Abstract
Objectives. We sought to validate previous reports of HCV prevalence in jails, identify HCV risk factors prevalence, and identify risk factors associated with HCV infection in this population.
Methods. Inmates at the Buzz Westfall Justice Center (BWJC) in St. Louis, Missouri, were offered risk factor screening for HCV and anti-HCV antibody testing from December 2012 through May 2013. Demographic and risk factor information were assessed for significant associations with positive HCV antibody results. Risk factors that were significantly associated in univariate analysis were assessed using binary logistic regression to model the relationship between positive HCV results and the risk factors and demographics.
Results. Fifty of 304 inmates were positive for HCV, with a prevalence of 16.4%. The risk factors significantly associated with increased risk for positive HCV antibody were age (odds ratio [OR] = 1.09; 95% confidence interval [CI] = 1.04, 1.15 for each year), injection drug use (OR = 53.87; 95% CI = 17.78, 163.21), sex with HCV-positive partner (OR = 7.35; 95% CI = 1.41, 38.20), and tattoos by a nonlicensed provider (OR = 2.62; 95% CI = 1.09, 6.33). Prevalence for women was 3 times that of men (38% vs 12%).
Conclusions. Prevalence of HCV at BWJC was similar to previous jail studies, which is lower than reported prison rates and higher than the general population.
HCV infection is one of the most common and deadly blood-borne infectious diseases in the United States.1–3 National Health and Nutrition Examination Survey (NHANES) data estimate that 1.6% of the US population, or about 4.1 million people are infected with HCV.2 This NHANES estimate is likely an underestimation because it did not sample several high prevalence populations; the true prevalence may be conservatively closer to 2% (5.2 million) or potentially as high as 2.8% (7.1 million).4 In 2010, approximately 17 000 new infections occurred with an incidence rate of 0.3 cases per 100 000 persons in the United States.5 Incidence rates have decreased significantly from 1992, but have been holding fairly steady over the past decade.3 Some authors predict the incidence will likely increase slightly with recent increases in injection drug use. The incidence of complications associated with HCV is expected to continue to increase as well.3,6
HCV infection is associated with significant morbidity, mortality, and cost. It is the most common chronic liver disease associated with hepatocellular carcinoma, present in close to half of all cases.7,8 It is the leading indication for liver transplantation in the United States, with a rate nearly double that of the second cause.9 HCV infection was listed as an underlying or contributing cause of more than 15 000 deaths in 2007.10 Patients who do not go on to develop cirrhosis or those in the 20- to 30-year window between infection and development of cirrhosis can also suffer social, emotional, and physical complications; experience a decreased quality of life; and require hospitalization.3,11,12 The yearly total health care costs associated with HCV infection were calculated to be $6.5 billion in 2007 and are predicted to peak at $9.1 billion in 2024 based on current trends and excluding the cost of antiviral treatments.6
In the general US population, the risk factors most associated with HCV infection are injection drug use (IDU), sexual contact with HCV-positive partners, receipt of blood and blood products prior to 1992, and needle sticks.2,5 According to data from NHANES, men have a higher prevalence of HCV infection than women (2.1% vs 1.1%), and non-Hispanic Blacks have a higher prevalence than non-Hispanic Whites or Mexican Americans (3%, 1.5%, and 1.3% respectively).2 The Centers for Disease Control and Prevention has recently added a recommendation to test all patients born between 1945 and 1965, as this birth cohort has a HCV prevalence rate of 3.25% and accounts for approximately 75% of HCV infections in the general US population.13 One recent analysis found that among those with a history of IDU, any past incarceration was significantly associated with HCV infection with an adjusted odds ratio (OR) of 2.6 (95% confidence interval [CI] = 1.2, 6.1).14
As prevalent as HCV infection is in the general population, it is nearly 10-fold higher in the incarcerated population. The prevalence of HCV infection in incarcerated individuals is estimated to be 23.1% to 41.2%.4 Individuals who are incarcerated are more likely to participate in high-risk behavior for HCV infection, including IDU, tattoos from nonlicensed providers, and prostitution. In addition to their increased risk prior to incarceration, inmates are also at higher risk for becoming infected during incarceration, mostly from tattoos received in prison and continued use of injection drugs while incarcerated. With increasing rates of IDU in the United States, rates of incarceration and HCV infection are predicted to increase as well.3
Although there is a significant amount of literature assessing HCV in the general population and incarcerated populations as a whole, most of the literature assessing incarcerated populations deals specifically with prison populations rather than jail populations. Jails are more dynamic environments than prisons and include people being released from custody in a short period of time as well as those destined to be imprisoned. Studies relating to HCV infection in a jailed population are much more limited. Only 1 previous study has specifically assessed only jailed populations.15 This study assessed the prevalence of HCV infection from a random sample of stored blood samples from 3 city jails and did not include any risk factor assessment directly from inmates, although it did link results to demographic information, previous incarceration status, hepatitis B virus (HBV) infection, and HIV infection status. This evaluation found the weighted prevalence of HCV to be 13% overall with 10% prevalence in San Francisco, California; 14% in Chicago, Illinois; and 15% in Detroit, Michigan. The study was not able to assess whether inmates were previously aware of their HCV infection.15 Another study assessed both jail and prison populations in Maryland.16 This study also assessed HCV rates on stored samples and was linked to demographic information, reasons for incarceration, syphilis infection, HBV infection, and HIV infection. Those enrollees labeled as “detainees,” meaning presentencing, had an HCV prevalence of 31.1%, higher than that in the prison population at 26.4%.
As pointed out in a 2012 editorial, jails may represent an ideal location to institute widespread screening programs for HCV.17 Jails may represent a higher-risk group than the general population. Identifying those at high risk for HCV infection in a jail could lead to education on risk reduction to those not already infected and could lead to earlier detection for those infected with HCV who did not previously know of their infection status. This detection could prevent the spread within communities for those jail inmates who are released from custody shortly after incarceration and could decrease the spread of HCV within prisons for those who are sentenced. In addition to slowing or preventing the spread of HCV, the detection of an infection in jails could lead to more frequent and earlier treatment, improving the health of the infected inmate and decreasing the morbidity and costs associated with late-stage HCV infections. This article also correctly points out, however, that the cost savings that may be realized because of early screening and intervention for HCV are unlikely to be realized directly by the same payers as the initial direct screening costs. Finding ways to better target testing expenditures would enable jails to provide a public health benefit without the costs associated with testing all those incarcerated.
The current project was undertaken to add to and validate previous reports of HCV prevalence in jailed populations, identify the HCV risk factors present in this population, and identify the risk factors most associated with HCV infection in the population.
METHODS
The Buzz Westfall Justice Center (BWJC) houses the Saint Louis County Jail. It is classified as a large jail with an average daily census of 1250 inmates. It houses inmates for Saint Louis County, Missouri, neighboring municipalities, and the United States Department of Justice. Health care at BWJC is provided through the Saint Louis County Department of Health. Participants were enrolled from December 2012 through May 2013.
Enrollment and Data Collection
In addition to the 14-day physical assessment mandated by the American Correctional Association, inmates at the BWJC receive the following screening tests: serum tests for HIV and syphilis, urine tests for gonorrhea and chlamydia, and a purified protein derivative test for tuberculosis. Traditionally, only selected inmates at the BWJC were screened for the risk of exposure to HCV and active HCV infection. The selection of testing candidates was left to the discretion of each provider.
This project offered risk factor screening and anti-HCV testing to inmates about to receive their assessment blood draws. Inmates were identified the day before their lab draw was scheduled. The primary investigator interviewed inmates individually in a private interview room to offer enrollment, started the informed consent process, provided brief HCV education, and delivered a demographic and risk factor screening questionnaire to those who consented to enrollment.
Demographic information collected from enrollees was self-identified race/ethnicity, age at the time of enrollment (which was verified on their identification bracelets), and gender.
Self-reported risk factors were any history of injection drug use, receipt of clotting factors prior to 1987, receipt of blood transfusion prior to 1992, any history of hemodialysis, any tattoo or body piercing from a noninspected or nonlicensed provider, receipt of blood or organ from a donor known or revealed to be HCV positive, any history of an accidental needle stick, any history of a positive HIV test, having a mother who was HCV positive at the time of enrollee’s birth, sexual contact with any partner known or revealed to be HCV positive, and self-reported previous HCV-positive test results. No distinction was made on risk factor behavior that occurred in a prison or jail versus while not incarcerated.
Inmates with positive anti-HCV tests were provided detailed education, counseling, and follow-up outside the parameters of this study.
Statistical Analysis
Investigators employed a consecutive sampling method to enroll approximately 300 inmates. This sample size was determined to provide at least 80% power to detect an effect size of 0.8 based on an HCV prevalence rate in the range of 15% to 40%.
Data were entered into SPSS Statistics version 19 (IBM, Somers, NY). We compared demographic information between HCV-positive and HCV-negative patients by using the χ2 test for race/ethnicity and gender, and the independent sample t-test for age. For HCV risk factors, we tabulated frequencies and compared them between HCV-positive and HCV-negative patients by using the χ2 test. An α level of 0.05 for significance was used for all tests.
Binary logistic regression was utilized to model the relationship between positive HCV antibody test results and the risk factors and demographic information. A backward stepwise deletion procedure was used with an α of 0.1 for removal. Model explanatory power and fit were assessed using the c-statistic and the Hosmer–Lemeshow lack-of-fit test. Adjusted ORs based on this model were calculated with 95% CIs.
RESULTS
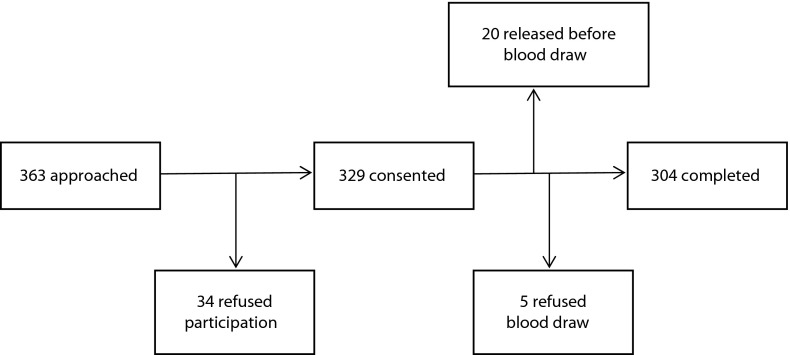
A total of 304 patients provided informed consent, completed the risk factor interview and provided blood samples. This represents 83.7% of those who were approached for participation and 92.4% of those who consented to enrollment. Figure 1 displays the recruitment, enrollment, and completion data.
FIGURE 1—
Recruitment algorithm: Buzz Westfall Justice Center; Saint Louis County, MO; December 2012–May 2013.
Table 1 lists the demographic and risk factor information for participants. Fifty patients had positive HCV antibody results, corresponding to a 16.4% prevalence rate in this population. Of those 50, 23 reported a previous positive result. Of the 254 patients who had negative HCV antibody results, 2 reported testing positive previously. The 2 most common risk factors present in all patients as well as those with positive antibody results were IDU and tattoo received from a nonlicensed provider. The prevalence in the 1945 to 1965 birth cohort (22%) was higher than the overall prevalence, but the 1966 to 1986 birth cohort also had a high prevalence of 22.5%.
TABLE 1—
Demographic and Risk Factor Distribution: Buzz Westfall Justice Center; Saint Louis County, MO; December 2012–May 2013
| Total (n = 304), Mean ±SD or No. (%) | Anti-HCV Positive (n = 50), Mean ±SD or No. (%) | Anti-HCV Negative (n = 254), Mean ±SD or No. (%) | P | |
| Age, y | 32.8 ±10.8 | 36.6 ±9.5 | 32.1 ±10.9 | .08a |
| Birth cohort | ||||
| 1945–1965 | 37 (12.2) | 8 (22) | 29 (78) | |
| 1966–1986 | 160 (52.6) | 36 (22.5) | 124 (77.5) | |
| 1987–1995 | 107 (35.2) | 6 (6) | 101 (94) | |
| Self-reported race/ethnicity | < .001b | |||
| African American/Black | 183 (60.2) | 16 (9) | 167 (91) | |
| Hispanic/Latino | 5 (1.6) | 0 | 5 (100) | |
| Non-Hispanic White | 107 (35.2) | 33 (31) | 74 (69) | |
| Asian/Pacific Islander | 2 (0.7) | 0 | 2 (100) | |
| Native American | 4 (1.3) | 1 (25) | 3 (75) | |
| Other | 3 (1) | 0 | 3 (100) | |
| Gender | < .001a | |||
| Female | 55 (18.1) | 21 (38) | 34 (62) | |
| Male | 249 (81.9) | 29 (12) | 220 (88) | |
| Injection drug use | < .001a | |||
| No | 227 (74.7) | 6 (3) | 221 (97) | |
| Yes | 77 (25.3) | 44 (57) | 33 (43) | |
| Blood transfusion before 1992 | .178a | |||
| No | 299 (98.4) | 48 (16) | 251 (84) | |
| Yes | 5 (1.6) | 2 (40) | 3 (60) | |
| Tattoo nonlicensed provider | < .001a | |||
| No | 207 (68.1) | 20 (10) | 187 (90) | |
| Yes | 97 (31.9) | 30 (31) | 67 (69) | |
| Accidental needle stick | .002a | |||
| No | 290 (95.4) | 43 (15) | 247 (85) | |
| Yes | 14 (4.6) | 7 (50) | 7 (50) | |
| HIV positive | .991a | |||
| No | 302 (99.3) | 50 (17) | 252 (83) | |
| Yes | 2 (0.7) | 0 | 2 (100) | |
| HCV-positive mother | .989a | |||
| No | 301 (99) | 50 (17) | 251 (83) | |
| Yes | 3 (1) | 0 | 3 (100) | |
| Sex with HCV-positive partner | < .001a | |||
| No | 277 (91.1) | 27 (10) | 250 (90) | |
| Yes | 27 (8.9) | 23 (85) | 4 (15) | |
| Previous HCV positive | < .001a | |||
| No | 279 (91.8) | 27 (10) | 252 (90) | |
| Yes | 25 (8.2) | 23 (92) | 2 (8) |
Note. The following risk factors were assessed, but had 0 “Yes” responses: clotting factor use before 1987, hemodialysis, receipt of HCV-positive blood or organ.
Positive vs negative.
African American vs White.
The univariate analysis showed 7 factors to be significantly associated with positive HCV antibody results: age, gender, IDU, history of an accidental needle stick, race/ethnicity, history of sexual contact with an HCV-positive partner, and history of a tattoo by an unlicensed provider. Table 1 lists the results of univariate analysis of the demographic characteristics and risk factors.
The overall multivariable logistic regression model was statistically significant (LR χ2 = 135.518; df = 4; P < .001) with a c-statistic of 0.929. Significant predictors in the model included age (OR = 1.09; 95% CI = 1.04, 1.15), indicating that older participants had significantly higher odds of having a positive antibody test than younger participants; IDU (OR = 53.87; 95% CI = 17.78, 163.21), signifying that participant who had ever injected drugs had significantly higher odds than those who had never injected; sex with an HCV-positive partner (OR = 7.35; 95% CI = 1.41, 38.20), showing that participants who had a history of sexual contact with a HCV-positive partner had significantly higher odds than those who had not; and tattoo from a nonlicensed provider (OR = 2.62; 95% CI = 1.09, 6.33), with participants who had received a tattoo from a nonlicensed provider having significantly higher odds those who had not. Table 2 lists the output from the logistic regression model. The Hosmer–Lemeshow lack of fit test (χ2 = 11.513; P = .173) indicated acceptable model fit to the data.
TABLE 2—
Logistic Regression Results: Buzz Westfall Justice Center; Saint Louis County, MO; December 2012–May 2013
| Variable | OR (95% CI) | P |
| Age (per y) | 1.093 (1.042, 1.147) | < .001 |
| IDU (yes vs no) | 53.869 (17.780, 163.205) | < .001 |
| Sex (yes vs no) | 7.345 (1.412, 38.204) | .018 |
| Tattoo (yes vs no) | 2.623 (1.087, 6.333) | .032 |
Note. CI = confidence interval; IDU = injection drug use; OR = odds ratio.
The prevalence results in this analysis showed some marked differences in terms of gender and race/ethnicity from the general population. In our population, the prevalence for women was more than 3 times the rate of men (38% vs 12%) whereas in the general population, men have nearly double the prevalence (2.1% vs 1.1%). Table 3 lists the risk factors that were significantly different between male and female participants. Injection drug use was also nearly 3 times higher in women than in men (52.7% vs 19.3%) and all of the women who were anti-HCV-positive reported injection drug use versus 79.3% of the positive men. Accidental needle stick rates were 4.5 times higher in the female population (12.7% vs 2.8%). Female participants were 6 times more likely to have had sex with HCV-positive partners than men (14.5% vs 2.4%). No information was gathered on the gender of the sexual partners who were HCV-positive or the sexual preferences of the participants in this study.
TABLE 3—
Risk Factor Prevalence by Gender: Buzz Westfall Justice Center; Saint Louis County, MO; December 2012–May 2013
| All Women (n = 55), No. (%) | Anti-HCV Positive Women (n = 21), No. (%) | All Men (n = 249), No. (%) | Anti-HCV Positive Men (n = 29), No. (%) | Pa | |
| Injection drug use | < .001 | ||||
| No | 26 (47.3) | 0 (0) | 201 (80.7) | 6 (20.7) | |
| Yes | 29 (52.7) | 21 (100) | 48 (19.3) | 23 (79.3) | |
| Accidental needle stick | .001 | ||||
| No | 48 (87.3) | 15 (71.4) | 242 (97.2) | 28 (96.6) | |
| Yes | 7 (12.7) | 6 (28.6) | 7 (2.8) | 1 (3.4) | |
| Sex with HCV-positive partner | < .001 | ||||
| No | 47 (85.5) | 14 (66.7) | 243 (97.6) | 26 (89.7) | |
| Yes | 8 (14.5) | 7 (33.3) | 6 (2.4) | 3 (10.3) |
Note. The following risk factors were assessed, but the differences were found to be nonsignificant: age, race/ethnicity, blood transfusion, tattoo from nonlicensed provider, HIV infection, born to HCV-positive mother.
All women vs all men.
In terms of self-reported race/ethnicity, participants who identified as White, non-Hispanic had a significantly higher prevalence than those who self-reported as African American or Black (31% vs 9%). In the general US population, Blacks have twice the prevalence of Whites (3% vs 1.5%). Table 4 lists the significant risk factors when comparing Blacks to Whites. Black participants were significantly more likely than White participants to be male (86.3% vs 73.8%), less likely to have engaged in injection drug use (10.4% vs 50.5%), less likely to have received a tattoo from a nonlicensed provider (25.1% vs 43%), less likely to have had an accidental needle stick (1.1% vs 10.3%), and less likely to have had sex with an HCV-positive partner (1.1% vs 10.3%).
TABLE 4—
Risk Factor Prevalence by Self-Reported Race/Ethnicity Buzz Westfall Justice Center; Saint Louis County, MO; December 2012–May 2013
| African American or Black (n = 183), No. (%) | Anti-HCV Positive African American or Black (n = 16), No. (%) | White, Non-Hispanic (n = 107), No. (%) | Anti-HCV Positive White (n = 33), No. (%) | P (African American vs White) | |
| Gender | .008 | ||||
| Female | 25 (13.7) | 4 (25) | 28 (26.2) | 16 (48.5) | |
| Male | 158 (86.3) | 12 (75) | 79 (73.8) | 17 (51.5) | |
| Injection drug use | < .001 | ||||
| No | 164 (89.6) | 4 (25) | 53 (49.5) | 2 (6.1) | |
| Yes | 19 (10.4) | 12 (75) | 54 (50.5) | 31 (93.9) | |
| Tattoo nonlicensed provider | .002 | ||||
| No | 137 (74.9) | 7 (43.8) | 61 (57) | 12 (36.4) | |
| Yes | 46 (25.1) | 9 (56.3) | 46 (43) | 21 (63.6) | |
| Accidental needle stick | < .001 | ||||
| No | 181 (98.9) | 15 (93.8) | 96 (89.7) | 27 (81.8) | |
| Yes | 2 (1.1) | 1 (6.3) | 11 (10.3) | 6 (18.2) | |
| Sex with HCV-positive partner | < .001 | ||||
| No | 181 (98.9) | 15 (93.8) | 96 (89.7) | 24 (72.7) | |
| Yes | 2 (1.1) | 1 (6.3) | 11 (10.3) | 9 (27.3) |
Note. The following risk factors were assessed, but the differences were found to be nonsignificant: age, blood transfusion, HIV infection, born to HCV-positive mother.
DISCUSSION
The overall prevalence rate of 16.4% was higher than that of the general population (1.6%–2.8%) and lower than that of the prison population (23%–41%). It is similar to the estimates from the previous study in a jail population (mean = 13%; range = 10%–15%). The reporting of risk factor prevalence and association with HCV antibody positivity is new for a jail population.
With such a high prevalence rate, universal screening for all jail inmates would make sense from a public health standpoint. Universal screening would also eliminate the potential for missed screening because of inmates not wanting to report illegal or potentially embarrassing risky behaviors. The largest barriers against universal screening are the cost of testing and deciding what next steps to take after diagnosis. It could be argued that because jails are a fluid population with mostly short lengths of stay, treatment would be difficult or impossible in addition to expensive. The benefits of identifying patients with HCV, though, surpass those of immediately treating infected person. Testing provides an opportunity to discuss risk mitigation in both positive and negative inmates. It can also potentially reduce the spread of HCV in prison and in the community. Testing in jails could lead to increased rates and earlier initiation of treatment when inmates transition to more stable living arrangements or, if coordinated correctly, could lead to bridging programs that start treatment in jails and follow patients to their next destination. The cost of testing and potentially for treatment would need to be assessed in the larger picture of better population health and potentially decreased morbidity, mortality, and cost for individual patients and the system as a whole to help justify the increased expenditures as those entities directly paying for the cost of testing are unlikely to see direct cost savings.
The differences found between our population and the general population in terms of gender and race/ethnicity are notable, but their significance cannot be fully explained with the current analysis. Because neither was significant after logistic regression, they would not seem to be particularly useful in developing a comprehensive screening tool, but may be useful when developing and targeting risk-reduction information and strategies. It would also be necessary to expand this analysis to other jail settings to see if these differences hold in the larger jail population or are unique to the current population.
Sex with an HCV-positive partner remained in the multivariable model as an independent risk factor. Because all HCV-positive women reported IDU, sex with an HCV-positive partner, independent of IDU, could be applicable only for men in our analysis. However, the relatively small number of HCV-positive patients overall, the subjective nature of risk-factor reporting, and the rare occurrence of sexual transmission of HCV in general make it impossible to determine that sex with an HCV-positive partner was the source of exposure for any of the patients.
The consent rate of 90.6% and completion rate of 83.7% of those approached indicates the results should be reliable when applied to this specific population. No reasons for refusal to participate were collected, so it is not possible to assess for the presence or direction of bias attributed to nonparticipation or noncompletion after consent was obtained. It is possible that there was a bias away from participation for those who were self-determined to be at low risk for HCV infection. This would lead to a higher estimated prevalence than in the whole jail population.
The results may also be affected by the self-reported nature of the risk-factor questions. Although some patients may have under- or over-reported their high-risk behaviors, this is unavoidable for this type of screening tool.
Future direction for this project is identifying the best way to utilize these data to screen patients prior to their assessment labs to allow for the highest sensitivity and specificity possible while providing a simple user-friendly screening tool for the screening medical staff to utilize. It is also clear that simply screening patients in the 1945 to 1965 birth cohort is not as applicable or beneficial to this high-risk population as it would be to the general population. It is likely that this population could be adequately screened without using the full screening questionnaire, with good sensitivity and specificity to balance clinical detection with cost of testing a large number of negative patients. Using only IDU, sex with an HCV-positive partner, and tattoo from nonlicensed provider as the screening tool (any 1 of the 3 would trigger a screening test) would have missed 3 positive patients (sensitivity of 94%) and would have tested 86 negative patients (specificity of 66%). Adding in age or birth cohort would potentially increase the sensitivity, but would also decrease the specificity unless a more complicated calculation were used rather than a simple rule of what age or birth cohort should be tested.
Acknowledgments
This publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This project was also funded by the Faculty Research Incentive Fund through the St. Louis College of Pharmacy.
The authors would like to thank Ling Chen for her help with statistical analysis and Jeffrey Peipert for his thoughtful review.
Human Participant Protection
This project was reviewed and approved by the Director of Research at the Saint Louis County Department of Health and the St. Louis College of Pharmacy institutional review board.
References
- 1.Alter MJ, Kruszon-Moran D, Nainan OV et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Hu DJ, Jiles R, Holmberg SD. Evolving epidemiology of hepatitis C virus in the United States. Clin Infect Dis. 2012;55(suppl 1):S3–S9. doi: 10.1093/cid/cis393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31(8):1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis - United States, 2010. Atlanta, GA: US Department of Health and Human Services; 2012. [Google Scholar]
- 6.Razavi H, Elkhoury AC, Elbasha E et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology. 2013;57(6):2164–2170. doi: 10.1002/hep.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37(suppl 2):S88–S94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 8.Di Bisceglie AM, Lyra AC, Schwartz M et al. Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol. 2003;98(9):2060–2063. doi: 10.1111/j.1572-0241.2003.t01-1-07641.x. [DOI] [PubMed] [Google Scholar]
- 9.Berg CL, Steffick DE, Edwards EB et al. Liver and intestine transplantation in the United States 1998-2007. Am J Transplant. 2009;9(4 Pt 2):907–931. doi: 10.1111/j.1600-6143.2009.02567.x. [DOI] [PubMed] [Google Scholar]
- 10.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 11.Moorman AC, Gordon SC, Rupp LB et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the chronic hepatitis cohort study. Clin Infect Dis. 2013;56(1):40–50. doi: 10.1093/cid/cis815. [DOI] [PubMed] [Google Scholar]
- 12.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5, suppl 1):S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 13.Smith BD, Morgan RL, Beckett GA et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1–32. [PubMed] [Google Scholar]
- 14.Latimer WW, Hedden SL, Floyd L et al. Prevalence and correlates of hepatitis C among injection drug users: the significance of duration of use, incarceration and race/ethnicity. J Drug Issues. 2009;39(4):893–904. doi: 10.1177/002204260903900406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennessey KA, Kim AA, Griffin V, Collins NT, Weinbaum CM, Sabin K. Prevalence of infection with hepatitis B and C viruses and co-infection with HIV in three jails: a case for viral hepatitis prevention in jails in the United States. J Urban Health. 2009;86(1):93–105. doi: 10.1007/s11524-008-9305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon L, Flynn C, Muck K, Vertefueille J. Prevalence of HIV, syphilis, hepatitis B, and hepatitis C among entrants to Maryland correctional facilities. J Urban Health. 2004;81(1):25–37. doi: 10.1093/jurban/jth085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spaulding AC, Thomas DL. Screening for HCV infection in jails. JAMA. 2012;307(12):1259–1260. doi: 10.1001/jama.2012.374. [DOI] [PMC free article] [PubMed] [Google Scholar]