Abstract
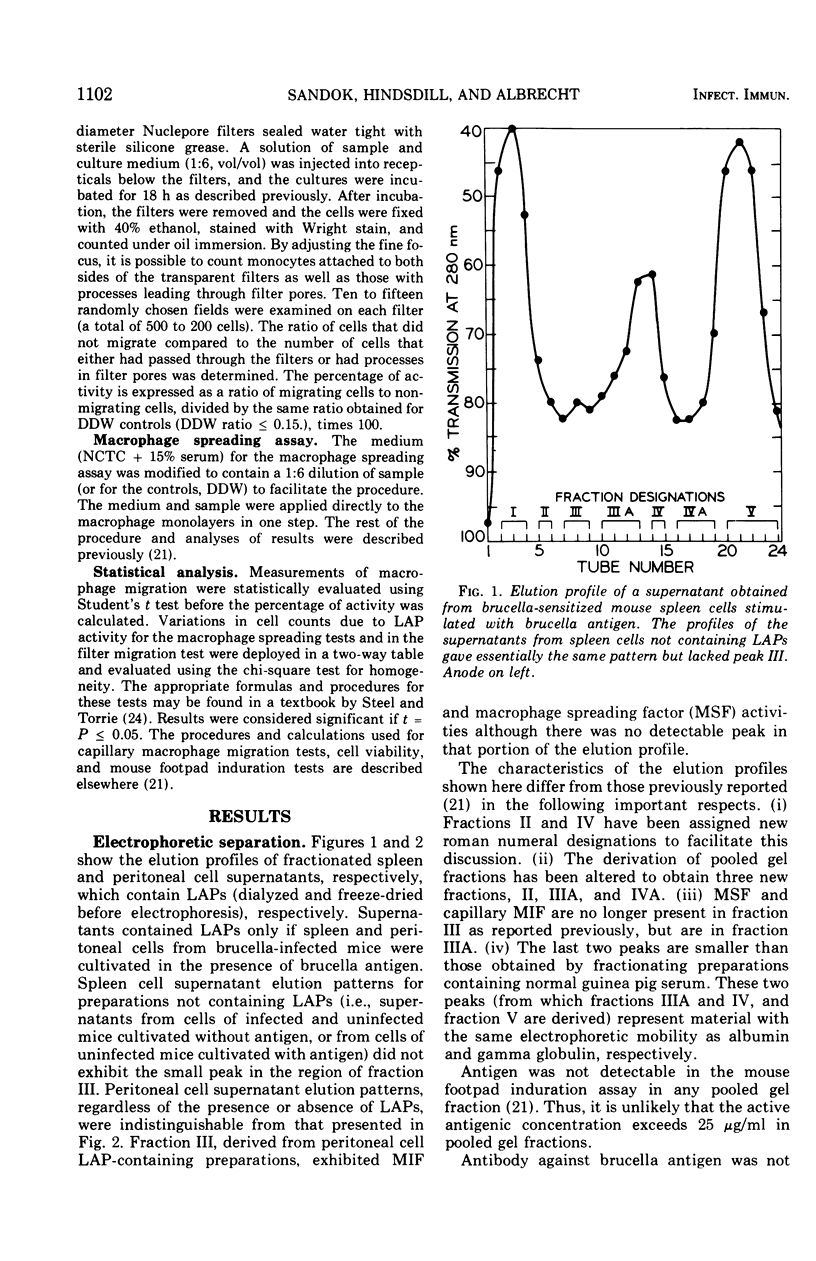
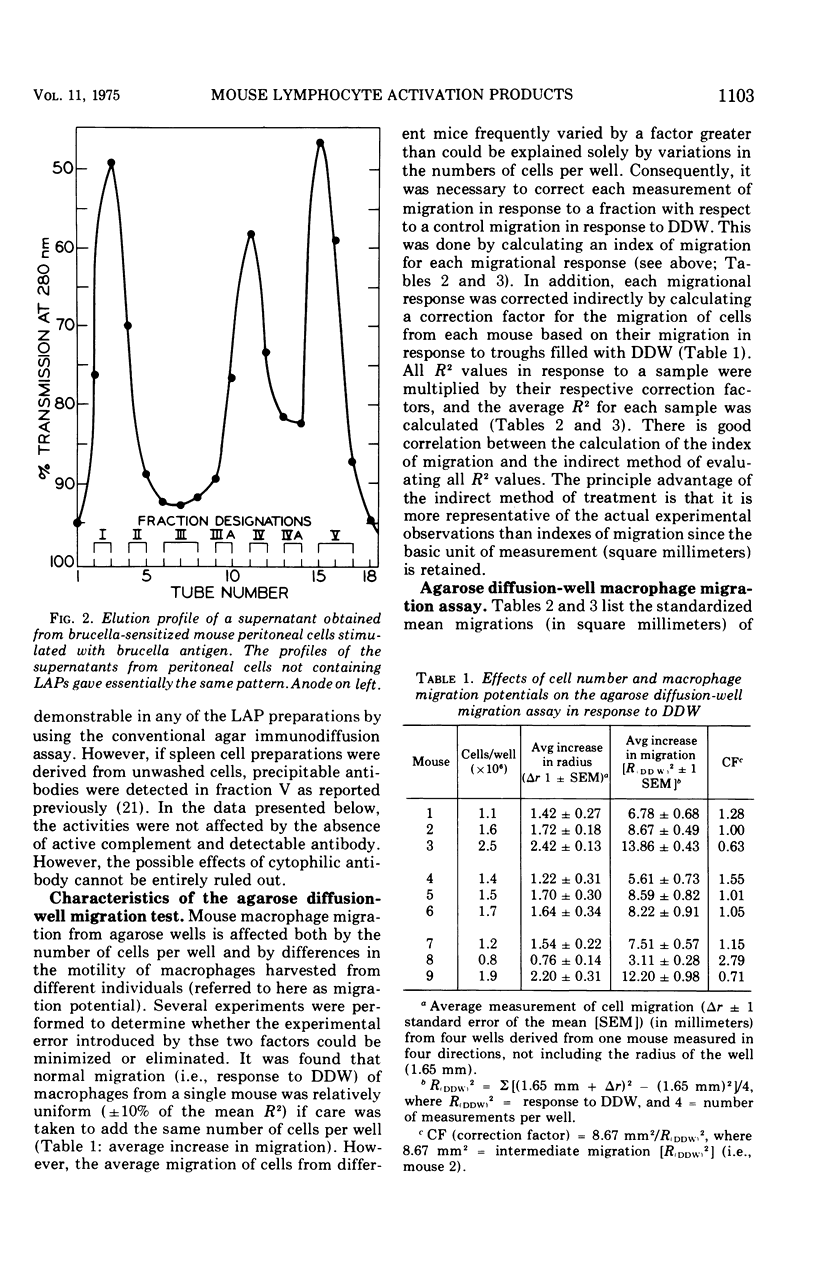
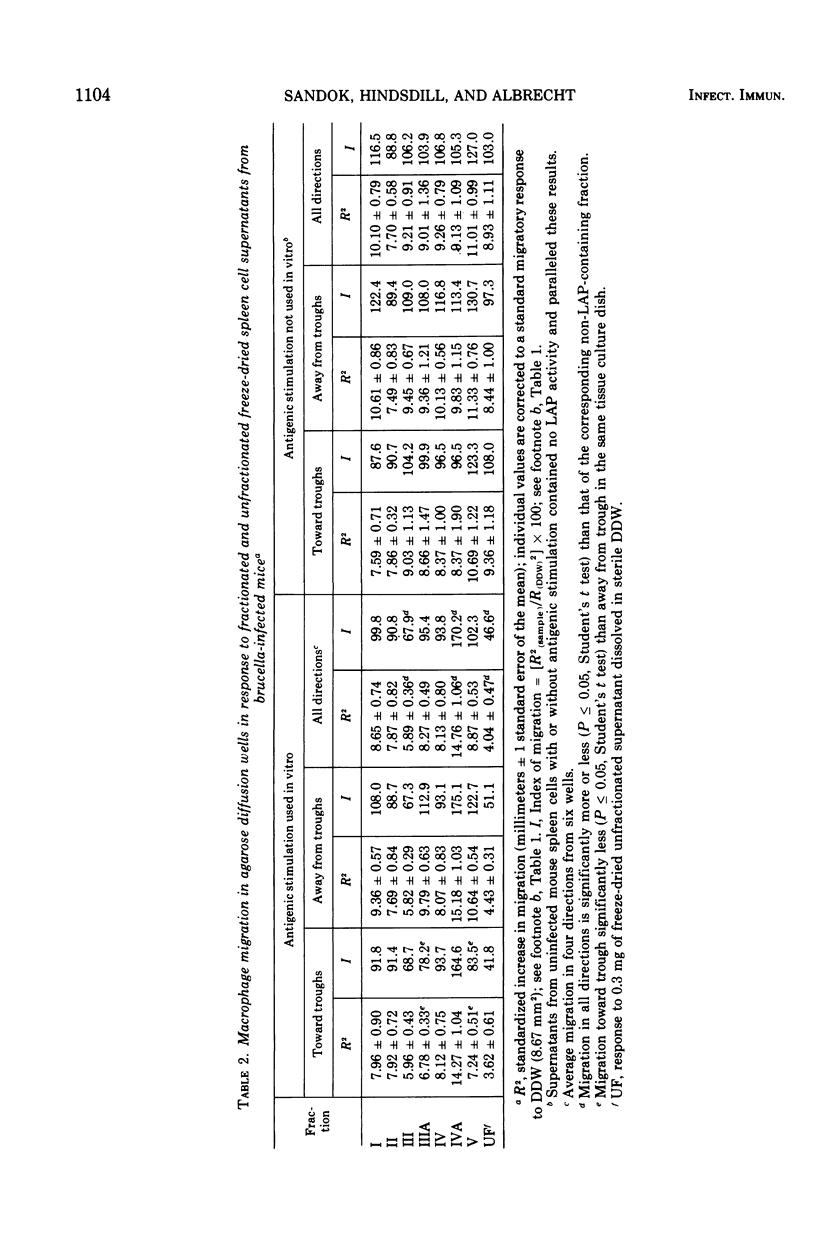
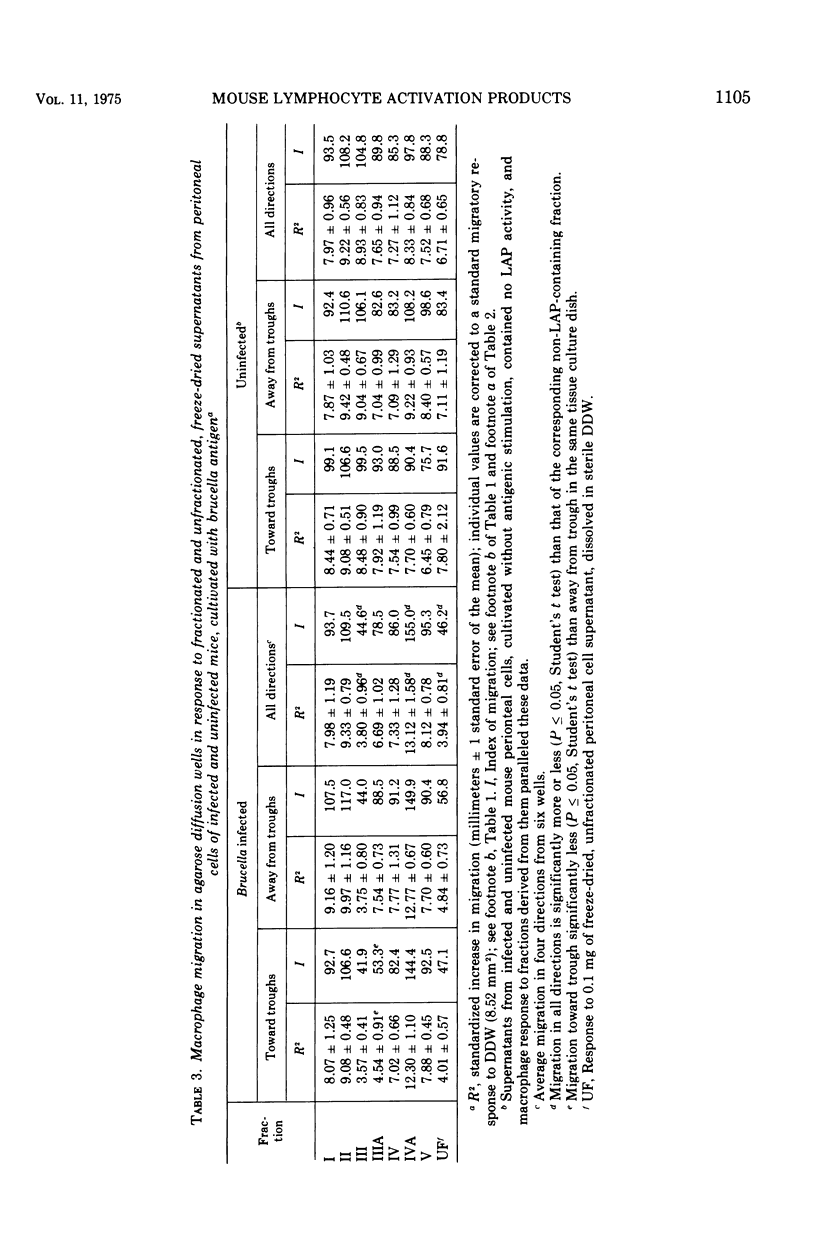
Supernatants from mouse spleen cell and peritoneal cell cultures were tested for the presence of lymphocyte activation products. Supernatants from mouse spleen cell and peritoneal cell cultures incubated with brucella antigens contained a macrophage migration inhibition factor(s) and a macrophage spreading factor(s) only if the cells were harvested from Brucella-infected mice. After dialysis and freeze-drying, the supernatants were fractionated by preparative acrylamide-gel electrophoresis. Three fractions with lymphocyte activation product activity were obtained from the fractionated supernatants of mouse spleen cells and peritoneal cells harvested from Brucella-infected mice and cultured with brucella antigen. One fraction inhibited mouse macrophage migration from capillary tubes but not from agarose wells. A second fraction not only inhibited macrophage migration from both agarose wells and capillary tubes, but also contained an activity(s) that stimulated macrophage migration through Nuclepore filters and induced macrophage spreading. A third fraction timulated macrophage migration from agarose wells and also contained an activity(s) that stimulated macrophage migration through Nuclepore filters. Fractionated supernatants of mouse spleen cells and peritoneal cells harvested from uninfected mice incubated with and without brucella antigen, as well as of cells harvested from infected mice and not incubated with antigen, did not contain detectable lymphocyte activation products.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht R. M., Hinsdill R. D., Sandok P. L., Mackenzie A. P., Sachs I. B. A comparative study of the surface morphology of stimulated and unstimulated macrophages prepared without chemical fixation for scanning EM. Exp Cell Res. 1972 Jan;70(1):230–232. doi: 10.1016/0014-4827(72)90203-0. [DOI] [PubMed] [Google Scholar]
- Altman L. C., Snyderman R., Oppenheim J. J., Mergenhagen S. E. A human mononuclear leukocyte chemotactic factor: characterization, specificity and kinetics of production by homologous leukocytes. J Immunol. 1973 Mar;110(3):801–810. [PubMed] [Google Scholar]
- Amos H. E., Lachmann P. J. The immunological specificity of a macrophage inhibition factor. Immunology. 1970 Feb;18(2):269–278. [PMC free article] [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton B. A., Magoc T. J., Aspinall R. L. The abrogation of macrophage migration inhibition by pretreatment of immune exudate cells with anti-theta antibody and complement. J Immunol. 1974 May;112(5):1741–1746. [PubMed] [Google Scholar]
- Fox R. A., Gregory D. S., Feldman J. D. Macrophage receptors for migration inhibitory factor (MIF), migration stimulatory factor (MSF), and agglutinating factor. J Immunol. 1974 May;112(5):1867–1872. [PubMed] [Google Scholar]
- Fox R. A., Gregory D. S., Feldman J. D. Migration inhibition factor (MIF) and migration stimulation factor (MSF) in fetal calf serum. J Immunol. 1974 May;112(5):1861–1866. [PubMed] [Google Scholar]
- Hinsdill R. D., Berman D. T. Antigens of Brucella abortus. I. Chemical and immunoelectrophoretic characterization. J Bacteriol. 1967 Feb;93(2):544–549. doi: 10.1128/jb.93.2.544-549.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In vitro methods in cell-mediated immunity: a progress report. Cell Immunol. 1973 Mar;6(3):331–347. doi: 10.1016/0008-8749(73)90034-8. [DOI] [PubMed] [Google Scholar]
- Kelly R. H., Wolstencroft R. A., Dumonde D. C., Balfour B. M. Role of lymphocyte activation products (LAP) in cell-mediated immunity. II. Effects of lymphocyte activation products on lymph node architecture and evidence for peripheral release of LAP following antigenic stimulation. Clin Exp Immunol. 1972 Jan;10(1):49–65. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Remold H. G., David J. R. Characterization of a lymphocyte factor which alters macrophage functions. J Exp Med. 1973 Feb 1;137(2):275–290. doi: 10.1084/jem.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. M., Carpenter C. B., Merrill J. P. Cellular immunity in the mouse. I. In vitro lymphocyte reactivity. Cell Immunol. 1972 Oct;5(2):235–248. doi: 10.1016/0008-8749(72)90050-0. [DOI] [PubMed] [Google Scholar]
- Pick E., Turk J. L. The biological activities of soluble lymphocyte products. Clin Exp Immunol. 1972 Jan;10(1):1–23. [PMC free article] [PubMed] [Google Scholar]
- Reeves J., Yoshida T., Cohen S. Quantitative aspects of the migration inhibition reaction. Immunol Commun. 1973;2(3):287–296. doi: 10.3109/08820137309022800. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E. Mediators associated with delayed hypersensitivity. J Reticuloendothel Soc. 1971 Jul;10(1):50–57. [PubMed] [Google Scholar]
- Rosenstreich D. L., Blake J. T., Rosenthal A. S. The peritoneal exudate lymphocyte. I. Differences in antigen responsiveness between peritoneal exudate and lymph node lymphocytes from immunized guinea pigs. J Exp Med. 1971 Nov 1;134(5):1170–1186. doi: 10.1084/jem.134.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandok P. L., Hinsdill R. D., Albrecht R. M. Isolation of substances responsible for lymphokine activity from sensitized mouse spleen cells. Infect Immun. 1974 Jun;9(6):1045–1050. doi: 10.1128/iai.9.6.1045-1050.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandok P. L., Hinsdill R. D., Albrecht R. M. Migration inhibition of mouse macrophages by Brucella antigens. Infect Immun. 1971 Oct;4(4):516–518. doi: 10.1128/iai.4.4.516-518.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Hausman M. S., Mergenhagen S. E. Human mononuclear leukocyte chemotaxis: a quantitative assay for humoral and cellular chemotactic factors. J Immunol. 1972 Mar;108(3):857–860. [PubMed] [Google Scholar]
- Wahl S. M., Altman L. C., Oppenheim J. J., Mergenhagen S. E. In vitro studies of a chemotactic lymphokine in the guinea pig. Int Arch Allergy Appl Immunol. 1974;46(5):768–784. doi: 10.1159/000231176. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Remold H. G., David J. R. Leukotactic factor produced by sensitized lymphocytes. Science. 1969 Mar 7;163(3871):1079–1081. doi: 10.1126/science.163.3871.1079. [DOI] [PubMed] [Google Scholar]
- Weisbart R. H., Bluestone R., Goldberg L. S., Pearson C. M. Migration enhancement factor: a new lymphokine. Proc Natl Acad Sci U S A. 1974 Mar;71(3):875–879. doi: 10.1073/pnas.71.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbart R. H., Cunningham J. E., Bluestone R., Goldberg L. S. A modified agarose method for detection of migration inhibitory factor and delineation of its antigen dependency. Int Arch Allergy Appl Immunol. 1973;45(4):612–619. doi: 10.1159/000231104. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Reisfeld R. A. Two fractions with macrophage migration inhibitory activity from sensitized lymphocyte cultures. Nature. 1970 May 30;226(5248):856–857. doi: 10.1038/226856a0. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



