Abstract
Pain is the defining symptom of osteoarthritis (OA), yet available treatment options, of which NSAIDs are the most common, provide inadequate pain relief and are associated with serious health risks when used long term. Chronic pain pathways are subject to complex levels of control and modulation, both in the periphery and in the central nervous system. Ongoing clinical and basic research is uncovering how these pathways operate in OA. Indeed, clinical investigation into the types of pain associated with progressive OA, the presence of central sensitization, the correlation with structural changes in the joint, and the efficacy of novel analgesics affords new insights into the pathophysiology of OA pain. Moreover, studies in disease-specific animal models enable the unravelling of the cellular and molecular pathways involved. We expect that increased understanding of the mechanisms by which chronic OA-associated pain is generated and maintained will offer opportunities for targeting and improving the safety of analgesia. In addition, using clinical and genetic approaches, it might become possible to identify subsets of patients with pain of different pathophysiology, thus enabling a tailored approach to pain management.
Introduction
Pain is the defining clinical presentation of osteoarthritis (OA). Joint symptoms were responsible for 15 million visits to US physician’s offices in 2004 and are closely corre lated with functional limitations, leading to decreased productivity and impaired quality of life.1 Current management of OA pain falls short of patient needs both in terms of providing adequate and sustained pain relief and in terms of undesirable adverse events and health risks. Relief from pain is what drives people to seek out care, but available treatments provide modest relief at best. Inadequately controlled pain is the major reason for total joint replacement (TJR).2 The efficacy of ‘first-line’ agents such as paracetamol is hard to distinguish from placebo, and our most common effective therapies, NSAIDs and opioids, when used to treat OA, generally have effect sizes of ~0.3, which is considered small to moderate.3 The fact that placebo itself has been advanced as a possible therapeutic approach,4 and the vast use of unproven over-the-counter remedies, speaks to the need for more effective approaches to pain manage ment in this condition. Safety concerns with the currently available drugs are legion. For example, NSAIDs have long been associated with gastro intestinal adverse events, both minor and life-threatening,5 and have more recently been implicated in thrombotic cardio vascular disease.6 With opioids, constipation, nausea, dizziness and confusion limit use, and the abuse potential when used chronically is serious.7
Recent efforts in OA management have largely focused on arresting or slowing structural disease progression;8 how such interventions might affect pain, if at all, is not well understood. The demonstration that non-opioid agents that act on the central nervous system (CNS), such as serotonin–noradrenaline reuptake inhibitors (SNRIs), can modulate musculoskeletal pain,9 coupled with the reports of efficacy of purely peripherally-acting antibody therapy against nerve growth factor (NGF),10 provides important new insights into the patho physiology of OA pain and opens up avenues for the exploration of novel therapeutic approaches.
A careful look at the clinical presentation of OA pain offers additional appreciation of its mechanisms. Joint pain associated with OA has a strong mechanical component, triggered by specific activities (for instance, climbing stairs elicits knee pain) and relieved by rest.11 The pain becomes more constant over time, and has been described as “dull, aching, throbbing, punctuated increasingly with shorter episodes of a more intense, often unpredictable, and emotionally draining pain”.12 In advanced disease, neuropathic traits can be present, such as a burning sensation and ‘pins and needles’.13,14 Mood changes, sleep disturbances and fatigue are all part of chronic pain states, and are present in OA as well.15
Clinical investigators have reported signs of central sensitization,16 including reduced pain pressure thresholds,17 in patients with OA. Moreover, knee OA is associated with loss of proprioception, and cross-sectional studies have suggested a correlation between loss of proprioception and pain severity.18 Loss of proprioceptive acuity was a modest predictor of worsening of knee joint pain in a 30-month prospective study (n = 2,243).19 Deficits in vibratory sensation can be associated with OA of the knee and hip, and in the latter scenario even vibratory deficits in the upper extremities have been documented.20,21 Thus, an emerging clinical picture suggests that pain and complex somatosensory changes constitute an integral part of progressive OA.
This Review provides a mechanistic overview of how musculoskeletal pain in the context of OA can be generated and of the factors that are important for its chronification. A more thorough understanding of the pathophysiology of chronic OA pain, including its heterogeneity in clinical presentation and response to analgesic treatment, might lead to the development of mechanism-based therapies. Selected representative agents are reviewed here to highlight progress with this rational approach.
Pain mechanisms in osteoarthritis
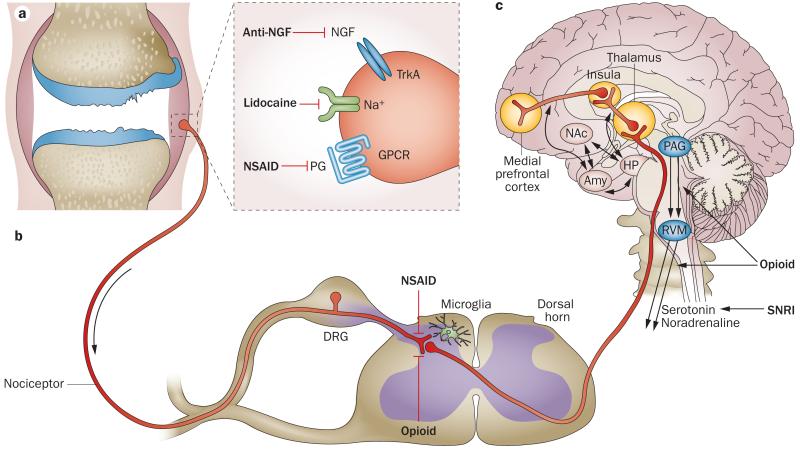
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”.22 Pain prompts behaviour to avoid situations that might lead to tissue damage (for example, withdrawal from a flame) and imposes immobilization of an injured tissue, which favours healing; thus, pain is vital to survival of the organism. In its most essential physiological form, pain equals nociception, occurring when afferent nociceptive neurons that innervate tissues are activated by a noxious (that is, high-threshold) stimulus of a mechanical, thermal or chemical nature (reviewed elsewhere23). Nociceptors are pseudo-unipolar cells, with an axonal stalk that extends from the cell body in the dorsal root ganglia (DRG) and splits into two terminals. The peripheral terminal innervates peripheral tissues and the central terminal extends to the dorsal horn of the spinal cord (Figure 1a,b). Thus, nociceptors can receive and send signals at both terminals. On the peripheral terminal, specific receptors—for example, heat receptors, chemoreceptors and mechanoreceptors—can detect specific stimuli. Activation of these receptors triggers a set of voltage-gated sodium and potassium channels that are essential for the generation and propagation of action potentials.23 These action potentials carry the ‘painful’ information along nociceptor axons, which are either medium-sized thinly myelinated Aδ-fibres or small unmyelinated C-fibres, to the dorsal horn of the spinal cord. Other neurons then transmit the signals further cephalad, to the brainstem, thalamus and cortex (Figure 1b,c). Detection, transmission and decoding of noxious stimuli are subject to complex control mechanisms at many levels in both the peripheral nervous system and the CNS, and are characterized by tremendous plasticity. Next, we provide an overview of this process, and describe how these levels and their controls are thought to be operating in OA.
Figure 1.
Neuroanatomy of the pain pathway and analgesic targets in OA. a | Pain signals are detected by nociceptors in the periphery and carried to the dorsal horn of the spinal cord. Various analgesics that are efficacious against joint pain act in the periphery by targeting receptors expressed at nociceptor peripheral terminals. b | The central terminals of the afferent nociceptors synapse with second-order neurons in the dorsal horn, in a stratified pattern that is anatomically very precisely organized. Second-order neurons are either interneurons (not shown) or projection neurons that cross to the contralateral side and carry the signal up the spinal cord. Central sensitization can occur through the strengthening of synapses and through the loss of inhibitory mechanisms. In addition, the activation of microglia contributes to enhanced pain sensitivity. Prostaglandins can also have a sensitizing effect in the dorsal horn, and NSAIDs can thus exert central analgesic actions, in addition to their peripheral actions. Opioids can inhibit incoming pain signals in the dorsal horn. c | Projection neurons relay pain signals along the spinothalamic tract to the thalamus, and along the spinoreticulothalamic tract to the brainstem. From there, the signals can be propagated to different areas of the brain, including the cortex. Descending pathways (black arrows), both facilitating and inhibitory, modulate pain transmission; descending inhibitory pathways release noradrenaline and serotonin onto the spinal circuits. SNRIs engage these descending inhibitory pathways. RVM neurons are opioid sensitive, and morphine has an analgesic effect through engaging descending inhibition. Abbreviations: Amy, amygdala; DRG, dorsal root ganglion; GPCR, G-protein-coupled receptor; HP, hippocampus; NAc, nucleus accumbens; NGF, nerve growth factor; PAG, peri-aqueductal grey; PG, prostaglandin; RVM, rostral ventromedial medulla; SNRI, serotonin–noradrenaline reuptake inhibitor.
Activation and sensitization of joint afferents
The joint is a densely innervated organ, and its sensory innervation is geared predominantly towards proprioception and nociception, indicating how vital positioning sense and awareness of potentially injurious movement are to proper joint function. In rats and cats, reportedly 80% of all afferent neurons in the knee joint are nociceptors.24 In humans, articular branches of the tibial nerve that innervate the posterior knee joint capsule contain 70–80% unmyelinated C-fibres and sympathetic nerves (which have also been associated with pain).25 Nociceptors are abundant in the joint capsule, ligaments, periosteum, menisci, subchondral bone and synovium.26–29 Thus, pain can originate from many articu lar tissues, and a study in 20 patients demonstrating that an intra-articular local anaesthetic abolishes OA knee pain suggests that these structures are in contact with the intra-articular environment.30 A fascinating experiment was reported by researchers who consciously mapped the pain sensation of intra-articular structures in the knee of an intrepid co-author.31 They found that the most sensitive areas were the anterior synovium, fat pad and joint capsule. Cartilage surfaces transmitted no pain, which is in line with cartilage being aneural.31
The fast-growing literature exploring the relationship between pain and structural changes in the joint is shedding more light on which structures in the OA joint might generate pain. The documented discordance between radiographic severity and pain, particularly for knee OA,32,33 has underscored the poor correlation between structural changes in the joint and the severity of pain.34 Several studies, however, are reporting stronger associations than previously proposed. For example, one study reported that in individuals with knee pain and symptoms, those with worse pain and disability were more likely to have radiographic OA.35 Another study showed that radiographic OA and individual radiographic features of OA were strongly associated with knee pain. Specifically, joint space narrowing was more strongly associated with pain than were osteophytes.36 Moreover, painful knees display greater rates of medial cartilage loss, as assessed longitudinally by MRI, than knees without pain, with or without adjustment or stratification for radiographic disease stage.37 Specific MRI-detectable features that have been related to knee pain include bone marrow lesions (BMLs), subarticular bone attrition and synovitis (reviewed elsewhere38,39). Patients with chronic symptomatic OA of the knee experi ence fluctuations in the presence and intensity of knee pain, and changes in BMLs and synovitis, as demonstrated by MRI, are associated with these fluctuations.40 A randomized placebo-controlled clinical trial of intravenous zoledronic acid in patients with knee OA (n = 59) demonstrated significant reduction of BML size after 6 months (−175.7 mm2, 95% CI −327.2 to −24.3), with a trend after 12 months (−146.5 mm2, 95% CI −307.5 to +14.5), and of pain, as assessed by a visual analogue scale (VAS) score, after 6 months (−14.5 mm, 95% CI −28.1 to −0.9) (although not after 3 or 12 months).41 Histological evidence of synovitis has also been correlated with symptoms in patients with early knee OA undergoing arthroscopic meniscectomy.42
It is now fully appreciated that OA is a disease of the entire joint, not just the cartilage.43 This fact implies that, during the course of progressive OA, nociceptors are exposed to the changing biochemical environment in the different joint tissues. These changes might contribute to their activation and sensitization, as discussed below.
Mechanical stimulation
Mammalian molecular mechanisms of mechanosensation—the process by which a cell converts a mechanical stimulus into an electrical signal—are poorly understood.23 Only a few studies have addressed how mechanical pain is sensed in the joint. One such study used an electrophysiological approach to demonstrate the presence of mechanogated ion channels on rat knee joint nociceptors, suggesting that these channels might have a role in pain sensing.44 In addition, experiments in the rat monoiodoacetate (MIA) model, which is frequently used to model OA pain pathways,45 have found a role for the voltage-gated sodium channel Nav1.8 in noxious mechanosensation in the joint.46 Nav1.8 is restricted in its expression to small primary afferent neurons47 and has been implicated in noxious mechanosensation in mice.48 Approximately 50% of C-fibres and 10% of Aδ-fibres in normal rats express Nav1.8,49 and expression levels were shown to be increased in afferent neurons that innervate inflamed rat knees.50
Inflammatory mediators in the joint
Nociceptors express a broad range of receptors for ligands that can change the properties of these neurons, such that they require lower thresholds to fire action potentials or even fire spontaneously when the receptors are engaged. These ligands include cytokines, chemokines, neuropeptides and prostaglandins,51 which can all form part of the biochemical milieu in the OA joint. As a result of this peripheral sensitization, joint movement within the normal range becomes painful (a phenomenon known as mechanical allodynia).
It has long been known that injecting proinflammatory agents into the joint cavity sensitizes afferent neurons.52,53 Now being established is how specific cytokines can sensitize neurons following intra-articular injection; such cytokines include TNF54 and IL-6,55 which cause prolonged mechanical hypersensitivity,56 and IL-17, which sensitizes joint nociceptors to mechanical stimuli.57 The proinflammatory cytokines IL-1β and TNF can directly affect sensory neurons and can also trigger hyperalgesia via downstream mediators such as other cytokines, chemokines, prostanoids, neurotrophins, nitric oxide, kinins, lipids, ATP and members of the complement pathway (reviewed elsewhere51). Nonetheless, how these processes operate in OA is still unclear.
In addition to increasing cytokine levels, inflammation enhances local levels of NGF, a major contributor to peripheral hypersensitivity.58 Indeed, NGF has been shown to elicit a pain response following injection in humans.59 The binding of NGF to its high-affinity receptor, TrkA, on peripheral nociceptors causes rapid potentiation of the thermosensitive ion channel TRPV1 (transient receptor potential cation channel, sub family V, member 1).60 TRPV1 is present in a subpopulation of primary afferent sensory neurons and is activated by noxious heat and by certain chemicals, including capsaicin.61 Sensitization of TRPV1 contributes to pain hypersensitivity associated with tissue injury.62 In addition, NGF taken up by peripheral nerve terminals is retrogradely transported to the cell body in the DRG, where it activates the mitogen-activated protein kinase p3863 and promotes expression of pronociceptive proteins, including substance P, TRPV1 and Nav1.8.63,64 Mast cells also express TrkA, and NGF binding to this receptor promotes mast-cell-mediated production of a range of additional proinflammatory molecules that can potentiate its activity.65
NGF can be produced by articular cartilage, meniscus and synovium, and increased levels have been reported in synovial fluid from patients with inflammatory arthritis.66–69 In a widely used surgical mouse model of OA, induced by destabilization of the medial meniscus (DMM),70 Ngf mRNA was present in joint extracts immediately after surgery (day 3) and again 16 weeks post-DMM, when the mice showed signs of pain, as assessed by hindlimb weight bearing.71 Injection of a soluble recombinant form of the NGF receptor, known as TrkAd5, suppressed pain in both phases.71 Blockade of NGF has been shown to be efficacious in many preclinical models of pain, including joint pain in rat autoimmune arthritis.72 However, results from studies with anti-NGF antibodies in preclinical models of OA are not available in the public domain.
Tissue damage and remodelling
Degradation and remodelling of joint tissues is the hallmark of OA pathology. In human OA and in animal models, blood vessels grow from the subchondral bone into the articular cartilage.73,74 Fine, unmyelinated nerves (C-fibres and sympathetic nerves) accompany these vessels.75 Moreover, this vascular and nerve growth has also been observed in the meniscus.76 Osteochondral and meniscal vascularization might thus be a source of arthritic pain and constitute a target for analgesic therapy.73
Unravelling the molecular mechanisms of nociceptor activation and sensitization in the OA joint will require more studies in animal models and patient cohorts. Scant data are available regarding the spatial and temporal changes in receptor expression on joint afferent neurons in OA. Furthermore, information on the expression and source of putative ligands that might sensitize these afferent neurons in the OA joint is also scarce. It is often noted that cartilage, as an aneural and avascular tissue, probably does not directly ‘hurt’. However, degrading cartilage is potentially a major source of factors that feed into the pain machinery, including cytokines, other mediators (such as H+ ions and adenosine) and possibly extra cellular matrix fragments. Nociceptors have been reported to express Toll-like receptors (TLRs), which are pattern-recognition receptors that recognize a variety of damage-associated molecular patterns (DAMPs) released during tissue injury and that contribute to pain generation.77–79 In OA, no information on the role of TLRs in pain generation is available, but it has been proposed that TLRs have a role in synovitis in OA.80,81 Thus, TLRs might contribute to pain in OA indirectly by activating synovial fibroblasts and macrophages,80 and potentially directly by sensitizing nociceptors.
Modulation in the DRG
Neuronal cell bodies in the DRG reside alongside small satellite glial cells and macrophages, and their interactions may promote the transition from acute to chronic pain (reviewed elsewhere82). In the slowly progressive mouse DMM model, the expression of the chemokine CCL2 (CC-chemokine ligand 2; also known as MCP1) and its receptor, CCR2 (CC-chemokine receptor 2), was increased in DRG neurons 8 weeks after surgery, but not at an earlier time point.83 This increase was accompanied by the infiltration of macrophages into the DRG and by the onset of pain behaviours.83 Ccr2-null mice did not develop these pain behaviours, and showed no macrophage infiltration in the DRG.83 DRG infiltration by macrophages also correlated with pain-related be haviours in rats with antigen-induced inflammatory arthritis.84 Evidence is growing that in the DRG, glial cells, neurons, and immune cells form an integrated network in which reciprocal signals dynamically modulate pain.82 However, in the context of OA or preclinical OA models, very little is known about the contribution of these DRG phenomena to chronic pain.
Central sensitization: spinal mechanisms
Central termini of afferent neurons enter the dorsal horn of the spinal cord and make their first synapse with interneurons or projection neurons (Figure 1b). Continued nociceptor input can lead to prolonged hyperexcitability of pain circuits in the CNS, a phe nomenon known as central sensitization.16 Many mechanisms contribute to central sensitization (reviewed in detail elsewhere85). Cellular processes involved are increased neuronal membrane excitability, synaptic facilitation, and disinhibition.85 Glial cells (microglia and astrocytes) also have a role in this process.86 Overall, central sensitization is the result of tremendous plasticity of the CNS, and it leads to increased spontaneous neuronal activity, reduced activation thresholds, and expansion of the receptive field, and is manifested as hyperalgesia (increased sensitivity to noxious stimuli) and allodynia (the interpretation of non-noxious stimuli as painful), even in areas outside the initial trigger zone.16
Central sensitization thus contributes to the lack of direct correlation between nociceptor activation and the pain experience, and this phenomenon is characteristic of chronic pain. Mounting evidence suggests that central sensitization phenomena are integral to OA pain. Indeed, several papers have reported tactile allodynia and lowered pain thresholds in OA.87–89 More recently, quantitative sensory testing has been more systematically explored in patients with OA. Findings have been extensively reviewed by Suokas et al.,17 who concluded that patients with OA display reduced pressure pain thresholds in both affected and unaffected sites.
Thus, central sensitization might contribute to the apparent disconnect between structural changes and pain. Indeed, a study reported that central sensitization in knee OA is especially apparent among patients with high levels of clinical pain in the absence of moderate-to-severe radiographic evidence.90 The exact mechanisms of central sensitization in OA are not known. However, as discussed, central sensitization occurs through plasticity of the CNS. Abnormalities in somatosensory perception are reversible after successful surgery or joint replacement, as reported for hip OA89 and knee OA.91 This reversibility further underlines the plasticity of the CNS and, mechanistically, implies that central sensitization is maintained by peripheral input from the joint.
Ascending pathways and supraspinal processing
From the dorsal horn, projection neurons relay pain signals to the thalamus and the brainstem, and onwards to higher brain centres (Figure 1c). The manner in which these signals are then processed differs substantially not only between acute and chronic pain but also among different chronic pain states. Indeed, neuroimaging studies of conditions such as chronic back pain, OA, post-herpetic neuropathy and pelvic pain all show distinct patterns of brain activity to either evoked or spontaneous pain; these conditions are particularly distinguished from acute pain by enhanced activation of the prefrontal cortex, an area of the brain engaged in emotional behaviour.92 A study in 14 patients with knee OA and 9 healthy controls revealed a dissociation between mechanically induced and spontaneous OA knee pain, the latter engaging brain regions involved in emotional assessment of the self.93 Thus, it can be concluded that spontaneous chronic pain involves emotional (affective) conditioning, and this verdict applies to chronic knee OA pain too.93,94 Brain anatomical changes, including loss of regional brain grey matter, have also been defined in chronic pain states.95,96 Interestingly, in OA, these anatomical changes have been shown to be reversible,96 reflecting brain plasticity. Associated with these anatomical changes are alterations in established brain functional networks (for example, the default mode network), which might reflect the importance of the alterations in functional connectivity observed in chronic pain states.97 Using these techniques, it might become possible to identify brain circuitry that identifies patients who are more prone to developing chronic pain, permitting better targeting of therapeutic interventions.98
In the past few years, attention has focused on a number of cortical and subcortical areas for their role in pain processing and the development of chronic pain states. In addition to the well-described anatomical changes in regional brain grey matter volume associated with chronic pain, changes in functional connectivity between cortical and subcortical areas have been defined that are important for pain chronification. In a longitudinal study of people with subacute lower back pain, which compared those whose pain persisted after 1 year with those who recovered, brain functional reorganization was shown to be coupled with grey matter changes and the persistence of pain.99 In this setting, functional connectivity between the nucleus accumbens and the medial prefrontal cortex was increased in individuals with persistent pain and, when assessed independently, was a predictor at baseline for the development of chronic pain. The nucleus accumbens is part of the mesolimbic system involved in emotion-driven reinforcement learning, and this learning circuitry is known to have a role in pain chronification.99 Thus, agents that target this and related pathways might provide an effective approach to pain management.
Descending pathways
In addition to the ascending pain pathways, descending pathways operate in the CNS, and these pathways can either facilitate or dampen pain.100,101 Descending signals come from the hypothalamus, amygdala and rostral an terior cingulate cortex, and radiate to the peri-aqueductal grey (PAG) in the midbrain and the rostral ventro medial medulla (RVM) in the brainstem. Neurons project from the RVM to the dorsal horn of the spinal cord and directly or indirectly enhance or dampen nociception. Descending inhibitory pathways modulate the response to pain through the release of noradrenaline and serotonin (also known as 5HT) (Figure 1c). RVM neurons are opioid sensitive, and engagement of descending inhibition by morphine accounts for a part of its analgesic effects (Figure 1c).
Conditioned pain modulation (CPM; formerly known as diffuse noxious inhibitory control) is a serotonergic mechanism, active in healthy individuals, whereby applying a noxious stimulus at a remote site inhibits pain at the initial site of pain.102 In patients with OA, as in many chronic pain sufferers, CPM is defective.103,104 However, this mechanism seems to be restored after successful surgery, when patients are in a pain-free state,103,104 which provides further evidence that chronic peripheral input maintains chronic pain-associated changes in the CNS.
Management options
Guidelines and/or recommendations for the management of OA have been updated and published by a number of professional groups, including the ACR,105 American Geriatrics Society,106 EULAR,107,108 UK National Institute for Health and Clinical Excellence,109 American Academy of Orthopaedic Surgeons110 and Osteoarthritis Research Society International (OARSI),3 among others. All of these sets of recommendations are based on results from existing clinical trials, which vary greatly in methodological rigour and quality, and they also include expert opinion to varying degrees. None of these recommendations is mechanism-based or gives substantial consideration to which part of the pain pathway might be modulated by treatment.
A wide variety of interventions have been evaluated in the management of OA, with greatest attention focused over the years on the effects of weight loss, exercise, acupuncture, nutraceuticals, paracetamol, NSAIDs, opioids and other centrally-acting drugs (such as antidepressants, and particularly SNRIs), as well as intra-articular therapy with glucocorticoid and hyaluronan preparations. The following discussion focuses on clinical data and pathophysiological correlates for three therapeutic approaches—NSAIDs, SNRIs and antibodies to NGF—to highlight how study of these agents has improved our understanding of pain mechanisms in OA.
NSAIDs
Clinical studies of NSAIDs have repeatedly demonstrated their efficacy, compared with placebo, in relieving pain and increasing function in people with OA.111,112 Their dose–response curve seems to be reasonably flat with regard to pain relief, such that only numerical and not statistically significant differences can be demonstrated for different doses of the same NSAID.113 Nevertheless, high doses are generally regarded as more efficacious and in clinical practice tend to be used to treat greater degrees of pain. However, only low doses of NSAIDs (specifically naproxen, ibuprofen and ketoprofen) are approved for sale without prescription in the USA to treat musculoskeletal pain for limited periods of time. Despite the widespread use of these drugs, their overall efficacy is remarkably limited. The effect size for NSAIDs in a recent OARSI meta-analysis (n = 14,523) was reported as 0.29 (95% CI 0.22–0.35),3 which signifies a small-to-moderate effect. More clinically meaningful is the evaluation of responders; that is, the percentage of patients meeting a pre-specified threshold of pain relief, often in the range of a 20–50% decrease in pain from baseline.114–116 This analysis permits the determination of the number-needed-to-treat (the number of patients who need to be treated for one to benefit), which can be more easily translated to the clinical setting than other measures of efficacy, such as effect size, mean change in WOMAC (Western Ontario and McMaster Universities Arthritis Index) or VAS pain scales. For NSAIDs, the number-needed-to-treat has not typically been reported in OA studies, but the values available are in the range of 3–5.117
Difficulty tolerating NSAID treatment owing to adverse effects including dyspepsia, nausea, vomiting, diarrhoea and rash is a common reason to discontinue use of these drugs, surpassing lack of efficacy in many reports.3 Serious NSAID-related adverse events—namely gastrointestinal bleeding and an increased incidence of myocardial infarctions—have resulted in a reassessment of how extensively, with which co-therapies and in which populations these drugs should be used. Individuals with a history of gastrointestinal ulcers or pre-existing cardiovascular disease should be treated with NSAIDs only if other therapies are not effective or appropriate, and only then with caution.105 The concomitant administration of gastroprotective agents has been shown to be effective at reducing the incidence of peptic ulcers.118 Whether aspirin co-therapy has a clinically meaningful effect on the thrombotic risk associated with NSAID use is not clear;119 however, data support a specific pharmacodynamic interaction between aspirin and ibuprofen, such that the beneficial anti-platelet effect of aspirin is significantly inhibited.120 This interaction is not seen with some other types of NSAID, namely selective cyclooxygenase 2 (COX2) inhibitors and diclofenac.121 These findings have led to the recommendation by the FDA that individuals on cardioprophylactic doses of aspirin requiring NSAID treatment carefully time the administration of both these drugs or consider treatment with either an analgesic that does not interfere with the anti-platelet effect of low-dose aspirin or a non-NSAID analgesic.122 Nevertheless, NSAIDs in general are and can be used safely in large numbers of individuals with OA, providing meaningful clinical benefit.
How do NSAIDs reduce pain in OA, and can our understanding of these mechanisms help to explain the limited efficacy reported? NSAIDs are potent inhibitors of the COX enzymes in humans, and thereby reduce the production of prostaglandins.123 Prostaglandins effectively sensitize peripheral nociceptors to painful stimuli, resulting in increased sensitivity to pain.124 In OA, considerable evidence shows that inflammatory processes known to result in prostaglandin production occur in the synovium, bone and surrounding joint tissues.125 Hence, in OA, NSAID activity might be based on the ability of these drugs to downregulate the peripheral production of neural sensitizers. In addition, it is now recognized that COX2 expression is upregulated in painful states not only in the periphery but also in spinal cord neurons;126 thus, NSAIDs, which can cross the blood–brain barrier, might also have a role centrally in modulating the pain response. Indeed, pilot data have demonstrated a relation ship between cerebrospinal fluid COX2 inhibitor levels and change in pain after NSAID treatment in patients with OA.93 Thus, NSAIDs might have dual sites of action, inhibiting the sensitization of peripheral nociceptors as well as acting centrally at the level of the spinal cord and brain (Figure 1a,b).
SNRIs
In contrast to early antidepressant drugs, which were selective serotonin reuptake inhibitors (SSRIs), newer antidepressants, including duloxetine, venlafaxine and milnacipran, inhibit both serotonin and noradrenaline reuptake in the CNS and have shown efficacy in the treatment of major depressive disorder and general anxiety disorder.127 Subsequent clinical trials with duloxetine also demonstrated its efficacy in reducing pain levels in patients with diabetic peripheral neuropathy as well as in individuals with fibromyalgia.128,129 Most recently, duloxetine has been shown to reduce pain in individuals with chronic back pain and in those with OA,130–132 leading to its approval in the USA by the FDA for the treatment of chronic musculoskeletal pain. In these OA and back pain studies, duloxetine at 60 mg twice daily resulted in statistically significant pain relief when used as either a monotherapy or an adjunctive therapy to existing analgesics, and titration to a higher dose did not result in greater efficacy.130–132 The magnitude of pain relief reported was similar to that observed for NSAIDs, and use of duloxetine in the OA study population resulted in the expected, known adverse effects of the drug, including an increase in blood pressure and heart rate, elevated liver function tests, nausea, dry mouth, fatigue, constipation, dizziness and increased sweating.133 Duloxetine offers an alternative to opioids for patients who are not able to take NSAIDs or who have a sub optimal response to NSAIDs and require adjunctive therapy. No head-to-head trials of duloxetine versus opioids or NSAIDs have been reported, so the relative efficacy, tolerability and safety of these drugs are not known.
As described above, duloxetine is thought to work by increasing local brain levels of serotonin and noradrenaline. The reduction in pain observed with duloxetine treatment has been ascribed to an increase in the tonic activity of the descending inhibitory pain pathways in the CNS (Figure 1c).100 Data on the pain-relieving effects of other SNRIs are sparse, although venlafaxine and milnacipran have been reported to be effective in some studies of fibromyalgia and neuropathic pain.134,135 No studies with these agents in musculoskeletal pain have been reported. The efficacy of duloxetine, a drug with activity in the CNS, in patients with OA supports the concept that OA pain pathways can respond to modulation within the CNS. This concept thus provides the rationale for targeting central pain pathways in the t reatment of OA pain.
Antibodies to NGF
Early clinical studies with antibodies to NGF in patients with OA, first reported in 2005,136 demonstrated the potential efficacy of this approach to treatment and led to a larger and more definitive phase II study of tanezumab (n = 450).10,136 This study provided clear data regarding the efficacy of this novel approach for reducing pain levels in OA, with a dose-dependent response evident for both efficacy and safety. Mean knee pain on walking was reduced by 45–62% with various doses of tanezumab as compared with 22% with placebo (P <0.001). The OMERACT–OARSI responder rate ranged from 74% to 93% in the tanezumab-treated groups versus 44% with placebo (P <0.001). The most common adverse effect possibly related to tanezumab treatment was paresthesias, reported in 7% of those treated. Several large phase III studies were then initiated with tanezumab,137–141 which confirmed the phase II efficacy data but also found an unexpected incidence of what seemed to be an accelerated OA process. This adverse event was much more common in study participants taking concomitant NSAIDs and was primarily limited to joints already affected by OA, leading to TJR in some patients.141 Because such findings were reported not only with tanezumab but also with other anti-NGF monoclonal antibodies in development,141 all clinical studies were put on hold by the FDA to allow further evaluation. An FDA advisory panel met in 2012 to review the data and concluded that additional clinical trials were warranted and could be undertaken with appropriate risk evaluation and mitigation programmes in place.141
A role for NGF in the pain pathway has been clearly demonstrated (see above) and, hence, reducing NGF levels is a rational approach to modulating pain. The mechanism(s) involved in NGF activity in pain have been extensively studied (see discussion above and elsewhere142,143). From a mechanistic perspective, what is important to note is that antibodies to NGF do not cross the blood–brain barrier and therefore function entirely in the periphery. The fact that a substantial subset of individuals report complete or almost complete pain relief after treatment with tanezumab, whereas others have little relief, supports the possibility that differing pain mechanisms are important in different individuals. Thus, further study of pain phenotypes might enable a more rational use of such targeted interventions.
Unresolved issues and clinical challenges
The clinical presentation of OA is heterogeneous, affecting different types and numbers of joints to varying degrees and resulting in pain that can be intermittent or persistent and can fluctuate with activity and in response to other external factors. The question arises as to whether, despite this heterogeneity of clinical presentations, it might be possible to define specific subsets of patients with OA on the basis of the pathophysiology of their pain, leading to a more focused and rational approach to therapeutic intervention.16 From the evidence discussed above, a defined group of patients with OA exists in whom central sensitization can be identified and in whom, presumably, this process might have a considerable role in their pain. The obvious next step would therefore seem to be to ask whether such patients are differentially responsive to centrally acting agents (such as SNRIs) versus peripherally acting agents (such as anti-NGF antibodies). Furthermore, we know from clinical trials of both SNRIs133 and antibodies to NGF137 that some individuals show a marked decrease in pain in response to treatment, whereas others have little or no response. It could be presumed that those individuals who are SNRI responders are those in whom central sensitization plays a more prominent part in pain potentiation and maintenance, whereas the pain of non-responders might be a consequence of a more purely acute, nociceptive type of pain input. The opposite might be the case for the pain response to anti-NGF antibodies137: responders have pain that is primarily peripherally driven, whereas non-responders have more central sensitization, which might have become less dependent on peripheral input for its maintenance. Further studies using these pharmacological agents as tools will help to answer questions such as these.
Maintenance of central sensitization in the apparent absence of peripheral input might also explain the finding that some patients report continued pain after what is an apparently technically successful TJR. Persistent pain occurs in 10–20% of patients following such surgery,144,145 with no explained aetiology. The magnitude of pain preoperatively is known to be a risk factor for poor outcome,146,147 whereas an inverse relationship between preoperative radiographic severity and postoperative pain after TJR has been reported.148 These data might point to the presence of a peripheral pain generator that is distinct from that typically observed in OA and that is not affected by joint replacement surgery. In addition, it is possible that, in a minority of indivi duals with long-standing chronic pain, irreversible changes in neural pain networks can occur. Whether such changes are related to the phenomenon that has been termed central sensitization is unknown. Variations exist in sensitivity to pain between individuals, and this variation might be genetically determined.149 Several genetic association studies in patients with symptomatic OA have been reported,150–155 but information on the genetic effect on OA pain is rather limited so far (Table 1). Nonetheless, in addition to potentially enabling the stratification of patients with OA, future genetic studies might shed light on molecular pathways of pain genesis in OA.
Table 1.
Genetic association studies of pain responses in OA
| Gene | Encoded protein |
Variant | Cohort | Association | Reference |
|---|---|---|---|---|---|
| TRPV1 | TRPV1 | 585 Ile–Ile* | Multiple OA cohorts (3,270 symptomatic knee OA cases, 1,098 asymptomatic cases, 3,852 controls) |
This variant (which has also been associated with reduced thermal pain sensitivity) was associated with a reduced risk of symptomatic knee OA |
Valdes et al.150 |
| PCSK6 | PACE4 | Noncoding | Multiple OA cohorts (2,068 symptomatic knee OA cases, 674 asymptomatic cases) |
Associated with protection against pain in knee OA |
Malfait et al.151 |
| P2RX7 | P2X7 | Hypofunctional Arg270His variant (impaired pore formation) |
One OA cohort (743 cases, 586 controls) |
Associated with reduced pain intensity |
Sorge et al.152 |
| COMT | COMT | Reduced activity Val158Met variant |
One OA cohort (n = 171) | Associated with increased pain | van Meurs et al.153 |
| SCN9A | Nav1.7 | Arg1150Trp variant is predicted to have increased activity |
One OA cohort (n = 578) Multiple OA cohorts (n = 1,854) |
Associated with higher pain scores No association with OA pain‡ |
Reimann et al.154 Valdes et al.155 |
An amino acid variant, Ile585Val, of TRPV1 has been reported to be associated with thermal pain sensitivity, with the Ile–Ile genotype corresponding to lower sensitivity to cold pain.
This variant was significantly associated with an increased risk of chronic widespread pain (CWP), which might imply that different mechanisms operate in CWP and in OA.155 Abbreviations: COMT, catechol O-methyltransferase; Nav1.7, voltage-gated sodium channel subunit α Nav1.7; OA, osteoarthritis; P2X7, P2X purinoceptor 7; PACE4, paired basic amino acid cleaving enzyme 4; TRPV1, transient receptor potential cation channel, subfamily V, member 1.
Conclusions
Current evidence supports the idea that OA pain is generated and maintained through continuous nociceptive input from the arthritic joint. During the progression of OA, chronic pain is modulated at different levels in the periphery and the CNS. Future research in human OA and in disease-specific animal models should enable us to elucidate how these cellular and molecular mechanisms operate at different stages of OA. This understanding should offer tremendous opportunity for targeted and safer analgesic intervention—perhaps tailored to subsets of patients depending on the stage of OA, the presence of central sensitization or the genetic makeup of the individual.
Key points.
Current evidence supports the idea that osteoarthritis (OA) pain is generated and maintained through continuous nociceptive input from the joint
Chronic OA pain is associated with changes in the central nervous system (CNS); these changes are reversible, reflecting the plasticity of the CNS and the requirement for continuous input from the periphery
Antibodies to nerve growth factor, which do not cross the blood–brain barrier and therefore act entirely through effects in the periphery, are effective at relieving OA pain
OA pain pathways can also respond to modulation centrally, as exemplified by data from OA pain trials with duloxetine, thus offering opportunity for the identification of new targets for pain relief
Heterogeneity in the clinical presentation of OA pain and in the response to analgesic therapies suggests that, in the future, distinct mechanism-based therapeutic approaches could be tailored to specific subsets of patients
Acknowledgements
A.-M. Malfait acknowledges funding from the US National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR060364 and R01AR064251) and from the Arthritis Foundation. The funding sources had no role in the preparation of this publication.
Footnotes
Competing interests
A.-M. Malfait declares an association with the following company: Allergan. T. J. Schnitzer declares associations with the following companies: Lilly, Janssen, Pfizer and Regeneron. See the article online for full details of the relationships.
Author contributions
Both authors contributed substantially to all aspects of the preparation of this manuscript.
Contributor Information
Anne-Marie Malfait, Department of Medicine, Section of Rheumatology, and Department of Biochemistry, Rush University Medical Center, 1611 W. Harrison Street, Chicago, IL 60612, USA.
Thomas J. Schnitzer, Department of Physical Medicine and Rehabilitation and Department of Internal Medicine, Northwestern University Feinberg School of Medicine, 710 N. Lake Shore Drive, Chicago, IL 60611, USA
References
- 1.Hing E, Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2004 summary. Advance Data. 2006;374:1–33. [PubMed] [Google Scholar]
- 2.Hawker GA. Differences between men and women in the rate of use of hip and knee arthroplasty. N. Engl. J. Med. 2000;342:1016–1022. doi: 10.1056/NEJM200004063421405. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Doherty M, Dieppe P. The “placebo” response in osteoarthritis and its implications for clinical practice. Osteoarthritis Cartilage. 2009;17:1255–1262. doi: 10.1016/j.joca.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N. Engl. J. Med. 1999;340:1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 6.Kearney PM. Do selective cyclooxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ. 2006;174:1589–1594. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Graverand-Gastineau M. Disease modifying osteoarthritis drugs: facing development challenges and choosing molecular targets. Curr. Drug Targets. 2010;11:528–535. doi: 10.2174/138945010791011893. [DOI] [PubMed] [Google Scholar]
- 9.Stahl SM, Grady MM, Moret C, Briley M. SNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectr. 2005;10:732–747. doi: 10.1017/s1092852900019726. [DOI] [PubMed] [Google Scholar]
- 10.Lane NE. Tanezumab for the treatment of pain from osteoarthritis of the knee. N. Engl. J. Med. 2010;363:1521–1531. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felson DT. Developments in the clinical understanding of osteoarthritis. Arthritis Res. Ther. 2009;11:203. doi: 10.1186/ar2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawker GA. Understanding the pain experience in hip and knee osteoarthritis—an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16:415–422. doi: 10.1016/j.joca.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Hochman JR, French MR, Bermingham SL, Hawker GA. The nerve of osteoarthritis pain. Arthritis Care Res. 2010;62:1019–1023. doi: 10.1002/acr.20142. [DOI] [PubMed] [Google Scholar]
- 14.Hochman JR, Gagliese L, Davis AM, Hawker GA. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage. 2011;19:647–654. doi: 10.1016/j.joca.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Hawker GA. Experiencing painful osteoarthritis: what have we learned from listening? Curr. Opin. Rheumatol. 2009;21:507–512. doi: 10.1097/BOR.0b013e32832e99d7. [DOI] [PubMed] [Google Scholar]
- 16.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suokas AK. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20:1075–1085. doi: 10.1016/j.joca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Sharma L. Proprioceptive impairment in knee osteoarthritis. Rheum. Dis. Clin. North Am. 1999;25:299–314. doi: 10.1016/s0889-857x(05)70069-7. [DOI] [PubMed] [Google Scholar]
- 19.Felson DT. The effects of impaired joint position sense on the development and progression of pain and structural damage in knee osteoarthritis. Arthritis Rheum. 2009;61:1070–1076. doi: 10.1002/art.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shakoor N, Agrawal A, Block JA. Reduced lower extremity vibratory perception in osteoarthritis of the knee. Arthritis Rheum. 2008;59:117–121. doi: 10.1002/art.23241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shakoor N, Lee KJ, Fogg LF, Block JA. Generalized vibratory deficits in osteoarthritis of the hip. Arthritis Rheum. 2008;59:1237–1240. doi: 10.1002/art.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International Association for the Study of Pain. IASP. [online], http://www.iasp-pain.org.
- 23.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDougall JJ. Arthritis and pain. Neurogenic origin of joint pain. Arthritis Res. Ther. 2006;8:220. doi: 10.1186/ar2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hines AE, Birn H, Teglbjaerg PS, Sinkjaer T. Fiber type composition of articular branches of the tibial nerve at the knee joint in man. Anat. Rec. 1996;246:573–578. doi: 10.1002/(SICI)1097-0185(199612)246:4<573::AID-AR18>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Freeman MA, Wyke B. The innervation of the knee joint. An anatomical and histological study in the cat. J. Anat. 1967;101:505–532. [PMC free article] [PubMed] [Google Scholar]
- 27.Hukkanen M. Innervation of bone from healthy and arthritic rats by substance P and calcitonin gene related peptide containing sensory fibers. J. Rheum. 1992;19:1252–1259. [PubMed] [Google Scholar]
- 28.Nixon AJ, Cummings JF. Substance P immunohistochemical study of the sensory innervation of normal subchondral bone in the equine metacarpophalangeal joint. Am. J. Vet. Res. 1994;55:28–33. [PubMed] [Google Scholar]
- 29.McDougall JJ, Bray RC, Sharkey KA. Morphological and immunohistochemical examination of nerves in normal and injured collateral ligaments of rat, rabbit, and human knee joints. Anat. Rec. 1997;248:29–39. doi: 10.1002/(SICI)1097-0185(199705)248:1<29::AID-AR4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 30.Creamer P, Hunt M, Dieppe P. Pain mechanisms in osteoarthritis of the knee: effect of intraarticular anesthetic. J. Rheum. 1996;23:1031–1036. [PubMed] [Google Scholar]
- 31.Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am. J. Sports Med. 1998;26:773–777. doi: 10.1177/03635465980260060601. [DOI] [PubMed] [Google Scholar]
- 32.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J. Rheum. 2000;27:1513–1517. [PubMed] [Google Scholar]
- 33.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet. Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365:965–973. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 35.Duncan R, et al. Symptoms and radiographic osteoarthritis: not as discordant as they are made out to be? Ann. Rheum. Dis. 2007;66:86–91. doi: 10.1136/ard.2006.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neogi T, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckstein F. Greater rates of cartilage loss in painful knees than in pain-free knees after adjustment for radiographic disease stage: data from the osteoarthritis initiative. Arthritis Rheum. 2011;63:2257–2267. doi: 10.1002/art.30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felson DT. Imaging abnormalities that correlate with joint pain. Br. J. Sports Med. 2011;45:289–291. doi: 10.1136/bjsm.2010.081398. [DOI] [PubMed] [Google Scholar]
- 39.Yusuf E, Kortekaas MC, Watt I, Huizinga TW, Kloppenburg M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann. Rheum. Dis. 2011;70:60–67. doi: 10.1136/ard.2010.131904. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 2011;63:691–699. doi: 10.1002/art.30148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laslett LL. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann. Rheum. Dis. 2012;71:1322–1328. doi: 10.1136/annrheumdis-2011-200970. [DOI] [PubMed] [Google Scholar]
- 42.Scanzello CR. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 2011;63:391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heppelmann B, McDougall JJ. Inhibitory effect of amiloride and gadolinium on fine afferent nerves in the rat knee: evidence of mechanogated ion channels in joints. Exp. Brain Res. 2005;167:114–118. doi: 10.1007/s00221-005-0040-z. [DOI] [PubMed] [Google Scholar]
- 45.Little CB, Zaki S. What constitutes an “animal model of osteoarthritis”—the need for consensus? Osteoarthritis Cartilage. 2012;20:261–267. doi: 10.1016/j.joca.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Schuelert N, McDougall JJ. Involvement of Nav 1.8 sodium ion channels in the transduction of mechanical pain in a rodent model of osteoarthritis. Arthritis Res. Ther. 2012;14:R5. doi: 10.1186/ar3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- 48.Raouf R. Sodium channels and mammalian sensory mechanotransduction. Mol. Pain. 2012;8:21. doi: 10.1186/1744-8069-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amaya F. Diversity of expression of the sensory neuron-specific TTX-resistant voltagegated sodium ion channels SNS and SNS2. Mol. Cell. Neurosci. 2000;15:331–342. doi: 10.1006/mcne.1999.0828. [DOI] [PubMed] [Google Scholar]
- 50.Strickland IT. Changes in the expression of NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. Eur. J. Pain. 2008;12:564–572. doi: 10.1016/j.ejpain.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb. Exp. Pharmacol. 2009;194:417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coggeshall RE, Hong KA, Langford LA, Schaible HG, Schmidt RF. Discharge characteristics of fine medial articular afferents at rest and during passive movements of inflamed knee joints. Brain Res. 1983;272:185–188. doi: 10.1016/0006-8993(83)90379-7. [DOI] [PubMed] [Google Scholar]
- 53.Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J. Neurophysiol. 1985;54:1109–1122. doi: 10.1152/jn.1985.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 54.Richter F. Tumor necrosis factor causes persistent sensitization of joint nociceptors to mechanical stimuli in rats. Arthritis Rheum. 2010;62:3806–3814. doi: 10.1002/art.27715. [DOI] [PubMed] [Google Scholar]
- 55.Brenn D, Richter F, Schaible HG. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum. 2007;56:351–359. doi: 10.1002/art.22282. [DOI] [PubMed] [Google Scholar]
- 56.Schaible HG. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann. NY Acad. Sci. 2010;1193:60–69. doi: 10.1111/j.1749-6632.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- 57.Richter F. Interleukin17 sensitizes joint nociceptors to mechanical stimuli and contributes to arthritic pain through neuronal interleukin17 receptors in rodents. Arthritis Rheum. 2012;64:4125–4134. doi: 10.1002/art.37695. [DOI] [PubMed] [Google Scholar]
- 58.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 59.Svensson P, Cairns BE, Wang K, Arendt-Nielsen L. Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain. 2003;104:241–247. doi: 10.1016/s0304-3959(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 60.Chuang HH. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 61.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 62.Huang J, Zhang X, McNaughton PA. Inflammatory pain: the cellular basis of heat hyperalgesia. Curr. Neuropharmacol. 2006;4:197–206. doi: 10.2174/157015906778019554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 64.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 65.Bullock ED, Johnson EM., Jr. Nerve growth factor induces the expression of certain cytokine genes and bcl2 in mast cells. Potential role in survival promotion. J. Biol. Chem. 1996;271:27500–27508. doi: 10.1074/jbc.271.44.27500. [DOI] [PubMed] [Google Scholar]
- 66.Gigante A. Expression of NGF, Trka and p75 in human cartilage. Eur. J. Histochem. 2003;47:339–344. [PubMed] [Google Scholar]
- 67.Iannone F. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology. 2002;41:1413–1418. doi: 10.1093/rheumatology/41.12.1413. [DOI] [PubMed] [Google Scholar]
- 68.Barthel C, et al. Nerve growth factor and receptor expression in rheumatoid arthritis and spondyloarthritis. Arthritis Res. Ther. 2009;11:R82. doi: 10.1186/ar2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Halliday DA, Zettler C, Rush RA, Scicchitano R, McNeil JD. Elevated nerve growth factor levels in the synovial fluid of patients with inflammatory joint disease. Neurochem. Res. 1998;23:919–922. doi: 10.1023/a:1022475432077. [DOI] [PubMed] [Google Scholar]
- 70.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 71.McNamee KE. Treatment of murine osteoarthritis with TrkAd5 reveals a pivotal role for nerve growth factor in non-inflammatory joint pain. Pain. 2010;149:386–392. doi: 10.1016/j.pain.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 72.Shelton DL, Zeller J, Ho WH, Pons J, Rosenthal A. Nerve growth factor mediates hyperalgesia and cachexia in auto-immune arthritis. Pain. 2005;116:8–16. doi: 10.1016/j.pain.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 73.Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 2012;8:390–398. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 74.Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone. 2012;51:204–211. doi: 10.1016/j.bone.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Suri S. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann. Rheum. Dis. 2007;66:1423–1428. doi: 10.1136/ard.2006.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ashraf S. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann. Rheum. Dis. 2011;70:523–529. doi: 10.1136/ard.2010.137844. [DOI] [PubMed] [Google Scholar]
- 77.Acosta C, Davies A. Bacterial lipopolysaccharide regulates nociceptin expression in sensory neurons. J. Neurosci. Res. 2008;86:1077–1086. doi: 10.1002/jnr.21565. [DOI] [PubMed] [Google Scholar]
- 78.Kim D, You B, Lim H, Lee SJ. Toll-like receptor 2 contributes to chemokine gene expression and macrophage infiltration in the dorsal root ganglia after peripheral nerve injury. Mol. Pain. 2011;7:74. doi: 10.1186/1744-8069-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 2012;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr. Opin. Rheumatol. 2008;20:565–572. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 81.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013;5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller RE. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proc. Natl Acad. Sci. USA. 2012;109:20602–20607. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Segond von Banchet G. Experimental arthritis causes tumor necrosis factor-α-dependent infiltration of macrophages into rat dorsal root ganglia which correlates with pain-related behavior. Pain. 2009;145:151–159. doi: 10.1016/j.pain.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 85.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Driscoll SL, Jayson MI. Proceedings: Pain threshold (PT) analysis in patients with osteoarthritis of the hip. Ann. Rheum. Dis. 1975;34:195–196. doi: 10.1136/ard.34.2.195-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bajaj P, Graven-Nielsen T, Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain. 2001;93:107–114. doi: 10.1016/S0304-3959(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 89.Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur. J. Pain. 2000;4:229–238. doi: 10.1053/eujp.2000.0175. [DOI] [PubMed] [Google Scholar]
- 90.Finan PH. Quantitative sensory tests of central sensitization are associated with discordance between pain and radiographic severity in knee osteoarthritis. Arthritis Rheum. 2013;65:363–372. doi: 10.1002/art.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Graven-Nielsen T, Wodehouse T, Langford RM, Arendt-Nielsen L, Kidd BL. Normalization of widespread hyperesthesia and facilitated spatial summation of deep-tissue pain in knee osteoarthritis patients after knee replacement. Arthritis Rheum. 2012;64:2907–2916. doi: 10.1002/art.34466. [DOI] [PubMed] [Google Scholar]
- 92.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog. Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parks EL. Brain activity for chronic knee osteoarthritis: dissociating evoked pain from spontaneous pain. Eur. J. Pain. 2011;15:843. doi: 10.1016/j.ejpain.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kulkarni B. Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum. 2007;56:1345–1354. doi: 10.1002/art.22460. [DOI] [PubMed] [Google Scholar]
- 95.Apkarian AV. Cortical pathophysiology of chronic pain. Novartis Found. Symp. 2004;261:239–245. [PubMed] [Google Scholar]
- 96.Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum. 2010;62:2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- 97.Farmer MA, Baliki MN, Apkarian AV. A dynamic network perspective of chronic pain. Neurosci. Lett. 2012;520:197–203. doi: 10.1016/j.neulet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Apkarian AV. The brain in chronic pain: clinical implications. Pain Management. 2011;1:577–586. doi: 10.2217/pmt.11.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baliki MN. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012;15:1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Millan MJ. Descending control of pain. Prog. Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 101.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J. Clin. Invest. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davis MP. In: Research and Development of Opioid-Related Ligands. Ko M-C, Husbands SM, editors. ACS; 2013. pp. 9–38. Ch. 3. [Google Scholar]
- 103.Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88:69–78. doi: 10.1016/S0304-3959(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 104.Arendt-Nielsen L. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 105.Hochberg MC. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64:465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 106.Pharmacological management of persistent pain in older persons. J. Am. Geriatr. Soc. 2009;57:1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 107.Jordan KM. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT) Ann. Rheum. Dis. 2003;62:1145–1155. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang W. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann. Rheum. Dis. 2005;64:669–681. doi: 10.1136/ard.2004.028886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.The National Collaborating Centre for Chronic Conditions Osteoarthritis: national clinical guideline for care and management in adults. NICE. 2008 [online], http://www.nice.org.uk/nicemedia/pdf/cg059fullguideline.pdf.
- 110.Richmond J. American Academy of Orthopaedic Surgeons clinical practice guideline on the treatment of osteoarthritis (OA) of the knee. J. Bone Joint Surg. Am. 2010;92:990–993. doi: 10.2106/JBJS.I.00982. [DOI] [PubMed] [Google Scholar]
- 111.Towheed T, Shea B, Wells G, Hochberg M. Analgesia and non-aspirin, non-steroidal anti-inflammatory drugs for osteoarthritis of the hip. Cochrane Database Syst. Rev. 2000:CD000517. doi: 10.1002/14651858.CD000517. [DOI] [PubMed] [Google Scholar]
- 112.Watson MC, Brookes ST, Kirwan JR, Faulkner A. Non-aspirin, non-steroidal anti-inflammatory drugs for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2000:CD000142. doi: 10.1002/14651858.CD000142. [DOI] [PubMed] [Google Scholar]
- 113.McQuay HJ, Moore RA. Dose-response in direct comparisons of different doses of aspirin, ibuprofen and paracetamol (acetaminophen) in analgesic studies. Br. J. Clin. Pharmacol. 2007;63:271–278. doi: 10.1111/j.1365-2125.2006.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pham T. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12:389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 115.Dworkin RH. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 116.Dworkin RH. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146:238–244. doi: 10.1016/j.pain.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 117.Osiri M, Suarez-Almazor ME, Wells GA, Robinson V, Tugwell P. Number needed to treat (NNT): implication in rheumatology clinical practice. Ann. Rheum. Dis. 2003;62:316–321. doi: 10.1136/ard.62.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rostom A. Prevention of NSAID-related upper gastrointestinal toxicity: a meta-analysis of traditional NSAIDs with gastroprotection and COX2 inhibitors. Drug Healthc. Patient Saf. 2009;1:47–71. doi: 10.2147/dhps.s4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Strand V. Are COX2 inhibitors preferable to non-selective non-steroidal anti-inflammatory drugs in patients with risk of cardiovascular events taking low-dose aspirin? Lancet. 2007;370:2138–2151. doi: 10.1016/S0140-6736(07)61909-6. [DOI] [PubMed] [Google Scholar]
- 120.Catella-Lawson F. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N. Engl. J. Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 121.Hohlfeld T, Saxena A, Schror K. High on treatment platelet reactivity against aspirin by non-steroidal anti-inflammatory drugs—pharmacological mechanisms and clinical relevance. Thromb. Haemost. 2013;109:825–833. doi: 10.1160/TH12-07-0532. [DOI] [PubMed] [Google Scholar]
- 122.US Food and Drug Administration Information for healthcare professionals: concomitant use of ibuprofen and aspirin. FDA. [online], http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm125222.htm.
- 123.Moncada S, Vane JR. Mode of action of aspirin-like drugs. Adv. Intern. Med. 1979;24:1–22. [PubMed] [Google Scholar]
- 124.Momin A, McNaughton PA. Regulation of firing frequency in nociceptive neurons by proinflammatory mediators. Exp. Brain Res. 2009;196:45–52. doi: 10.1007/s00221-009-1744-2. [DOI] [PubMed] [Google Scholar]
- 125.Adatia A, Rainsford KD, Kean WF. Osteoarthritis of the knee and hip. Part I: aetiology and pathogenesis as a basis for pharmacotherapy. J. Pharm. Pharmacol. 2012;64:617–625. doi: 10.1111/j.2042-7158.2012.01458.x. [DOI] [PubMed] [Google Scholar]
- 126.Vardeh D. COX2 in CNS neural cells mediates mechanical inflammatory pain hypersensitivity in mice. J. Clin. Invest. 2009;119:287–294. doi: 10.1172/JCI37098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gupta S, Nihalani N, Masand P. Duloxetine: review of its pharmacology, and therapeutic use in depression and other psychiatric disorders. Ann. Clin. Psychiatry. 2007;19:125–132. doi: 10.1080/10401230701333319. [DOI] [PubMed] [Google Scholar]
- 128.Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurol. 2008;8:29. doi: 10.1186/1471-2377-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy or chronic pain. Cochrane Database Syst. Rev. 2009:CD007115. doi: 10.1002/14651858.CD007115.pub2. [DOI] [PubMed] [Google Scholar]
- 130.Chappell AS. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain. 2009;146:253–260. doi: 10.1016/j.pain.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 131.Skljarevski V. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine. 2010;35:E578–E585. doi: 10.1097/BRS.0b013e3181d3cef6. [DOI] [PubMed] [Google Scholar]
- 132.Chappell AS. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Pract. 2011;11:33–41. doi: 10.1111/j.1533-2500.2010.00401.x. [DOI] [PubMed] [Google Scholar]
- 133.Hochberg MC, Wohlreich M, Gaynor P, Hanna S, Risser R. Clinically relevant outcomes based on analysis of pooled data from 2 trials of duloxetine in patients with knee osteoarthritis. J. Rheum. 2012;39:352–358. doi: 10.3899/jrheum.110307. [DOI] [PubMed] [Google Scholar]
- 134.Lee YC, Chen PP. A review of SSRIs and SNRIs in neuropathic pain. Expert Opin. Pharmacother. 2010;11:2813–2825. doi: 10.1517/14656566.2010.507192. [DOI] [PubMed] [Google Scholar]
- 135.Derry S, Gill D, Phillips T, Moore RA. Milnacipran for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2012;3:CD008244. doi: 10.1002/14651858.CD008244.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lane NE, et al. RN624 (anti-NGF) improves pain and function in subjects with moderate knee osteoarthritis: a phase I study. Arthritis Rheum. 2005;52:S461. [Google Scholar]
- 137.Brown MT. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J. Pain. 2012;13:790–798. doi: 10.1016/j.jpain.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 138.Balanescu AR, et al. Efficacy and safety of tanezumab added on to diclofenac sustained release in patients with knee or hip osteoarthritis: a double-blind, placebo-controlled, parallel-group, multicentre phase III randomised clinical trial. Ann. Rheum. Dis. doi: 10.1136/annrheumdis-2012-203164. http://dx.doi.org/10.1136/annrheumdis-2012-203164. [DOI] [PubMed]
- 139.Brown MT. Tanezumab reduces osteoarthritic hip pain: results of a randomized, double-blind, placebo-controlled phase III trial. Arthritis Rheum. 2013;65:1795–1803. doi: 10.1002/art.37950. [DOI] [PubMed] [Google Scholar]
- 140.Spierings EL, et al. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain. doi: 10.1016/j.pain.2013.04.035. http://dx.doi.org/10.1016/j.pain.2013.04.035. [DOI] [PubMed]
- 141.FDA Center For Drug Evaluation And Research Arthritis Advisory Committee Meeting: March 12, 2012. FDA. [online], http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM307880.pdf.
- 142.Hefti FF. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol. Sci. 2006;27:85–91. doi: 10.1016/j.tips.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 143.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu. Rev. Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 144.Bonnin MP, Basiglini L, Archbold HA. What are the factors of residual pain after uncomplicated TKA? Knee Surg. Sports Traumatol. Arthrosc. 2011;19:1411–1417. doi: 10.1007/s00167-011-1549-2. [DOI] [PubMed] [Google Scholar]
- 145.Hofmann S, Seitlinger G, Djahani O, Pietsch M. The painful knee after TKA: a diagnostic algorithm for failure analysis. Knee Surg. Sports Traumatol. Arthrosc. 2011;19:1442–1452. doi: 10.1007/s00167-011-1634-6. [DOI] [PubMed] [Google Scholar]
- 146.Fortin PR. Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999;42:1722–1728. doi: 10.1002/1529-0131(199908)42:8<1722::AID-ANR22>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 147.Lingard EA, Katz JN, Wright EA, Sledge CB. Predicting the outcome of total knee arthroplasty. J. Bone Joint Surg. Am. 2004;86A:2179–2186. doi: 10.2106/00004623-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 148.Valdes AM. Inverse relationship between preoperative radiographic severity and postoperative pain in patients with osteoarthritis who have undergone total joint arthroplasty. Semin. Arthritis Rheum. 2012;41:568–575. doi: 10.1016/j.semarthrit.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 149.Diatchenko L, Nackley AG, Tchivileva IE, Shabalina SA, Maixner W. Genetic architecture of human pain perception. Trends Genet. 2007;23:605–613. doi: 10.1016/j.tig.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 150.Valdes AM. The Ile585Val TRPV1 variant is involved in risk of painful knee osteoarthritis. Ann. Rheum. Dis. 2011;70:1556–1561. doi: 10.1136/ard.2010.148122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Malfait AM. A role for PACE4 in osteoarthritis pain: evidence from human genetic association and null mutant phenotype. Ann. Rheum. Dis. 2012;71:1042–1048. doi: 10.1136/annrheumdis-2011-200300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sorge RE. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat. Med. 2012;18:595–599. doi: 10.1038/nm.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.van Meurs JB. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628–629. doi: 10.1002/art.24175. [DOI] [PubMed] [Google Scholar]
- 154.Reimann F, et al. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc. Natl Acad. Sci. USA. 2010;107:5148–5153. doi: 10.1073/pnas.0913181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Valdes AM. Role of the Nav1.7 R1150W amino acid change in susceptibility to symptomatic knee osteoarthritis and multiple regional pain. Arthritis Care Res. 2011;63:1440–1444. doi: 10.1002/acr.20375. [DOI] [PubMed] [Google Scholar]