Abstract
Inadequate control of serum phosphate in chronic kidney disease can lead to pathologies of clinical importance. Effectiveness of on-market phosphate binders is limited by safety concerns and low compliance due to high pill size/burden and gastrointestinal (GI) discomfort. VS-501 is a nonabsorbed, calcium- and aluminum-free, chemically modified, plant-derived polymer. In vitro studies show that VS-501 has a high density and a low swell volume when exposed to simulated gastric fluid (vs. sevelamer). When male Sprague–Dawley (SD) rats on normal diet were treated with VS-501 or sevelamer, serum phosphate was not significantly altered, but urinary phosphate levels decreased by >90%. VS-501 had no effect on serum calcium (Ca) or urinary Ca, while 3% sevelamer significantly increased serum and urine Ca. In 5/6 nephrectomized (NX) uremic SD rats on high-phosphate diet, increasing dietary phosphate led to an increase in serum and urine phosphate, which was prevented in rats treated with VS-501 or sevelamer (0.2–5% in food). High-phosphate diet also increased serum fibroblast growth factor-23 and parathyroid hormone in 5/6 NX rats that was prevented by VS-501 or sevelamer. VS-501 or sevelamer increased fecal phosphate in a dose-dependent manner. More aortic calcification was observed in 5/6 NX rats treated with 5% sevelamer, while VS-501 and sevelamer did not show significant effects on cardiac parameters, fibrosis, intestine histology, and intestinal sodium-dependent phosphate cotransporter gene expression. These results suggest that VS-501 is effective in binding phosphate with no effects on calcium homeostasis, and may have improved pill burden and GI side effects.
Keywords: Chronic kidney disease, hyperphosphatemia, phosphate, phosphate binder, phosphorus, VS-501
Introduction
Chronic kidney disease (CKD) is a serious public health problem. According to the National Kidney Foundation, 26 million people in America (∼13% of the US population) have CKD and millions more are at an increased risk. CKD progresses through five stages; Stage 5 CKD requires renal replacement therapy (dialysis or transplantation). From information provided by the National Kidney Foundation, CKD triggers many other health care issues such as anemia, cardiovascular diseases, hyperphosphatemia, secondary hyperparathyroidism, and other complications. The 5-year average mortality rate is ∼33% (Tonelli et al. 2006), and the mortality risk increases with disease progression (Go et al. 2004).
Inadequate control of serum phosphate levels in CKD can lead to various pathologies of clinical importance such as further deterioration of kidney function, cardiovascular complications, renal osteodystrophy, and increased mortality. Numerous studies have shown that there is a robust association between serum phosphorous levels and all-cause mortality in dialysis-dependent individuals (Block et al. 2004; Kalantar-Zadeh et al. 2006). In predialysis CKD, clinical evidence also demonstrates that elevated serum phosphate is linked to an adverse effect on renal/cardiovascular function and an increased mortality risk, independent of other traditional risk factors (Foley et al. 2005; Eddington et al. 2010; Bellasi et al. 2011). Eddington et al. (2010) further showed that Stage 3/4 predialysis CKD patients who had serum phosphate below the targets recommended in the K/DOQI (2003) guidelines had the best survival even though guidelines for serum phosphate in CKD were devised using only studies involving dialysis patients (Eddington et al. 2010).
A majority of currently available oral phosphate binders, that is calcium-containing and calcium-free phosphate binders, work by binding phosphate in the gastrointestinal (GI) tract, leading to less phosphate to be absorbed into the body. Current therapies (Calciumacetat-Nefro, Renagel, PhosLo, Fosrenol, etc.) have the following shortcomings: (1) suboptimal and inefficient phosphate binding, (2) high pill burden (large number of pills per day and large pill size), unpalatable and hence low compliance, (3) side effects in the GI tract, and (4) safety concerns such as hypercalcemia, aluminum toxicity, negative influence on other medication, and accumulation in organs (Chiu et al. 2009; Wang et al. 2013). Patient compliance with on-market drugs is a significant clinical management issue because of GI tolerability and pill burden (size and number). Considering the importance of controlling phosphate metabolism in CKD patients, there is a need for improved phosphate-controlling drugs. To this end, we have discovered VS-501 that is derived from a natural polymer commonly used in the food industry. VS-501 is nonabsorbed, calcium- and aluminum-free, and effectively binds phosphate in the GI tract. The preclinical studies summarized in this report show that VS-501 is effective in binding phosphate with no effects on calcium homeostasis, and may have potential in reducing pill burden and Gl side effects.
Materials and Methods
Materials
VS-501 was made by Vidasym (Chicago, IL). The synthesis has been published previously (Wu-Wong 2013). Other reagents were of analytical grade.
In vitro polymer characterization
The density of the compressed powder was determined by a helium pycnometer. For swelling volume determination, VS-501 or sevelamer at 0.1 g (dry powder) was incubated with 5 mL of simulated gastric fluid (0.2% [w/v] NaCl, 0.7% [v/v] hydrochloride [HCl], without pepsin) at 37°C for different periods of time. To determine phosphate-binding capacity in vitro, VS-501 at 0.1 g was incubated with 10 mL of a 20 mmol/L phosphate solution (1.37 mL of 85% phosphoric acid, 3.18 g of sodium carbonate and 4.68 g of NaCl in 1 L of water) at room temperature at different pH (as indicated) for 24 h. In separate studies, VS-501 at 0.1 g was incubated with a phosphate solution containing different phosphate concentrations (as indicated) and sodium carbonate and NaCl as described above at neutral pH for 24 h at room temperature. The samples were centrifuged and the supernatant collected for phosphate determination using a phosphate colorimetric assay (Catalog #K410-500; BioVision, Milpitas, CA).
Normal rat studies
Male Sprague–Dawley (SD) rats were fed a normal diet (containing 1% calcium and 0.7% phosphorus in powder form) containing VS-501 or sevelamer-HCl (concentrations as indicated) in food for 6 days. On the first (before dosing) and last days of treatment, rats were placed in metabolic cages with one rat per cage. Urine and/or feces samples were collected for 24 h. Blood samples were collected from each rat for serum preparation. Physiological parameters were determined as described below. This and all other animal studies were conducted under the auspice of the Office of Animal Care and Institutional Biosafety, University of Illinois at Chicago. The study conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 1996).
5/6 nephrectomized uremic rat studies
The 5/6 nephrectomized (NX) rats were prepared and handled as previously described (Wu-Wong et al. 2011, 2013a). Briefly, nephrectomy was performed on male, Sprague–Dawley rats weighing ∼200 g with a standard two-step surgical ablation procedure. At 6 weeks after the second surgery when uremia was firmly established (as indicated by elevated serum creatinine and blood urea nitrogen [BUN] levels), rats were fed a high-phosphate diet (normal diet containing 1% calcium and 0.7% phosphorus in powder form plus additional KH2PO4 at 0.67% and K2HPO4 at 0.33% by dry weight in food) and treated with VS-501 or sevelamer carbonate (concentrations as indicated) in food for 4 weeks. Sevelamer carbonate was tested in these studies to gain additional information on the newer version of sevelamer. Rats were placed in metabolic cages with one rat per cage on Days 0 (predosing), 14 (Week 2), and 28 (Week 4); urine and feces samples were collected during a period of 24 h. Blood samples were collected from each rat for serum preparation. Physiological parameters were determined as described below.
Measurements of physiological parameters
Serum and urine calcium (Ca) was measured using a Stanbio LiquiColor calcium assay kit (Boerne, TX). Serum parathyroid hormone (PTH) was measured using a rat intact PTH ELISA kit obtained from Immutopics (San Clemente, CA). Serum fibroblast growth factor (FGF)-23 was determined using a rat/mouse FGF-23 (C-Term) ELISA kit obtained from Immutopics. The serum and urine phosphorus/phosphate (Pi) levels were determined using a phosphate colorimetric assay (Catalog #K410-500; BioVision). Serum creatinine and BUN concentrations were measured using a chemistry analyzer.
For fecal phosphate determination, samples of 2 gm from each feces sample were ashed at 800°C for 30 min. Ash was extracted with 5 mL of 12N HCl by vortexing and shaking at room temperature for ∼60 min. The supernatant was collected by centrifugation and neutralized using an equal volume of 12N NaOH. The mixture was again centrifuged and the supernatant was collected for phosphate determination by the BioVision phosphate colorimetric assay. Total urinary and fecal phosphate levels during a 24-h period were calculated.
Tissue preparation and staining
Tissue samples were fixed in formalin for 1–3 days, and then transferred to 70% alcohol. Samples were embedded in wax and cut into 5-μm sections. Sections were stained with hematoxylin-eosin (H-E). For fibrosis, sections were stained with Masson Trichrome reagent, and imaged and analyzed using a Vectra Intelligent Multispectral Slide Analysis System (Perkin-Elmer, Waltham, MA). For calcification, aorta sections were stained by the von Kossa method and counterstained with nuclear fast red (Wu-Wong et al. 2007).
Echocardiographic assessment
Animals were sedated with isoflurane (1.5%, inhaled), placed in the decubitus position on a warming pad to maintain normothermia and the chest shaved/depilated. Transthoracic echocardiography was conducted using a 17.5 MHz high-resolution transducer plus an integrated system (Vevo 770 High-Resolution Imaging System, VisualSonics, Toronto, Canada), and B-mode, M-mode, pulsed Doppler, and tissue Doppler images were obtained. All cardiac parameters were calculated using VisualSonics Vevo 770 analysis software (v. 3.0.0) with a cardiac measurements package.
Measurement of GI calcium transport
Duodenal calcium absorption was measured ex vivo as described previously (Nakane et al. 2007; Wu-Wong et al. 2013b). Briefly, segments of proximal small intestine were removed from each rat, everted, and filled with incubation buffer (125 mmol/L NaCl, 10 mmol/L fructose, 0.25 mmol/L CaCl2, 30 mmol/L Tris, pH 7.4 at 37°C). Gut sacs were incubated for 90 min in incubation buffer at 37°C with occasional shaking. At the end of the incubation period, calcium concentration in the serosal and mucosal compartments was measured and the serosal/mucosal calcium ratio was calculated.
Real-time reverse transcription PCR
Real-time reverse transcription PCR (Real-time RT-PCR) was performed with an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Each sample consisted of a final volume of 25 μL containing 200 ng of mRNA, 100 nmol/L (final concentration) each of the forward and reverse PCR primers and 250 nmol/L (final concentration) of the TaqMan™ probe (Applied Biosystems). Temperature conditions consisted of a step of 30 min at 48°C and a step of 10 min at 95°C, followed by 45 cycles of 60°C for 1 min and 95°C for 15 sec. Data were collected during each extension phase of the PCR reaction and analyzed with a software package (Applied Biosystems). Threshold cycles were determined for each gene.
Data analysis
Differences among different groups were assessed using a one-way analysis of variance (ANOVA) followed by a Dunnett’s post hoc test. Statistical comparisons between two treatment groups were performed by unpaired t-test with 95% confidence intervals of difference.
Results
In vitro characterization
As mentioned above, large pill size/number is one of the reasons causing patient compliance issues for phosphate binders such as sevelamer to effectively control hyperphosphatemia (Chiu et al. 2009; Wang et al. 2013). The density of a polymer will have a significant impact on its pill size. Thus, we compared the density of VS-501 versus sevelamer. The density of the compressed powder was determined by a helium pycnometer to be 1.91 g/cm3 for VS-501, and 1.27 g/cm3 for sevelamer carbonate (Table 1).
Table 1.
Density and swell volume determination
| Parameters | VS-501 | Sevelamer* |
|---|---|---|
| Density of compressed powder determined by helium pycnometer (g/cm3) | 1.91 | 1.27 |
| Swell volume in simulated gastric fluid at 37°C for 20–180 min (cm3/0.1 g) | 0.2 | 4.0 |
Sevelamer carbonate was used in the study.
GI discomfort is another reason causing patient compliance issues for some on-market phosphate binders such as sevelamer. Larger swelling volume is often associated with more GI discomfort. The swell volume of VS-501 versus sevelamer was determined at different time points after exposure to simulated gastric fluid. As shown in Table 1, the swell volume of VS-501 is much less than that of sevelamer.
We then determined the in vitro Pi-binding capacity of VS-501. Figure 1A shows that VS-501 binds phosphate with an estimated maximal binding capacity at 1.3 mmol/g and Kd at 10 mmol/L. Figure 1B shows that VS-501 binds phosphate within a wide physiologically relevant pH range.
Figure 1.
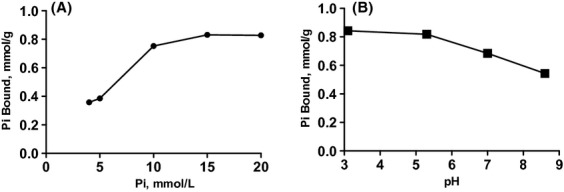
In vitro phosphate binding. (A) VS-501 at 0.1 g was incubated with 10 mL of a phosphate solution containing different phosphate concentrations (as indicated) and sodium carbonate and NaCl as described in Methods at neutral pH for 24 h at room temperature. The graph is representative of two independent experiments. (B) VS-501 at 0.1 g was incubated with 10 mL of a 20 mmol/L phosphate solution as described in Methods at different pH (as indicated) for 24 h at room temperature. The samples were processed for phosphate determination as described in Methods. The graph is representative of three independent experiments.
Normal rats on normal diet
Normal rats were chosen to screen compounds because they are easier to handle than the kidney disease uremic rat model (discussed below). We compared the efficacy of VS-501 versus sevelamer in normal rats on normal diet. Serum phosphate (Fig. 2A) was not significantly altered, but urinary phosphate levels (Fig. 2B) were decreased significantly in the VS-501 and sevelamer-HCl treatment groups. VS-501 had no effect on serum/urinary Ca while sevelamer-HCl increased serum/urinary Ca (Fig. 2C and D). Figure 2E shows that sevelamer at 3% significantly decreased serum PTH, while VS-501 had a modest effect.
Figure 2.
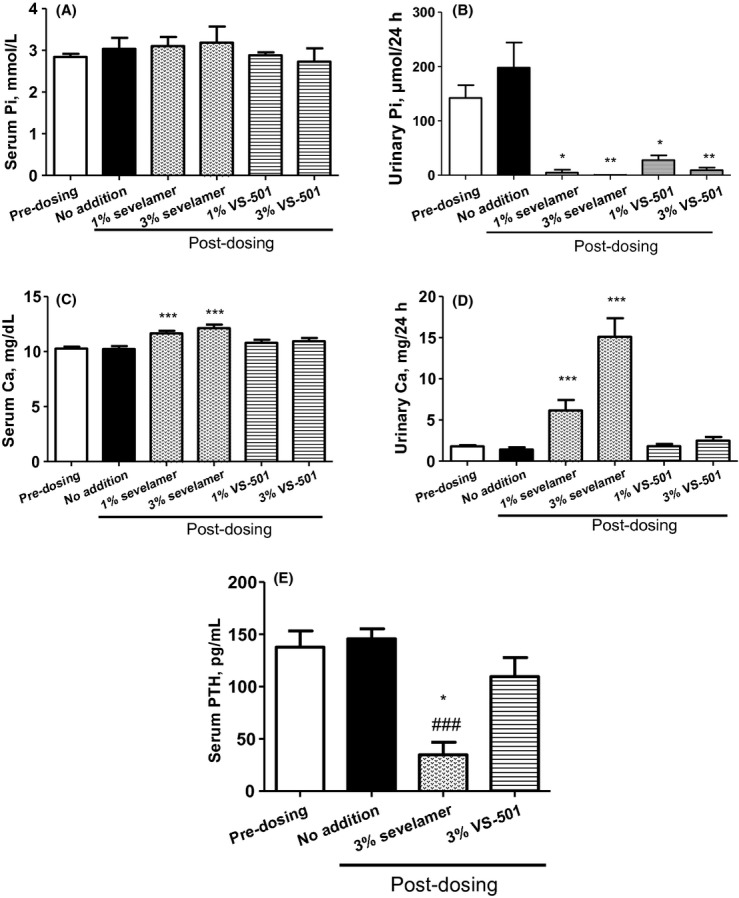
Serum and urinary physiological parameters in normal rats on normal diet. Male Sprague–Dawley (SD) rats were fed a normal diet (containing 1% calcium and 0.7% phosphorus in powder form) and VS-501 or sevelamer (concentrations as indicated) in food for 6 days. Blood and urine samples were collected at two different time points (predosing and on Day 6 after dosing) for Pi, Ca and PTH determination as described in Methods. Mean ± SE was calculated for each group (n = 6–12). (A) Serum Pi. (B) Urinary Pi in 24 h. (C) Serum Ca. (D) Urinary Ca in 24 h. (E) Serum PTH. One-way ANOVA and Dunnett test with 95% confidence intervals of difference was performed for statistical comparisons. *P < 0.05, **P < 0.01, ***P < 0.001 versus predosing. ###P < 0.001 versus no addition (untreated).
As an attempt to assess the impact of VS-501 on drinking and eating patterns, daily water and food consumption trends were tracked, and urine volume and fecal weight were determined in the high-dose groups. Figure 3A and B show the results from daily tracking of water and food consumptions in the 3% VS-501 and sevelamer groups; water consumption was consistently higher in the sevelamer group. Figure 3C and D show that sevelamer-HCl at 3% significantly increased urine volume and fecal weight, while VS-501 had a modest effect.
Figure 3.
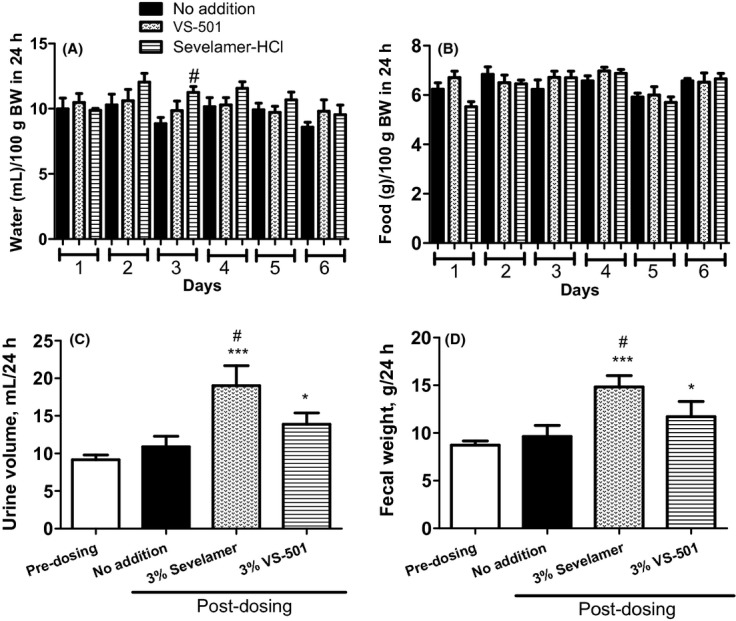
Water and food consumption versus urinary volume and fecal weight in normal rats on normal diet. Male SD rats were on normal diet plus 3% VS-501 or sevelamer-HCl as described in Figure 2. Water and food consumption for each rat was tracked daily. Urine and feces samples were collected for 24 h at two different time points (predosing and on Day 6 after dosing) as described in Methods. Mean ± SE was calculated for each group. (A) Water consumption in 24 h normalized by body weight. (B) Food consumption in 24 h normalized by body weight. (C) Urine volume in 24 h. (D) Fecal weight in 24 h. One-way ANOVA and Dunnett test with 95% confidence intervals of difference was performed for statistical comparisons. *P < 0.05, ***P < 0.001 versus predosing. #P < 0.05 versus no addition (untreated).
Efficacy in 5/6 NX rats on high-phosphate diet
Since VS-501 is intended for treating hyperphosphatemia associated with CKD, it is important to evaluate the efficacy of VS-501 in a CKD animal model. The CKD field has the advantage of the 5/6 NX uremic rat model that, albeit a difficult model to handle, is highly predictive of the human conditions. In addition, the 5/6 NX rats, similar to human CKD patients, also develop cardiovascular complications such as left ventricular hypertrophy, which makes them useful for assessing a compound’s cardiovascular protective effects.
Consistent with our previous studies in 5/6 NX rats (Wu-Wong et al. 2010, 2011, 2013a,b), serum BUN and creatinine levels were elevated significantly in 5/6 NX rats, indicating established uremia even at 6 weeks after surgery (Table 2). Treatment with VS-501 or sevelamer carbonate had no significant effects.
Table 2.
Serum BUN and creatinine in 5/6 nephrectomized uremic rats
| Serum BUN (mg/dL) | Serum creatinine (mg/dL) | |||||
|---|---|---|---|---|---|---|
| Parameters | Predosing | Week 2 | Week 4 | Predosing | Week 2 | Week 4 |
| Sham | 19.64 ± 0.88 | 18.65 ± 1.07 | 19.97 ± 0.78 | 0.41 ± 0.04 | 0.46 ± 0.03 | 0.38 ± 0.01 |
| NX-1% vehicle | 45.15 ± 2.40a | 46.20 ± 2.71a | 50.73 ± 5.79a | 0.79 ± 0.08a | 1.02 ± 0.04a | 0.96 ± 0.20a |
| NX-5% vehicle | 48.28 ± 3.64a | 46.14 ± 3.69a | 46.20 ± 3.92a | 0.93 ± 0.02a | 0.94 ± 0.05a | 0.87 ± 0.09a |
| 0.2% sevelamer | 39.82 ± 3.09a | 42.09 ± 4.43a | 40.78 ± 6.00a | 0.77 ± 0.11b | 0.74 ± 0.04b | 0.85 ± 0.18a |
| 1% sevelamer | 47.54 ± 2.06a | 41.87 ± 1.48a | 48.83 ± 2.86a | 0.80 ± 0.03a | 0.95 ± 0.02a | 0.91 ± 0.10a |
| 5% sevelamer | 51.85 ± 2.92a | 47.55 ± 2.83a | 50.93 ± 4.61a | 0.93 ± 0.03a | 1.03 ± 0.04a | 1.02 ± 0.13a |
| 0.2% VS-501 | 42.70 ± 2.42a | 37.43 ± 1.37a | 38.54 ± 1.92a | 0.66 ± 0.03c | 0.70 ± 0.03b | 0.67 ± 0.03c |
| 1% VS-501 | 46.76 ± 1.81a | 41.52 ± 2.66a | 47.23 ± 2.46a | 0.75 ± 0.03a | 0.96 ± 0.02a | 0.76 ± 0.04a |
| 5% VS-501 | 52.85 ± 3.03a | 47.42 ± 2.99a | 53.04 ± 3.37a | 0.89 ± 0.03a | 1.04 ± 0.03a | 1.02 ± 0.08a |
Data are mean ± SE. One-way ANOVA and Dunnett test with 95% confidence intervals of difference was performed for statistical comparisons.
P < 0.001, bP < 0.01, cP < 0.05 versus sham, predosing. Predosing: Week 6 after surgery. Week 2: Week 8 after surgery with 2 weeks of dosing. Week 4: Week 10 after surgery with 4 weeks of dosing. Vehicle: unprocessed plant polymer. Sham rats were on high-Pi diet with no addition of vehicle or binders.
As shown in Figure 4, increasing dietary phosphate led to a modest increase in serum phosphate and a significant increase in urinary phosphate levels in sham and in the 5/6 NX rat groups treated with vehicle (unmodified polymer). The increase in phosphate levels was observed at both time points (Week 2 and Week 4 after dosing). VS-501 or sevelamer effectively reduced serum phosphate and also reduced urinary phosphate in a dose-dependent manner. The effects of the drugs were also observed at both time points (Week 2 and Week 4 after dosing). VS-501 or sevelamer at 5% seemed to over-suppress serum and urinary phosphate. No significant changes in serum chloride, potassium, and sodium levels were observed (data not shown).
Figure 4.
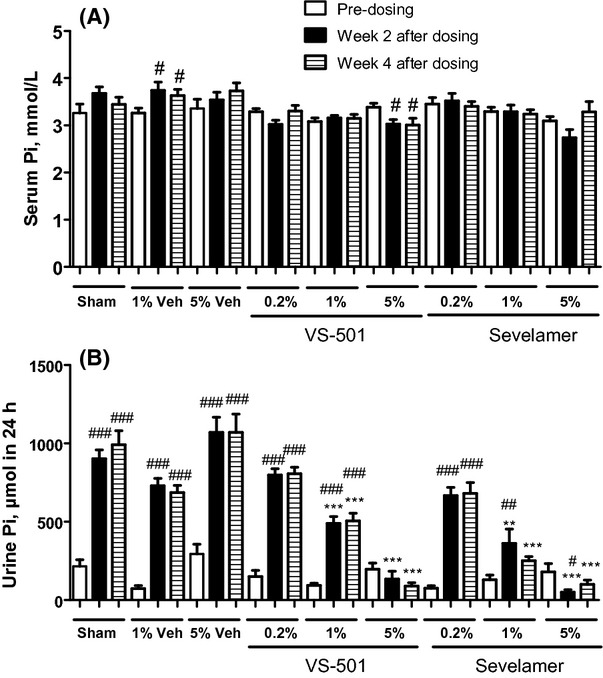
Serum and urinary phosphate in 5/6 NX rats on high-phosphate diet. NX rats were dosed with vehicle (Veh: unprocessed polymer at 1 or 5%), VS-501 or sevelamer carbonate (concentrations at 0.2, 1 or 5%) in food for 4 weeks. Sham rats were on high-Pi diet with no addition of vehicle or binders. Blood and urine samples were collected at three different time points (predosing, at Week 2 after dosing, and at Week 4 after dosing) for Pi determination as described in Methods. Mean ± SE was calculated for each group (n = 7–12). (A) Serum Pi. (B) Urinary Pi in 24 h. One-way ANOVA and Dunnett test with 95% confidence intervals of difference was performed for statistical comparisons. #P < 0.05, ##P < 0.01, ###P < 0.001 versus predosing, same group. **P < 0.01, ***P < 0.001 versus Sham, same time point. Open bar: predosing. Solid bar: Week 2 after dosing. Pattern bar: Week 4 after dosing.
As shown in Figure 5, increasing dietary phosphate led to an increase in fecal phosphate levels in sham and in the groups treated with vehicle (unmodified polymer). The increase in phosphate levels was observed at both time points (Week 2 and Week 4 after dosing). VS-501 and sevelamer further increased fecal phosphate in a dose-dependent manner, suggesting that these polymers carried phosphate into feces.
Figure 5.

Fecal phosphate in 5/6 NX rats on high-phosphate diet. NX rats were dosed with vehicle (Veh: unprocessed polymer at 1 or 5%), VS-501 or sevelamer carbonate (concentrations at 0.2, 1 or 5%) in food for 4 weeks. Sham rats were on high-Pi diet with no addition of vehicle or binders. Fecal samples were collected at three different time points (predosing, at Week 2 after dosing, and at Week 4 after dosing) for Pi determination as described in Methods. Mean ± SE was calculated for each group. One-way ANOVA and Dunnett test with 95% confidence intervals of difference was performed for statistical comparisons. #P < 0.05, ##P < 0.01, ###P < 0.001 versus predosing, same group. *P < 0.05, **P < 0.01, ***P < 0.001 versus sham, same time point. Open bar: predosing. Solid bar: Week 2 after dosing. Pattern bar: Week 4 after dosing.
Figure 6 shows that serum PTH and FGF-23 levels were significantly higher in 5/6 NX rats (vs. sham). Increasing phosphate in the diet further elevated serum FGF-23 and PTH levels over time. VS-501 or sevelamer at 1% and 5% effectively prevented the progressive rise over time in serum PTH and FGF-23 levels induced by a high-phosphate diet.
Figure 6.
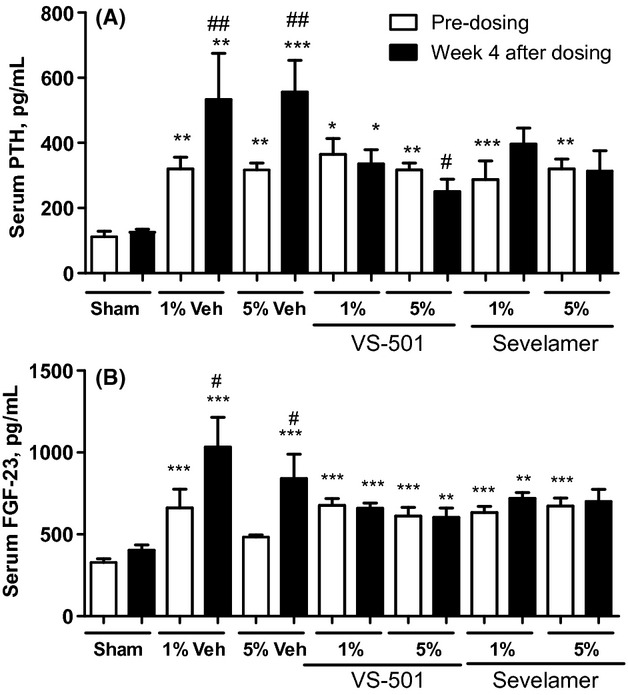
Serum PTH and FGF-23 in 5/6 NX rats on high-phosphate diet. NX rats were dosed with vehicle (Veh: unprocessed polymer at 1 or 5%), VS-501 or sevelamer carbonate (concentrations at 1 or 5%) in food for 4 weeks. Sham rats were on high-Pi diet with no addition of vehicle or binders. Serum samples were collected for PTH and FGF-23 determination as described in Methods. Mean ± SE was calculated for each group. (A) Serum PTH. (B) Serum FGF-23. Statistical comparisons between two groups were performed by unpaired t-test with 95% confidence intervals of difference. #P < 0.05, ##P < 0.01 versus predosing, same group. *P < 0.05, **P < 0.01, ***P < 0.001 versus sham, same time point. Open bar: predosing. Solid bar: Week 4 after dosing.
As shown in Table 3, increasing dietary phosphate had no significant effects on serum and urinary calcium levels in sham and in the groups treated with vehicle (unmodified polymer) or VS-501. Interestingly, although serum Ca levels were not affected by sevelamer at 1–5%, urinary Ca levels trended higher in a dose-dependent manner.
Table 3.
Serum and urinary calcium in 5/6 nephrectomized uremic rats
| Serum Ca (mg/dL) | Urinary Ca (mg in 24 h) | |||||
|---|---|---|---|---|---|---|
| Parameters | Predosing | Week 2 | Week 4 | Predosing | Week 2 | Week 4 |
| Sham | 10.95 ± 0.19 | 11.14 ± 0.25 | 10.89 ± 0.16 | 0.74 ± 0.09 | 0.79 ± 0.09 | 0.60 ± 0.06 |
| NX-1% vehicle | 10.91 ± 0.20 | 11.90 ± 0.32 | 11.07 ± 0.34 | 1.86 ± 0.28 | 1.48 ± 0.33 | 1.54 ± 0.27 |
| NX-5% vehicle | 11.18 ± 0.23 | 10.46 ± 0.14 | 9.86 ± 0.40 | 1.70 ± 0.23 | 1.91 ± 0.34 | 1.63 ± 0.29 |
| 0.2% sevelamer | 11.37 ± 0.22 | 11.82 ± 0.41 | 11.98 ± 0.27 | 1.40 ± 0.15 | 2.22 ± 0.63 | 2.12 ± 0.65 |
| 1% sevelamer | 11.00 ± 0.16 | 10.91 ± 0.18 | 11.28 ± 0.14 | 1.72 ± 0.28 | 2.10 ± 0.28 | 2.33 ± 0.24 |
| 5% sevelamer | 11.39 ± 0.27 | 11.05 ± 0.29 | 10.52 ± 0.29 | 1.97 ± 0.23 | 3.38 ± 0.73a | 3.32 ± 0.74a |
| 0.2% VS-501 | 11.14 ± 0.16 | 11.48 ± 0.12 | 11.82 ± 0.22 | 0.95 ± 0.08 | 0.90 ± 0.06 | 1.14 ± 0.10 |
| 1% VS-501 | 11.06 ± 0.16 | 11.02 ± 0.36 | 11.30 ± 0.13 | 1.48 ± 0.14 | 1.12 ± 0.10 | 1.23 ± 0.11 |
| 5% VS-501 | 10.82 ± 0.18 | 11.25 ± 0.20 | 11.06 ± 0.18 | 1.90 ± 0.22 | 2.01 ± 0.33 | 2.02 ± 0.33 |
Data are mean ± SE. A t-test was used to assess differences between predosing and post dosing in the same group.
P < 0.05 versus predosing, same group. Predosing: Week 6 after surgery. Week 2: Week 8 after surgery with 2 weeks of dosing. Week 4: Week 10 after surgery with 4 weeks of dosing. Vehicle: unprocessed plant polymer. Sham rats were on high-Pi diet with no addition of vehicle or binders.
Cardiovascular parameters in 5/6 NX rats
Since cardiovascular complication is a serious concern in CKD, it is of interest to investigate whether phosphate control by VS-501 or sevelamer affects cardiovascular parameters.
Previously we have shown that, at 8 weeks after the renal ablation surgery, the left-ventricle weight (LVW) versus body weight (BW) ratio as a percentage of control was significantly higher in 5/6 NX rats (vs. sham), and treatment with vitamin D analogs reduced the LVW/BW ratio in a dose-dependent manner (Wu-Wong et al. 2011, 2013a). Consistent with our previous reports, the LVW/BW ratio was elevated in 5/6 NX rats, but VS-501 or sevelamer had no significant effects (data not shown).
The E/A ratio, a parameter representative of diastolic cardiac function, was significantly reduced in NX rats at 6 weeks after the renal ablation, and further reduced after 4 weeks on high-phosphate diet. Sevelamer and VS-501 at 5% prevented the further reduction in the E/A ratio, but did not restore the parameter to the level observed in the sham group (Fig. 7A).
Figure 7.
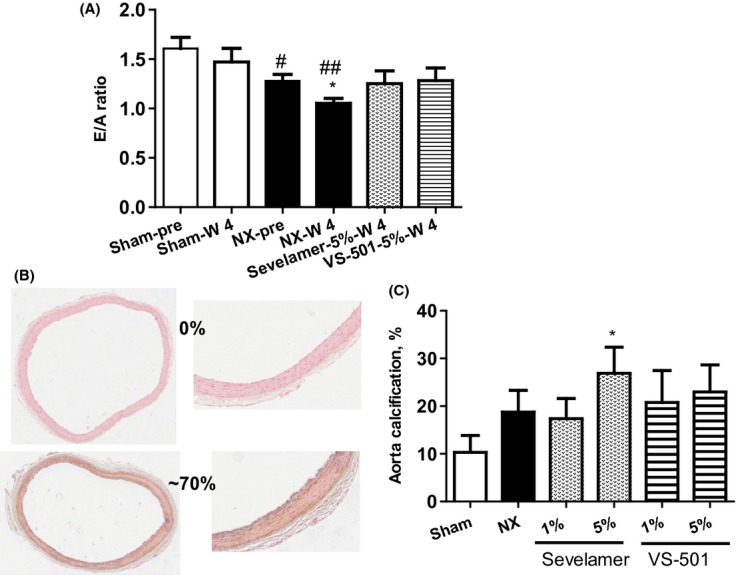
Cardiovascular parameters in 5/6 NX rats on high-phosphate diet. NX rats were treated with 5% vehicle (unprocessed polymer), 5% sevelamer-carbonate, or 5% VS-501 in food for 4 weeks as described above. (A) E/A ratio: Cardiac function was determined at predosing (pre) and Week 4 after dosing (W4) as described in Methods. Statistical comparisons between two groups were performed by unpaired t-test with 95% confidence intervals of difference. #P < 0.05, ##P < 0.01 versus Sham-predosing. *P < 0.05 versus NX-predosing. (B) Calcification staining: Aorta samples were randomly selected from each 5/6 NX group with n = 16 (four aorta samples per rat, four rats per group) and stained by the von Kossa method. The staining was scored separately by two investigators in a blinded manner and the scores were averaged for each sample. Two representative samples indicating 0% and 70% positive staining of the aortic cross-sectional area are shown. (C) Quantification of calcification: The percentages of positive staining in each treatment group were calculated and expressed as mean ± SE. Statistical comparisons between two groups were performed by unpaired t-test with 95% confidence intervals of difference.*P < 0.05 versus Sham.
Results from fibrosis staining by Masson Trichrome are consistent with our previous observations (Wu-Wong et al. 2011, 2013a) that, compared to sham, a significant increase in collagen deposition was observed in the heart in 5/6 NX group. However, unlike our previous observations that vitamin D analogs significantly reduce fibrosis (Wu-Wong et al. 2011, 2013a), neither VS-501 nor sevelamer had any significant effects (data not shown).
To investigate vascular calcification, aorta samples were randomly selected from each 5/6 NX group and stained for calcification. The staining was scored separately by two investigators in a blinded manner. Ultimately, the staining assessment by two investigators was similar. Two representative samples indicating 0% and 70% positive staining of the aortic cross-sectional area are shown in Figure 7B. The averaged percentage of positive staining in each treatment group is shown in Figure 7C; the results demonstrate a significant increase in aortic calcification in the 5% sevelamer group (vs. NX-vehicle).
GI parameters in 5/6 NX rats
As a measure to investigate whether chronic dosing of phosphate binders affects intestine physiology, the intestinal integrity was first evaluated by H-E staining of intestinal samples. There was no significant difference across the different treatment groups (data not shown).
To investigate further whether VS-501 and sevelamer affects intestine physiology, duodenal calcium absorption was measured ex vivo in 5/6 NX rats. Unlike our previous observations that vitamin D analogs such as calcitriol and paricalcitol increase intestinal calcium transport (Nakane et al. 2007; Wu-Wong et al. 2013b), no significant difference was observed across different groups (data not shown).
Consistent with the intestinal calcium transport results, and unlike our previous observations that vitamin D analogs such as calcitriol and paricalcitol induce the expression of intestinal Calb3 (the gene encoding calbindin D9K) and TRPV6 (the gene encoding CaT1 and ECaC2) that are involved in intestinal calcium transport (Nakane et al. 2007; Wu-Wong et al. 2013b), no significant difference was observed across the different groups (data not shown). The expression of the intestinal type II sodium-dependent phosphate cotransporter (NPT2b) gene was also determined. Figure 8 shows that there was no significant difference across different groups.
Figure 8.
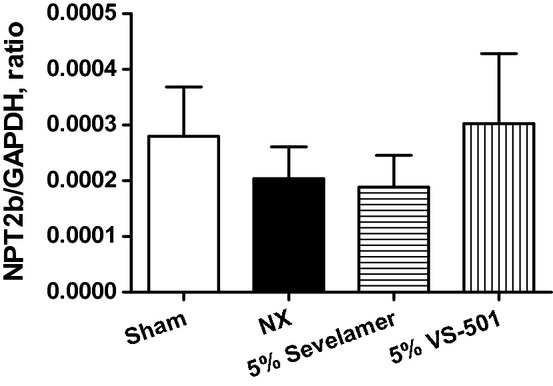
Intestinal type II sodium-dependent phosphate cotransporter (NPT2b) gene expression. NX rats were treated with 5% vehicle (unprocessed polymer), 5% sevelamer carbonate, or 5% VS-501 in food for 4 weeks as described above. RNA samples were prepared from small intestines using the standard RNA isolation procedure. The real-time RT-PCR was performed as described in Methods. NPT2b values were normalized with GAPDH. Statistical comparisons between two groups were performed by unpaired t-test with 95% confidence intervals of difference. Mean ± SE was calculated for each group.
Discussion
We have demonstrated in this report that VS-501, a novel phosphate binder derived from a natural plant polymer, has the potential to overcome some of the issues associated with current phosphate binders used for treating CKD patients. VS-501 has a significantly higher density (vs. sevelamer), suggesting its potential for reduced pill size and pill number. Furthermore, VS-501 has a significantly lower swell volume when exposed to simulated gastric fluid, and this characteristic may lead to a lower incidence of GI discomfort than sevelamer. It is of interest to note that VS-501 binds phosphate within a wide physiologically relevant pH range, which suggests that VS-501 binds phosphate at different sites in the GI tract. These unique attributes of VS-501 may improve patient compliance, which is one of the critical factors preventing current phosphate binders from effectively controlling hyperphosphatemia in CKD.
From tracking daily water and food consumption in normal rats on normal diet, we observed that water consumption was consistently higher in the sevelamer group, which may explain the higher urine volume in those rats. At the same time, a decrease in food consumption on Day 1 in the sevelamer group may be related to the taste of sevelamer. No significant change in food consumption was observed in VS-501-treated normal rats. Higher fecal weight in the sevelamer group is consistent with results from the in vitro swell volume studies.
Our data show that, while VS-501 has no effects on serum or urinary Ca in normal rats on normal diet, sevelamer significantly increases both measures. It has been reported previously that sevelamer may reduce serum PTH (Fournier, 2000; Burke et al., 2003), whereas other investigators showed that switching from Ca-containing Pi binders such as CaCO3 to sevelamer decreased the serum levels of calcium, resulting in the elevation of iPTH levels (Sato et al., 2005; Iwata et al., 2007). Although both sevelamer and VS-501 prevented the additional increase in serum PTH induced by a high-phosphate diet in 5/6 NX rats, sevelamer did not suppress PTH in a manner similar to that observed in normal rats on a normal diet, which coincides with the observation that sevelamer did not affect serum calcium in 5/6 NX rats. Our results suggest that the impact of sevelamer on serum PTH in normal rats is likely linked to its effect on calcium homeostasis.
Normal rats fed a normal diet do not develop vascular calcification, but increased aortic calcification was noted in 5/6 NX uremic rats on a high-phosphate diet, which was further increased in the high-dose sevelamer group. A report by Block et al. (2012) shows that all phosphate binders currently used in clinical settings including sevelamer potentially increase vascular calcification although the mechanism of action is not known. Our observations offer a possible explanation that sevelamer may disturb calcium homeostasis, leading to increased vascular calcification.
FGF-23 is a phosphorus regulating factor (Wolf 2010). FGF-23 levels increase progressively beginning in early stages of kidney disease in order to maintain normophosphatemia despite decreased nephron mass (Gutierrez et al. 2005), and abnormal FGF-23 levels are associated with increased cardiovascular events and mortality in CKD (Gutierrez et al. 2008). Results from this study that phosphate binders prevent the increase in FGF-23 in uremic rats induced by high phosphate diet are in line with the clinical observations made in human CKD patients (Gupta et al. 2004; Martin and Gonzalez 2011; Karczmarewicz et al. 2012). Although no improvement in fibrosis or cardiac function was observed in uremic rats treated with VS-501 or sevelamer, the phosphate binders seem to prevent the continued deterioration of cardiac diastolic function observed in vehicle-treated rats. It may be worth noting that, while both sevelamer and VS-501 prevented the further increase in FGF-23, sevelamer caused more aortic calcification. Whether there is a direct link between FGF-23 and vascular calcification is still being debated (Moldovan et al. 2013; Ozkok et al. 2013). Our results suggest that disturbance of calcium homeostasis may contribute to vascular calcification independent of FGF-23.
It is not known whether the long-term use of phosphate binder therapy alters NPT2b gene expression and/or GI physiology in humans, and whether different phosphate binders might exhibit different effects on these parameters. Although the animal data may not be directly applicable to humans, the results from this study suggest that 1 month of dosing with VS-501 or sevelamer in 5/6 NX rats had no significant effects on intestinal histology and NPT2b gene expression.
In conclusion, this study suggests that VS-501 is effective in binding phosphate with low swell volume and without an effect on calcium homeostasis. In addition, due to its high density, the pill size/number can be significantly reduced compared to sevelamer.
Acknowledgments
The project described was supported by grant number SBIR 1R43DK096698-01 from the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH awarding component. A portion of this study was conducted in the Center for Cardiovascular Research Physiology Core facility via the Research Resources Center at the University of Illinois at Chicago. This manuscript is original work not previously published in any substantial part, and is not under consideration of publication elsewhere. The manuscript has been read and approved for submission by all authors. The signature of the corresponding author is on behalf of all the authors.
Glossary
- ANOVA
analysis of variance
- BW
body weight
- Ca
Calcium
- CKD
chronic kidney disease
- GI
gastrointestinal
- HCl
hydrochloride
- H-E
hematoxylin-eosin
- LVW
left-ventricle weight
- NX
nephrectomized
- Pi
phosphate
- PTH
parathyroid hormone
- Real-time RT-PCR
Real-time reverse transcription PCR
- SD
Sprague–Dawley
Disclosure
Chen, Wessale, and Wu-Wong work for Vidasym.
References
- Bellasi A, Mandreoli M, Baldrati L, Corradini M, Di Nicolo P, Malmusi G, et al. Chronic kidney disease progression and outcome according to serum phosphorus in mild-to-moderate kidney dysfunction. Clin J Am Soc Nephrol. 2011;6:883–891. doi: 10.2215/CJN.07810910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, et al. Effects of Phosphate Binders in Moderate CKD. J Am Soc Nephrol. 2012;23:1407–1415. doi: 10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SK, Dillon MA, Hemken DE, Rezabek MS, Balwit JM. Meta-analysis of the effect of sevelamer on phosphorus, calcium, PTH, and serum lipids in dialysis patients. Adv Ren Replace Ther. 2003;10:133–145. doi: 10.1053/jarr.2003.50016. [DOI] [PubMed] [Google Scholar]
- Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4:1089–1096. doi: 10.2215/CJN.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddington H, Hoefield R, Sinha S, Chrysochou C, Lane B, Foley RN, et al. Serum phosphate and mortality in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:2251–2257. doi: 10.2215/CJN.00810110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley RN, Wang C, Collins AJ. Cardiovascular risk factor profiles and kidney function stage in the US general population: the NHANES III study. Mayo Clin Proc. 2005;80:1270–1277. doi: 10.4065/80.10.1270. [DOI] [PubMed] [Google Scholar]
- Fournier A. Greater PTH suppression in dialysis patients taking sevelamer hydrochloride (Renagel) Clin Nephrol. 2000;54:81–82. [PubMed] [Google Scholar]
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- Gupta A, Winer K, Econs MJ, Marx SJ, Collins MT. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab. 2004;89:4489–4492. doi: 10.1210/jc.2004-0724. [DOI] [PubMed] [Google Scholar]
- Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- K/DOQI. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201. [PubMed] [Google Scholar]
- Iwata Y, Wada T, Yokoyama H, Toyama T, Kitajima S, Okumura T, et al. Effect of sevelamer hydrochloride on markers of bone turnover in Japanese dialysis patients with low biointact PTH levels. Intern Med. 2007;46:447–452. doi: 10.2169/internalmedicine.46.6338. [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- Karczmarewicz E, Czekuc-Kryskiewicz E, Lorenc RS. Pathologies of calcium-phosphate homeostasis. Postepy Biochem. 2012;58:474–477. [PubMed] [Google Scholar]
- Martin KJ, Gonzalez EA. Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: what is normal, when to start, and how to treat? Clin J Am Soc Nephrol. 2011;6:440–446. doi: 10.2215/CJN.05130610. [DOI] [PubMed] [Google Scholar]
- Moldovan D, Moldovan I, Rusu C, Kacso I, Patiu IM, Gherman-Caprioara M. FGF-23, vascular calcification, and cardiovascular diseases in chronic hemodialysis patients. Int Urol Nephrol. 2013;46:121–128. doi: 10.1007/s11255-013-0422-2. [DOI] [PubMed] [Google Scholar]
- Nakane M, Ma J, Rose AE, Osinski MA, Wu-Wong JR. Differential effects of Vitamin D analogs on calcium transport. J Steroid Biochem Mol Biol. 2007;103:84–89. doi: 10.1016/j.jsbmb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ozkok A, Kekik C, Karahan GE, Sakaci T, Ozel A, Unsal A, et al. FGF-23 associated with the progression of coronary artery calcification in hemodialysis patients. BMC Nephrol. 2013;14:241. doi: 10.1186/1471-2369-14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Fukazawa S, Yasuda T, Akatsuka T, Tozawa S, Niida Y, et al. Treatment with phosphate binder (sevelamer hydrochloride, calcium carbonate) based on PTH. Clin Calcium. 2005;15(Suppl. 1):23–28. discussion 28–29. [PubMed] [Google Scholar]
- Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- Wang S, Alfieri T, Ramakrishnan K, Braunhofer P, Newsome BA. Serum phosphorus levels and pill burden are inversely associated with adherence in patients on hemodialysis. Nephrol Dial Transplant. 2013 doi: 10.1093/ndt/gft280. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. J Am Soc Nephrol. 2010;21:1427–1435. doi: 10.1681/ASN.2009121293. [DOI] [PubMed] [Google Scholar]
- Wu-Wong JR. 2013. Iron-fiber composition, preparation and uses thereof. WO 2013/056085 A1 (published 18 April 2013)
- Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE. Elevated phosphorus modulates vitamin D receptor-mediated gene expression in human vascular smooth muscle cells. Am J Physiol Renal Physiol. 2007;293:F1592–F1604. doi: 10.1152/ajprenal.00492.2006. [DOI] [PubMed] [Google Scholar]
- Wu-Wong JR, Nakane M, Gagne GD, Brooks KA, Noonan WT. Comparison of the pharmacological effects of paricalcitol and doxercalciferol on the factors involved in mineral homeostasis. Int J Endocrinol. 2010;2010:1–8. doi: 10.1155/2010/621687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Wong JR, Kawai M, Chen YW, Nakane M. VS-105: a novel vitamin D receptor modulator with cardiovascular protective effects. Br J Pharmacol. 2011;164:551–560. doi: 10.1111/j.1476-5381.2011.01473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Wong JR, Kawai M, Chen YW, Wessale JL, Huang CJ, Wu MT, et al. Two novel vitamin D receptor modulators with similar structures exhibit different hypercalcemic effects in 5/6 nephrectomized uremic rats. Am J Nephrol. 2013a;37:310–319. doi: 10.1159/000348755. [DOI] [PubMed] [Google Scholar]
- Wu-Wong JR, Nakane M, Chen YW. Mapping the time-dependent effects of paricalcitol on serum calcium, phosphorus and parathyroid hormone levels in 5/6 nephrectomized uremic rats. Life Sci. 2013b;92:161–166. doi: 10.1016/j.lfs.2012.11.018. [DOI] [PubMed] [Google Scholar]


