Abstract
Context:
The ingestion of carbohydrate (CHO) before and during exercise and at halftime is commonly recommended to soccer players for maintaining blood glucose concentrations throughout match play. However, an exercise-induced rebound glycemic response has been observed in the early stages of the second half of simulated soccer-specific exercise when CHO-electrolyte beverages were consumed regularly. Therefore, the metabolic effects of CHO beverage consumption throughout soccer match play remain unclear.
Objective:
To investigate the blood glucose and blood lactate responses to CHOs ingested before and during soccer match play.
Design:
Crossover study.
Setting:
Applied research study.
Patients or Other Participants:
Ten male outfield academy soccer players (age = 15.6 ± 0.2 years, height = 1.74 ± 0.02 m, mass = 65.3 ± 1.9 kg, estimated maximal oxygen consumption = 58.4 ± 0.8 mL·kg−1·min−1).
Intervention(s):
Players received a 6% CHO-electrolyte solution or an electrolyte (placebo) solution 2 hours before kickoff, before each half (within 10 minutes), and every 15 minutes throughout exercise. Blood samples were obtained at rest, every 15 minutes during the match (first half: 0–15, 15–30, and 30–45 minutes; second half: 45–60, 60–75, and 75–90 minutes) and 10 minutes into the halftime break.
Main Outcome Measure(s):
Metabolic responses (blood glucose and blood lactate concentrations) and markers of exercise intensity (heart rate) were recorded.
Results:
Supplementation influenced the blood glucose response to exercise (time × treatment interaction effect: P ≤ .05), such that glucose concentrations were higher at 30 to 45 minutes in the CHO than in the placebo condition. However, in the second half, blood glucose concentrations were similar between conditions because of transient reductions from peak values occurring in both trials at halftime. Blood lactate concentrations were elevated above those at rest in the first 15 minutes of exercise (time-of-sample effect: P < .001) and remained elevated throughout exercise. Supplementation did not influence the pattern of response (time × treatment interaction effect: P = .49).
Conclusions:
Ingestion of a 6% CHO-electrolyte beverage before and during soccer match play did not benefit blood glucose concentrations throughout the second half of exercise.
Key Words: supplementation, sports drinks, football, intermittent exercise, rebound hypoglycemia
Key Points
A 6% carbohydrate-electrolyte beverage ingested before and during soccer match play did not maintain blood glucose concentrations in the second half of match play.
The efficacy of carbohydrate-fluid supplementation regimes that are recommended for high-intensity intermittent team sports players can be improved, and more effective carbohydrate-supplementation strategies can be developed to maintain blood glucose concentrations throughout soccer match play.
Soccer is a high-intensity intermittent team sport played over 90 minutes, with two 45-minute halves separated by a halftime recovery period. In adult players, researchers have reported that over the duration of a match, muscle glycogen can become compromised in specific muscle fibers1 and blood glucose concentrations can fall to levels that might impair cognitive function.2 Consequently, players often are encouraged to consume carbohydrates (CHOs) throughout play to spare muscle glycogen and maintain blood glucose concentrations for the duration of the match. The effects of CHO supplementation on the metabolic responses to simulated and actual soccer match play have been reviewed.3–7 Investigators6–8 generally have suggested that blood glucose concentrations are maintained better in the latter stages of exercise when a CHO supplement is consumed. However, as highlighted in a review of the topic,3 methodologic concerns relating to the exercise protocol (eg, the use of simulated or actual match play, inclusion or omission of skilled actions throughout exercise, or presence of a halftime period) and the fact that few blood samples are taken during soccer-specific exercise mean that the pattern of blood glucose response during soccer match play is unclear.
Consuming a high–glycemic-index CHO in the hour before exercise can lower blood glucose levels 15 to 30 minutes after starting exercise.9–11 Free fatty acid inhibition is a likely contributor to increased CHO use during an isolated bout of exercise completed soon after CHO ingestion.9–11 However, the glycemic response to successive exercise bouts that are separated by a period of recovery has received little attention. This is somewhat surprising, as this pattern of activity reflects the demands of several sports (eg, tennis, boxing), especially team sports with halftime breaks separating consecutive periods of play.
Halftime often is considered crucial in team sports for primarily tactical reasons; however, this break also can be viewed as a recovery period after the first half, a period of preparation before the second half, an opportunity to provide exogenous fluids, and a transition between the 2 halves of play.12 Despite the potential for a period of recovery between 2 bouts of exercise to influence selected physiologic responses, such as indices of acid-base balance5,13 and blood glucose concentrations,5 researchers14,15 have not commonly included halftime recovery periods during exercise simulations that replicate the demands of high-intensity intermittent sports, such as soccer. Therefore, the validity of studies aiming to replicate the demands of team sports might be improved by including a halftime break.16
Russell et al17 reported that blood glucose concentrations decreased sharply with the onset of a soccer-specific exercise protocol after a halftime recovery period while players routinely ingested a CHO-free fluid-electrolyte beverage. Although the mechanisms causing this response are unclear, increased glucose uptake due to the translocation of GLUT-4 transporters to the muscle membrane probably caused the rate of disappearance of blood glucose to exceed the rate of appearance; the net effect was a transient decrease in blood glucose concentration (exercise-induced rebound glycemic response) at the onset of exercise with the start of the second half.5 The consequences of a rapid reduction in blood glucose concentration remain unclear; however, given that CHO ingestion has been found to improve indices of skilled performance,18 such a response could negatively affect the skilled actions involved in soccer match play.
In summary, compromising glucose availability has the potential to negatively influence the motor skills and cognitive function that are vital for optimal performance in intermittent sports, such as soccer. Although soccer players often are encouraged to consume CHO-electrolyte solutions, CHO supplementation recently has been reported to result in a transient lowering of blood glucose concentrations throughout the duration of a match while players completed a soccer-specific exercise protocol.18 However, data with sufficient resolution are not available to establish the existence of this phenomenon during soccer match play. Therefore, the purpose of our study was to investigate metabolic responses to CHO ingestion before and during soccer match play.
METHODS
Participants
Ten male soccer players (age = 15.6 ± 0.2 years, height = 1.74 ± 0.02 m, mass = 65.3 ± 1.9 kg, estimated maximal oxygen consumption = 58.4 ± 0.8 mL·kg−1·min−1) from a British Championship club representing the second tier of professional soccer within the United Kingdom volunteered and completed all study requirements. After approval by the Swansea University Ethics Committee, the players were informed about the potential risks of the study and provided written informed consent; parental consent was obtained for players who were less than 18 years of age. We recruited players on the basis that they had no injuries, did not have diabetes, and had participated in training with a soccer team of academy standard for at least 12 months before the start of the study.
Experimental Design
Players attended a preliminary session during which the procedures of the main trials were explained and maximal oxygen uptake was estimated using the multistage fitness test.19 Two main trials (CHO and placebo [PL]) that were separated by 1 week were completed in a counterbalanced, randomized, double-blind, crossover fashion. Players were instructed to refrain from strenuous physical activity and caffeine consumption in the 2 days preceding all testing sessions. Additionally, players recorded all food consumed in the 2 days before each main trial. Food records subsequently were analyzed using commercially available software (CompEat version 5.8.0; Nutrition Systems, Colsterworth, Grantham, United Kingdom). All players gave oral confirmation that they had complied with these instructions upon completion of the study.
Main Trial Procedures
On arrival at the laboratory at 5:00 pm, the players emptied their bowels and provided a midflow urine sample. Urine osmolality subsequently was measured by freezing-point depression (model Cryoscopic Osmometer Osmomat 030; Gonotec GmbH, Berlin, Germany; intra-assay coefficient of variance = 0.2%), with a threshold of greater than 900 mOsmol·kg−1 H2O used to indicate hypohydration.20,21 A resting blood sample was taken at 5:30 pm before players consumed a standardized 1470-kJ meal (energy content = 62% CHOs, 25% fats, 13% proteins) and 500 mL of the treatment beverage. Body mass (model 770; Seca Ltd, Birmingham, United Kingdom) and stature (Portable Stadiometer; Holtain Ltd, Wales, United Kingdom) were measured. In agreement with the participants' usual practices, players remained in a rested state for approximately 90 minutes, which is a period wherein researchers have observed changes in blood glucose concentrations in response to the ingestion of a similar CHO-containing beverage.18 Thereafter, a 30-minute standardized warm-up consisting of high-intensity running, dynamic stretching, and ball skills preceded the match kickoff at 7:30 pm. The main trial procedures are illustrated in Figure 1.
Figure 1.
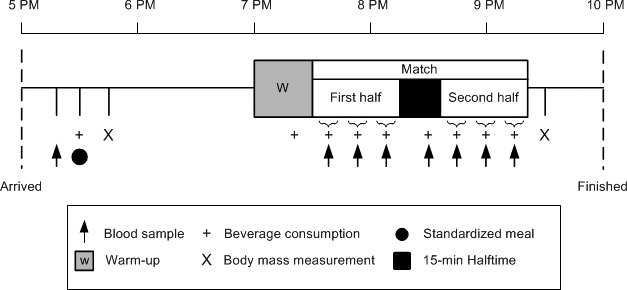
Schematic diagram of the main trial procedures.
Soccer Match
Two soccer matches between an academy team (test team) and an opposing team similar in playing standard were arranged for the purpose of this study. Each match lasted 90 minutes and consisted of two 45-minute halves that were separated by a 15-minute passive recovery period (halftime). Players from both teams and the referee were consistent in both main trials, and players also maintained the same positional role between games. Matches were played on a floodlit grass pitch measuring 95 × 68 m that conformed to Football Association regulations, and official balls (Total 90 Aerow; size 5; Nike Inc, Beaverton, OR) were used.
The outfield players of the test team (N = 10) were subject to periodic blood sampling and left the pitch on 6 occasions during match play (ie, once every 15 minutes). To ensure minimal disruption to the match, players were removed individually and in an order that remained consistent among time points and between matches. Matches were divided into six 15-minute periods (first half: 0–15, 15–30, and 30–45 minutes; second half: 45–60, 60–75, and 75–90 minutes). Two minutes into each of these periods, the first player left the pitch for blood sampling (ie, after 2, 17, 32, 47, 62, and 77 minutes of match play). Players were removed from match play for 1.5 ± 0.1 minutes each time a blood sample was taken. When the first player returned to the match, the second player left the pitch; this sequence continued until the 10 players had been sampled within each 15-minute period. To maintain the standard number of players on the pitch at all times, 1 utility player, who was deemed by coaching staff as able to fulfill all positional demands, served as a substitute. The substitute changed positions as different players left the pitch for blood sampling. He was not subject to any analyses and did not play for the first 2 minutes of each 15-minute period of the match.
Heart rate (HR) was recorded continuously throughout each match using Team Polar transmitters (Polar Electro Oy, Kempele, Finland), and values were categorized into 4 HR zones (HR zone 1 < 70% maximal HR [HRmax], HR zone 2 = 70%–79% HRmax, HR zone 3 = 80%–89% HRmax, and HR zone 4 = 90%–100% HRmax) to represent the proportion of match play spent at each relative exercise intensity. Environmental conditions were measured at kickoff (model ETHG-912 thermo hygrometer; Oregon Scientific, Tualatin, OR).
Experimental Beverages
Participants consumed an initial bolus of the treatment beverage (500 mL) at 5:30 pm with the standardized meal. Thereafter, they consumed fluid at a rate of 14 mL·kg−1·h−1 of body mass, with equal volumes consumed within 10 minutes of commencing each half and on each occasion that the players left the pitch for blood sampling during match play. During the CHO trial, a CHO-electrolyte beverage that contained 6% sucrose, 23 mmol·L−1 sodium, and 14 mmol·L−1 chloride was consumed. The PL beverage was equimolar in terms of electrolytes but void of CHO. Sweetness of the PL beverage was provided by an artificial energy-free sodium-saccharin–based sweetener (Sweetex; Reckitt Benckiser Ltd, Swindon, United Kingdom). Both the CHO-electrolyte and PL beverages were flavored with a commercially available fruit cordial (CHOs < 0.15 g/L). The drinks were indistinguishable by taste and texture and consumed from identical containers. After completion of the second main trial, the players reported that they were unable to distinguish between the experimental beverages. Other than the standardized meal and the experimental beverages consumed, no further sources of CHOs were ingested throughout the trials.
Blood Sampling and Analyses
Fingertip blood samples were taken at rest, 10 minutes into halftime, and every 15 minutes during match play (first half: 0–15, 15–30, and 30–45 minutes; second half: 45–60, 60–75, and 75–90 minutes). Glucose concentrations were analyzed immediately from whole blood (Medisense Optium Xceed glucose monitor; Abbott Laboratories, Abbott Park, IL). Blood lactate concentrations were analyzed using a portable microvolume lactate analyzer (Lactate-Pro; Arkray, Inc, Kyoto City, Nakagyo-ward, Japan). Before both trials, the analyzers were calibrated in accordance with the manufacturers' guidelines. The interassay coefficients of variance for blood glucose and lactate concentrations were 2.6% and 4.8%, respectively.
Statistical Analyses
Paired-samples t tests were used to compare environmental conditions, nutritional intake, urine osmolality, and HR variables. Two-way repeated-measures analyses of variance with the within-subjects factors of treatment × time of sample were used when the data contained multiple time points during each main trial. We consulted the Mauchly test and applied the Greenhouse-Geisser correction if the assumption of sphericity was violated. If we identified an interaction effect (time × treatment), CHO supplementation was deemed to have influenced the exercise response, and simple main-effect analyses were performed. Main effects of time (time of sample) were investigated further using multiple pairwise comparisons with Bonferroni confidence interval adjustments. Statistical analysis was carried out using SPSS (version 16.0; SPSS, Inc, Chicago, IL). All data were reported as the mean ± SEM. The α level was set at ≤ .05.
RESULTS
Environmental conditions were similar during both trials (ambient temperature = 5.5°C ± 0.1°C, barometric pressure = 761 ± 1 mm Hg, humidity = 64% ± 4%). Urine osmolality analysis indicated that players were euhydrated on arrival for the CHO (570 ± 140 mOsmol·kg−1 H2O) and PL (420 ± 130 mOsmol·kg−1 H2O) trials (P = .35). The calculated daily diet comprised 11.6 ± 0.6 MJ·d−1 (2761 ± 152 kcal·d−1) of which 53% ± 2%, 31% ± 2%, and 16% ± 1% of energy intake was obtained from CHOs, fats, and proteins, respectively.
Physiologic Demand and Exercise Intensity
Mean and peak HR values were not influenced by supplementation, and no differences existed between trials for the percentage of time spent in each HR zone during match play (Table). The mean volume of fluid ingested during each trial was 1874 ± 40 mL (0.47 ± 0.01 L·h−1), and average mass losses over the duration of the trials were similar between the CHO (1.2 ± 0.1 kg) and PL (1.0 ± 0.1 kg) conditions (P = .29).
Table.
Heart-Rate (HR) Responses to Soccer Match Play During the Carbohydrate and Placebo Trials
| Trial |
|||
| Heart-Rate Response |
Carbohydrate |
Placebo |
P Valuea |
| Peak HR, beats/min | 196 ± 3 | 193 ± 2 | .15 |
| Mean HR, beats/min | 158 ± 3 | 157 ± 2 | .64 |
| HR zone 1, %b | 12 ± 2 | 13 ± 4 | .66 |
| HR zone 2, %b | 25 ± 4 | 28 ± 6 | .14 |
| HR zone 3, %b | 47 ± 4 | 45 ± 8 | .53 |
| HR zone 4, %b | 17 ± 5 | 14 ± 6 | .56 |
P value from paired-samples t test.
Heart-rate zone values present the percentage of match play spent in each HR zone. Heart-rate zones were derived from age-predicted maximal HR values, where HR zone 1 < 70% maximal HR, HR zone 2 = 70%–79% maximal HR, HR zone 3 = 80%–89% maximal HR, and HR zone 4 = 90%–100% maximal HR.
Blood Metabolites
Supplementation influenced the pattern of response in blood glucose concentrations (time × treatment interaction effect: F7,63 = 2.182, P = .048). Carbohydrate ingestion elevated blood glucose concentration by 30% ± 12% above rest at the end of the first half (30–45 minutes), with differences between CHO (123.42 ± 7.57 mg·dL−1 [6.85 ± 0.42 mmol·L−1]) and PL (97.12 ± 10.63 mg·dL−1 [5.39 ± 0.59 mmol·L−1]) conditions evident at this time point (P = .03; Figure 2). However, compared with peak values, reductions in blood glucose concentrations were observed in both CHO (P = .001) and PL (P = .007) trials at halftime. From halftime onward, blood glucose concentrations were similar between conditions (P > .05).
Figure 2.
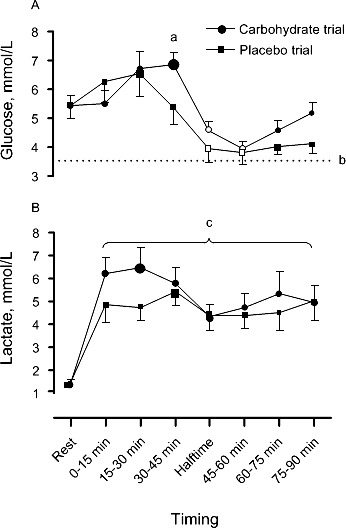
A, Blood glucose concentrations and B, blood lactate concentrations during the carbohydrate and placebo trials. The large data point indicates peak value, and the hollow data point indicates the within-trials difference (P ≤ .05) from peak value. a Indicates a difference between trials at the corresponding time point (P ≤ .05). b Broken horizontal line represents the hypoglycemic threshold (63.06 mg/dL [3.5 mmol/L]). c Indicates a time effect relative to rest (P ≤ .05).
Blood lactate concentrations were elevated above rest during the first 15 minutes of exercise (time-of-sample effect: F7,63 = 9.748, P < .001; Figure 2) and remained elevated throughout exercise. Supplementation did not influence the pattern of response (time × treatment interaction effect: F7,63 = 0.934, P = .49; Figure 2). In the final 15 minutes of the match, lactate concentrations were 45.05 ± 6.31 mg·dL−1 (5.0 ± 0.7 mmol·L−1).
DISCUSSION
The provision of exogenous CHOs before and during 90 minutes of soccer match play in the form of a 6% CHO–electrolyte solution initially elevated blood glucose concentration at the end of the first half of exercise. However, a passive halftime period caused reductions in blood glucose concentrations of approximately 30% in both trials. Consequently, from halftime onward, blood glucose concentrations were similar between conditions for the remainder of match play. We believe that we are the first to document a transient lowering of blood glucose concentrations while players were routinely ingesting a CHO–electrolyte beverage during competitive play of a team sport, such as soccer.
The metabolic responses to the ingestion of CHOs differ according to whether they are consumed during exercise or resting states.22 This is especially true for the actions of insulin. For example, in nonexercising conditions, the normal physiologic response to ingesting CHOs that increase blood glucose concentrations is an upregulation in the synthesis and secretion of insulin within the beta cells of the islets of Langerhans. Insulin causes decreased lipolysis and increased glucose uptake in liver, skeletal muscle, and fat cells. Conversely, counterregulatory hormones, including cortisol, growth hormone, and catecholamines, are stimulated during high-intensity exercise and exert hyperglycemic responses.22 Given that CHOs were consumed before and during the first half of match play in periods that incorporated high-intensity actions (ie, the warm-up and the first half of exercise), it is plausible that the effects of epinephrine, which stimulates glycogenolysis and increased liver glucose output and directly inhibits insulin release,23 explain the elevated blood glucose concentrations observed in the CHO trial until 30 to 45 minutes.
Transient reductions in blood glucose concentrations occurred in both trials during the 15-minute passive halftime period. Researchers5 have speculated that an increased glucose uptake by the previously active muscles, lowered catecholamine concentrations, and reduced stimulation of liver glycogenolysis can cause a transient reduction in blood glucose concentrations at the onset of the second half. Although logistical issues associated with the modality of exercise used in our study prevented the measurement of specific hormones, similarities in the physiologic responses to both matches (ie, HR and blood lactate concentrations) allow speculation that locally mediated factors responsible for the insulin-independent uptake of glucose were also similar between trials. Consequently, based on our data, we question the efficacy of strategies that advocate the consumption of a 6% CHO-electrolyte beverage before kickoff, during match play, and throughout a passive halftime period to maintain elevated blood glucose concentrations throughout a match. These findings are supported by those of Bangsbo et al.5 They presented preliminary data from 3 soccer players that demonstrated reduced blood glucose concentrations in the initial stages of the second half of soccer-specific exercise; however, they did not report the fluid-ingestion strategies of these players.5
The effects of insulin and counterregulatory hormones on the glycemic response to intermittent match play after a halftime break (passive recovery) have not previously been reported. We are not aware of any other investigators who have identified the pattern of blood glucose response that we observed when CHOs were consumed before and during competitive match play of a team sport, such as soccer. Using a similar fluid-ingestion pattern, Russell et al18 identified that reduced blood glucose concentrations, negating the benefit of CHO supplementation, occurred at 60 minutes in simulated match play. Although the explanation for this glycemic response in our work may relate to the frequency of blood samples taken (ie, every 15 minutes during match play and at halftime) or the inclusion of a halftime period, we do not know why a lack of agreement exists in the timing of this response between actual (ie, halftime) and simulated (ie, 60 minutes) match play. Methodologic differences in the timing of blood samples between study protocols may afford an explanation. Moreover, given that energy expenditure has not been compared between simulated and actual match play, the glycemic response to soccer-specific exercise may be influenced if the energy demands of the 2 modes of exercise differ; however, this has not been confirmed.
The consumption of a high–glycemic-index CHO within the hour of an isolated bout of exercise can result in blood glucose responses that reach hypoglycemic levels (63.06 mg·dL−1 [3.5 mmol·L−1]) in the first 15 to 30 minutes of exercise.9–11 Although rebound hypoglycemia has not been specifically reported to occur during the onset of high-intensity intermittent sports, consuming a high–glycemic-index CHO within 30 minutes of starting this type of activity is likely to elicit a similar glycemic response, as rebound hypoglycemia does not depend on exercise intensity if a prolonged period of recovery separates CHO ingestion and the start of exercise.10 Whereas the minimal mean blood glucose concentrations that we observed (ie, 70.99 ± 4.86 mg·dL−1 [3.94 ± 0.27 mmol·L−1]) exceeded the 63.06 mg·dL−1 (3.5 mmol·L−1) threshold previously defined as hypoglycemic24 (Figure 2), analysis of individual data identified that 30% of the participants sampled at 45 to 60 minutes of match play experienced blood glucose concentrations less than 54.05 mg·dL−1 (3.0 mmol·L−1) in the CHO trial. Supporting these data and using a protocol designed to elicit rebound hypoglycemia, Moseley et al11 observed considerable individuality in the hypoglycemic response even though the average plasma glucose concentrations of the 8 participants remained above the hypoglycemic threshold. Mechanisms causing transient reductions in blood glucose responses remain to be established, but researchers11 have speculated that the incidence of hypoglycemia is increased in some individuals according to a critical threshold of insulin exposure.
No convincing evidence has suggested that transient reductions in blood glucose concentrations are associated with reduced performance.25 However, the effects of rebound hypoglycemia primarily have been investigated from a physical perspective (eg, time-trial performance).10,11 The principal energy source for cerebral metabolism is blood glucose.26 Boyle et al27 proposed that cerebral glucose uptake begins to decline when blood glucose concentration falls below 64.86 mg·dL−1 (3.6 mmol·L−1). Given the beneficial effects that have been demonstrated in skilled performances executed during the latter stages of simulated soccer match play after the ingestion of CHO–containing beverages,18 it is plausible to speculate that skilled performers may be negatively affected by transient reductions in blood glucose concentrations. Moreover, Bandelow et al28 demonstrated that higher blood glucose concentrations were associated with faster visual discrimination, fine-motor speed, and psychomotor speed after soccer match play in the heat. Nevertheless, the relationship between blood glucose concentrations and skill performance during competitive soccer match play remains to be determined.
The final match scores reflected the competitive nature of both matches, with 1 goal separating the teams on both occasions. Average HR and blood lactate responses were comparable with previously published values for soccer match play,1 and the proportion of time spent in the relative exercise intensities (HR zones 1–4) also agreed with previously reported values.29 However, lactate concentrations collected during soccer match play largely represented the pattern of activity in the immediate presampling period,4 and thus such data should be interpreted with caution. Nevertheless, given that our supplementation regime was similar to published nutritional guidelines on fluid replacement,30 our findings suggested that current CHO–fluid-replacement strategies do not increase blood glucose concentrations in the second half of soccer match play when the halftime recovery period is passive.
CONCLUSIONS
A 6% CHO-electrolyte beverage ingested 2 hours before and throughout soccer match play initially elevated blood glucose concentrations relative to ingestion of PL throughout the first half of exercise. However, a sharp decline in blood glucose concentrations occurred in both trials during the passive rest period, and blood glucose concentrations were similar between conditions throughout the second half. These findings suggest that the efficacy of CHO–fluid-supplementation regimes that are recommended for high-intensity intermittent-activity sports teams could be improved. Therefore, more effective CHO-supplementation strategies could be developed to maintain elevated blood glucose concentrations throughout the full duration of soccer match play.
ACKNOWLEDGMENTS
We thank Gary Richards and Anthony Pennock of Swansea City Football Club for their cooperation throughout the study.
REFERENCES
- 1.Krustrup P, Mohr M, Steensberg A, Bencke J, Kjaer M, Bangsbo J. Muscle and blood metabolites during a soccer game: implications for sprint performance. Med Sci Sports Exerc. 2006;38(6):1165–1174. doi: 10.1249/01.mss.0000222845.89262.cd. [DOI] [PubMed] [Google Scholar]
- 2.Shephard RJ, Leatt P. Carbohydrate and fluid needs of the soccer player. Sports Med. 1987;4(3):164–176. doi: 10.2165/00007256-198704030-00002. [DOI] [PubMed] [Google Scholar]
- 3.Russell M, Kingsley M. Influence of exercise on skill proficiency in soccer. Sports Med. 2011;41(7):523–539. doi: 10.2165/11589130-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Bangsbo J. The physiology of soccer: with special reference to intense intermittent exercise. Acta Physiol Scand Suppl. 1994;619:1–155. [PubMed] [Google Scholar]
- 5.Bangsbo J, Iaia FM, Krustrup P. Metabolic response and fatigue in soccer. Int J Sports Physiol Perform. 2007;2(2):111–127. doi: 10.1123/ijspp.2.2.111. [DOI] [PubMed] [Google Scholar]
- 6.Coyle EF. Fluid and fuel intake during exercise. J Sports Sci. 2004;22(1):39–55. doi: 10.1080/0264041031000140545. [DOI] [PubMed] [Google Scholar]
- 7.Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American College of Sports Medicine position stand: exercise and fluid replacement. Med Sci Sports Exerc. 2007;39(2):377–390. doi: 10.1249/mss.0b013e31802ca597. [DOI] [PubMed] [Google Scholar]
- 8.Coyle EF, Montain SJ. Carbohydrate and fluid ingestion during exercise: are there trade-offs? Med Sci Sports Exerc. 1992;24(6):671–678. [PubMed] [Google Scholar]
- 9.Costill DL, Coyle E, Dalsky G, Evans W, Fink W, Hoopes D. Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. J Appl Physiol. 1977;43(4):695–699. doi: 10.1152/jappl.1977.43.4.695. [DOI] [PubMed] [Google Scholar]
- 10.Achten J, Jeukendrup AE. Effects of pre-exercise ingestion of carbohydrate on glycaemic and insulinaemic responses during subsequent exercise at differing intensities. Eur J Appl Physiol. 2003;88((4–5)):466–471. doi: 10.1007/s00421-002-0730-1. [DOI] [PubMed] [Google Scholar]
- 11.Moseley L, Lancaster GI, Jeukendrup AE. Effects of timing of pre-exercise ingestion of carbohydrate on subsequent metabolism and cycling performance. Eur J Appl Physiol. 2003;88((4–5)):453–458. doi: 10.1007/s00421-002-0728-8. [DOI] [PubMed] [Google Scholar]
- 12.Sugiura K, Kobayashi K. Effect of carbohydrate ingestion on sprint performance following continuous and intermittent exercise. Med Sci Sports Exerc. 1998;30(11):1624–1630. doi: 10.1097/00005768-199811000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Russell M, Kingsley MI. Changes in acid-base balance during simulated soccer match play. J Strength Cond Res. 2012;26(9):2593–2599. doi: 10.1519/JSC.0b013e31823f284e. [DOI] [PubMed] [Google Scholar]
- 14.Nicholas CW, Williams C, Lakomy HK, Phillips G, Nowitz A. Influence of ingesting a carbohydrate-electrolyte solution on endurance capacity during intermittent, high-intensity shuttle running. J Sports Sci. 1995;13(4):283–290. doi: 10.1080/02640419508732241. [DOI] [PubMed] [Google Scholar]
- 15.Drust B, Reilly T, Cable NT. Physiological responses to laboratory-based soccer-specific intermittent and continuous exercise. J Sports Sci. 2000;18(11):885–892. doi: 10.1080/026404100750017814. [DOI] [PubMed] [Google Scholar]
- 16.Kingsley MI, Wadsworth D, Kilduff LP, McEneny J, Benton D. Effects of phosphatidylserine on oxidative stress following intermittent running. Med Sci Sports Exerc. 2005;37(8):1300–1306. doi: 10.1249/01.mss.0000175306.05465.7e. [DOI] [PubMed] [Google Scholar]
- 17.Russell M, Rees G, Benton D, Kingsley M. An exercise protocol that replicates soccer match-play. Int J Sports Med. 2011;32(7):511–518. doi: 10.1055/s-0031-1273742. [DOI] [PubMed] [Google Scholar]
- 18.Russell M, Benton D, Kingsley M. Influence of carbohydrate supplementation on skill performance during a soccer simulation. J Sci Med Sport. 2012;15(4):348–354. doi: 10.1016/j.jsams.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Ramsbottom R, Brewer J, Williams C. A progressive shuttle run test to estimate maximal oxygen uptake. Br J Sports Med. 1988;22(4):141–144. doi: 10.1136/bjsm.22.4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirreffs SM, Maughan RJ. Urine osmolality and conductivity as indices of hydration status in athletes in the heat. Med Sci Sports Exerc. 1998;30(11):1598–1602. doi: 10.1097/00005768-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Maughan RJ, Shirreffs SM. Development of individual hydration strategies for athletes. Int J Sport Nutr Exerc Metab. 2008;18(5):457–472. doi: 10.1123/ijsnem.18.5.457. [DOI] [PubMed] [Google Scholar]
- 22.Astrand PO, Rodahl K. Textbook of Work Physiology: Physiological Bases of Exercise. 3rd ed. New York, NY: McGraw Hill; 1986. [Google Scholar]
- 23.Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43(5):533–549. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- 24.DeMarco HM, Sucher KP, Cisar CJ, Butterfield GE. Pre-exercise carbohydrate meals: application of glycemic index. Med Sci Sports Exerc. 1999;31(1):164–170. doi: 10.1097/00005768-199901000-00025. [DOI] [PubMed] [Google Scholar]
- 25.Hargreaves M, Hawley JA, Jeukendrup A. Pre–exercise carbohydrate and fat ingestion: effects on metabolism and performance. J Sports Sci. 2004;22(1):31–38. doi: 10.1080/0264041031000140536. [DOI] [PubMed] [Google Scholar]
- 26.Duelli R, Kuschinsky W. Brain glucose transporters: relationship to local energy demand. News Physiol Sci. 2001;16:71–76. doi: 10.1152/physiologyonline.2001.16.2.71. [DOI] [PubMed] [Google Scholar]
- 27.Boyle PJ, Nagy RJ, O'Connor AM, Kempers SF, Yeo RA, Qualls C. Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc Natl Acad Sci U S A. 1994;91(20):9352–9356. doi: 10.1073/pnas.91.20.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandelow S, Maughan R, Shirreffs S, et al. The effects of exercise, heat, cooling and rehydration strategies on cognitive function in football players. Scand J Med Sci Sports. 2010;20((suppl 3)):148–160. doi: 10.1111/j.1600-0838.2010.01220.x. [DOI] [PubMed] [Google Scholar]
- 29.Tauler P, Ferrer MD, Sureda A, et al. Supplementation with an antioxidant cocktail containing coenzyme Q prevents plasma oxidative damage induced by soccer. Eur J Appl Physiol. 2008;104(5):777–785. doi: 10.1007/s00421-008-0831-6. [DOI] [PubMed] [Google Scholar]
- 30.Convertino VA, Armstrong LE, Coyle EF, et al. American College of Sports Medicine position stand: exercise and fluid replacement. Med Sci Sports Exerc. 1996;28(1):i–vii. doi: 10.1097/00005768-199610000-00045. [DOI] [PubMed] [Google Scholar]


