Abstract
Objectives. We aimed to assess the value of school-based eating disorder (ED) screening for a hypothetical cohort of US public school students.
Methods. We used a decision-analytic microsimulation model to model the effectiveness (life-years with ED and quality-adjusted life-years [QALYs]), total direct costs, and cost-effectiveness (cost per QALY gained) of screening relative to current practice.
Results. The screening strategy cost $2260 (95% confidence interval [CI] = $1892, $2668) per student and resulted in a per capita gain of 0.25 fewer life-years with ED (95% CI = 0.21, 0.30) and 0.04 QALYs (95% CI = 0.03, 0.05) relative to current practice. The base case cost-effectiveness of the intervention was $9041 per life-year with ED avoided (95% CI = $6617, $12 344) and $56 500 per QALY gained (95% CI = $38 805, $71 250).
Conclusions. At willingness-to-pay thresholds of $50 000 and $100 000 per QALY gained, school-based ED screening is 41% and 100% likely to be cost-effective, respectively. The cost-effectiveness of ED screening is comparable to many other accepted pediatric health interventions, including hypertension screening.
Eating disorders (EDs), including anorexia nervosa, bulimia nervosa, and binge-eating disorder, are prevalent among adolescents.1 Approximately 3.8% of females and 1.5% of males aged 13 to 18 years have an ED,2 and 16.3% of US 9th to 12th graders report engaging in disordered eating behaviors such as fasting or vomiting to lose weight.3 Although efficacious treatments for EDs exist,4 services for these conditions are underused.5 Seventy-eight percent to 88% of adolescents with EDs have contact with a health provider; of these youths, however, only 3% to 28% received treatment specifically for eating problems.1 Left untreated, EDs can significantly affect the length and quality of adolescent lives.6,7 ED medical complication, hospitalization, and mortality rates are the highest of any psychiatric disorder.8–11 Like many other chronic mental heath disorders, EDs can be costly to treat and place a considerable burden on patients and their caregivers. Estimates of the annual impact of EDs on health care costs and economic productivity in Australia and England range from US $1.8 billion to $19.2 billion.12–14 With early diagnosis and timely treatment, we may be able to decrease the economic and health burden of EDs.
The American Academy of Pediatrics suggests that schools are a viable setting for health screening.15 Scoliosis, hearing, body mass index, and other health screenings are currently conducted in US public schools or required for enrollment.16 Policies designed to identify secondary school students with ED have been introduced in several states (Figure A, available as a supplement to the online version of this article at http://www.ajph.org). As of September 2013, only 1 state passed legislation aimed at improving detection of EDs, requiring schools to educate parents on how to recognize symptoms of an ED. Three states are currently considering ED-related legislation and ED screening legislation has failed in 2 states (Taryn O’Brien, written communication, September 2013).
The impact of school-based screening on ED diagnosis and treatment duration is unknown. No studies have evaluated the health or economic impact of screening for EDs in school-based settings. Given the high proportion of EDs that remain undetected and the fact that no states currently mandate ED screening, experimentally evaluating the benefits of such screening programs in the real world would be resource intensive and may underestimate the potential benefits of screening. However, simulation models can be used to estimate the cost-effectiveness of screening with few constraints.17 We used a decision-analytic simulation model to evaluate the cost-effectiveness of a theoretical school-based ED screening program.
METHODS
ED definitions have evolved over time; the subtypes considered in this analysis are based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V).18 We considered 4 DSM-V ED subtypes: anorexia nervosa (AN), bulimia nervosa (BN), binge-eating disorder (BED), and other specified feeding or eating disorders (OSFED). To simplify our analysis, we categorized any children with subthreshold AN, BN, or BED as OSFED. Together, BED and OSFED make up a substantial portion of cases of EDs previously referred to as eating disorder not otherwise specified (ED-NOS) in the DSM-IV.
We evaluated the cost-effectiveness of a theoretical school-based ED screening program relative to the current practice of no screening. We developed our theoretical program following a review of existing and proposed obesity and ED screening programs and ED screening instruments. The theoretical screening program targeted a hypothetical cohort of 15.2 million 10- to 17-year-old males and females enrolled in US public schools.19 In this program, students would be asked to complete the SCOFF, a self-administered, easily scored, 5-question ED screening instrument that has been validated among multiple populations.20,21 We assumed that school staff would score the SCOFF and refer positive cases to a clinician for further evaluation and, if necessary, treatment. We assumed that screening would occur annually until age 18 years. We consulted representatives from the National Eating Disorders Association, a US community-based nonprofit organization, to review the feasibility and acceptability of the theoretical program (Taryn O’Brien, oral communication, October 2013).
Decision-Analytic Model
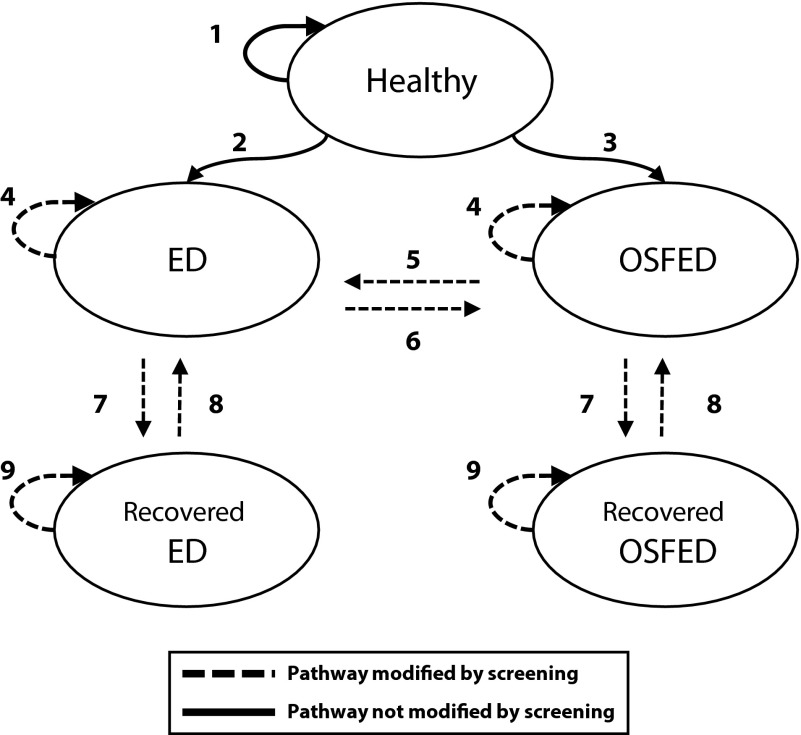
We developed a Markov-based decision-analytic microsimulation model in TreeAge (TreeAge Pro Healthcare; TreeAge Software Inc, Williamstown, MA) to estimate the potential costs and health benefits of the program in the intervention (screening) and the current practice (no screening) arms of the study. We modeled cases of AN, BN, BED, and OSFED that would develop and be treated over a 10-year horizon in each study arm, the cost of screening and diagnosis in the intervention arm, and the cost of ED treatment in both study arms. As seen in the simplified model schematic in Figure 1, we modeled transitions between 5 health states: being healthy, having a specified ED (AN, BN, or BED), having OSFED, and being recovered from a specified ED or OSFED. We assumed that students could only develop 1 specified ED in their lifetimes; for example, an adolescent who developed AN could never develop BN or BED. A more detailed schematic of the decision tree used in the model can be found in Figures B and C (available as supplements to the online version of this article at http://www.ajph.org).
FIGURE 1—
Schematic of Markov microsimulation model used to estimate the potential costs and health benefits of school-based eating disorder screening.
Note. ED = specified eating disorder; OSFED = other specified feeding or eating disorders. “Specified” ED represents anorexia nervosa, bulimia nervosa, or binge-eating disorder. Students move between each health state (circle) based on probabilities derived from the literature. Arrows indicate pathways between heath states. Dashed lines indicate pathways modified by screening: (1) stay healthy for another year, (2) develop ED, (3) develop OSFED, (4) remain ill for another year, (5) progress to ED, (6) partially recover from ED, (7) recover, (8) relapse, and (9) remain recovered for another year.
The simulation model automatically assigned each student a baseline age of 10 to 17 years, based on the distribution of ages in the US public school population.19 Students in the intervention arm of the model were screened annually until they reached age 18, at which point their probability of seeking clinical treatment of ED was the same as that of a person in the current practice arm.
Model Input Data
Transitions between health states were governed by epidemiological data on ED prevalence, diagnosis rates, treatment probabilities, and recovery rates. Table 1 describes model input parameters. For this analysis, we converted multiyear estimates to annual estimates.43
TABLE 1—
Model Input Parameters Used in Determining the Potential Cost-Effectiveness of School-Based Eating Disorder Screening
| Parameter | Mean Value (95% CI) | Statistical Distribution | Source |
| SCOFF, % | Cotton et al.22 | ||
| Sensitivity | 76 (62, 93) | Triangular | |
| Specificity | 88 (84, 93) | Triangular | |
| 12-mo prevalence of ED, % | National Comorbidity Survey, Swanson et al.1 | ||
| AN | 0.2 (0.10, 0.30) | Triangulara | |
| BN | 0.6 (0.29, 0.91) | Triangulara | |
| BED | 0.9 (0.59, 1.21) | Triangulara | |
| OSFED | 1.1 (0.86, 1.33) | Triangulara | |
| Probability of seeking clinical treatment (no screen), % | National Comorbidity Survey, Swanson et al.1 | ||
| AN | 27.5 (4.9, 50.1) | Triangulara | |
| BN | 21.5 (10.6, 32.4) | Triangulara | |
| BED | 11.4 (5.6, 17.2) | Triangulara | |
| OSFED | 3.4 (1.0, 5.8) | Triangulara | |
| Probability of full recovery from specified ED with therapy, % | Herzog et al.,23 Milos et al.,24 Lock et al.,25 Steinhausen26 | ||
| AN | 18.2 (7.2, 35.0) | Triangular | |
| BN | 23.9 (18.1, 29.7) | Triangular | |
| BEDc | 28.1 (25.4, 30.8) | Uniform | |
| Probability of partial recovery from specified ED with therapy, % | Milos et al.24 | ||
| AN | 13.4 (8.7, 17.9) | Uniform | |
| BN | 15.2 (11.9, 18.3) | Uniform | |
| BEDd | 14.2 (10.3, 18.1) | Uniform | |
| OSFED progression and recovery probability, % | Milos et al.24 | ||
| Progress from OSFED to AN with treatment | 11.4 (6.1, 16.9) | Uniform | |
| Progress from OSFED to BN with treatment | 12.8 (1.9, 23.5) | Uniform | |
| Progress from OSFED to BED with treatment | 20.7 (14.1, 27.3) | Uniform | |
| OSFED full recovery with treatment | 28.1 (25.4, 30.8) | Uniform | |
| Relative recovery probability without treatmentb | 50 (26, 74) | Uniform | Assumption |
| Probability of relapse with therapy, % | |||
| AN | Variese (NA) | Nonparametric | Strober et al.,27 Olmsted et al.,28 Birchall et al.29 |
| BN | Variese (NA) | Nonparametric | Herzog et al.,23 Grilo et al.,30 Keller et al.,31 Birchall et al.29 |
| AN or BN (sensitivity analysis) | Variese (NA) | Nonparametric | Wang et al.,32 Eddy et al.33 |
| BED or OSFED | 10.8 (NR) | NA | Milos et al.24 |
| Utility values (scale = 0–1) | |||
| AN | 0.72 (NR) | NA | Vos and Mathers34 |
| Recovered from AN | 0.91 (NR) | NA | de la Rie et al.35 |
| BN | 0.8 (NR) | NA | Pohjolainen et al.36 |
| Recovered from BN | 0.94 (NR) | NA | de la Rie et al.35 |
| BED or OSFED | 0.77 (NR) | NA | Grenon et al.37 |
| Recovered from BED or OSFED | 0.91 (NR) | NA | de la Rie et al.35 |
| Screening costs, $ | |||
| Cost per SCOFF instrument | 0.03 (0.01, 0.05) | Uniform | Assumption |
| Teachers’ hourly wage | 27.74 (NA) | NA | US National Compensation Survey38 |
| Cost of initial diagnostic consultation, $ | 134 (123, 144) | Normal | 2010 US Medical Expenditure Panel Survey39 |
| Cost of standard therapy, $ | Striegel-Moore et al.40 | ||
| AN | 11 375 (271, 65 818)f | Lognormal | |
| BN | 5 574 (207, 30 066)f | Lognormal | |
| BEDg | 6 035 (113, 36 715)f | Lognormal | |
| Cost of low-intensity therapy, $ | Striegel-Moore et al.40 | ||
| AN | 4 411 (160, 23 672)f | Lognormal | |
| BN | 3 541 (207, 17 167)f | Lognormal | |
| BED or OSFEDg | 2 977 (223, 13 310)f | Lognormal |
Note. AN = anorexia nervosa; BED = binge-eating disorder; BN = bulimia nervosa; CI = confidence interval; ED = eating disorder; NA = not applicable; NR = not reported; OSFED = other specified feeding or eating disorder. SCOFF is a self-administered, 5-question ED screening instrument.20,21 We inflated all costs to 2012 dollars using the medical care component of the Consumer Price Index.41
The primary source reported means and standard deviations for these estimates. We calculated the 95% confidence interval from these estimates assuming a normal distribution, and a triangular distribution was used in the model to avoid sampling negative probabilities in uncertainty analyses.
We assumed that without treatment, recovery rates would be 25% to 75% lower than recovery rates with treatment. This value was randomly sampled from a uniform distribution.
Derived from eating disorder not otherwise specified (ED-NOS) recovery probability.
Assumed to be the average of AN and BN partial recovery probabilities.
Probability varies on the basis of number of years of treatment received. In the base case analysis, AN and BN recovery probabilities ranged from 2.3% to 17.1% and 4.9% to 48.5%, respectively, over 10 years. In the sensitivity analysis, recovery probabilities were 0%, 19%, 29%, 40%, 43%, 47%, and 50% for the first 7 years of treatment, respectively, and 100% afterward.
A lognormal distribution was used to model the 95% confidence interval to represent the positively skewed distribution that is typical of cost data. The mean and standard deviation that were reported in the literature were transformed into the mean of logs and standard deviation of logs to simulate the lognormal distribution.42
Reported as ED-NOS in the original study. As BED was formerly part of ED-NOS,18 we used the same treatment costs for BED and OSFED.
Eating disorder recovery and relapse.
Probabilities of ED recovery and relapse can vary widely for some ED subtypes. Using estimates in the literature (Table A, available as a supplement to the online version of this article at http://www.ajph.org), we estimated the annual probabilities of full recovery for AN and BN using triangular distributions with minimum, mean, and maximum values of 5.2%, 9.4%, and 40% for AN23–25 and 16.4%, 24.1%, and 31.4% for BN,23,24 respectively. We estimated BED and OSFED full recovery probabilities using a uniform distribution, informed by data on ED-NOS recovery probabilities (25.3%–31%).24 We estimated partial recovery probabilities (i.e., going from a specified ED to OSFED) using data from a study that analyzed the stability of ED diagnoses over time.24
We also derived annual ED relapse probabilities from the literature. AN and BN relapse probabilities ranged from 2.3% to 17.1%27,28 and 4.9% to 48.5%,23,30,31 respectively; the more years spent recovered, the lower the probability of relapse. We modeled BED and OSFED relapse probabilities using estimates of ED-NOS relapse.24 In a sensitivity analysis, we considered relapse probabilities for AN and BN that were used in a previous cost-effectiveness analysis.32 All recovery and relapse probabilities are detailed in Table 1 and Table A. We assumed that without clinical treatment, recovery probabilities would be 25% lower and relapse probabilities 75% higher, encompassing the modest to strong treatment effects observed for similar mental health interventions.44
Utility values.
We derived the health-related quality of life, or utility, associated with each ED from the literature (Table 1).34,36,37 We used the relative difference in health-related quality of life between current and former ED patients to inflate the utility values for a current ED patient to that for a former ED patient.35 de la Rie et al. estimated that former AN, BN, and BED or OSFED patients would see a 26.4%, 17.5%, and 18% increase in their health-related quality of life when recovered.35
Diagnosis and treatment costs.
We used data from a nationally representative survey of health care expenditures to estimate the cost of a diagnostic visit with a pediatrician.39 We reviewed ED treatment costs published in the literature (Table B, available as a supplement to the online version of this article at http://www.ajph.org). We used data from an analysis of mean inpatient and outpatient treatment costs for females with EDs to estimate standard therapy treatment costs for specified EDs in our base case analysis40 (Table 1). We used outpatient treatment costs for females to estimate the cost of low-intensity therapy for recovered ED or OSFED patients.40
Program costs.
Program costs represent the cost for the school system to screen students. Estimated program costs include the cost of printing the SCOFF instrument, the cost for school staff to score the instrument, and the cost for schools to notify parents about screening and screening results via mail. We assumed that staff could score 1 or 2 instruments per minute, and we estimated the cost of staff time to score questionnaires by using the 2012 average hourly wage for high school teachers obtained from the US National Compensation Survey (Table 1).38
Outcome Measures
Effectiveness.
Our primary effectiveness outcome measure was the number of life-years spent with an ED in each study arm over a 10-year horizon. The secondary effectiveness measure was the quality-adjusted life-year (QALY), a universal health metric that accounts for disease morbidity and health-related quality of life.17 We calculated QALYs in each study arm over a 10-year horizon by multiplying the life-years with an ED by the utility value associated with the ED (Table 1).
Costs.
We conducted the analysis from a payer perspective, estimating program costs in the intervention arm and the cost for ED diagnosis and treatment in both study arms. We conducted the analysis in “steady state,” considering only the marginal costs related to screening and treatment and excluding startup costs related to staff training or program development. As there are no estimates of the burden of EDs on patient and family time (e.g., missed school or work days or travel to appointments) or out-of-pocket expenditures in the United States, we were unable to conduct the analysis from a societal perspective. All costs used in the model are detailed in Table 1. We adjusted costs for inflation using the medical care component of the Consumer Price Index41 and report them in 2012 US dollars. We discounted all future costs, life-years, and QALYs at an annual rate of 3.5%.17
Cost-effectiveness analysis.
We calculated net costs (difference in total costs between the screening and current practice arms) and net effectiveness (difference in life-years with ED or QALYs between the screening and current practice arms) over a 10-year horizon for the target population. We used net costs and net effectiveness to calculate an incremental cost-effectiveness ratio (ICER), which is net costs divided by net effectiveness.17
Sensitivity and Uncertainty Analyses
We conducted a multivariate sensitivity analysis on AN and BN treatment recovery rates using alternative published estimates (Table 1) to assess whether results were robust to model assumptions.32,33 We also conducted 1-way sensitivity analyses on several input parameters, including the relative probability of recovery and relapse for adolescents who are not treated for ED (0.1–1.0), treatment costs ($1000–$30 000), hourly wages for school staff ($12.71 [teacher’s assistant] to $43.97 [nurse practitioner]), the cost of a clinical visit for ED diagnosis ($100–$500), ED utility values (0.5–0.99 on a scale of 0–1), and the clinical referral noncompliance rate (0%–75%).
We ran 10 000 trials in each microsimulation to capture variability in model parameters; input parameters in each trial were sampled from specified distributions. We conducted a probabilistic sensitivity analysis, running the microsimulation 5000 times to capture uncertainty related to sampling bias. We used probabilistic sensitivity analyses to construct 95% confidence intervals around cost-effectiveness outcomes and generate cost-effectiveness acceptability curves. Values and distributions of model inputs used in uncertainty analyses are listed in Table 1.
RESULTS
Results are shown in Table 2. Screening resulted in 0.91 life-years with ED (95% confidence interval [CI] = 0.87, 0.95) and 8.40 QALYs (95% CI = 8.39, 8.41) per capita over 10 years. Students in the no-screen arm accrued 1.16 total life-years with ED (95% CI = 1.11, 1.21) and 8.36 QALYs (95% CI = 8.35, 8.37) over 10 years. On average, students in the screening arm spent significantly less time with AN (0.11 vs 0.46 years, or 1.3 vs 5.5 months) and significantly more time with OSFED (0.28 vs 0.17 years, or 3.4 vs 2.0 months) than those in the no-screen arm. There were no significant differences in life-years with BN or BED between groups. Students in the screening arm spent significantly more time getting treatment than students in the no-screen arm (0.95 vs 0.25 years receiving standard therapy) and, as a result, spent significantly more time recovered from ED (0.51 vs 0.26 years, or 6.1 vs 3.1 months).
TABLE 2—
Results of Analysis of Potential Cost-Effectiveness of School-Based Eating Disorder Screening
| Variable | Screen (95% CI) | No Screen (95% CI) |
| Cost per child, $ | 3971 (3579, 4479) | 1710 (1473, 2020) |
| QALYs per child | 8.40 (8.39, 8.41) | 8.36 (8.35, 8.37) |
| LY with ED per child | ||
| Any ED | 0.91 (0.87, 0.95) | 1.16 (1.11, 1.21) |
| Anorexia | 0.11 (0.10, 0.13) | 0.46 (0.43, 0.49) |
| Bulimia | 0.21 (0.19, 0.23) | 0.22 (0.20, 0.24) |
| Binge-eating disorder | 0.31 (0.28, 0.34) | 0.30 (0.28, 0.33) |
| OSFED | 0.28 (0.25, 0.30) | 0.17 (0.17, 0.18) |
| LY recovered from ED per child | ||
| Any ED | 0.51 (0.48, 0.54) | 0.26 (0.24, 0.28) |
| Anorexia | 0.04 (0.03, 0.05) | 0.10 (0.09, 0.11) |
| Bulimia | 0.09 (0.08, 0.10) | 0.04 (0.03, 0.05) |
| Binge-eating disorder | 0.20 (0.17, 0.22) | 0.11 (0.10, 0.13) |
| OSFED | 0.18 (0.16, 0.20) | 0.005 (0.002, 0.008) |
| Time spent in treatment, y | ||
| Standard therapy | 0.95 (0.90, 1.0) | 0.25 (0.24, 0.27) |
| Low-intensity therapy | 0.33 (0.31, 0.35) | 0.02 (0.02, 0.02) |
| Net mean cost, $ | 2260 (1892, 2668) | |
| Net mean difference in LY with ED, y | −0.25 (−0.30, −0.21) | |
| Net mean difference in QALYs | 0.04 (0.03, 0.05) | |
| Incremental cost-effectiveness ratio (ICER; net costs per net benefits) | ||
| ICER LY with ED avoided, $/LY | 9041 (6617, 12 344) | |
| ICER QALY gained, $/QALY | 56 500 (38 805, 71 250) |
Note. CI = confidence interval; ED = eating disorder; ICER = incremental cost-effectiveness ratio; LY = life-years; OSFED = other specified feeding or eating disorder; QALY = quality-adjusted life-year. Numbers may not add up perfectly because of rounding.
In the base case scenario, the per capita cost of the intervention was $3791 (95% CI = $3579, $4479) versus $1710 (95% CI = $1473, $2020) in the no-screen arm, for a net cost of $2260 (95% CI = $1892, $2668). Excluding ED treatment costs, the screening program cost approximately $0.35 per child. The net difference in life-years with ED was 0.25 (95% CI = 0.21, 0.30) between groups and the net difference in QALYs was 0.04 (95% CI = 0.03, 0.05). ICERs were $9041 per life-year of ED avoided (95% CI = $6617, $12 344) and $56 500 per QALY gained (95% CI = 38 805, 71 250). At willingness-to-pay thresholds of $50 000 and $100 000 per QALY gained, the intervention is 41% and 100% likely to be cost-effective, respectively (Figure D, available as a supplement to the online version of this article at http://www.ajph.org).
Alternative assumptions about recovery and relapse rates improved ICERs. In the sensitivity analysis that considered alternative recovery rates for AN and BN,30, 31 the ICER was $32 359 per QALY gained (95% CI = $22 671, $44 266). In the series of sensitivity analyses conducted on parameter inputs, the model was most sensitive to ED utility weights, treatment costs, and referral rates. When the utility values for AN and OSFED were near that of perfect health, the intervention was not cost-effective. When 75% of screen-positive students were noncompliant with clinical referral recommendations, the ICER was $89 654 per QALY gained (95% CI = $44 930, $119 449). The model was somewhat sensitive to relative recovery and relapse probabilities for adolescents who were not treated for ED; ICERs for a relative probability of 0.1 to 1.0 ranged from $42 664 to $64 019, respectively (Figure 2).
FIGURE 2—
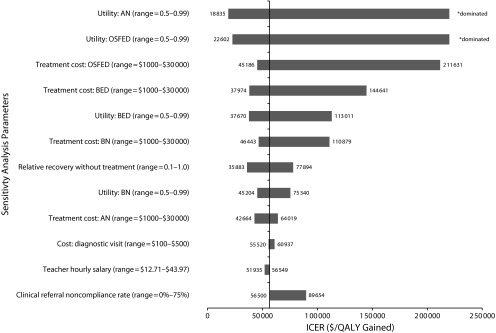
Sensitivity analyses of the potential cost-effectiveness of school-based eating disorder screening.
Note. AN = anorexia nervosa; BED = binge-eating disorder; BN = bulimia nervosa; ICER = incremental cost-effectiveness ratio; OSFED = other specified feeding or eating disorders; QALY = quality-adjusted life-year. This figure reports ICERs for a series of 1-way sensitivity analyses on key input parameters. ICERs that are dominated represent scenarios in which screening was more expensive, but slightly less effective, than usual care.
DISCUSSION
This study modeled the cost-effectiveness of a theoretical school-based ED screening program. Economic analyses of ED treatment or prevention programs have been previously conducted,32,45,46 but this is the first to evaluate the cost-effectiveness of an ED screening program. The net cost of the intervention was $2260 per student (95% CI = $1892, $2668), and the intervention resulted in a net 0.25 fewer life-years with ED (95% CI = 0.21, 0.30) and a net 0.04 more QALYs (95% CI = 0.03, 0.05) per child over 10 years. The ICERs for the intervention compared with current practice were $9041 per life-year of ED avoided (95% CI = $6617, $12 344) and $56 500 per QALY gained (95% CI = $38 805, $71 250). Results were sensitive to ED utility values and treatment costs.
EDs are some of the most costly and burdensome adolescent psychiatric disorders.8,11,47 Population-based screening for EDs is feasible,5 and early diagnosis can have a meaningful impact on adolescent quality of life and ED mortality rates.7,15 The per capita cost of administering the SCOFF was $0.35, far lower than the $8.58 cost of the self-administered, 50-minute-long emotional and mental health screening instruments in the Developmental Pathways Screening Program.41,48 Mental health screening in New York schools was estimated to cost $232 per child, but this estimate includes the cost of clinical diagnosis.41,49 Most of our intervention costs come not from screening or diagnosis but from increased per capita treatment costs in the intervention versus current practice groups. Early detection and longer treatment (0.95 vs 0.25 years in standard therapy) reduces the duration of disease.
The cost-effectiveness of any intervention can be judged by comparing the ICERs to thresholds that detail society’s willingness to pay for health improvements. There are no established thresholds for how much society is willing to pay to avoid a life-year with an ED. Therefore, although this outcome may be of interest to decision-makers, we cannot objectively assess the value of ED screening using this metric. However, we can make such a judgment for QALY gains. An intervention with an ICER at or below $50 000 per QALY gained would generally be considered cost-effective.50 Unlike in countries with nationalized health care systems, however, the choice of the $50 000-per-QALY threshold in the United States is somewhat arbitrary.50 At a more liberal threshold of $100 000 per QALY gained, school-based ED screening is 100% likely to be cost-effective, on par with many other public health interventions.50
We can also judge the cost-effectiveness of an intervention relative to that of interventions for similar populations. Although school-based ED screening is less cost-effective than most pediatric interventions, which cost a mean $11 000 per QALY gained,51 it is cost-effective relative to other health screening programs for female adolescents. Blood pressure screening, for example, costs a mean $58 000 per QALY gained and cervical cancer screening after HPV vaccination costs a mean $51 000 per QALY gained over a lifetime horizon.41,52,53 ED screening is less cost-effective than Planet Health, an obesity prevention program found to prevent ED, which was more effective than current practice and cost-saving over a 10-year horizon.52
By targeting a disease that is prevalent among adolescents and for which early intervention is crucial, ED screening meets the American Academy of Pediatrics criteria for successful school-based screening programs.16 A recent poll reports that 53% of US adults support school-based ED screening54 suggesting that, like body mass index screening,55 school-based ED screening will be accepted by stakeholders. One limitation of body mass index screening is the burden placed on school nurses,55,56 but the SCOFF is self-administered and avoids such constraints. Currently, no states mandate school-based mental health screening, but stakeholders acknowledge the importance of screening for early intervention.57 School-based mental health screening programs like Columbia University’s TeenScreen program58 and the Developmental Pathways Screening Program48 have improved access to services. Still, states will have to think carefully about programmatic issues such as obtaining consent to screen, parent notification of screening results, student privacy, and staff training when implementing ED screening.55–57
In addition to short-term effects, EDs can lead to osteoporosis, dental complications, prolonged depression and anxiety, and infertility in the long term.59 Because of limited data inputs, we were unable to model the impact of early ED identification and treatment on health over a lifetime horizon. Our results may be conservative because we used a shorter time horizon, did not model mortality, and excluded societal costs such as those related to lost economic productivity and out-of-pocket expenditures. The Congressional National Eating Disorders Awareness Caucus has only recently called for a review of the economic impact of ED in the United States.60
School-based ED screening may reduce disparities in access to care. Low-income students have been found to benefit from ED screening more than high-income students.61 Moreover, symptomatic minority, overweight, and male adolescents may be less likely than underweight White females to be clinically evaluated or treated for ED, in part because of biases in symptom recognition.5,62,63 School-based screening can improve equity by reaching adolescents in all demographic, weight, and socioeconomic groups.
Limitations
This study has some limitations. First, we assumed that adolescents could develop only 1 ED subtype in their lifetime, which may not concur with the clinical course of disease.33 We simplified the model in this way because of limited data on ED crossover rates and changing definitions of EDs over time.18 The model should be updated as better and more recent data on ED crossover become available. Additionally, some parents may be unwilling to seek clinical treatment after a positive SCOFF screen because of the perceived high cost of treatment or lack of insurance coverage, but the intervention was still relatively cost-effective when the referral rate was low. In mental health screening studies, socioeconomic status has not affected access to care, and recent changes to US health insurance policies as a result of the Affordable Care Act may decrease actual and perceived barriers to ED treatment.64
Finally, this study was limited by the quality of input parameters. Many studies had small sample sizes and defined EDs inconsistently. Additionally, although the utility values used to calculate QALYs were all rooted in accepted preference-based quality of life elicitation methods (i.e., person trade-off, the 15D, and the EQ-5D), different elicitation methods can give different results.65,66 We accounted for uncertainty around model inputs by using parameters that considered probabilities from multiple studies and conducting sensitivity analyses. Finally, the recovery rates modeled may underestimate the true effectiveness of ED treatment in light of newer, more efficacious treatment modalities such as family-based treatment of adolescents with AN.4
Conclusions
In addition to informing decision-makers about the value of public health strategies, decision-analytic models like this one can highlight gaps in the literature. To improve future economic analyses, epidemiological studies should evaluate the progression of EDs using larger sample sizes and should track the course of EDs and related comorbidities over a lifetime horizon using secondary data. Moreover, researchers should collect health state utilities for eating disorders using preference-based experiments or multiattribute utility instruments like the EQ-5D or Health Utilities Index.66
We used a decision-analytic simulation model to estimate the cost-effectiveness of school-based ED screening for 10- to 17-year-old US public school students. We conservatively estimated the cost-effectiveness estimates of school-based ED screening to be $9041 per life-year with ED avoided and $56 500 per QALY gained. Compared with other screening interventions, school-based ED screening may be a cost-effective public health intervention. There is some uncertainty around our results because of limited data in the literature. Future research should assess the long-term progression of and consequences of EDs.
Acknowledgments
This research was supported by the Ellen Feldberg Gordon Fund for Eating Disorders Research. S. B. Austin and K. R. Sonneville are supported by the Maternal and Child Health Bureau, Health Resources and Services Administration (training grant T76-MC00001) and the Leadership Education in Adolescent Health Project (training grant 6T71-MC00009).
Abstracts containing preliminary results from this article were presented at the XIXth Annual Meeting of the Eating Disorders Research Society; September 2013; Baltimore, MD; and at the AcademyHealth 2014 Annual Research Meeting; June 2014; San Diego, CA.
Human Participant Protection
No institutional review board approval was necessary because data were obtained from secondary sources.
References
- 1.Swanson SA, Crow SJ, Le Grange D, Swendsen J, Merikangas KR. Prevalence and correlates of eating disorders in adolescents. Results from the national comorbidity survey replication adolescent supplement. Arch Gen Psychiatry. 2011;68(7):714–723. doi: 10.1001/archgenpsychiatry.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merikangas KR, He JP, Burstein M et al. Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton DK, Kann L, Kinchen S et al. Youth risk behavior surveillance—United States, 2011. MMWR Surveill Summ. 2012;61(4):1–162. [PubMed] [Google Scholar]
- 4.Brown TA, Keel PK. Current and emerging directions in the treatment of eating disorders. Subst Abuse. 2012;6:33–61. doi: 10.4137/SART.S7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austin SB, Ziyadeh NJ, Forman S, Prokop LA, Keliher A, Jacobs D. Screening high school students for eating disorders: results of a national initiative. Prev Chronic Dis. 2008;5(4):A114. [PMC free article] [PubMed] [Google Scholar]
- 6.Padierna A, Quintana JM, Arostegui I, Gonzalez N, Horcajo MJ. The health-related quality of life in eating disorders. Qual Life Res. 2000;9(6):667–674. doi: 10.1023/a:1008973106611. [DOI] [PubMed] [Google Scholar]
- 7.Arcelus J, Mitchell A, Wales J, Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch Gen Psychiatry. 2011;68(7):724–731. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- 8.O’Herlihy A, Worrall A, Lelliott P, Jaffa T, Hill P, Banerjee S. Distribution and characteristics of in-patient child and adolescent mental health services in England and Wales. Br J Psychiatry. 2003;183:547–551. doi: 10.1192/bjp.183.6.547. [DOI] [PubMed] [Google Scholar]
- 9.Klump KL, Bulik CM, Kaye WH, Treasure J, Tyson E. Academy for eating disorders position paper: eating disorders are serious mental illnesses. Int J Eat Disord. 2009;42(2):97–103. doi: 10.1002/eat.20589. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan P. Mortality in anorexia nervosa. Am J Psychiatry. 1995;152(7):1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell JE, Crow S. Medical complications of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry. 2006;19(4):438–443. doi: 10.1097/01.yco.0000228768.79097.3e. [DOI] [PubMed] [Google Scholar]
- 12.Paying the Price: The Economic and Social Impact of Eating Disorders in Australia. Melbourne, Australia: Butterfly Foundation for Eating Disorders; 2012. [Google Scholar]
- 13.Henderson J. Costs of Eating Disorders in England: Economic Impacts of Anorexia Nervosa, Bulimia Nervosa and Other Disorders, Focussing on Young People. London, UK: ProBono Economics; 2012. [Google Scholar]
- 14.International Comparison Program Database. Washington, DC: World Bank; 2012. [Google Scholar]
- 15.Committee on School Health. School Health Policy & Practice. Elk Grove Village, IL: American Academy of Pediatrics; 2004. [Google Scholar]
- 16.School health assessments. Committee on School Health. American Academy of Pediatrics. Pediatrics. 2000;105(4 pt 1):875–877. [PubMed] [Google Scholar]
- 17.Gold M, Siegel J, Russell L, Weinstein MC. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 18.Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 19.Digest of Education Statistics. Washington, DC: US Dept of Education; 2012. [Google Scholar]
- 20.Hill LS, Reid F, Morgan JF, Lacey JH. SCOFF, the development of an eating disorder screening questionnaire. Int J Eat Disord. 2010;43(4):344–351. doi: 10.1002/eat.20679. [DOI] [PubMed] [Google Scholar]
- 21.Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: assessment of a new screening tool for eating disorders. BMJ. 1999;319(7223):1467–1468. doi: 10.1136/bmj.319.7223.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotton MA, Ball C, Robinson P. Four simple questions can help screen for eating disorders. J Gen Intern Med. 2003;18(1):53–56. doi: 10.1046/j.1525-1497.2003.20374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzog DB, Dorer DJ, Keel PK et al. Recovery and relapse in anorexia and bulimia nervosa: a 7.5-year follow-up study. J Am Acad Child Adolesc Psychiatry. 1999;38(7):829–837. doi: 10.1097/00004583-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Milos G, Spindler A, Schnyder U, Fairburn CG. Instability of eating disorder diagnoses: prospective study. Br J Psychiatry. 2005;187:573–578. doi: 10.1192/bjp.187.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lock J, Couturier J, Agras WS. Costs of remission and recovery using family therapy for adolescent anorexia nervosa: a descriptive report. Eat Disord. 2008;16(4):322–330. doi: 10.1080/10640260802115969. [DOI] [PubMed] [Google Scholar]
- 26.Steinhausen HC. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. 2002;159(8):1284–1293. doi: 10.1176/appi.ajp.159.8.1284. [DOI] [PubMed] [Google Scholar]
- 27.Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. Int J Eat Disord. 1997;22(4):339–360. doi: 10.1002/(sici)1098-108x(199712)22:4<339::aid-eat1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 28.Olmsted MP, Kaplan AS, Rockert W. Rate and prediction of relapse in bulimia nervosa. Am J Psychiatry. 1994;151(5):738–743. doi: 10.1176/ajp.151.5.738. [DOI] [PubMed] [Google Scholar]
- 29.Birchall H, Palmer RL, Waine J, Gadsby K, Gatward N. Intensive day programme treatment for severe anorexia nervosa—the Leicester experience. Psychiatr Bull. 2002;26:334–336. [Google Scholar]
- 30.Grilo CM, Pagano ME, Skodol AE et al. Natural course of bulimia nervosa and of eating disorder not otherwise specified: 5-year prospective study of remissions, relapses, and the effects of personality disorder psychopathology. J Clin Psychiatry. 2007;68(5):738–746. doi: 10.4088/jcp.v68n0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller MB, Herzog DB, Lavori PW, Ott IL, Bradburn IS, Mahoney EM. High rates of chronicity and rapidity of relapse in patients with bulimia nervosa and depression. Arch Gen Psychiatry. 1989;46(5):480–481. doi: 10.1001/archpsyc.1989.01810050094018. [DOI] [PubMed] [Google Scholar]
- 32.Wang LY, Nichols LP, Austin SB. The economic effect of Planet Health on preventing bulimia nervosa. Arch Pediatr Adolesc Med. 2011;165(8):756–762. doi: 10.1001/archpediatrics.2011.105. [DOI] [PubMed] [Google Scholar]
- 33.Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. Diagnostic crossover in anorexia nervosa and bulimia nervosa: implications for DSM-V. Am J Psychiatry. 2008;165(2):245–250. doi: 10.1176/appi.ajp.2007.07060951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vos T, Mathers CD. The burden of mental disorders: a comparison of methods between the Australian burden of disease studies and the Global Burden of Disease study. Bull World Health Organ. 2000;78(4):427–438. [PMC free article] [PubMed] [Google Scholar]
- 35.de la Rie SM, Noordenbos G, van Furth EF. Quality of life and eating disorders. Qual Life Res. 2005;14(6):1511–1522. doi: 10.1007/s11136-005-0585-0. [DOI] [PubMed] [Google Scholar]
- 36.Pohjolainen V, Rasanen P, Roine RP, Sintonen H, Wahlbeck K, Karlsson H. Cost-utility of treatment of bulimia nervosa. Int J Eat Disord. 2010;43(7):596–602. doi: 10.1002/eat.20754. [DOI] [PubMed] [Google Scholar]
- 37.Grenon R, Tasca GA, Cwinn E et al. Depressive symptoms are associated with medication use and lower health-related quality of life in overweight women with binge eating disorder. Womens Health Issues. 2010;20(6):435–440. doi: 10.1016/j.whi.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Office of Compensation Levels and Trends. National Compensation Survey—Wages. Washington, DC: Bureau of Labor Statistics; 2012. [Google Scholar]
- 39.Medical Expenditure Panel Survey [data set] Rockville, MD: Healthcare Research and Quality; 2010. [Google Scholar]
- 40.Striegel-Moore RH, Leslie D, Petrill SA, Garvin V, Rosenheck RA. One-year use and cost of inpatient and outpatient services among female and male patients with an eating disorder: evidence from a national database of health insurance claims. Int J Eat Disord. 2000;27(4):381–389. doi: 10.1002/(sici)1098-108x(200005)27:4<381::aid-eat2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 41.Bureau of Labor Statistics. Consumer Price Index. Washington, DC: US Dept of Labor; 2012. [Google Scholar]
- 42.Spiegelhalter D, Abrams K, Myles J. Bayesian Approaches to Clinical Trials and Health-Care Evaluation. West Sussex, UK: John Wiley & Songs; 2004. [Google Scholar]
- 43.Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. Pharmacoeconomics. 2007;25(1):3–6. doi: 10.2165/00019053-200725010-00002. [DOI] [PubMed] [Google Scholar]
- 44.Hofmann SG, Asnaani A, Vonk IJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res. 2012;36(5):427–440. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byford S, Barrett B, Roberts C et al. Economic evaluation of a randomised controlled trial for anorexia nervosa in adolescents. Br J Psychiatry. 2007;191:436–440. doi: 10.1192/bjp.bp.107.036806. [DOI] [PubMed] [Google Scholar]
- 46.Stuhldreher N, Konnopka A, Wild B et al. Cost-of-illness studies and cost-effectiveness analyses in eating disorders: a systematic review. Int J Eat Disord. 2012;45(4):476–491. doi: 10.1002/eat.20977. [DOI] [PubMed] [Google Scholar]
- 47.Agras WS. The consequences and costs of the eating disorders. Psychiatr Clin North Am. 2001;24(2):371–379. doi: 10.1016/s0193-953x(05)70232-x. [DOI] [PubMed] [Google Scholar]
- 48.Kuo E, Vander Stoep A, McCauley E, Kernic MA. Cost-effectiveness of a school-based emotional health screening program. J Sch Health. 2009;79(6):277–285. doi: 10.1111/j.1746-1561.2009.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chatterji P, Caffray CM, Crowe M, Freeman L, Jensen P. Cost assessment of a school-based mental health screening and treatment program in New York City. Ment Health Serv Res. 2004;6(3):155–166. doi: 10.1023/b:mhsr.0000036489.50470.cb. [DOI] [PubMed] [Google Scholar]
- 50.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8(2):165–178. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 51.Ladapo JA, Neumann PJ, Keren R, Prosser LA. Valuing children’s health: a comparison of cost-utility analyses for adult and paediatric health interventions in the US. Pharmacoeconomics. 2007;25(10):817–828. doi: 10.2165/00019053-200725100-00002. [DOI] [PubMed] [Google Scholar]
- 52.Wang YC, Cheung AM, Bibbins-Domingo K et al. Effectiveness and cost-effectiveness of blood pressure screening in adolescents in the United States. J Pediatr. 2011;158(2):257–264. doi: 10.1016/j.jpeds.2010.07.058. e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldhaber-Fiebert JD, Stout NK, Salomon JA, Kuntz KM, Goldie SJ. Cost-effectiveness of cervical cancer screening with human papillomavirus DNA testing and HPV-16,18 vaccination. J Natl Cancer Inst. 2008;100(5):308–320. doi: 10.1093/jnci/djn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puhl R, Neumark-Sztainer D, Austin SB, Luedicke J, King KM. Setting policy priorities to address eating disorders and weight stigma: views from the field of eating disorders and the US general public. BMC Public Health. 2014;14(1):524. doi: 10.1186/1471-2458-14-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nihiser AJ, Lee SM, Wechsler H et al. BMI measurement in schools. Pediatrics. 2009;124(suppl 1):S89–S97. doi: 10.1542/peds.2008-3586L. [DOI] [PubMed] [Google Scholar]
- 56.Ikeda JP, Crawford PB, Woodward-Lopez G. BMI screening in schools: helpful or harmful. Health Educ Res. 2006;21(6):761–769. doi: 10.1093/her/cyl144. [DOI] [PubMed] [Google Scholar]
- 57.Weist MD, Rubin M, Moore E, Adelsheim S, Wrobel G. Mental health screening in schools. J Sch Health. 2007;77(2):53–58. doi: 10.1111/j.1746-1561.2007.00167.x. [DOI] [PubMed] [Google Scholar]
- 58.Scott MA, Wilcox HC, Schonfeld IS et al. School-based screening to identify at-risk students not already known to school professionals: the Columbia Suicide Screen. Am J Public Health. 2009;99(2):334–339. doi: 10.2105/AJPH.2007.127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crow SJ. Medical complications of eating disorders. In: Wonderlich S, Mitchell J, de Zwaan M, Steiger H, editors. Eating Disorders Review, Part 1. Abingdon, UK: Radcliffe Publishing Ltd; 2005. pp. 127–136. [Google Scholar]
- 60.New York, NY: National Eating Disorders Association; February 28, 2014. Congressional National Eating Disorders Awareness Caucus calls for study on eating disorders [press release] [Google Scholar]
- 61.Austin SB, Ziyadeh NJ, Forman S, Keliher A, Jacobs D. Eating Disorders Referral Rates Improved by Community-Led Nationwide Screening in US High Schools. Boston, MA: Children’s Hospital Boston; 2011. [Google Scholar]
- 62.Austin SB, Penfold RB, Johnson RL, Haines J, Forman S. Clinical identification of youth abusing over-the-counter products for weight control in a large US integrated health system. J Eat Disord. 2013;1:40–46. doi: 10.1186/2050-2974-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marques L, Alegria M, Becker AE et al. Comparative prevalence, correlates of impairment, and service utilization for eating disorders across US ethnic groups: implications for reducing ethnic disparities in health care access for eating disorders. Int J Eat Disord. 2011;44(5):412–420. doi: 10.1002/eat.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis K, Abrams M, Stremikis K. How the Affordable Care Act will strengthen the nation’s primary care foundation. J Gen Intern Med. 2011;26(10):1201–1203. doi: 10.1007/s11606-011-1720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinstein MC, Torrance G, McGuire A. QALYs: the basics. Value Health. 2009;12(suppl 1):S5–S9. doi: 10.1111/j.1524-4733.2009.00515.x. [DOI] [PubMed] [Google Scholar]
- 66.Drummond MF, Sculpher MJ, Torrance GW, O'Briend BJ, Stodart GL. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. New York, NY: Oxford University Press; 2005. [Google Scholar]