adapt to ongoing reductions in ocean pH. If the ammonium ion is the preferred substrate, ocean acidification may actually promote the growth of AOA, influencing oceanic production of N2O now associated primarily with their activities (9, 10).
Light has long been implicated as a major factor controlling the activity and distribution of both ammonia oxidizers and nitrite oxidizers in the water column. The inhibitory effect of light on cultured AOB has been known for decades and attributed to photooxidative damage of the copper-containing ammonia monooxygenase (52–54). Archaeal photosensitivity was recently reported for marine, soil and fresh water AOA strains, including SCM1 (21, 55). At a light intensity similar to the base of the euphotic zone (15 µE⋅m−2⋅s−1), Merbt et al. (2012) reported the growth of SCM1 was almost completely inhibited with no evidence of dark recovery during an imposed diurnal cycle (55). In contrast, we found only a marginal inhibitory effect of a comparable diurnal light regime on strain SCM1 (2.5% at 15 µE⋅m−2⋅s−1) and a similarly low inhibition of novel strains HCA1 and PS0 to this light regime. Even under continuous illumination, the growth rate of SCM1 was reduced by only 11.8% at this light flux. At higher intensities (40 and 60 µE⋅m−2⋅s−1) these isolates were capable of rapid dark recovery from photoinhibition, suggesting that ammonia oxidation in upper regions of the euphotic zone could follow a diurnal cycle (56, 57). Although we have no explanation for the disparity between this and the previous study, these data provide physiological explanation for the high abundance and activity of AOA near the bottom of the euphotic zone as inferred by relating amoA gene and transcript abundance to in situ ammonia oxidation rate measurements (6–8, 36).
The requirement of the new isolates for organic carbon amplifies growing appreciation of the importance of mixotrophy in marine food webs and provided direct physiological support for previous isotopic studies suggesting both organic and inorganic carbon sources were assimilated by marine Thaumarchaeota (4, 25, 32, 33). There are obvious energetic advantages to the use of organic carbon to supplement or alleviate carbon fixation. In addition, organic material might serve as an alternative source of reductant, other than electrons derived solely from ammonia, for the ammonia monooxygenase. Our demonstration that isolate PS0 is capable of using urea as an alternative energy source also provided the first (to our knowledge) direct physiological confirmation of an activity implicated by marine genomic datasets (28–30). If the AOA have an affinity for urea comparable to their remarkably low Ks for ammonia, they may also effectively compete with phytoplankton for this reduced form of nitrogen, a question that can now be addressed using this new isolate.
The physiological data presented begin to define ecotype variation within the AOA and to disentangle environmental variables influencing the abundance and activity of populations now thought to play a fundamental role in the shaping of the marine nitrogen cycle. For example, the requirement of the new isolates for organic carbon suggests that substrates other than ammonia could limit the distribution of some environmental populations. Characterized AOB are unable to growth at pH values significantly below 7 (40, 58, 59). This has fostered speculation that the AOA may suffer similar inhibition at acidic pH. However, our demonstration of significant variation in pH adaptation among closely related AOA suggests that ongoing ocean acidification is more likely to change the distribution of competing AOA lineages and, as a consequence, alter the marine nitrogen cycle in unexpected ways. Finally, because these studies encompassed only a small and genetically closely circumscribed set of AOA, relative to the much greater genetic diversity revealed by ongoing metagenomic surveys, they point to a rich and mostly unexplored physiological diversity of the marine AOA.
Materials and Methods
Enrichment and Isolation of Marine AOA.
Low added ammonia (2 µM) was used to selectively enrich and isolate AOA from seawater samples collected from the Puget Sound estuary in Washington (SI Materials and Methods). Pure cultures were obtained by filtration of the enrichment cultures through a syringe filter, and diluting the filtrate to extinction. For details, see SI Materials and Methods.
Growth Experiments.
All materials and methods for physiology experiments are described in detail in SI Materials and Methods.
Transmission Electron Microscopy.
Preparation of the novel AOA isolates for examination by transmission electron microscopy is described in SI Materials and Methods.
Sequencing and Phylogenetic Analysis.
Sequences of AOA 16S rRNA and amoA genes were obtained using an ABI 3730xl sequencer. Phylogenetic trees were generated using MEGA5 as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank B. Schneider and the Fred Hutchinson Cancer Research Center EM staff for performing transmission electron microscopy, and the captain and crew of the R/V Clifford A. Barnes for their assistance with sample collection. We thank the technical assistance of Sun-Li Beatteay. This work was funded by National Science Foundation Grants MCB-0604448 and Dimensions of Biodiversity Program OCE-1046017.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. KF957663–KF957666).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1324115111/-/DCSupplemental.
References
- 1.Karner MB, DeLong EF, Karl DM. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature. 2001;409(6819):507–510. doi: 10.1038/35054051. [DOI] [PubMed] [Google Scholar]
- 2.Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci USA. 2005;102(41):14683–14688. doi: 10.1073/pnas.0506625102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sintes E, Bergauer K, De Corte D, Yokokawa T, Herndl GJ. Archaeal amoA gene diversity points to distinct biogeography of ammonia-oxidizing Crenarchaeota in the ocean. Environ Microbiol. 2013;15(5):1647–1658. doi: 10.1111/j.1462-2920.2012.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agogué H, Brink M, Dinasquet J, Herndl GJ. Major gradients in putatively nitrifying and non-nitrifying Archaea in the deep North Atlantic. Nature. 2008;456(7223):788–791. doi: 10.1038/nature07535. [DOI] [PubMed] [Google Scholar]
- 5.Prosser JI, Nicol GW. Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ Microbiol. 2008;10(11):2931–2941. doi: 10.1111/j.1462-2920.2008.01775.x. [DOI] [PubMed] [Google Scholar]
- 6.Santoro AE, Casciotti KL, Francis CA. Activity, abundance and diversity of nitrifying archaea and bacteria in the central California Current. Environ Microbiol. 2010;12(7):1989–2006. doi: 10.1111/j.1462-2920.2010.02205.x. [DOI] [PubMed] [Google Scholar]
- 7.Beman JM, Popp BN, Alford SE. Quantification of ammonia oxidation rates and ammonia-oxidizing archaea and bacteria at high resolution in the Gulf of California and eastern tropical North Pacific Ocean. Limnol Oceanogr. 2012;57(3):711–726. [Google Scholar]
- 8.Horak REA, et al. Ammonia oxidation kinetics and temperature sensitivity of a natural marine community dominated by Archaea. ISME J. 2013;7(10):2023–2033. doi: 10.1038/ismej.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoro AE, Buchwald C, McIlvin MR, Casciotti KL. Isotopic signature of N2O produced by marine ammonia-oxidizing archaea. Science. 2011;333(6047):1282–1285. doi: 10.1126/science.1208239. [DOI] [PubMed] [Google Scholar]
- 10.Löscher CR, et al. Production of oceanic nitrous oxide by ammonia-oxidizing archaea. Biogeosciences. 2012;9(7):2419–2429. [Google Scholar]
- 11.Walker CB, et al. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA. 2010;107(19):8818–8823. doi: 10.1073/pnas.0913533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amin SA, et al. Copper requirements of the ammonia-oxidizing archaeon Nitrosopumilus maritimus SCM1 and implications for nitrification in the marine environment. Limnol Oceanogr. 2013;58(6):2037–2045. [Google Scholar]
- 13.Metcalf WW, et al. Synthesis of methylphosphonic acid by marine microbes: A source for methane in the aerobic ocean. Science. 2012;337(6098):1104–1107. doi: 10.1126/science.1219875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karl DM, et al. Aerobic production of methane in the sea. Nat Geosci. 2008;1(7):473–478. [Google Scholar]
- 15.Tourna M, et al. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci USA. 2011;108(20):8420–8425. doi: 10.1073/pnas.1013488108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosier AC, Lund MB, Francis CA. Ecophysiology of an ammonia-oxidizing archaeon adapted to low-salinity habitats. Microb Ecol. 2012;64(4):955–963. doi: 10.1007/s00248-012-0075-1. [DOI] [PubMed] [Google Scholar]
- 17.Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc Natl Acad Sci USA. 2011;108(38):15892–15897. doi: 10.1073/pnas.1107196108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santoro AE, Casciotti KL. Enrichment and characterization of ammonia-oxidizing archaea from the open ocean: Phylogeny, physiology and stable isotope fractionation. ISME J. 2011;5(11):1796–1808. doi: 10.1038/ismej.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JG, et al. Cultivation of a highly enriched ammonia-oxidizing archaeon of thaumarchaeotal group I.1b from an agricultural soil. Environ Microbiol. 2012;14(6):1528–1543. doi: 10.1111/j.1462-2920.2012.02740.x. [DOI] [PubMed] [Google Scholar]
- 20.Hatzenpichler R, et al. A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc Natl Acad Sci USA. 2008;105(6):2134–2139. doi: 10.1073/pnas.0708857105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French E, Kozlowski JA, Mukherjee M, Bullerjahn G, Bollmann A. Ecophysiological characterization of ammonia-oxidizing archaea and bacteria from freshwater. Appl Environ Microbiol. 2012;78(16):5773–5780. doi: 10.1128/AEM.00432-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Torre JR, Walker CB, Ingalls AE, Könneke M, Stahl DA. Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ Microbiol. 2008;10(3):810–818. doi: 10.1111/j.1462-2920.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 23.Könneke M, et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature. 2005;437(7058):543–546. doi: 10.1038/nature03911. [DOI] [PubMed] [Google Scholar]
- 24.Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature. 2009;461(7266):976–979. doi: 10.1038/nature08465. [DOI] [PubMed] [Google Scholar]
- 25.Ingalls AE, et al. Quantifying archaeal community autotrophy in the mesopelagic ocean using natural radiocarbon. Proc Natl Acad Sci USA. 2006;103(17):6442–6447. doi: 10.1073/pnas.0510157103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouverney CC, Fuhrman JA. Marine planktonic archaea take up amino acids. Appl Environ Microbiol. 2000;66(11):4829–4833. doi: 10.1128/aem.66.11.4829-4833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teira E, van Aken H, Veth C, Herndl GJ. Archaeal uptake of enantiomeric amino acids in the meso- and bathypelagic waters of the North Atlantic. Limnol Oceanogr. 2006;51(1):60–69. [Google Scholar]
- 28.Alonso-Sáez L, et al. Role for urea in nitrification by polar marine Archaea. Proc Natl Acad Sci USA. 2012;109(44):17989–17994. doi: 10.1073/pnas.1201914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yakimov MM, et al. Contribution of crenarchaeal autotrophic ammonia oxidizers to the dark primary production in Tyrrhenian deep waters (Central Mediterranean Sea) ISME J. 2011;5(6):945–961. doi: 10.1038/ismej.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konstantinidis KT, Braff J, Karl DM, DeLong EF. Comparative metagenomic analysis of a microbial community residing at a depth of 4,000 meters at station ALOHA in the North Pacific subtropical gyre. Appl Environ Microbiol. 2009;75(16):5345–5355. doi: 10.1128/AEM.00473-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollibaugh JT, Gifford S, Sharma S, Bano N, Moran MA. Metatranscriptomic analysis of ammonia-oxidizing organisms in an estuarine bacterioplankton assemblage. ISME J. 2011;5(5):866–878. doi: 10.1038/ismej.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herndl GJ, et al. Contribution of Archaea to total prokaryotic production in the deep Atlantic Ocean. Appl Environ Microbiol. 2005;71(5):2303–2309. doi: 10.1128/AEM.71.5.2303-2309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansman RL, et al. The radiocarbon signature of microorganisms in the mesopelagic ocean. Proc Natl Acad Sci USA. 2009;106(16):6513–6518. doi: 10.1073/pnas.0810871106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Church MJ, Wai B, Karl DM, DeLong EF. Abundances of crenarchaeal amoA genes and transcripts in the Pacific Ocean. Environ Microbiol. 2010;12(3):679–688. doi: 10.1111/j.1462-2920.2009.02108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caffrey JM, Bano N, Kalanetra K, Hollibaugh JT. Ammonia oxidation and ammonia-oxidizing bacteria and archaea from estuaries with differing histories of hypoxia. ISME J. 2007;1(7):660–662. doi: 10.1038/ismej.2007.79. [DOI] [PubMed] [Google Scholar]
- 36.Beman JM, Popp BN, Francis CA. Molecular and biogeochemical evidence for ammonia oxidation by marine Crenarchaeota in the Gulf of California. ISME J. 2008;2(4):429–441. doi: 10.1038/ismej.2007.118. [DOI] [PubMed] [Google Scholar]
- 37.Beman JM, et al. Global declines in oceanic nitrification rates as a consequence of ocean acidification. Proc Natl Acad Sci USA. 2011;108(1):208–213. doi: 10.1073/pnas.1011053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urakawa H, Martens-Habbena W, Stahl DA. High abundance of ammonia-oxidizing Archaea in coastal waters, determined using a modified DNA extraction method. Appl Environ Microbiol. 2010;76(7):2129–2135. doi: 10.1128/AEM.02692-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huguet C, Martens-Habbena W, Urakawa H, Stahl DA, Ingalls AE. Comparison of extraction methods for quantitative analysis of core and intact polar glycerol dialkyl glycerol tetraethers (GDGTs) in environmental samples. Limnol Oceanogr Methods. 2010;8:127–145. [Google Scholar]
- 40.Ward BB. Kinetic-studies on ammonia and methane oxidation by Nitrosococcus-oceanus. Arch Microbiol. 1987;147(2):126–133. [Google Scholar]
- 41.Prosser JI. Autotrophic nitrification in bacteria. Adv Microb Physiol. 1989;30:125–181. doi: 10.1016/s0065-2911(08)60112-5. [DOI] [PubMed] [Google Scholar]
- 42.Hallam SJ, et al. Genomic analysis of the uncultivated marine crenarchaeote Cenarchaeum symbiosum. Proc Natl Acad Sci USA. 2006;103(48):18296–18301. doi: 10.1073/pnas.0608549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blainey PC, Mosier AC, Potanina A, Francis CA, Quake SR. Genome of a low-salinity ammonia-oxidizing archaeon determined by single-cell and metagenomic analysis. PLoS One. 2011;6(2):e16626. doi: 10.1371/journal.pone.0016626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helder W, Devries RTP. Estuarine nitrite maxima and nitrifying bacteria (Ems-Dollard estuary) Neth J Sea Res. 1983;17(1):1–18. [Google Scholar]
- 45.Feely RA, et al. The combined effects of ocean acidification, mixing, and respiration on pH and carbonate saturation in an urbanized estuary. Estuar Coast Shelf Sci. 2010;88(4):442–449. [Google Scholar]
- 46.Jung MY, et al. Enrichment and characterization of an autotrophic ammonia-oxidizing archaeon of mesophilic crenarchaeal group I.1a from an agricultural soil. Appl Environ Microbiol. 2011;77(24):8635–8647. doi: 10.1128/AEM.05787-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore SK, et al. A descriptive analysis of temporal and spatial patterns of variability in Puget Sound oceanographic properties. Estuar Coast Shelf Sci. 2010;87(1):174. [Google Scholar]
- 48.Newell SE, Fawcett SE, Ward BB. Depth distribution of ammonia oxidation rates and ammonia-oxidizer community composition in the Sargasso Sea. Limnol Oceanogr. 2013;58(4):1491–1500. [Google Scholar]
- 49.Zeebe RE. History of seawater carbonate chemistry, atmospheric CO2, and ocean acidification. Annu Rev Earth Planet Sci. 2012;40:141–165. [Google Scholar]
- 50.Huesemann MH, Skillman AD, Crecelius EA. The inhibition of marine nitrification by ocean disposal of carbon dioxide. Mar Pollut Bull. 2002;44(2):142–148. doi: 10.1016/s0025-326x(01)00194-1. [DOI] [PubMed] [Google Scholar]
- 51.Kitidis V, et al. Impact of ocean acidification on benthic and water column ammonia oxidation. Geophys Res Lett. 2011;38(21):L21603. [Google Scholar]
- 52.Hooper AB, Terry KR. Photoinactivation of ammonia oxidation in Nitrosomonas. J Bacteriol. 1974;119(3):899–906. doi: 10.1128/jb.119.3.899-906.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyman MR, Arp DJ. 14C2H2- and 14CO2-labeling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase. J Biol Chem. 1992;267(3):1534–1545. [PubMed] [Google Scholar]
- 54.Shears JH, Wood PM. Spectroscopic evidence for a photosensitive oxygenated state of ammonia mono-oxygenase. Biochem J. 1985;226(2):499–507. doi: 10.1042/bj2260499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merbt SN, et al. Differential photoinhibition of bacterial and archaeal ammonia oxidation. FEMS Microbiol Lett. 2012;327(1):41–46. doi: 10.1111/j.1574-6968.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- 56.Hollibaugh JT, et al. Seasonal variation in the metatranscriptomes of a Thaumarchaeota population from SE USA coastal waters. ISME J. 2014;8(3):685–698. doi: 10.1038/ismej.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pedneault E, Galand PE, Polvin M, Tremblay JE, Lovejoy C. Archaeal amoA and ureC genes and their transcriptional activity in the Arctic Ocean. Sci Rep. 2014;4:4661. doi: 10.1038/srep04661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki I, Dular U, Kwok SC. Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J Bacteriol. 1974;120(1):556–558. doi: 10.1128/jb.120.1.556-558.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allison SM, Prosser JI. Ammonia oxidation at low pH by attached populations of nitrifying bacteria. Soil Biol Biochem. 1993;25(7):935–941. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.