Significance
Both natural killer (NK) cells and γδT cells, classified as innate immune cells, recently have been shown to have features of memory cells. However, after activation, a memory fate of invariant NK T cells (iNKT cells) has not been identified. Here we show the presence of effector memory-like KLRG1+ (Killer cell lectin-like receptor subfamily G, member 1–positive) iNKT cells in the lung. The KLRG1+ iNKT cells are able to recognize and respond to an antigen in the context of CD1d and can persist for a long time and then mount a potent secondary response upon encountering with the same antigen months later. In addition, we suggest that the KLRG1+ iNKT cells could contribute extensively to immune surveillance, especially in preparation for a possible encounter with tumor diseases.
Abstract
Immunological memory has been regarded as a unique feature of the adaptive immune response mediated in an antigen-specific manner by T and B lymphocytes. However, natural killer (NK) cells and γδT cells, which traditionally are classified as innate immune cells, have been shown in recent studies to have hallmark features of memory cells. Invariant NKT cell (iNKT cell)–mediated antitumor effects indicate that iNKT cells are activated in vivo by vaccination with iNKT cell ligand-loaded CD1d+ cells, but not by vaccination with unbound NKT cell ligand. In such models, it previously was thought that the numbers of IFN-γ–producing cells in the spleen returned to the basal level around 1 wk after the vaccination. In the current study, we demonstrate the surprising presence of effector memory-like iNKT cells in the lung. We found long-term antitumor activity in the lungs of mice was enhanced after vaccination with iNKT cell ligand-loaded dendritic cells. Further analyses showed that the KLRG1+ (Killer cell lectin-like receptor subfamily G, member 1–positive) iNKT cells coexpressing CD49d and granzyme A persisted for several months and displayed a potent secondary response to cognate antigen. Finally, analyses of CDR3β by RNA deep sequencing demonstrated that some particular KLRG1+ iNKT-cell clones accumulated, suggesting the selection of certain T-cell receptor repertoires by an antigen. The current findings identifying effector memory-like KLRG1+ iNKT cells in the lung could result in a paradigm shift regarding the basis of newly developed extrathymic iNKT cells and could contribute to the future development of antitumor immunotherapy by uniquely energizing iNKT cells.
Invariant natural killer T cells (iNKT cells) express an invariant T-cell antigen receptor (TCR) α-chain and recognize a complex of the antigen-presenting the MHC-like molecule CD1d and a glycolipid (1–3). The activation of iNKT cells depends on the ligand delivery system. α-Galactosylceramide (α-GalCer), a well-known potent synthetic iNKT ligand, can be loaded onto CD1d-expressing cells, such as dendritic cells (DCs), CD1d-transfected tumor cells, and fibroblasts (4, 5). When activated by these α-GalCer–loaded CD1d+ cells in vivo, iNKT cells are capable of releasing large quantities of IFN-γ (4, 5). Clinical studies using α-GalCer–loaded monocyte-derived DCs have demonstrated the safety and efficacy of iNKT cell therapy (6, 7). On the other hand, we and others have shown that iNKT cells become contracted after activation and become anergic when unbound α-GalCer is administered (4, 8, 9). The different fates of iNKT cells’ response in the two models (i.e., one group of mice treated with unbounded α-GalCer and the other group of mice treated with α-GalCer–loaded CD1d+ cells), particularly the fate of iNKT cells after the vaccination with CD1d+ cells loaded with α-GalCer, have not been fully characterized.
Innate lymphocytes, which mount rapid effector responses, have been thought to be short-lived. However, NK cells (10) and γδT cells (11) with memory-type characteristics have been identified recently. In this study, we demonstrate an effector memory-like KLRG1 (Killer cell lectin-like receptor subfamily G, member 1)-expressing iNKT cell response in mice immunized with CD1d+ cells loaded with the iNKT-cell ligand. KLRG1 was used as a surrogate marker of terminally differentiated short-lived effector CD8+ T cells or effector memory-type CD8+ T cells (12). The contribution of the KLRG1+ T-cell subset to immunity has been defined recently: KLRG1+CD4+ and CD8+ T cells exhibited strong antitumor effects compared with KLRG1− T cells (13, 14). In addition, KLRG1+CD8+ T cells isolated from influenza virus-infected mice survive for a long period, proliferate well, and participate in a recall response (15). KLRG1 also has been identified as a surface marker on mature NK (16) and memory NK cells (10). Memory NK cells express KLRG1hi and CD62Llow and produce high IFN-γ after stimulation, much like the effector memory CD8+ T cells. In view of these findings, our current study investigating the fate of KLRG1+ iNKT cells induced by CD1d+ cells loaded with an iNKT cell ligand would have potential implications for future iNKT-cell–based therapies.
Results
Prolonged Activation of iNKT Cells in the Lung by an Injection of α-GalCer–Loaded DCs.
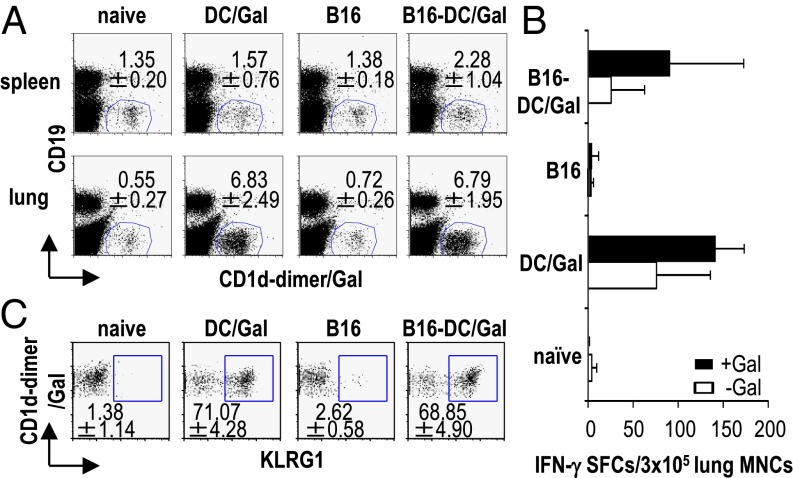
We previously reported that α-GalCer–loaded DCs (DC/Gal) induced an expansion of the IFN-γ–producing iNKT cell population for 2–4 d in the mouse spleen and that their number quickly returned to a basal level 1 wk later (4). In the current study we found that the number of IFN-γ–producing iNKT cells increased in the lung for at least 1 wk after an injection of DC/Gal in WT or tumor-bearing mice (Fig. 1 A and B and Fig. S1A). Also, lung mononuclear cells (MNCs) in the DC/Gal-injected mice (Fig. S1B), but not in the DC/Gal-injected NK1.1+ cell-depleted or IFN-γ−/− mice (Fig. S1 C and D), displayed the cytotoxic activity against tumor cells. Thus, DC/Gal treatment induced a prolonged antitumor effect in the lung mediated by IFN-γ–producing NKT cells. Initially, to search for a specific marker of these iNKT cells, we decided to examine phenotypical the changes in the iNKT cells in the spleen soon after return to the baseline level. The gene expression of iNKT cells in mice 1 wk before and 1 wk after immunization with DC/Gal was analyzed and compared by gene array (Fig. S1E). KLRG1 expression was detected in iNKT cells in lung from DC/Gal-injected mice (Fig. 1C) but not from naive mice (Fig. S1F).
Fig. 1.
Prolonged activation of iNKT cells in the lung by an injection of DC/Gal. B16 melanoma cells (2 × 105 cells per mouse) were administered i.v. to C57BL/6 mice. On day 7, B16-bearing or naive mice were immunized with 1 × 106 DC/Gal. (A) The frequency of iNKT cells in spleen and lung was assessed in the four groups of mice on day 14 (n = 4; data are shown as mean ± SEM). (B) The lung MNCs were isolated on day 14 from the four groups of mice and cultured for 16 h in the presence or absence of α-GalCer (100 ng/mL). IFN-γ production by lung MNCs was measured by ELISPOT assay (n = 4–6; data are shown as mean ± SEM). (C) KLRG1 expression on lung iNKT cells was analyzed by gating on CD19−CD1d-dimer/Gal+ cells (n = 5; data are shown as mean ± SEM).
Long-Term Persistence of Effector Memory-Like iNKT Cells.
To assess the longevity of transferred DC/Gal in vivo, we injected mice i.v. with the PKH-labeled DCs and observed the labeled DC/Gal in the lung for up to ∼48 h in vivo (Fig. S2A). Next, we detected an increased number of KLRG1+ iNKT cells after injection of DC/Gal and also CD1d-transfected B16 melanoma cells loaded with α-GalCer (CD1d-B16/Gal), CD1d-tranfected NIH 3T3 cells loaded with α-GalCer (CD1d-NIH 3T3/Gal), or CD1d-HEK293/Gal (Fig. S2B). However, we could not detect these cells in mice given DC or CD1d-NIH 3T3 alone. These results suggested that α-GalCer–loaded CD1d+ cells play an important role in this study. Because an injection of CD1d-B16/Gal could simultaneously induce both antigen-specific T-cell and the iNKT-cell response (5, 17), thus complicating the interpretation of our experiments, we decided to use DC/Gal as the main immunization regimen in this study.
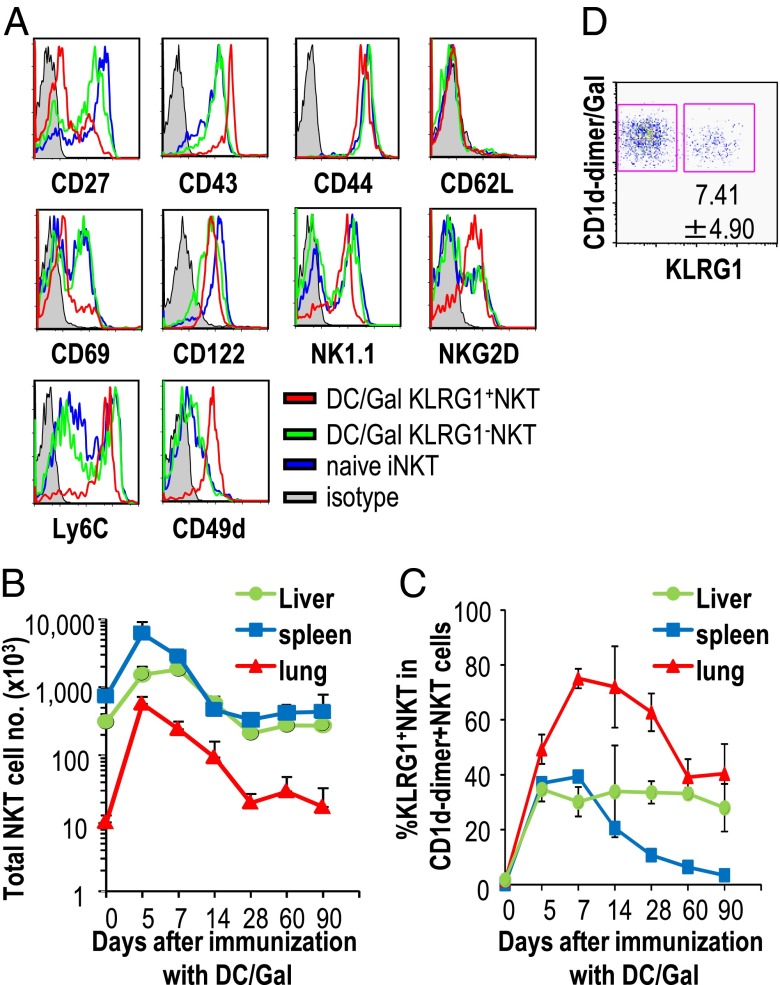
The KLRG1+ iNKT cells could be distinguished from naive iNKT cells based on the expression of several other surface markers. The KLRG1+ iNKT cells expressed more CD43, CD49d, Ly6C, NK1.1, and NKG2D and less CD27 and CD69 than the naive iNKT cells (Fig. 2A). These phenotypic characteristics of KLRG1+ iNKT cells may be partly similar to those of effector memory T cells and memory NK cells. These three types of cells commonly express KLRG1+, Ly6Chi, CD62Llow, and CD27low (18). In addition, the KLRG1+ iNKT cells express NKG2D+ and CD43hi, which are shared with memory NK cells (10).
Fig. 2.
Surface phenotype of KLRG1+ iNKT cells in DC/Gal-injected mice. (A) The surface phenotype on lung iNKT cells from naive mice or KLRG1+ and KLRG1− iNKT cells from DC/Gal-immunized mice were assessed by flow cytometry (n = 5). (B and C) The absolute number of total iNKT cells (B) and the percentages of KLRG1+ iNKT cells (C) in each organ were analyzed from days 5–90 (n = 4–6; data are shown as mean ± SEM). (D) The frequency of KLRG1+ iNKT cells in the lung was analyzed 8 to 9 mo after an immunization with DC/Gal (n = 5; data are shown as mean ± SEM).
Next, we sought to investigate the kinetics of the proliferative ability of KLRG1+ iNKT cells, which began to be detected 2 d after DC/Gal administration (Fig. S3). The absolute number of total iNKT cells increased and then returned to the baseline level (Fig. 2B). Interestingly, although the expansion and contraction of KLRG1+ iNKT cells was completed by 30 d after DC/Gal administration in spleen, the frequency of KLRG1+ iNKT cells in the lung and liver was maintained for 90 d after the contraction phase (Fig. 2C). Furthermore, we evaluated the presence of KLRG1+ iNKT cells in the lung at later phases and found that they persisted for up to 8 to 9 mo after an administration of DC/Gal in Fig. 2D.
Characterization of KLRG1+ iNKT Cells.
The phenotypes of long-lived KLRG1+ iNKT cells were determined by quantitative real-time RT-PCR for the expression levels of cytokine, chemokine, and cytotoxic molecules. The KLRG1+ iNKT cells expressed specific transcripts compared with naive iNKT cells, such as ccl3, ccl4, and granzyme A and expressed higher levels of IFN-γ and fasL transcripts than naive iNKT or KLRG1− iNKT cells (Fig. 3A).
Fig. 3.
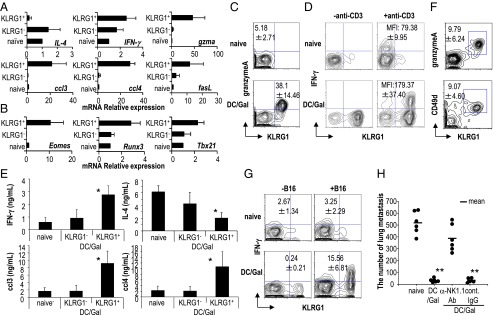
Characterization of KLRG1+ iNKT cells in DC/Gal-injected mice. C57BL/6 mice were immunized with DC/Gal. One month later, KLRG1+ and KLRG1− iNKT cells in the lung were purified by FACS Aria. As a control, iNKT cells in the lung of naive mice were shown as naive iNKT cells. (A and B) Quantitative analyses of gene expression were performed by quantitative real-time PCR using the primer sets shown in Table S1 (n = 4; data are shown as mean ± SEM). (C and D) To examine the functional features of KLRG1+ iNKT cells, the expression of granzyme A (C) and the production IFN-γ (D) in iNKT cells in the lung were analyzed. For intracellular staining of IFN-γ, lung MNCs were stimulated with anti-CD3 Ab plus anti-CD28 Ab for 2 h in the presence of brefeldin A. Analysis gates were set on CD19−CD1d-dimer/Gal+ cells (n = 4–6; data are shown as mean ± SEM). (E) The supernatants from the cultures with anti-CD3 Ab plus anti-CD28 Ab for 24 h were analyzed for IFN-γ, IL-4, CCL3, and CCL4 (n = 4; data are shown as mean ± SEM). *P < 0.05 naive iNKT or KLRG1− iNKT cells versus KLRG1+ iNKT cells. (F) The expression of granzyme A and CD49d of KLRG1+ iNKT cells in the lung was analyzed 6 mo after immunization with DC/Gal (n = 4; data are shown as mean ± SEM). (G and H) Mice that had been immunized with DC/Gal were challenged with B16 melanoma cells 4 mo later. In some experiments, the mice were treated with control rat IgG or anti-NK1.1 Ab. IFN-γ secretion assay for B16 reactive iNKT cells was performed 12 h later in mice vaccinated with or without DC/Gal (G). Antitumor effects were evaluated 2 wk later by counting the number of metastases in the lungs (H) (n = 4–6; data are shown as mean ± SEM). **P < 0.01 naive versus DC/Gal, anti-NK1.1 Ab treatment (DC/Gal) versus control rat IgG treatment (DC/Gal).
Several transcription factors, such as T-bet (Tbx21) and Eomesodermin (eomes) have been studied for their roles in the development of iNKT cells in the thymus (19) and also have been reported as factors generating memory T cells (20). In contrast, there has been no study evaluating these transcription factors of activated iNKT cells in the peripheral organs. We found that the expression levels of eomes, tbx21, and runx3 were higher in KLRG1+ iNKT cells than in naive iNKT cells (Fig. 3B).
We verified at the protein level that the KLRG1+I NKT cells from DC/Gal-injected mice, but not from naive mice, expressed a high level of granzyme A (Fig. 3C). We then sought to determine the function of KLRG1+ iNKT cells by assessing the response of KLRG1+ iNKT cells compared with that of naive iNKT cells. When the level of IFN-γ produced by iNKT cells from DC/Gal-injected or naive mice was assessed 2 h after the stimulation with anti-CD3 and anti-CD28 in vitro, the IFN-γ mean fluorescence intensity (MFI) of KLRG1+ iNKT cells was much higher than that of naive iNKT cells (Fig. 3D). Also, KLRG1+ iNKT cells produced higher amounts of IFN-γ, CCL3, and CCL4 than naive iNKT cells, while producing lower IL-4 (Fig. 3E). To verify the ligand dependency, we used CD1d-NIH 3T3/Gal cells instead of using DCs, because they do not express costimulatory molecules and IL-12. After the stimulation with CD1d-NIH 3T3/Gal in vitro, KLRG1+ iNKT cells showed an IFN-γ MFI higher than that of naive iNKT cells (Fig. S4A).
Thus, based on the characterization of transcription factors and cytokine production, the KLRG1+ iNKT cells belong to Th1-type polarized iNKT cells. These KLRG1+ iNKT cells also showed more potent and rapid response than naive iNKT cells to α-GalCer (Fig. S4A) and to anti-CD3Ab (Fig. 3 D and E) a prominent function that distinguishes them from naïve iNKT cells.
In addition to the findings described above, we verified the long persistence of KLRG1+ iNKT cells in the lung; these cells coexpressed granzyme A and CD49d 6 mo after immunization (Fig. 3F). To determine the antitumor activity of KLRG1+ iNKT cells, we performed a study in which DC/Gal-treated mice were challenged with the B16 melanoma i.v. 4 mo later. We first determined that the frequency of IFN-γ–producing KLRG1+ iNKT cells in lung was increased 12 h after B16 challenge (Fig. 3G). Then, for the tumor challenge, we counted the number of tumor metastases in the lung 2 wk after B16 melanoma challenge. We found that fewer metastases developed in mice given DC/Gal (Fig. 3H). Furthermore, to determine if NK1.1+ cells were responsible for the observed protection against B16, we treated mice with anti-NK1.1 Ab just before challenge with B16 at 4 mo after DC/Gal immunization. The number of B16 metastases in the lung was increased, suggesting that the antitumor effects are mediated by KLRG1+ iNKT cells and also to some extent by NK cells.
The iNKT-Cell Recall Response.
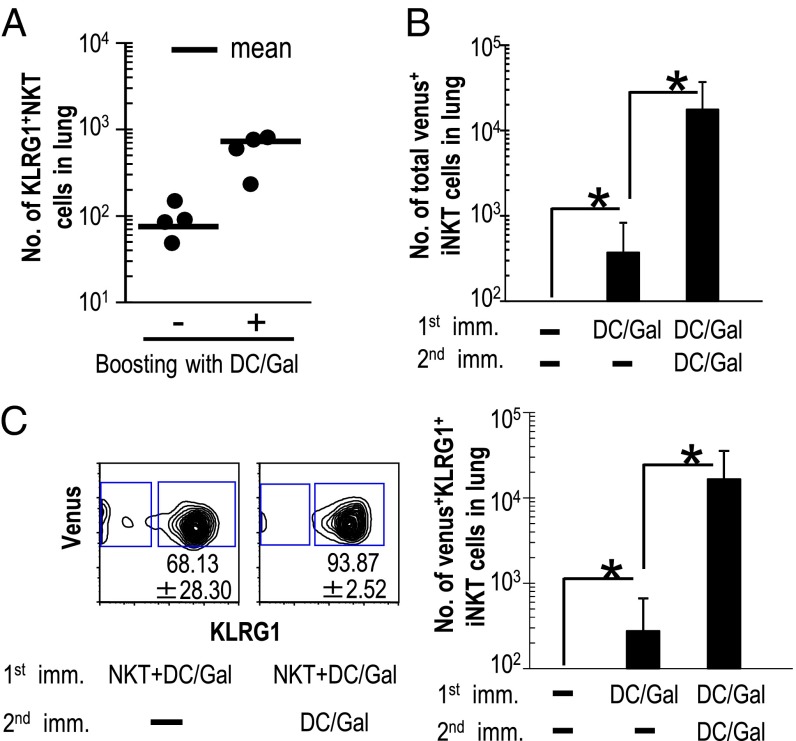
To monitor how long KLRG1+ iNKT cells can persist in vivo, we adoptively transferred KLRG1+ iNKT cells into Jα18−/− mice. The KLRG1+ iNKT cell population was sorted from DC/Gal-injected Vα14+venus+ iNKT cloned (Vα14NT) mice, which we had established previously (21). The use of KLRG1+ iNKT cells derived from DC/Gal-injected Vα14NT mice permitted us to track the fate of transferred cells as CD1d-dimer/Gal+venus+ cells in recipient mice (Fig. S5A). Before conducting this study, we verified that naive iNKT cells in Vα14NT mice did not express KLRG1. The level of expression of KLRG1 in DC/Gal-injected Vα14NT mice was similar to that in DC/Gal-injected WT mice, and KLRG1+Vα14+venus+ iNKT cells from immunized mice produced more IFN-γ than naive Vα14+venus+ iNKT cells (Fig. S5B). The KLRG1+Vα14+venus+ iNKT cells that had been transferred into Jα18−/− mice were expanded when they re-encountered DC/Gal (Fig. 4A). This result suggests that KLRG1+ iNKT cells are capable of eliciting a recall antigen response.
Fig. 4.
Maintenance and secondary response of KLRG1+ iNKT cells in the lung following administration of DC/Gal. (A) KLRG1+Vα14+venus+ iNKT cells were sorted from Vα14NT mice (Vα14+ iNKT cloned mice) that had been immunized with DC/Gal 1 mo previously. The KLRG1+ iNKT cells (1 × 105 per mouse) were transferred into Jα18−/− mice. Four weeks later the mice were injected with or without DC/Gal. The frequency of KLRG1+ iNKT cells in the lung was analyzed 1 wk later (n = 4 per group). (B and C) A total of 1 × 105 naive iNKT cells from Vα14NT mice was transferred into C57BL/6 mice, and the mice were immunized with DC/Gal on the same day. Twelve weeks later, the mice were rechallenged with or without DC/Gal. (B) The total number of Vα14+venus+ iNKT in the lung in all three groups was assessed 1 wk later. (C) The frequency (Left) and number (Right) of KLRG1+ iNKT cells was analyzed 1 wk later. Data are representative of two separate experiments (n = 6 per group); *P < 0.05.
Next, to reflect the physiological condition better, a small number of naive Vα14+venus+ iNKT cells were transferred into C57BL/6 mice. Adoptive transfer of naive Vα14+venus+ iNKT cells from Vα14NT mice into WT mice allowed us to distinguish between antigen-experienced iNKT cells and iNKT cells newly developed from the thymus. WT mice were transferred with naive Vα14+venus+ iNKT cells, followed by DC/Gal immunization on the same day, and the frequency and the number of KLRG1+Vα14+venus+ iNKT cells were ascertained 12 wk later. The Vα14+venus+ iNKT cells were almost undetectable in WT mice that received those cells without being immunized with DC/Gal. In contrast, Vα14+venus+ iNKT cells could be detected even 12 wk later in DC/Gal-immunized mice that had received Vα14+venus+ iNKT cells (Fig. 4B). The number of Vα14+venus+ iNKT cells was increased by rechallenging with DC/Gal 12 wk later (Fig. 4B) while maintaining the expression of KLRG1 phenotype (Fig. 4C). These results suggest that KLRG1+ iNKT cells can be long-lived in the periphery and participate in a recall antigen response.
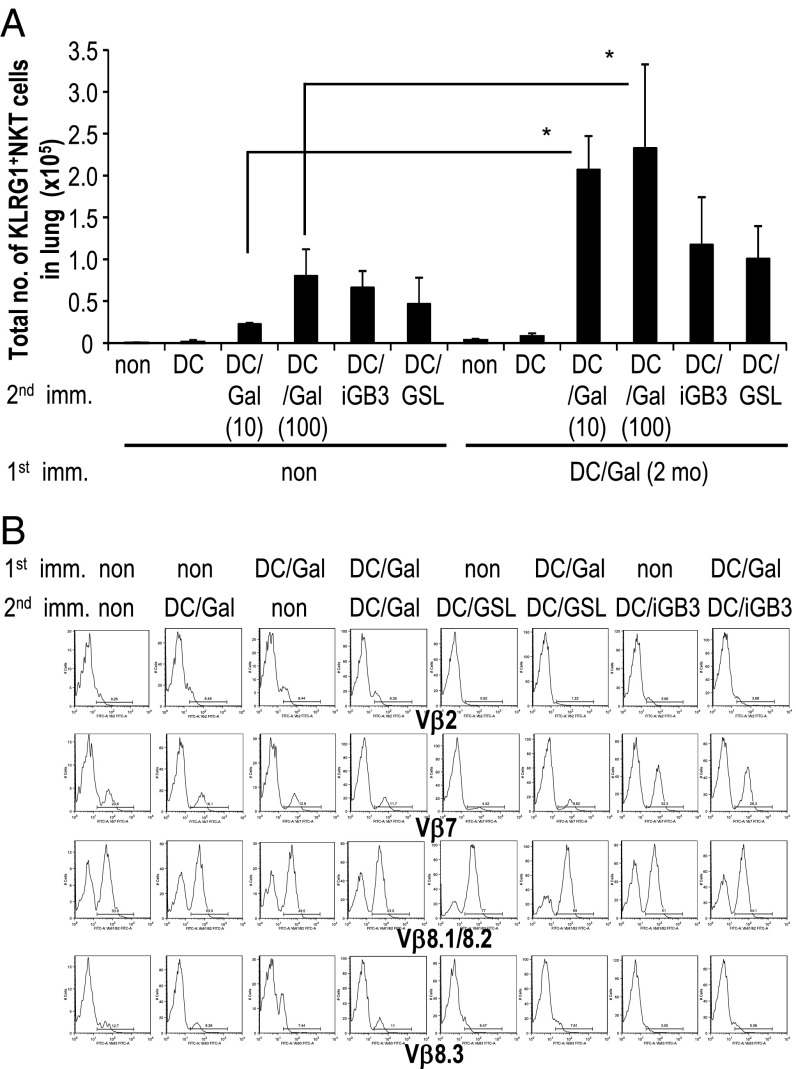
Next, we sought to determine whether an antigen-specific secondary response could occur in DC/Gal-immunized WT mice without receiving iNKT cell transfer. The KLRG1+ iNKT cells in the lung were boosted efficiently with DC/Gal 2 mo after the first immunization. The total number of KLRG1+ iNKT cells in mice after the boost with DC pulsed with a low dose (10 ng/mL) of α-GalCer was almost 10-fold higher than in mice primed with the same DC pulsed with a low dose of α-GalCer but that did not receive the DC/Gal boost. As expected, KLRG1+ iNKT cells failed to expand in mice that received DC alone (Fig. 5A). It is noteworthy that when DC/Gal-immunized mice were boosted with DC pulsed with isoglobotrihexosylceramide (iGB3) or glycosphingolipid (GSL), known endogenous ligands, we were not able to detect a secondary boosting effect. That is, the KLRG1+ iNKT cells in the lung were increased, but the level was similar to the primary response in mice upon administration of DC/iGB3 or DC/GSL (Fig. 5A). Interestingly, when the mice that had been immunized with DC/Gal 1 y previously were boosted with CD1d-NIH 3T3/Gal, the number of KLRG1+ iNKT cells in the lung was greater than the number induced during the primary response to the administration of CD1d-NIH/Gal cells (Fig. S4B).
Fig. 5.
The KLRG1+ iNKT cell recall response. (A) Mice were immunized with or without DC/Gal (100) as a first immunization. Two months later, the mice were administered unloaded DC or α-GalCer–loaded DC (DC/Gal; 10 ng/mL), DC/Gal (100 ng/mL), iGB3 (10 μg/mL)-loaded DC (DC/iGB3), or GSL (10 μg/mL)-loaded DC (DC/GSL) as a second immunization. The number of KLRG1+ iNKT cells in the lung was measured 7 d after the second immunization by flow cytometry after gating on CD19−CD1d-dimer/Gal+ cells. (B) As in A, but immunized mice were analyzed for iNKT cell TCR Vβ using TCR Vβ-FITC. Analysis gates were set on CD19−CD1d-dimer/Gal+KLRG1− cells in the nonimmunized group and CD19−CD1d-dimer/Gal+KLRG1+ cells in the immunized groups. Data are representative of two separate experiments (mean ± SEM); n = 4–6 per group. *P < 0.05 DC/Gal (10) versus DC/Gal-DC/Gal (10) and DC/Gal (100) versus DC/Gal-DC/Gal (100).
Collectively, these results show that KLRG1+ iNKT cells in the lung are able to recognize and respond specifically to cognate antigen and that KLRG1+ iNKT cells are long-lived and can mount a potent secondary response.
Analysis of the TCR Repertoire of KLRG1+ iNKT Cells.
It is well known that the α chain of the iNKT cell TCR is invariant; however, there is more variability in the β chain, although it is restricted mainly to Vβ7, Vβ8, and Vβ2 (22). We next used flow cytometry to evaluate the TCRVβ repertoire of iNKT cells in naive mice, DC/Gal-injected mice, and DC/Gal-DC/Gal–injected mice. We did not find any accumulation of a specific Vβ repertoire in KLRG1+iNKT cells of DC/Gal-injected or DC/Gal-DC/Gal–injected mice compared with naive iNKT cells. However, there was an increase in TCRVβ8.1+/8.2+ iNKT cells, accompanied by a decrease of other TCRVβ+ iNKT cells in DC/GSL or DC/Gal-DC/GSL–boosted mice, whereas TCRVβ7+ iNKT cells increased in both DC/iGB3-injected and DC/Gal-DC/iGB3–boosted mice (Fig. 5B). In accordance with previous reports (23), iGB3 is recognized predominantly by Vβ7+ iNKT cells. GSL was found to be recognized predominantly by Vβ8+ iNKT cells (Fig. 5B). These data indicate that the recall antigen response of KLRG1+ NKT cells depends on the ligand rather than on the DCs alone.
Next, we used massively parallel RNA sequencing from a group of pooled iNKT cells to analyze the Vβ complementary-determining regions1β (CDR1β), CDR2β, and CDR3β in detail. To look carefully for evidence of antigen selection of the effector memory-like KLRG1+ iNKT cells, we prepared iNKT cells from four types of mice, i.e., naive NKT cells from naive mice, KLRG1+ iNKT cells from mice that had been administered DC/Gal 7 d or 28 d previously, and KLRG1+ iNKT cells harvested from mice that had been injected with DC/Gal and boosted with DC/Gal 28 d later (DC/Gal-DC/Gal) (Fig. 6). When we analyzed the RNA sequences in detail after clustering of CDR1 and CDR2, the CDR1β and CDR2β of the KLRG1+ iNKT cells matched those of naive iNKT cells almost completely (Fig. S6).
Fig. 6.
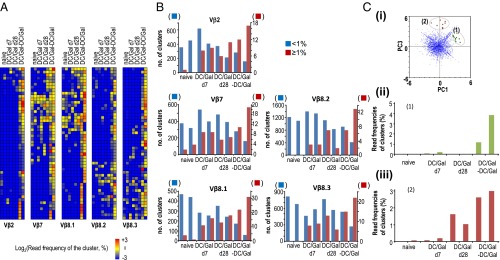
TCR repertoire analyses of KLRG1+ iNKT cells. The naive iNKT cells and KLRG1+ iNKT cells were sorted and tested for TCR RNA-seq analysis. In brief, pooled naive or KLRG1+ iNKT cells were prepared from five or six naive or DC/Gal-immunized mice on day 7 or day 28. In some experiments, DC/Gal-injected mice were boosted with DC/Gal 28 d after the first immunization (DC/Gal-DC/Gal). The CDR3β of naive iNKT cells or KLRG1+ iNKT cells of DC/Gal-injected mice (at day 7 or day 28) or DC/Gal-DC/Gal–boosted mice was assessed by a GS Junior system (Roche) in two independent experiments at each stage. (A) The clusters of high-frequency (top50) CDR3 in each Vβ repertoire in the boosted mice were selected and then clustered among those in naive mice, DC/Gal-injected mice (at day 7 or day 28), or DC/Gal-DC/Gal–boosted mice. The results are shown as heatmaps indicating the cluster sizes [log2(frequency of the sequence reads in the cluster) (%)]. (B) The size distributions of CDR3β clusters in each Vβ repertoire of each group of mice. The numbers of clusters that are larger or smaller than 1% of the total sequence reads are shown by red and blue bars, respectively. (C) (i) Principal component analysis was performed for whole clusters of CDR3. Two groups of CDR3 clusters were identified as groups 1 and 2. (ii and ii) The cluster size distributions of KLRG1+ iNKT cells in groups 1 and 2 are shown as bar graphs in ii and iii, respectively.
Analysis of CDR3β of KLRG1+ iNKT Cells by RNA Sequencing.
We then analyzed the CDR3β sequences of KLRG1+ iNKT cells, because the TCR Vβ repertoire in the iNKT cells became more skewed in the effector or memory phases. Both the CDR3 composition of the Vβ chains and the association with different Jβ segments make the junctional diversity of CDR3β quite heterogeneous in naive mice. We found that even after clustering the common CDR1 and CDR2, the CDR3βs of KLRG1+ iNKT cells were somewhat heterogeneous in DC/Gal-injected mice; however, the CDR3β sequences were distinctly different from those of naive iNKT cells (Fig. 6A). The pattern of CDR3 regions would be clonal in some cases. In addition, such clones accumulated in clusters and became more prominent after boosting (Fig. 6A). Interestingly, further analysis of the clusters of CDR3 reads demonstrated that naive iNKT cells are comprised of many different small read clusters (<1%) and very few large read clusters (>1%), whereas activated KLRG1+ iNKT cells at day 7, effector memory-like KLRG1+ iNKT cells at day 28, and boosted effector memory-like KLRG1+ iNKT cells were all selected to make specific large clusters in all the Vβ families (Fig. 6B), possibly leading to memory-like patterns in a CDR3β-dependent manner.
Next, we performed principal component analysis of whole CDR3 clusters of KLRG1+ iNKT cells and successfully identified such CDR3 clusters (Fig. 6 C, i). Group 1 contained CDR3 clusters in which any reads in CDR3β could be detected in the effector and the boosting phase (Fig. 6 C, ii), whereas the group 2 was composed of CDR3 clusters in which some reads in CDR3β could be detected in the effector phase, but more could be detected in the memory and the boosting phase (Fig. 6 C, iii). The TCR clone reads in the groups 1 and 2 were enriched in Vβ8.2 and Vβ8.3 and in Vβ7 and Vβ8.3, respectively. These results indicate that the iNKT cells may be negatively selected by activation-induced cell death and that some of them survive with memory-like function in a CDR3β-dependent manner. RNA sequencing of the CDR3 Vβ repertoire of iNKT TCRβs may provide a good biomarker of the iNKT-cell response during or after iNKT-cell–based antitumor therapy and possibly during infection with pathogens.
Discussion
One of the key findings in our current study is that lung-resident KLRG1+ iNKT cells induced by CD1d+ cells loaded with a potent NKT cell ligand, α-GalCer, show unique features that distinguish them from resting, naive iNKT cells. They have distinct phenotypes, such as CD49d and granzyme A, and up-regulated expression of CD43 and NKG2D molecules and are long-lived in the periphery. In addition, the effector memory-like iNKT cells respond rapidly as a secondary response and show a prominent function that distinguishes them from naive iNKT cells. Last, the TCRs of KLRG1+ iNKT cells accumulate some particular CDR3β, most likely as the result of clonal expansion.
Another key finding is that KLRG1+ iNKT cells have biological properties similar to those of memory NK and T cells (12–14, 18, 24). Specifically, KLRG1+ NKT cells are able to exhibit a potent secondary response upon encountering the cognate iNKT-cell ligand even after several weeks or months; these responses resemble KLRG1+ memory NK (11) and T-cell responses (12–14, 18, 24). However, the kinetics of KLRG1+ iNKT cells (i.e., expansion fold) resemble the kinetics of memory CD4+ T cells rather than those of memory CD8+ T cells (25, 26).
The evidence in this study suggests a previously unidentified role of KLRG1+ iNKT cells, which are able to persist locally in the lung and patrol and conduct immune surveillance in preparation for a possible encounter with and fight against tumor and infections. The strategy for the selective expansion of KLRG1+ iNKT cells could be explored in the future. Recently, we have successfully generated induced pluripotent stem cell (iPS)-reprogrammed iNKT cells that showed potential antitumor activity (27). Therefore, establishing a strategy for the effective generation of KLRG1+ iNKT cells by selectively expanding iNKT cells or by using the iPS-reprogrammed iNKT approach ultimately would allow us to engage in novel therapeutic interventions against human infections and cancer.
Materials and Methods
DCs of mice were generated from bone marrow as described previously (4). In antitumor studies, mice were immunized with or without 1 × 106 DC/Gal. Four months later, the mice were injected with 2 × 105 B16 melanoma i.v. Fourteen days later, mice were sacrificed and the number of lung metastases were analyzed (4). Detailed information on materials and methods used in this study is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan [26460583 (to K.S. and S.F.)], City Area Program (Kazusa/Chiba Area) MEXT (Japan) (to S.F., M. Tanuguchi, and O.O.), and by National Institutes of Health Grant AI070258 (to M. Tsuji).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406240111/-/DCSupplemental.
References
- 1.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9(1):28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 2.Fujii S, et al. Adjuvant activity mediated by iNKT cells. Semin Immunol. 2010;22(2):97–102. doi: 10.1016/j.smim.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: Natural killer T cell defects and human disease. Nat Rev Immunol. 2011;11(2):131–142. doi: 10.1038/nri2904. [DOI] [PubMed] [Google Scholar]
- 4.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3(9):867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu K, Goto A, Fukui M, Taniguchi M, Fujii S. Tumor cells loaded with α-galactosylceramide induce innate NKT and NK cell-dependent resistance to tumor implantation in mice. J Immunol. 2007;178(5):2853–2861. doi: 10.4049/jimmunol.178.5.2853. [DOI] [PubMed] [Google Scholar]
- 6.Chang DH, et al. Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201(9):1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motohashi S, et al. A phase I-II study of α-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009;182(4):2492–2501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]
- 8.Parekh VV, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115(9):2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uldrich AP, et al. NKT cell stimulation with glycolipid antigen in vivo: Costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175(5):3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheridan BS, et al. γδ T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity. 2013;39(1):184–195. doi: 10.1016/j.immuni.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 13.Hirschhorn-Cymerman D, et al. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J Exp Med. 2012;209(11):2113–2126. doi: 10.1084/jem.20120532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson JA, McDonald-Hyman C, Jameson SC, Hamilton SE. Effector-like CD8+ T cells in the memory population mediate potent protective immunity. Immunity. 2013;38(6):1250–1260. doi: 10.1016/j.immuni.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye F, Turner J, Flaño E. Contribution of pulmonary KLRG1(high) and KLRG1(low) CD8 T cells to effector and memory responses during influenza virus infection. J Immunol. 2012;189(11):5206–5211. doi: 10.4049/jimmunol.1200137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huntington ND, et al. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol. 2007;178(8):4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii S. Cross-presentation of glycolipid from tumor cells loaded with α-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells. J Exp Med. 2007;204(11):2641–2653. doi: 10.1084/jem.20070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune “memory”. J Immunol. 2011;186(4):1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen NR, et al. ImmGen Project Consortium Shared and distinct transcriptional programs underlie the hybrid nature of iNKT cells. Nat Immunol. 2013;14(1):90–99. doi: 10.1038/ni.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6(12):1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 21.Watarai H, et al. Generation of functional NKT cells in vitro from embryonic stem cells bearing rearranged invariant Valpha14-Jalpha18 TCRalpha gene. Blood. 2010;115(2):230–237. doi: 10.1182/blood-2009-04-217729. [DOI] [PubMed] [Google Scholar]
- 22.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12(12):845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei DG, Curran SA, Savage PB, Teyton L, Bendelac A. Mechanisms imposing the Vbeta bias of Valpha14 natural killer T cells and consequences for microbial glycolipid recognition. J Exp Med. 2006;203(5):1197–1207. doi: 10.1084/jem.20060418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curran MA, et al. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J Exp Med. 2013;210(4):743–755. doi: 10.1084/jem.20121190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacLeod MK, et al. CD4 memory T cells divide poorly in response to antigen because of their cytokine profile. Proc Natl Acad Sci USA. 2008;105(38):14521–14526. doi: 10.1073/pnas.0807449105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLeod MK, Kappler JW, Marrack P. Memory CD4 T cells: Generation, reactivation and re-assignment. Immunology. 2010;130(1):10–15. doi: 10.1111/j.1365-2567.2010.03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watarai H, et al. Murine induced pluripotent stem cells can be derived from and differentiate into natural killer T cells. J Clin Invest. 2010;120(7):2610–2618. doi: 10.1172/JCI42027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.