Abstract

We measured mercury (Hg) isotope ratios in sediments and various estuarine organisms (green crab, blue mussel, killifish, eider) to investigate methylmercury (MMHg) sources and exposure pathways in five Northeast coast (U.S.) estuaries. The mass independent Hg isotopic compositions (MIF; Δ199Hg) of the sediments were linearly correlated with the sediment 1/Hg concentrations (Δ199Hg: r2 = 0.77, p < 0.05), but the mass dependent isotope compositions (MDF; δ202Hg) were not (r2 = 0.26, p = 0.16), reflecting inputs of anthropogenic Hg sources with varying δ202Hg. The estuarine organisms all display positive Δ199Hg values (0.21 to 0.98 ‰) indicating that MMHg is photodegraded to varying degrees (5–12%) prior to entry into the food web. The δ202Hg and Δ199Hg values of most organisms can be explained by a mixture of MMHg and inorganic Hg from sediments. At one contaminated site mussels have anomalously high δ202Hg, indicating exposure to a second pool of MMHg, compared to sediment, crabs and fish. Eiders have similar Δ199Hg as killifish but much higher δ202Hg, suggesting that there is an internal fractionation of δ202Hg in birds. Our study shows that Hg isotopes can be used to identify multiple anthropogenic inorganic Hg and MMHg sources and determine the degree of photodegradation of MMHg in estuarine food webs.
Introduction
Monomethylmercury (MMHg) is a toxic and bioaccumulative organometallic compound that poses serious health risks to both humans and wildlife.1 Humans are primarily exposed to MMHg via the consumption of marine fish and shellfish, with over 90% of marine fisheries products originating from estuarine and open ocean areas.2,3 Many estuarine organisms currently contain elevated MMHg levels, which can cause health problems in humans,4,5 but there is considerable uncertainty concerning the relative importance of the sources and exposure pathways of MMHg to estuarine food webs.
Sediments have long been suggested as the dominant MMHg source to estuarine food webs. Estuarine sediments act as an important sink for Hg, receiving Hg via atmospheric deposition,6 industrial runoff7 and riverine input,8 and provide geochemical conditions that promote biotic methylation.9 Past studies have also reported strong linkages between total Hg (THg) concentrations in sediments and THg in estuarine forage fish in San Francisco Bay.10,11 In contrast, Chen et al.12,13 recently documented a strong positive association between pelagic forage fish (Fundulus heteroclittus and Menidia menidia) MMHg concentrations and water column particulate MMHg concentrations, but not with sediments, across multiple estuaries on the Northeast coast, U.S. Based on this observation, the authors suggested that the MMHg accumulated into water column particulates may be a more dominant MMHg source to pelagic organisms than sediment MMHg. Monomethylmercury can enter the water column via diffusion, advection and resuspension from sediments.14 Inflowing fluvial and tidal waters have been suggested as important external MMHg sources to pelagic food webs in the Bay of Fundy15 and the Hudson River estuary,16 with coastal sediments being a less important source. At Chesapeake Bay17 and Long Island Sound,8 both sediments and external sources were found to be important MMHg sources to these systems. Given these diverse sources, tools that can differentiate MMHg sources and exposure pathways are expected to help highlight the most important biogeochemical processes affecting MMHg in estuarine food webs.
Studies of the natural abundance of Hg isotopes have enhanced understanding of the sources and biogeochemical processing of Hg in natural environments. Mercury isotopes undergo mass-dependent fractionation (MDF, reported as δ202Hg) and mass-independent fractionation (MIF, reported as Δ199Hg and Δ201Hg).18 While MDF has been documented in various environmentally relevant reactions such as biotic methylation,19 demethylation,20 thiol–ligand exchange21 and photochemical reactions,22 MIF in natural systems is thought to occur primarily in odd-mass number isotopes via photochemical reduction and degradation of inorganic Hg (IHg) and MMHg. Recently, Δ199Hg has been applied as a biological and ecological tracer for understanding processes such as bioaccumulation and trophic transfer23,24 as well as transfers of MMHg between ecosystems.25 The ratio of Δ199Hg/Δ201Hg has also become a valuable tool for distinguishing between photochemical reduction of IHg (Δ199Hg/Δ201Hg = 1.00) and degradation of MMHg (Δ199Hg/Δ201Hg = 1.2–1.4).22
Mercury isotope ratios have recently been used to provide insight into the sources and exposure pathways of MMHg to diverse marine food webs.10,24,26,27 Based on these studies, we can make predictions as to how Hg isotope ratios might be useful for understanding the sources and biogeochemical processing of MMHg in estuaries. For instance, high positive Δ199Hg values have been documented in pelagic fish (>1‰) compared to coastal fish (<1‰), indicating that MMHg that has been subjected to extensive photochemical degradation in the open ocean water column is the dominant MMHg source to pelagic food webs.26,27 In shallow coastal environments it has been suggested that MMHg produced in the sediment, that has undergone relatively little photochemical degradation, enters the base of the food web.27 In another relevant study, Gehrke et al.10 documented relatively low Δ199Hg values (<1‰) in intertidal forage fish (i.e., topsmelt and silverside) that feed on epibenthic invertebrates across the San Francisco Bay Estuary. Fish δ202Hg values were found to be consistently higher by 0.6 ‰ compared to nearby sediments at multiple sites, which led to the conclusion that the fractionation imparted during production and degradation pathways of MMHg in the sediment or during trophic transfer was responsible for these δ202Hg offsets. Fish feeding experiments,23,24 have now documented an absence of Hg isotope fractionation during trophic transfer of MMHg to fish, and therefore the isotopic offset is thought to provide insight into the biogeochemical pathways of MMHg prior to introduction to the food web. We also expect that the isotopic offset between fish and sediments may differ between sites that vary in Hg biogeochemical cycling, as well as between different feeding guilds among estuarine organisms.
In this study, we investigated the sources and exposure pathways of MMHg in food webs from five estuaries located across the northeastern coast of the U.S. Sediments and organisms from these sites have been previously studied for IHg and MMHg concentrations by Chen et al.12,13 The Northeast coast of the U.S. is one of the most productive marine ecosystems in the world and supports valuable commercial and recreational fisheries. In the last century, this area has become severely impacted by an increase in population, industrial activity, and emission of anthropogenic pollutants including Hg.28 Here we characterize the Hg isotopic compositions of estuarine sediments across sites on the Northeast coast to identify Hg sources in these regions. The sources and exposure pathways of MMHg were evaluated across food webs consisting of epibenthose (green crab; Carcinus maenas, killifish; Fundulus heteroclittus), filter feeders (blue mussel; Mytilus edulis, ribbed mussels; Geukensia demissa), and consumers (common eider; Somateria mollissima). We determined the Hg isotopic composition of the estuarine organisms and estimated the isotopic composition of MMHg to identify the dominant MMHg source and exposure pathway to the estuarine food webs. The MMHg isotopic compositions were compared across sites and between feeding guilds (i.e., groups of species that exploit the same resources) to assess potential spatial and ecological variability in MMHg sources. To our knowledge, this is the first attempt to use Hg isotope ratios to compare MMHg sources to varying marine feeding guilds across multiple ecosystems.
Materials and Methods
Site Description
We sampled five estuarine sites along the northeastern coast of the U.S. (Maine; ME, Massachusetts; MA, Rhode Island, RI; Connecticut, CT, New Jersey; NJ) in the summers of 2008 and 2010 (Supporting Information (SI) Figure S1). All sites were adjacent to coastal marsh habitats except for Bold Point, RI (BOLD), which was located in an unvegetated area of the Providence River Estuary. The Webhannet Estuary in Wells, ME (WELLS) and Buzzards Bay, MA (BUZZ) are characterized by sandy beaches and are sparsely populated. Both sites receive primarily atmospherically deposited Hg from nonpoint sources and the inputs are relatively small compared to the other Northeast coast sites.29 BOLD is situated in urbanized Providence, RI, and receives some atmospheric point source Hg from local waste incinerators (10% of the total Hg budget).29 BOLD is impacted by local wastewater treatment facilities (21% of Hg input) and industrially impacted rivers (69% of Hg input).30 Barn Island, CT (BARN) is located in the southeastern tip of Connecticut and also receives Hg via local wastewater treatment facilities and industrially impacted rivers—the Connecticut River (59% of Hg input) and the East River (25% of Hg input).8 Mill Creek, NJ (MILL) is impacted by the Hackensack River, which has been heavily contaminated by landfills, and a Hg recovery plant that discharged 30–400 tons of Hg including elemental Hg, mercuric oxide, and other forms of oxidized Hg byproducts during its operation between 1929 and 1974.31
Sampling and Analysis
The estuarine sediments were measured for THg and MMHg concentrations at the University of Connecticut Department of Marine Sciences and were reported previously13 (n = 9). Four types of biota were analyzed for this study: green crabs (Carcinus maenas) (n = 6), blue mussels (Mytilus edulis) (n = 5) or ribbed mussels (Geukensia demissa) (n = 2), killifish (Fundulus heteroclittus) (n = 9), and eider (Somateria mollissima) (n = 5) (referred to as crab, mussel, fish, and bird hereafter). The methods for sampling and processing of the sediments and aquatic biota are described in Chen et al.13 The aquatic biota were measured for stable nitrogen and carbon isotopic composition at the Stable Isotope Laboratory, Dartmouth College to characterize the trophic positions and feeding guilds. THg and MMHg analyses were conducted for most samples at the Trace Element Analysis Laboratory, Dartmouth College. These results were previously reported in Chen et al.13 Additional samples of mussels (n = 4) and crabs (n = 3) were measured for THg concentrations at the University of Michigan. These samples were freeze-dried prior to removing the shells, and homogenized in a zirconium grinding mill (Retsch, Mixer Mill MM 301). The mussels and crabs were measured for THg using Atomic Absorption Spectroscopy (AAS) following combustion of samples at 800 °C using a Nippon Instruments MA-2000 Hg analyzer. Standard reference material NIST-3133 was used to generate calibration curves and for quality control. Standard reference material TORT-3 (n = 4) was analyzed as an external standard and agreed within 5% of certified values.
Samples of bird blood were collected near WELLS and BUZZ between January and April 2010 (SI Figure S1). The birds were captured using floating mist nets and the blood samples were collected by following the standard tissue collection protocol described in Evers et al.32 The blood samples were drawn nonlethally by venipuncture from either the cutaneous ulnar or tarsal vein using needles and syringes. The blood samples were stored in either clean heparinized capillary tubes or microtainers. The samples were sealed on both ends with Critocaps, placed in 10 cc plastic vacutainers, and frozen at −25 °C prior to the analyses for THg concentrations at the Wildlife Mercury Research Lab at the Biodiversity Research Institute, Maine, U.S. The THg concentrations were determined using a direct Hg analyzer (DMA 80, Milestone Incorporated). The standard reference materials DOLT-4 and DORM-4 were used to generate calibration curves and for quality control. DOLT-4 (n = 5) and DORM-3 (n = 5) were analyzed with samples and agreed within 10% of certified values. The relative standard deviations of duplicate analyses of samples were within 5%. The THg concentrations of sediments and aquatic biota are reported in dry weight and the aquatic biota represent whole body tissues. The bird blood analyses are reported in wet weight.32
Hg Isotope Analysis
The sediment and biota samples were measured for stable Hg isotopic composition at the University of Michigan. Blood samples were thawed and oven-dried at 50–60 °C in acid washed ceramic boats. Samples of aquatic biota were weighed and loaded into ceramic boats with sodium carbonate and aluminum oxide powders. Offline two-stage combustion furnace systems were used to release Hg (as Hg0) from the samples. The ceramic boats were loaded into the first combustion compartment and heated to 750 °C over a 6-h period. Released Hg0 was directed to the second combustion compartment (1000 °C) via a stream of Hg-free oxygen and subsequently oxidized in a trap solution (1% KMnO4 in 10% trace metal grade H2SO4). The solutions containing Hg2+ were neutralized with hydroxylamine, reduced back to Hg0 with SnCl2 and purged into a new trap solution to remove combustion product matrix components from the sample.
Procedural blanks were prepared in the same manner as the samples (but with no sample placed in the ceramic boat) and measured for THg before and after the transfer steps. The procedural blanks had an average THg of 0.2 ± 0.1 ng (n = 6). The THg concentrations of the samples as well as the standard reference materials ERM CE 464 (n = 3), TORT-2 (n = 1), and MESS-3 (n = 2) were measured before and after the transfer step to monitor the recoveries of THg during the combustion and transfer processes. The recoveries of the combustion and transfer steps of the samples and standard reference materials ranged between 89–100%, and 92–106%, respectively.
Stable Hg isotope ratios were measured using a Nu Instruments multicollector inductively coupled plasma mass spectrometer (MC-ICP-MS). The trap solutions were neutralized using hydroxylamine. To match the THg concentration of the sample to the standard, the trap solutions containing the sample were diluted to between 1 and 5 ng/g using the same neutralized trap solution matrix. Mercury was introduced to the MC-ICP-MS as Hg0 by reducing Hg2+ in solution with SnCl2, and separating Hg0 using a frosted glass tip phase separator. On-peak zero corrections were applied. Instrumental mass bias was corrected using an internal Tl standard (NIST SRM 997) and by bracketing each sample with NIST SRM 3133 matched to sample THg concentrations and matrix composition. MDF is reported as δ202Hg in permil (‰) referenced to NIST SRM 3133:18
| 1 |
MIF represents the difference between the measured δxxxHg value and the value predicted based on MDF and the δ202Hg value.18 MIF is reported as Δ199Hg and Δ201Hg in permil (‰). The calculation is based on an approximation valid for δ <10‰:
| 2 |
| 3 |
Analytical uncertainty at 2 SD is estimated based on either replicate analysis of a standard solution (UM-Almáden) or replicate analyses of standard reference materials. We used ERM CE 464 to report analytical uncertainty since it had a larger uncertainty. UM-Almáden (n = 30) had mean values (±2 SD) of δ202Hg = −0.57 ± 0.06 ‰, Δ201Hg = −0.04 ± 0.04 ‰, and Δ199Hg = −0.02 ± 0.05 ‰. Standard reference material ERM CE 464 (n = 3) had mean values of δ202Hg = 0.66 ± 0.08 ‰, Δ201Hg = 1.91 ± 0.06 ‰, and Δ199Hg = 2.31 ± 0.09 ‰; TORT-2 (n = 1) had values of δ202Hg = 0.10 ‰, Δ201Hg = 0.57 ‰, and Δ199Hg = 0.79 ‰; and MESS-3 (n = 2) had mean values of δ202Hg = −1.81 ± 0.08 ‰, Δ201Hg = −0.05 ± 0.05 ‰, and Δ199Hg = 0.01 ± 0.03 ‰.
Results and Discussion
Hg Concentrations and Isotopic Compositions in the Sediments
The THg concentrations of the Northeast coast sediments are quite low at BUZZ (5.70 ng/g) and WELLS (9.43 ng/g) and higher at the other three sites (BARN, BOLD, MILL) (42.0 to 2961 ng/g) (SI Table S1). Due to the low THg values at the BUZZ and WELLS sites (typical of uncontaminated sites),33,34 we designate WELLS and BUZZ as “background sites” and due to the relatively higher values at BOLD, BARN, and MILL we designate them as “contaminated sites.” The contaminated sites are associated with Hg point sources (see site description) and have at least 6 times higher THg concentration compared to the background sites.
Overall, the sediments displayed ranges of δ202Hg values between −0.89 ‰ and −0.38 ‰, and Δ199Hg values between −0.04 ‰ and 0.19 ‰, and were within the range of values reported previously in other coastal marine sediments.7,27,35,36 We plotted 1/THg concentration against δ202Hg and Δ199Hg to linearize mixing relationships and to characterize the Hg isotopic compositions of the sediments in relation to the Hg contamination. We observed a weak negative (r2 = 0.26, p = 0.16) and significant positive (r2 = 0.77, p < 0.05) relationship between 1/THg concentration and δ202Hg and Δ199Hg, respectively (Figure 1). The Δ199Hg values of the sediments were distinct between the background and contaminated sites with the contaminated sediments exhibiting uniform Δ199Hg ≈ 0 ‰, and slightly elevated Δ199Hg in the background sediments. The sediments displayed ranges of negative δ202Hg values and were less well correlated with the sediment THg concentrations.
Figure 1.
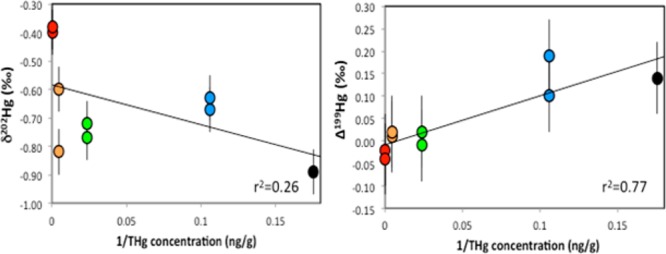
Plot of 1/THg concentration (ng/g) versus δ202Hg and Δ199Hg values of the Northeast coast estuary sediments. Each site is represented with different colors; BUZZ (black), WELLS (blue), BARN (green), BOLD (orange), and MILL (red). The solid lines represent linear regressions. Analytical uncertainty is indicated by the error bar (2 SD).
The fact that the Δ199Hg values of the Northeast coast sediments are correlated with the THg concentrations suggests that these sediments reflect inputs from multiple Hg sources associated with the Northeast coast estuaries. When we compared the background sediments to the contaminated sediments, the sites designated as “background” displayed negative δ202Hg values with Δ199Hg values that were distinctly positive compared to the contaminated sediments. Coastal and marine sediments from other studies that were characterized as background, or those that are preanthropogenic in age, display low THg concentrations, negative δ202Hg and slightly elevated Δ199Hg.7,35,36 The background sediments from this study are consistent with the δ202Hg and Δ199Hg values reported in those previous studies. The positive Δ199Hg values in the background sediments are likely the result of fractionation caused during partial photochemical reduction of IHg, which may occur either through deposition of photochemically reduced IHg from surface water,27 periodic exposure of surface sediments to sunlight during tidal fluctuation of water levels (1–2 m),7,37 or deposition of photochemically reduced IHg from rainwater.38 Photochemical reduction of IHg has been used to explain the positive Δ199Hg values documented in shallow and relatively undisturbed marshes (∼0.66 ‰)37 and coastal and intertidal sediments (∼0.08 ‰)7,27 and we suggest that positive Δ199Hg values may be imparted in background sediments that lack significant inputs from anthropogenic Hg sources.
In the contaminated sediments, we observed similar ranges of negative δ202Hg values but uniform Δ199Hg ≈ 0 ‰, reflecting the dominance of anthropogenic Hg sources in these sediments.7,35,36,39 Various industrial Hg sources have been characterized by wide ranges of negative δ202Hg and zero Δ199Hg values,22,39,40 and the input of industrial Hg sources likely explains the low Δ199Hg values, high THg concentrations, and wide ranges in δ202Hg values observed in the contaminated sediments. The range of δ202Hg values for the contaminated sites that are impacted by local industrial Hg sources (BARN, BOLD; −0.60 to −0.82 ‰) were also consistent with values for Hg0 used in gold mining in the San Francisco Bay Estuary (−0.59 to −0.78 ‰)7. Thus, the Hg isotopic compositions and the THg concentrations of the sediments appear to be consistent with the presence of multiple anthropogenic Hg sources in these regions.
Hg Concentrations and Isotopic Compositions in Biota
The THg concentrations of the estuarine biota increase in the order: crabs (55.3 ± 24 ng/g) < fish (138 ± 188 ng/g) < mussels (145 ± 52 ng/g) < bird blood (863 ± 724 ng/g) (SI Table S1). The fraction of THg that is in the form of MMHg (referred to as % MMHg hereafter) for the aquatic biota increases in the order: mussels (57.9 ± 4.5%) < crabs (83.7 ± 3.0%) < fish (91.4 ± 5.9%) and these values follow the same order as δ15N values (7.4 ± 1.0‰, 10.3 ± 3.0‰, 11.3 ± 3.2‰; SI Table S1), representing the approximate trophic position. The % MMHg values of the birds were estimated from Wayland et al.,42 who reported over 98% MMHg in blood. The increasing % MMHg with trophic position (δ15N) is consistent with the pattern of MMHg biomagnification documented in many aquatic food webs.43,44 We designate the feeding guilds of the estuarine biota based on the δ13C values and the detailed feeding behaviors are shown in SI Table S1 and Chen et al.12,13 The δ15N (13.2 ‰) and δ13C (−17.4 ‰) values for the birds (SI Table S1) were estimated from Hobson et al.45 who measured the same species in the Arctic. This estimate was adequate for approximating the relative trophic position and feeding guild of these birds, which suggests a slightly higher trophic position compared to the fish measured in this study.
Across the Northeast coast estuarine study sites, we observed wide ranges of δ202Hg values but relatively narrow ranges of Δ199Hg values in the estuarine biota (SI Table S1). At each study site the δ202Hg and Δ199Hg values of the estuarine biota displayed an increasing trend with trophic position—following the order mussels, crabs, fish, and birds (Figure 2a,b). Similar trends have previously been attributed to the varying extent of MMHg bioaccumulation with trophic position.23,24 We plotted % MMHg against δ202Hg and Δ199Hg of the sediment and aquatic biota (without the birds) and observed significant positive relationships with Δ199Hg (p < 0.05) across all sites; (WELLS; r2 = 0.86, BUZZ; r2 = 0.70, BOLD; r2 = 0.72, BARN; r2 = 0.89) and of δ202Hg at all sites except BOLD; (WELLS; r2 = 0.68, p < 0.05, BUZZ; r2 = 0.71, p < 0.05, BOLD; r2 = 0.52, p = 0.08, BARN; r2 = 0.80, p < 0.05). Due to the significant positive relationships observed at most sites, the Hg isotopic composition reflecting the bioaccumulated MMHg was estimated using a linear regression of % MMHg against δ202Hg and Δ199Hg. The δ202Hg and Δ199Hg values estimated for MMHg demonstrated relatively narrow ranges across the Northeast coast estuarine food webs; −0.22 to 0.28 ‰ and 0.39 to 1.00 ‰, respectively (Figure 2a,b). At sites where birds were measured (in close proximity to WELLS and BUZZ), the birds (containing >98% MMHg) had similar Δ199Hg but much higher δ202Hg compared to the fish (containing >90% MMHg) measured at the same locations (bird δ202Hg; 0.59 to 1.39 ‰, fish δ202Hg; −0.34 to 0.23 ‰). At BOLD, the mussels displayed significantly higher δ202Hg values compared to other aquatic biota.
Figure 2.
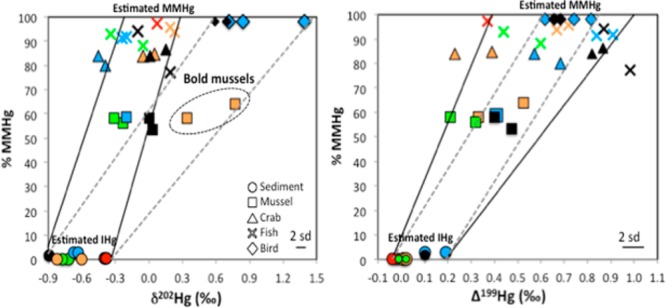
Plot of % MMHg versus δ202Hg (a) and Δ199Hg values (b) of estuarine sediments and biota. Each site is represented by a different color; BUZZ (black), WELLS (blue), BARN (green), BOLD (orange), and MILL (red). The solid lines represent the range of estimated MMHg isotopic composition needed to explain sediments, crabs, and fish. The dotted lines represent the range of estimated MMHg isotopic composition needed to explain mussels from BOLD and birds from BUZZ and WELLS. The “estimated MMHg” and “estimated IHg” represent the ranges of Hg isotopic composition extrapolated for 100% MMHg and 100% IHg, respectively, based on the linear regression of % MMHg vs δ202Hg and Δ199Hg. Analytical uncertainty is indicated by the error bar (2 SD).
Overall, we find strong evidence for an increasing trend in the δ202Hg and Δ199Hg values of estuarine biota with % MMHg (trophic position) in the Northeast coast estuarine food webs. This trend has also been documented in other aquatic food webs.27,46−48 While metabolic fractionation was previously proposed as a potential mechanism,46 recent fish feeding experiments showed that metabolic fractionation does not occur during trophic transfer to fish.23,24 Instead, the differences in Hg isotopic composition between MMHg and IHg and the varying extent of MMHg versus IHg bioaccumulation with trophic position provide a consistent explanation for the Hg isotopic composition variability in food webs. Thus, the absence of MDF and MIF during MMHg trophic transfer implies that the estuarine biota mainly reflect the isotopic composition of MMHg incorporated at the base of the food web. The anomalously high δ202Hg values observed in the WELLS and BUZZ birds and the BOLD mussels suggest that these organisms may be accumulating MMHg from an additional source. Below we assess the dominant MMHg source and exposure pathways in the Northeast coast estuarine food webs using the Hg isotopic composition estimated for MMHg and provide an explanation for the δ202Hg values found in the birds and the BOLD mussels.
Sources and Biogeochemical Pathways of MMHg
The Northeast coast estuarine biota analyzed in this study collectively displayed moderately positive Δ199Hg values (0.2–1.0 ‰) (SI Table S1). This suggests that the bioaccumulated MMHg was subjected to photochemical degradation prior to incorporation into the estuarine food web. In fish, which have consistently high % MMHg and for which we have isotope data at each site, we found lower Δ199Hg in the contaminated sites (0.56 ± 0.15 ‰) compared to the uncontaminated sites (0.90 ± 0.06 ‰). This indicates that the fish at the contaminated sites are either exposed to MMHg that was subjected to a lesser degree of photochemical degradation compared to those found at the uncontaminated sites or MMHg originated from anthropogenic IHg sources which have low Δ199Hg (≈0 ‰). The slope of Δ199Hg/ Δ201Hg has been used to distinguish between photochemical degradation and reduction of MMHg versus IHg in natural aquatic ecosystems.22 We plotted Δ199Hg against Δ201Hg for all estuarine biota, and used a York regression to estimate the slope of Δ199Hg/ Δ201Hg, which is 1.22 ± 0.07 (r2 = 0.90, p < 0.05). This slope is consistent with the slopes reported in other marine fish (∼1.2) exposed to photochemically degraded MMHg from their respective environments.10,26,27,41,49,50 On a Δ199Hg versus δ202Hg diagram we plot values for sediments and biota and the experimentally derived slopes representing the expected changes in the MMHg isotopic composition caused by photochemical degradation of MMHg (Δ199Hg = 4.79 ± 0.33 × δ202Hg at 10 mg/L DOC)22 (Figure 3; dotted lines). These slopes are derived from previously published MMHg photochemical degradation experiments that employed aquatic solutions spiked with MMHg and DOC, and natural sunlight,22 and are only rough estimates since experimental conditions were different from those in the natural setting we studied. Based on values for fish, we estimate that MMHg is photochemically degraded to varying degrees ranging from 5 to 12% across the Northeast coast sites prior to entering the food webs.
Figure 3.
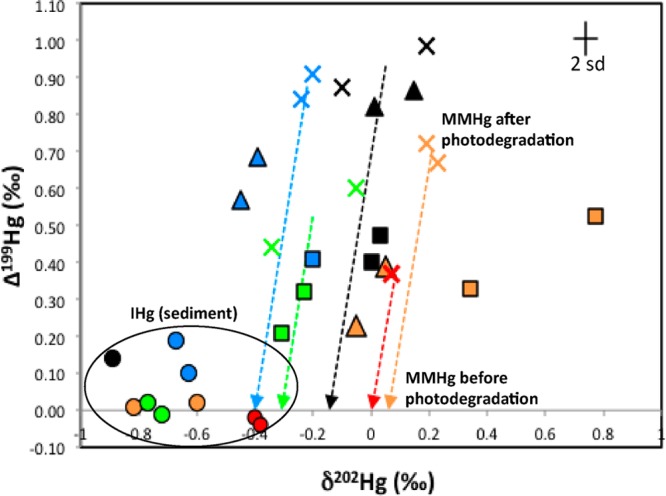
Plot of δ202Hg and Δ199Hg values of all sediments and aquatic biota. Each site is represented with different colors; BUZZ (black), WELLS (blue), BARN (green), BOLD (orange), and MILL (red). Symbols are the same as Figure 2. The arrows represent the experimentally derived photochemical degradation slope for MMHg. Analytical uncertainty is indicated by the error bar (2 SD).
The δ202Hg values for MMHg prior to photochemical degradation were estimated following the approach of Gehrke et al.10 and Sherman and Blum51 and were all higher than the corresponding δ202Hg values of THg measured in the sediments (Figure 3). Given that the sediment is mainly composed of IHg, we suggest that MMHg that is bioaccumulated is mass dependently fractionated compared to the IHg in the sediment. This has been observed in previous studies10,51 and has been attributed to the net effect of microbial Hg methylation and demethylation, which has increased δ202Hg values prior to photochemical degradation. The degree of the offset in δ202Hg between the MMHg (before photodegradation) and IHg are somewhat variable across the sites but generally consistent with previous studies. An alternate explanation for this offset in δ202Hg between IHg and MMHg is that it is caused by introduction of MMHg from a different source than the local sediment, which has higher δ202Hg (e.g., methylation in upstream marshes).
The proportion of MMHg photochemical degradation estimated here is comparable with many shallow coastal regions10,27 and high turbidity lakes,51 but much lower than open ocean environments.26,27 The simplest explanation for the source of the MMHg to which estuarine biota are exposed is that it is derived from the IHg in sediments and has subsequently undergone small amounts of photochemical degradation in the water column, perhaps becoming attached to particles and subsequently redeposited to the sediment. In a study of fish and sediment in the Gulf of Mexico it was observed that the Hg isotopic composition of coastal fish was consistent with exposure to sediment-derived MMHg that had undergone small amounts of photochemical degradation, whereas open ocean fish had much higher Δ199Hg values indicative of much higher degrees of photodegradation.27 Day et al.49 also found ranges of Δ199Hg values in the eggs of epipelagic seabirds with the individuals feeding near shallow coastal embayments displaying significantly lower Δ199Hg values compared to those feeding on open ocean species.
The positive offsets in δ202Hg between IHg (in sediment) and MMHg (in biota) are consistent with previous studies suggesting that the local sediments may be the primary site for MMHg production prior to photochemical degradation in the water column and bioaccumulation in the food webs. It has been shown that microbial methylation causes the fractionation of δ202Hg, leading to lower δ202Hg in the product-MMHg compared to the reactant.19 A portion of Hg that has been methylated could subsequently be microbially demethylated, resulting in higher δ202Hg values in the remaining MMHg,20 and the net effect of these processes provides an explanation for the δ202Hg values in the sediments. Large positive offsets in δ202Hg have been documented in estuarine sediments,10 and to a lesser extent in rocky streambeds25 where MMHg production in sediments is less likely to occur. The site-specific variation in the offsets in δ202Hg suggests that the relative degree of fractionation due to microbial methylation versus demethylation differs across the Northeast coast study sites. The demethylation activities in these regions are poorly understood but Schartup et al.52 recently documented variable methylation rates in sediment across the Northeast coast estuarine sites.
While our results are consistent with the previous findings that suggested sediments as the primary site for MMHg production, it is possible that MMHg derived from various external sources may also play an important role in the Northeast coast estuarine food webs. Methylmercury produced within wetlands and river watersheds have been shown to supply significant amounts of MMHg to aquatic ecosystems in the Northeast U.S.15,17,29,53 Chen et al.13 recently documented a lack of relationship between MMHg concentrations in sediments and in the water column across the same sites of the Northeast coast estuaries that we studied and suggested that sediments may be a minor contributor to MMHg found in water column. Given that the MMHg flux via sedimentation exceeds that due to resuspension at our study sites,13 it is possible that external MMHg sources deposited in the sediment via settling particles may provide a viable alternative explanation for the observed offsets in δ202Hg between IHg (sediment) and the bioaccumulated MMHg. It is difficult to make a clear differentiation between in situ mass dependent fractionation of sediment-produced MMHg versus mixing of external MMHg sources in this study. Further investigation will be required to characterize the isotopic composition of various external MMHg sources and biogeochemical processes affecting MMHg production in sediments.
In summary, our results are consistent with either the production of MMHg from IHg in sediments or derivation of MMHg externally with deposition to the sediments. This MMHg is then subjected to a small amount of photodegradation in the water column before entering the food web and being passed to the various organisms at the study sites (with one exception described below). The use of Hg isotope ratios in this study provides additional insight into the sources and exposure pathways of MMHg studied by Chen et al.12,13 in the same Northeast coast estuarine food webs. Chen et al.12,13 documented a strong positive association between pelagic fish (silverside, killifish) MMHg concentrations and water column particulate MMHg concentrations, but not with sediment MMHg concentrations. Mussels and crabs did not show significant correlations with sediment and water column concentrations of MMHg. Based on these observations, it was suggested that while sediments may be the main repository for Hg, sediment MMHg concentrations are not an accurate predictor for MMHg bioaccumulation in estuarine biota. The authors also argued that the MMHg exposure via the water column may be the result of either complex interactions between water and sediment MMHg or input of external MMHg sources from offshore. Given that the estuarine biota studied demonstrate different feeding behaviors and variable MMHg uptake routes, the consistent MMHg isotopic composition observed in various feeding guilds from each study location suggest that there may be extensive MMHg cycling between the sediment and water column via deposition and resuspension cycles without significant changes in the Hg isotopic compositions of the MMHg. In other words, even if the fish derive MMHg primarily from the water column, the MMHg in the water column may be isotopically similar to the small proportion of MMHg in the sediments.
Ecological Variability in MMHg Sources
While it appears that most aquatic organisms in this study acquire sediment-associated MMHg via trophic transfer, the high positive δ202Hg values found in the WELLS and BUZZ birds and the BOLD mussels suggest that these organisms are exposed to a different (or additional) MMHg source that has considerably higher δ202Hg values (Figure 2). Considering the mussels first, the main difference between the BOLD mussels and the mussels sampled in the other Northeast coast estuaries is that while the other mussels have a Hg isotopic composition consistent with a mixture of IHg and MMHg from the sediment, this pattern was observed only for Δ199Hg, but not δ202Hg in the BOLD mussels (Figure 2). Thus, we infer that the BOLD mussels may be exposed to an additional MMHg source, possibly from the water column, due to their active filter feeding mechanism. The δ13C values confirm that they feed on both pelagic and benthic resources,12,13 which is clearly different from the crabs and fish that depend dominantly on benthic and epibenthic resources closely associated with the sediments.
We have identified two different scenarios that might explain how the BOLD mussels are exposed to Hg with anomalously high positive δ202Hg values. First, to achieve high positive δ202Hg values, the sediment-associated MMHg would have to be subjected to additional nonphotochemical degradation and accompanying fractionation, causing the remaining MMHg to shift to higher δ202Hg values. We speculate that resuspension of sediment-associated MMHg followed by microbial demethylation20 in the water column could produce MMHg with high positive δ202Hg that is available for uptake by the mussels. BOLD is characterized by the highest TSS and particulate MMHg concentrations,13 and this region may be particularly susceptible to additional biogeochemical processing of Hg in the water column. Second, it is also possible that the BOLD mussels were exposed to a second, and possibly unrelated external MMHg source that either has high δ202Hg values inherited from an anthropogenic source or has undergone extensive microbial demethylation prior to being released into the estuary. BOLD is surrounded by an unvegetated area and diverse point sources of Hg, which may expose this site to extensive runoff of industrial Hg sources.29,53 Moreover, based on recent evidence that suggests inflowing fluvial MMHg can serve as an important external MMHg source to pelagic food webs,15 we presume that exposure to an externally derived MMHg source may be a viable alternate explanation for the high δ202Hg in the BOLD mussels.
In the birds, we documented similar Δ199Hg but much higher δ202Hg compared to fish from the same sites, which are the highest trophic level aquatic organisms measured in this study (Figure 2). It is possible that the high δ202Hg MMHg is caused by the differences in food sources obtained from their respective sampling habitats compared to those of WELLS and BUZZ (SI Figure S1) or by their selective feeding behavior given that they feed dominantly on benthic invertebrates including mussels.54 Thus, if we assume that the birds analyzed in this study feed primarily on mussels, we might expect a significant positive relationship between % MMHg and δ202Hg, and between % MMHg and Δ199Hg of the sediments, mussels, and birds due to the trophic transfer of MMHg. We plotted % MMHg against the δ202Hg and Δ199Hg values of the sediments, mussels, and birds and observed significant positive relationships at WELLS (δ202Hg; r2 = 0.87, p < 0.05, Δ199Hg = 0.93, p < 0.05) and BUZZ (δ202Hg; r2 = 0.99, p < 0.05, Δ199Hg; r2 = 0.97, p < 0.05). However, the trophic transfer of MMHg from the sediment, to mussels, to birds would indicate that the crabs and fish are exposed to a different MMHg source than the mussels, and this is not what we observed, except at the BOLD site. Moreover, given that the species of bird analyzed in this study have a relatively large feeding habitat (∼60 km2),55 the small difference in the feeding habitat probably cannot account for the high δ202Hg in the birds. Instead, we attribute the high δ202Hg values in the birds to an internal metabolic fractionation of δ202Hg. While metabolic fractionation of δ202Hg has not been observed in fish,23,24 internal demethylation has been proposed to occur in the liver of many species of bird56,57 and this may lead to the fractionation of δ202Hg in birds. Previous studies have documented 1–2 ‰ higher δ202Hg values in birds (egg contents), and in mammals including seals, whales, and human hair compared to their respective diets41,48,49,58,59 and attributed this pattern to the kinetic fractionation of δ202Hg via internal demethylation.
Our study demonstrates that the measurement of Hg isotope ratios provides new insight into sources and exposure pathways of MMHg in estuarine food webs. Past studies that utilized Hg isotope ratios to decipher MMHg sources and exposure pathways in coastal food webs have only examined fish, and over smaller coastal regions.10,27 We found evidence for multiple Hg sources across the Northeast coast sediments, and the MMHg associated with the sediments appears to serve as the dominant MMHg source to the estuarine food webs. There has been a long-standing debate over the relative importance of MMHg derived from sediment or from the water column as a source to estuarine organisms. Complex biogeochemical processes affecting Hg bioavailability between sediment and water column however make it difficult to trace the dominant MMHg sources in the estuarine food webs based on Hg concentration alone. This study suggests that MMHg associated with sediments probably acts as the dominant source to many estuarine organisms, but that certain feeding guilds and certain localities are more susceptible to accumulating additional external MMHg sources. While further investigation is necessary to characterize various external MMHg sources, our study demonstrates that the measurement of Hg isotope ratios can be a valuable tool for deciphering MMHg sources and exposure pathways in diverse aquatic food webs and assessing the ecological variability of MMHg sources.
Acknowledgments
We thank M. Johnson for the MC-ICP-MS operation, L. Savoy and other BRI staff for eider sample collection, D. Bugge, J. Williams, G. Bernier, T. Hollweg, and K. Labrum for collection of aquatic samples, and P. Balcom, B. Jackson, and V. Taylor for mercury analysis. This project was supported in part by NIH Gran P42 ES007373 from the NIEHS, and NSF grant EAR 0952108.
Supporting Information Available
Supporting figure (Figure S1) and table (Table S1). This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Mergler D.; Anderson H. A.; Chan L. H. M.; Mahaffey K. R.; Murray M.; Sakamoto M.; Stern A. H. Methylmercury exposure and health effects in humans: A worldwide concern. Ambio. 2007, 36, 3–11. [DOI] [PubMed] [Google Scholar]
- Ryther J. H. Photosynthesis and fish production in the sea. Science 1969, 166, 72–76. [DOI] [PubMed] [Google Scholar]
- Sunderland E. M. Mercury exposure from domestic and imported estuarine and marine fish in the U.S. seafood market. Environ. Health Perspect. 2007, 115, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens W.; Leermakers M.; Papina T.; Saprykin A.; Brion N.; Noyen J.; De Geiter M.; Elskens M.; Goyens L. Bioconcentration and biomagnification of mercury and methylmercury in North Sea and Scheldt Estuary fish. Arch. Environ. Contam. Toxicol. 2003, 45, 498–508. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T.; Porter D.; Bushek D.; Jones B. Mercury in the American oyster Crassostrea virinica in South Carolina, USA, and public health concerns. Mar. Pollut. Bull. 1999, 38, 324–327. [Google Scholar]
- Engle M. A.; Tate M. T.; Krabbenhoft D. P.; Shauer J. J.; Kolker A.; Shanley J. B.; Bothner M. H. Comparison of atmospheric mercury speciation and deposition at nine sites across central and eastern North America. J. Geophys. Res. Atmos. 2010, 115, 1–13. [Google Scholar]
- Gehrke G. E.; Blum J. D.; Marvin-DiPasquale M. Sources of mercury to San Francisco Bay surface sediment as revealed by mercury stable isotopes. Geochim. Cosmochim. Acta 2009a, 75, 691–705. [Google Scholar]
- Balcom P. H.; Fitzgerald W. F.; Vandal G. M.; Lamborg C. H.; Rolfhus K. R.; Langer C. S.; Hammerschmidt C. R. Mercury sources and cycling in the Connecticut River and Long Island Sound. Mar. Chem. 2004, 90, 53–74. [Google Scholar]
- Benoit J. M.; Gilmour C. C.; Heyes A.; Mason R. P.; Miller C. L.. Geochemical and biological controls over methylmercury production and degradation in aquatic ecosystems. In Biogeochemistry of Environmentally Important Trace Elements; Cai Y., Braids O. C., Eds.; American Chemical Society: Washington DC, 2003; Vol. 835, pp 262.-. [Google Scholar]
- Gehrke G. E.; Blum J. D.; Slotton D. G.; Greenfield B. K. Mercury isotope link mercury in San Francisco Bay forage fish to surface sediments. Environ. Sci. Technol. 2011, 45, 1264–1270. [DOI] [PubMed] [Google Scholar]
- Greenfield B. K.; Jahn A. Mercury in San Francisco Bay forage fish. Environ. Pollut. 2010, 158, 2716–2724. [DOI] [PubMed] [Google Scholar]
- Chen C. Y.; Dionne M.; Mayes B. M.; Ward D. M.; Sturup S.; Jackson B. P. Mercury bioavailability and bioaccumulation in estuarine food webs in the Gulf of Maine. Environ. Sci. Technol. 2009, 43, 1804–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y.; Borsuk M. E.; Bugge D. M.; Hollweg T.; Balcom P. H.; Ward D. M.; Williams J.; Mason R. P. Benthic and pelagic pathways of methylmercury bioaccumulation in estuarine food webs of the Northeast United States. Plos One. 2014, 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammershmidt C. R.; Fitzgerald W. F. Sediment-water exchange of methylmercury determined from shipboard benthic flux chambers. Mar. Chem. 2008, 109, 86–97. [Google Scholar]
- Sunderland E. M.; Dalziel J.; Heyes A.; Branfireun B. A.; Krabbenhoft D. P.; Gobas F. A. P. C. Response of a macrotidal estuary to changes in anthropogenic mercury loading between 1850 and 2000. Environ. Sci. Technol. 2010, 44, 1698–1704. [DOI] [PubMed] [Google Scholar]
- Balcom P. H.; Hammerschmidt C. R.; Fitzgerald W. F.; Lamborg C. H.; O’Conner J. S. Seasonal distributions and cycling of mercury and methylmercury in the waters of New York/New Jersey Harbor Estuary. Mar. Chem. 2008, 109, 1–17. [Google Scholar]
- Mason R. P.; Lawson N. M.; Lawrence A. L.; Leaner J. J.; Lee J. G.; Sheu G. R. Mercury in the Chesapeake Bay. Mar Chem. 1999, 65, 77–96. [Google Scholar]
- Blum J. D.; Bergquist B. A. Reporting of variations in the natural isotopic composition of mercury. Anal. Bioanal. Chem. 2007, 388, 353–359. [DOI] [PubMed] [Google Scholar]
- Rodriguez-gonzalez P.; Epov V. N.; Bridou R.; Tessier E.; Guyoneud R.; Monperrus M.; Amouroux D. Species-specific stable isotope fractionation of mercury during Hg(II) methylation by an anaerobic bacteria (Desulfobulbus propionicus) under dark conditions. Envrion Sci. Technol. 2009, 43, 9183–9188. [DOI] [PubMed] [Google Scholar]
- Kritee K.; Barkay T.; Blum J. D. Mass dependent stable isotope fractionation of mercury during mer mediated microbial degradation of monomethylmercury. Geochim. Cosmochim. Acta 2009, 73, 1285–1296. [Google Scholar]
- Wiederhold J. C.; Cramer C. J.; Daniel K.; Infante I.; Bourdon B.; Kretzschmar R. Equilibrium mercury isotope fractionation between dissolved Hg(II) species and thiol-bound Hg. Environ. Sci. Technol. 2010, 44, 4191–4197. [DOI] [PubMed] [Google Scholar]
- Bergquist B. A.; Blum J. D. Mass-dependent and -independent fractionation of Hg isotopes by photoreduction in aquatic systems. Science 2007, 318, 417–420. [DOI] [PubMed] [Google Scholar]
- Kwon S. Y.; Blum J. D.; Carvan M. J.; Basu N.; Head J. A.; Madenjian C. P.; David S. R. Absence of fractionation of mercury isotopes during trophic transfer of methylmercury to freshwater fish in captivity. Environ. Sci. Technol. 2012, 46, 7527–7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S. Y.; Blum J. D.; Chirby M. A.; Chesney E. J. Application of mercury isotopes for tracing trophic transfer and internal distribution of mercury in marine fish feeding experiments. Environ. Toxicol. Chem. 2013, 23, 2322–2330. [DOI] [PubMed] [Google Scholar]
- Tsui M. T. K.; Blum J. D.; Kwon S. Y.; Finlay J. C.; Balogh S. J.; Nollet Y. H. Sources and transfers of methylmercury in adjacent river and forest food webs. Environ. Sci. Technol. 2012, 46, 10957–10964. [DOI] [PubMed] [Google Scholar]
- Blum J. D.; Popp B. N.; Drazen J. C.; Choy C. A.; Johnson M. W. Methylmercury production below the mixed layer in the North Pacific Ocean. Nat. Geosci. 2013, 6, 879–884. [Google Scholar]
- Senn D. B.; Chesney E. J.; Blum J. D.; Bank M. S.; Maage A.; Shine J. P. Stable isotope (N, C, Hg) study of methylmercury sources and trophic transfer in the Northern Gulf of Mexico. Environ. Sci. Technol. 2010, 44, 1630–1637. [DOI] [PubMed] [Google Scholar]
- Long E. R.; Robertson A.; Wolfe D. A.; Hameedi J. W.; Sloane G. M. Estimates of the spatial extent of sediment toxicity in major US estuaries. Environ. Sci. Technol. 1996, 30, 3585–3592. [Google Scholar]
- Driscoll C. T.; Han Y. J.; Chen C. Y.; Evers D. C.; Lambert K. F.; Holsen T. M.; Kamman N. C.; Munson R. K. Mercury contamination in forest and freshwater ecosystems in the northeastern United States. BioScience 2007, 57, 17–28. [Google Scholar]
- Vandal G. M.; Fitzgerald W. F. A preliminary mercury budget for Narragansett Bay (Rhode Island, USA). Water, Air, Soil Pollut. 1995, 80, 679–682. [Google Scholar]
- Cardona-Marek T.; Schaefer J.; Ellickson K.; Barkay T.; Reinfelder J. R. Mercury speciation, reactivity, and bioavailability in a highly contaminated estuary, Berry’s Creek, New Jersey Meadowlands. Environ. Sci. Technol. 2007, 41, 8268–8274. [DOI] [PubMed] [Google Scholar]
- Evers D. C.; Burgess N.; Champoux L.; Hoskins B.; Major A.; Goodale W.; Taylor R.; Roppenga R.; Daigle T. Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology 2005, 14, 193–222. [DOI] [PubMed] [Google Scholar]
- Kannan K.; Smith R. G. Jr; Lee R. F.; Windom H. L.; Heitmuller P.T.; Macauley J. M.; Summer J. K. Distribution of total mercury and methyl mercury in water, sediment, and fish from south Florida estuaries. Arch. Environ. Contam. Toxicol. 1998, 34, 109–118. [DOI] [PubMed] [Google Scholar]
- Sunderland E. M.; Gobas F. A. P. C.; Heyes A.; Branfireun B. A.; Bayer A. K.; Cranston R. E.; Parson M. B. Speciation and bioavailability of mercury in well-mixed estuarine sediments. Mar. Chem. 2004, 90, 91–105. [Google Scholar]
- Donovan P. M.; Blum J. D.; Yee D.; Gehrke G. E.; Singer M. B. An isotopic record of mercury in San Francisco Bay sediment. Chem. Geol. 2013, 349–350, 87–98. [Google Scholar]
- Foucher D.; Ogrinc N.; Hintelmann H. Tracing mercury contamination from the Idrija mining region (Slovenia) to the Gulf of Trieste using Hg isotope ratio. Environ. Sci. Technol. 2009, 43, 33–39. [DOI] [PubMed] [Google Scholar]
- Das R.; Bizimis M.; Wilson A. M. Tracing mercury seawater vs. atmospheric inputs in a pristine SE USA salt march system: Mercury isotope evidence. Chem. Geol. 2012, 336, 50–61. [Google Scholar]
- Gratz L. E.; Keeler G. J.; Blum J. D.; Sherman L. S. Isotopic composition and fractionation of mercury in Great Lakes precipitation and ambient air. Environ. Sci. Technol. 2010, 44, 7754–7770. [DOI] [PubMed] [Google Scholar]
- Mil-Homens M.; Blum J.; Canario J.; Caetano M.; Costa A. M.; Lebreiro S. M.; Trancoso M.; Richter T.; de Stigter H.; Johnson M.; Branco V.; Cesario R.; Mouro F.; Mateus M.; Boer W.; Melo Z. Tracing anthropogenic Hg and Pb input using stable Hg and Pb isotope ratios in sediments of the central Portuguese Margin. Chem. Geol. 2012, 336, 62–71. [Google Scholar]
- Gehrke G. E.; Blum J. D.; Meyers P. A. The geochemical behavior and isotopic composition of Hg in a mid-Pleistocene western Mediterranean sapropel. Geochim. Cosmochim. Acta 2009, 73, 1651–1665. [Google Scholar]
- Laffont L.; Sonke J. E.; Maurice L.; Monrroy S. L.; Chincheros J.; Amouroux D.; Behra P. Hg speciation and stable isotope signature in human hair as a tracer for dietary and occupational exposure to mercury. Environ. Sci. Technol. 2011, 45, 9910–9916. [DOI] [PubMed] [Google Scholar]
- Wayland M.; Garcia-Fernandez A. J.; Neugebauer E.; Gilchrist H. G. Concentrations of cadmium, mercury and selenium in blood, liver and kidney of common eider ducks from the Canadian arctic. Environ. Monit. Assess. 2001, 71, 255–267. [DOI] [PubMed] [Google Scholar]
- Mason R. P.; Sullivan K. A. Mercury in Lake Michigan. Environ. Sci. Technol. 1997, 31, 942–947. [Google Scholar]
- Watras C. J.; Bloom N. S. Mercury and methylmercury in individual zooplankton: Implications for bioaccumulation. Limol. Oceanogr. 1992, 37, 1313–1318. [Google Scholar]
- Hobson K. A. Trophic relationships among high Arctic seabirds: Insights from tissue-dependent stale-isotope models. Mar. Ecol.: Prog. Ser. 1993, 95, 7–18. [Google Scholar]
- Das R.; Salters V. J. M.; Odom A. L. A case for in vivo mass-independent fractionation of mercury isotopes in fish. Geochem. Geophys. Geosyst. 2009, 10, Q11012. [Google Scholar]
- Gantner N.; Hintelmann H.; Zheng W.; Muir D. C. Variations in stable isotope fractionation of Hg in food webs of Arctic lakes. Environ. Sci. Technol. 2009, 43, 9148–9154. [DOI] [PubMed] [Google Scholar]
- Perrot V.; Pastukhov M. V.; Epov V. N.; Husted S.; Donard O. F. X.; Amouroux D. Higher mass-independent isotope fractionation of methylmercury in the pelagic food web of Lake Baikal (Russia). Environ. Sci. Technol. 2012, 46, 5902–5911. [DOI] [PubMed] [Google Scholar]
- Day R. D.; Roseneau D. G.; Berail S.; Hobson K. A.; Donard O. F. X.; Vander Pol S. S.; Pugh R. S.; Moors A. J.; Long S. E.; Becker P. R. Mercury stable isotopes in seabird eggs reflect a gradient from terrestrial geogenic to oceanic mercury reservoirs. Environ. Sci. Technol. 2012, 46, 5327–5335. [DOI] [PubMed] [Google Scholar]
- Point D.; Sonke J. E.; Day R. D.; Roseneau D. G.; Hobson K. A.; Vander Pol S. S.; Moors A. J.; Pugh R. S.; Donard O. F. X.; Becker P. R. Methylmercury photodegradation influenced by sea-ice cover in Arctic marine ecosystems. Nat. Geosci. 2011, 4, 188–194. [Google Scholar]
- Sherman L. S.; Blum J. D. Mercury stable isotopes in sediments and largemouth bass from Florida lakes, USA. Sci. Total Environ. 2013, 448, 163–175. [DOI] [PubMed] [Google Scholar]
- Schartup A. T.; Mason R. P.; Balcom P. H.; Hollweg T. A.; Chen C. Y. Methylmercury production in estuarine sediments: Role of organic matter. Environ. Sci. Technol. 2012,, 47, 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis I. F.; Clair T. A.; Driscoll C. T.; Kamman N.; Chalmercs A.; Shanley J.; Norton S. A.; Kahl S. Distribution patterns of mercury in lakes and rivers of northeastern North America. Ecotoxicol. 2005, 14, 113–123. [DOI] [PubMed] [Google Scholar]
- Guillemette M.; Ydenberg R. C.; Himmelman J. H. The role of energy intake rate in prey and habitat selection of common eiders Somateria mollissima in winter: A risk-sensitive interpretation. J. Anim. Ecol. 1992, 61, 599–610. [Google Scholar]
- Merkel F. R.; Mosbech A.; Sonne C.; Flagstad A.; Falk K.; Jamieson S. E. Local movements, home ranges and body condition of Common Eider Somateria mollissima wintering in southwest Greenland. Ardea 2006, 94, 639–650. [Google Scholar]
- Eagles-Smith C. A.; Ackerman J. T.; Yee J.; Adelsbach T. L. Mercury demethylation in waterbird livers: Dose-response thresholds and differences among species. Environ. Toxicol. Chem. 2009, 28, 568–577. [DOI] [PubMed] [Google Scholar]
- Kim E. Y.; Murakami T.; Saeki K.; Tatsukawa R. Mercury levels and its chemical form in tissues and organs of seabirds. Arch. Environ. Contam. Toxicol. 1996, 30, 259–266. [Google Scholar]
- Li M.; Sherman L. S.; Blum J. D.; Granjean P.; Mikkelsen B.; Sunderland E. C.; Weihe P.; Shine J. Assessing sources of human methylmercury exposure using stable mercury isotopes. Environ. Sci. Technol. 2014, 10.1021/es500340r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. S.; Blum J. D.; Franzblau A.; Basu N. New insight into biomarker of human mercury exposure using naturally occurring mercury stable isotopes. Environ. Sci. Technol. 2013, 47, 3403–3409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


