Abstract
Objectives
This study examined whether dispositional optimism would be associated with reduced levels of cortisol secretion among individuals who perceive stress levels that are either higher than their normal average (i.e., within-person associations) or higher than the stress levels of other individuals (i.e., between-person associations).
Methods
Stress perceptions and four indicators of diurnal cortisol (AUC, awakening, afternoon/evening, and CAR levels) were assessed on 12 different days over six years in a sample of 135 community-dwelling older adults.
Results
Hierarchical linear models showed that while pessimists secreted relatively elevated AUC, awakening, and afternoon/evening levels of cortisol (but not CAR) on days they perceived stress levels that were higher than their normal average, optimists were protected from these stress-related elevations in cortisol. However, when absolute stress levels were compared across participants, there was only a significant effect for predicting CAR (but not the other cortisol measures), indicating that optimism was associated particularly strongly with a reduced CAR among participants who experienced high levels of stress.
Conclusions
Dispositional optimism can buffer the association between stress perceptions and elevated levels of diurnal cortisol when individuals perceive higher-than-normal levels of stress, and it may predict a reduced CAR among individuals who generally perceive high stress levels. Research should examine relative, in addition to absolute, levels of stress to identify the personality factors that help individuals adjust to psychological perceptions of stress.
Keywords: dispositional optimism, perceived stress, cortisol
Research has shown that optimists are more likely than pessimists to adjust successfully to stressful life circumstances and maintain their physical health (Rasmussen, Scheier, & Greenhouse, 2009). Although such health benefits could occur, at least in part, because optimism ameliorates the secretion of cortisol, research has failed to show that optimism consistently modulates stress-related alterations in cortisol (e.g., Taylor, Burklund, Eisenberger et al., 2008). The available literature on optimism, however, has examined inter-individual differences in stress and cortisol. This approach is based on comparing a person’s stress level to other individuals and thus leaves unexamined the possibility that optimism could prevent cortisol dysregulation in circumstances when individuals experience stress levels that are higher than their typical level of stress. To examine the latter possibility, within-person research is needed to assess stress levels over time and capture deviations from a person’s typical level of stress. Such an approach may be particularly fruitful because it controls for each person’s average level of stress and thus rules out the possibility that associations between stress and cortisol could be attenuated if cortisol secretion among some individuals have become habituated to high levels of stress (Miller, Chen, & Zhou, 2007). Here we test this hypothesis by examining the influence of dispositional optimism on the within-person and between-person associations of stress perceptions and diurnal cortisol in a community sample of older adults. We expected that optimism would be associated with a buffering of the stress-cortisol link and becomes paramount when individuals perceive stress that is higher than their normal average.
Optimism, Perceived Stress, and Diurnal Cortisol
Dispositional optimism is conceptualized as a relatively stable, continuous, and bipolar individual difference variable, reflecting a person’s generalized expectations about future life events across different domains (Scheier & Carver, 1985). While optimists hold expectancies for positive outcomes, pessimists tend to expect negative outcomes. A large body of research has shown that optimism ameliorates the adverse consequences of stressful life experiences on individuals’ well-being and health. For example, optimists cope more effectively with stress and report higher levels of subjective well-being than pessimists (Carver, Scheier, & Segerstrom, 2010; Wrosch & Scheier, 2003). In addition, stress-related benefits of optimism have been associated with adaptive immune responses (Brydon, Walker, Wawrzyniak et al., 2009; Ironson et al., 2005; Segerstrom, Taylor, Kemeny, & Fahey, 1998)1 and physical health outcomes (e.g., physical symptoms, cardiovascular incidents, or survival, Boehm & Kubzansky, 2012; Rasmussen, Scheier, & Greenhouse, 2009).
A biological mechanism that could be associated with these beneficial consequences of optimism is related to individuals’ cortisol secretion. Cortisol is a hormone that is secreted by the HPA axis and follows a diurnal rhythm across the day (peaking shortly after awakening and subsequently declining until bedtime, Van Cauter & Turek, 1994). Research suggests that the psychological perception of stress and associated negative affect can release cortisol into the circulation (Cohen, Janicki-Deverts, & Miller, 2007).2 While cortisol may facilitate the short-term management of stressful circumstances (Taylor et al., 2000), it also serves regulatory functions in different bodily systems and through these processes could compromise physical health (e.g., dysregulation of immune, metabolic, or nervous systems, Bjoerntorp & Rosmond, 1999; Cohen et al., 2007). In support of this possibility, increased cortisol output has been associated with aging, physical health problems, and mortality (Otte et al., 2005; Sephton, Sapolsky, Kraemer, & Spiegel, 2000; Wrosch, Miller, & Schulz, 2009), although both elevated and blunted forms of cortisol may affect physical health (Segerstrom & Miller, 2004).
The previous discussion makes it likely that optimism is also associated with cortisol secretion. In particular, the behavioral and emotional benefits of optimism may prevent individuals who perceive high levels of psychological stress from exhibiting an elevated cortisol response. Surprisingly, however, research examining the role of dispositional optimism in the stress-cortisol link shows inconsistent results. While some studies found optimism to be associated with a lower cortisol awakening response (Endrighi, Hamer, & Steptoe, 2011; Lai, Evan, Ng et al., 2005) and reduced cortisol output after a stress induction (Brydon et al., 2009), several other studies suggest that optimism is unrelated to cortisol level across the day (Endrighi et al., 2011; Minton, Hertzhog, Barron et al., 2009), cortisol awakening levels (Ebrecht et al., 2004), and stress-induced cortisol response (Endrighi, et al., 2011; Taylor et al., 2008).
A review of the extant literature indicates that this research has relied on between-person designs. In particular, the studies examined how either inter-individual differences in levels of naturally occurring or experimentally induced stress are associated with cortisol output among optimists versus pessimists (e.g., Minton et al., 2009; Taylor et al., 2008). While this approach compares each individual’s stress level to the mean of a sample of different individuals, it does not consider that optimism may protect individuals against elevations in cortisol when they are faced with stress that is higher than their personal average. To examine the latter possibility, however, within-person research is needed to measure perceptions of stress repeatedly over time.
We think that such an approach could contribute to a more comprehensive understanding of the role of dispositional optimism in the stress-cortisol link. Most importantly, a within-person approach would address a potential problem that may arise from the fact that pessimists typically perceive higher levels of stress than optimists (Carver et al., 2010). In this regard, these differences in absolute levels of perceived typical stress could attenuate a buffering effect of optimism on the association between perceived stress and cortisol secretion. This could be the case because individuals’ physiological system can habituate to stress over time and sustained exposure to severe stress may result in lower levels of cortisol (Miller et al., 2007; Wüst, Fedorenko, vanRossum et al., 2005). Thus, given that pessimists typically perceive higher levels of stress than optimists, pessimists may also be particularly likely to become physiologically habituated to their typically higher levels of stress, which may at times result in a relatively low secretion of cortisol. As a consequence, pessimists’ stress-related cortisol responses might not always be distinguishable from their optimistic counterparts. We should be clear about what it is that we think habituates. Specifically, we believe that it is the response of the HPA axis to perceptions of stress, and not necessarily the perception of stress itself. Thus, pessimists might perceive higher levels of stress than optimists, but still not exhibit increased levels of cortisol.
Nonetheless, differences in cortisol output between optimists and pessimists may be reliably observed if perceptions of stress exceed individuals’ typical stress levels. In such circumstances, pessimists are less likely to be habituated to the stress experienced and should exhibit an associated increase in their cortisol levels, while the beneficial behavioral and emotional effects of optimists’ positive outcome expectancies may ameliorate stress-related cortisol output. Further, such differences in stress-related cortisol secretion between optimists and pessimists should be particularly evident in within-person research, as this approach accounts for habituation effects by examining deviations from a person’s typical stress level.
The Present Study
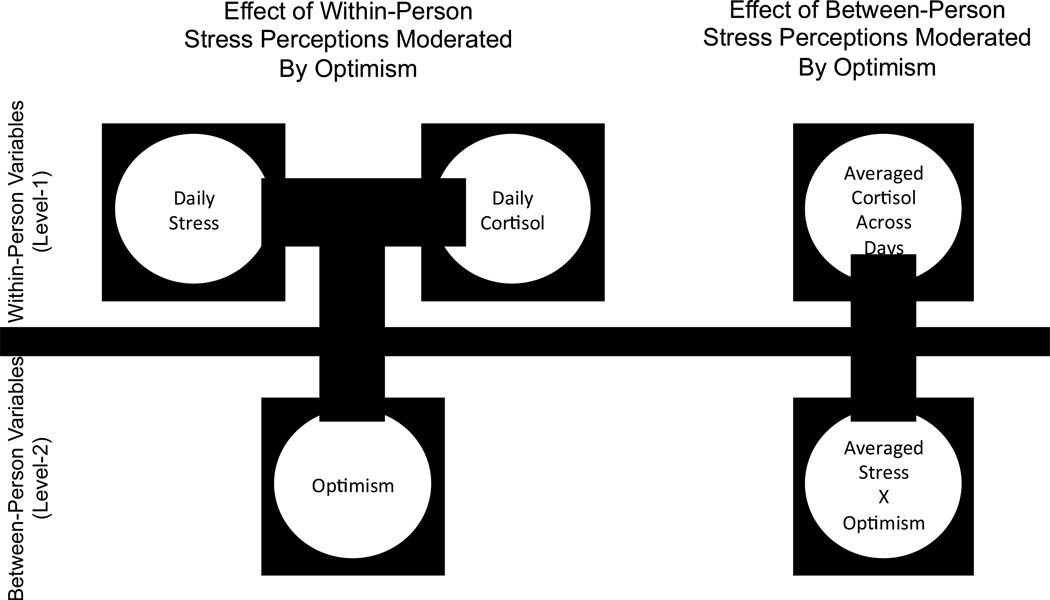
We examined whether dispositional optimism would moderate the within-person and/or between-person associations of psychological perceptions of stress and four indicators of diurnal cortisol secretion (area-under-the-curve [AUC], awakening levels, afternoon/evening levels, and cortisol awakening response [CAR]). To this end, we analyzed data from a heterogeneous and community-dwelling sample of older adults, which included measures of stress perceptions and diurnal cortisol secretion on twelve different days across six years of study. This normative study of older adults was particularly well-suited to test our hypothesis, as aging is commonly associated with both incidence of age-normative problems and dysregulation of cortisol (McEwen & Stellar, 1993; Wrosch & Schulz, 2008). We hypothesized that participants would exhibit higher levels of indicators of cortisol secretion on days they perceived higher, as compared to lower, stress. In addition, we hypothesized that this effect would appear only among pessimists, and not among optimists. Finally, we analyzed the same data points in between-person analyses by averaging the 12 daily measures of stress and cortisol. Given the aforementioned mixed literature, we explored whether optimism would also be associated with a buffering of the stress-cortisol link in between-person analyses (see Figure 1 for the conceptual framework that guides the research).
Figure 1.
Graphical representation of the potential moderating role of dispositional optimism in the within-person and between-person associations between perceptions of stress and cortisol secretion. In the within-person analyses perceptions of stress was a Level-1 predictor, whereas in the between-person analyses perceptions of stress were aggregated across twelve measurement points and used as a Level-2 predictor.
Method
Participants
This study was based on a heterogeneous sample of community-dwelling older adults who participated in the Montreal Aging and Health Study (Wrosch, Schulz, Miller, Lupien, & Dunne, 2007). Following a baseline assessment in 2004 (N = 215), subsequent waves of the study were conducted approximately two years (M = 1.89, SD = 0.08, range = 1.72 to 2.13 years; n = 184), four years (M = 3.78, SD = 0.24, range = 3.28 to 4.77 years; n = 164) and six years after baseline (M = 6.05, SD = 0.20, range = 5.52 to 6.40 years; n = 137). Attrition over six years of study was associated with refusal to participate further (n = 9), inability to locate participants (n =19), presence of other personal problems (n = 27), and death (n = 23). Participants who dropped out of the study were significantly older at baseline (M = 73.82, SD = 6.78) than those who remained in the study (M = 71.61, SD = 5.21; t[129.14] = 2.49, p = 0.01). Study attrition was not significantly associated with any of the other baseline variables used in this study or the earliest measure of dispositional optimism (i.e., 2-year follow-up). Two of those 137 subjects who participated in the 6-year follow-up were further excluded from the analyses because they provided cortisol samples on less than 50% of the sampling days, resulting in a final sample of 135 subjects.
Procedure
Participants were recruited through newspaper advertisements. In order to obtain a normative sample, the only inclusion criterion was that participants had to be older than 60 years. In each wave of the study, they were either visited in their homes or invited to the laboratory and responded to a main questionnaire. On three non-consecutive and typical days during the week following the initial appointment, participants collected saliva and responded to daily questionnaires including the perception of stress. Across waves, this procedure resulted in twelve assessments of daily cortisol and stress perceptions over six years of study.
Materials
Perceptions of stress were assessed in each wave over three days by asking participants at bedtime to rate how 1) stressed and 2) overwhelmed they felt during each of three days, using 5-point Likert-type scales (0 = very slightly or not at all to 4 = extremely). For each day, we computed a sum score of the two items to obtain daily measures of stress perceptions (rs = .20 to .60, ps < .01; average r [based on z-transformation] = .44, p < .01). Because some subjects did not participate in all waves, 85 out of 1620 potential stress values (5.25%) were replaced with the respective sample mean3. Perceptions of stress showed some stability within waves (average r = .58, p < .01) and exerted an average 2-year stability across waves of r = .28, p < .01. We also computed an overall score of stress perceptions by averaging stress scores across all twelve assessments.
Diurnal cortisol secretion was also assessed across waves on three days. Participants used salivettes to collect five saliva samples throughout the day: at awakening, 30 minutes after awakening, 2 PM, 4 PM, and bedtime. They were instructed not to brush their teeth or eat thirty minutes prior to saliva collection to prevent contamination with food or blood. Participants took the first saliva sample when they awoke. To collect the second saliva sample thirty minutes after awakening, they were provided with a timer. Participants were contacted by phone to facilitate compliance with the afternoon saliva collection (i.e., at 2 PM and 4 PM). They collected the last saliva sample by themselves at the time they went to bed. The exact time of day of each sample collected was recorded by the participants. Samples were stored in participants’ home refrigerators until they were returned to the lab 2–3 days after collection was completed, and they were frozen until completion of each wave. Cortisol analysis was performed at the University of Trier using a time-resolved fluorescence immunoassay with a cortisol-biotin conjugate as a tracer. The intra-assay coefficient of variation was less than 5%, and the inter-assay variability from cortisol analyses performed at the University of Trier has been found to be routinely below 10%.
We collected 7815 cortisol samples from the 135 participants (96.48% of possible samples). Ninety-four samples (1.2%) deviated 3 standard deviations or more from the mean cortisol level for a given time of day and were excluded from the analyses. To obtain a reliable CAR, 72 samples (4.67%) were further excluded because they deviated more than 10 minutes from 30-minutes after awakening, and thus could compromise and accurate measurement of CAR. We calculated cortisol indicators only for days during which participants provided at least four usable cortisol scores, resulting in cortisol scores for 95.19% of the 1620 sampled days. For days on which participants had one single cortisol score missing (8.95%), the missing value was replaced with the respective sample mean. Additional missing values for single days (4.81%) were also replaced by the respective sample mean. Across waves, samples were on average collected .51 (SD = .02), 7.04 (SD = .96), 9.11 (SD = .97), and 15.82 hours (SD = .94) after awaking. The cortisol scores were log-transformed to stabilize variance. They formed a typical diurnal rhythm, including high awakening levels (M = 1.06, SD = .15), increasing 30-minutes levels (M = 1.16, SD = .17), as well as declining levels at 2 PM (M = .76, SD = .12), 4 PM (M = .69, SD = .12), and bedtime (M = .54, SD = .14).
We calculated four different indicators of cortisol secretion for each assessment day. To examine overall cortisol volume, area-under-the-curve (AUC) across day was computed using the trapezoidal method based on hours after awakening. The 30-minutes measure was excluded from AUC because early morning increase of cortisol has been shown to be relatively independent from overall cortisol volume (Chida & Steptoe, 2009). In addition, we analyzed awakening levels (by using the first measure of the day) and afternoon/evening levels of cortisol (by averaging the last three measures of the day) to explore whether differences in overall cortisol volume would relate to morning levels and/or later levels of cortisol secretion. Finally, we calculated the cortisol awakening response (CAR) by computing the difference between the 30-minutes and the awakening measures. All indicators of cortisol secretion showed some stability within waves (average rs = .26 to .56, ps < .01) and across waves (average 2-year stability: rs = .22 to .35, ps < .01).
Dispositional optimism was assessed in waves 2, 3, and 4, using the 6-item Life Orientation Test-Revised, which is a reliable and well-validated measure of dispositional optimism (LOT-R, Scheier, Carver, & Bridges, 1994). Participants were asked to indicate their agreement with each of the six items, using 5-point Likert-type scales (0 = strongly disagree, to 4 = strongly agree). The LOT-R includes three optimism items (e.g., I am always optimistic about my future) and three pessimism items (e.g., If something can go wrong for me, it will). For each wave, we computed a sum score of the six items after reverse coding the pessimism items. Measures of optimism demonstrated good internal consistency (αs = .72 to .79), were correlated (average 2-yr stability: r = .73, p < .01), and did not change significantly across waves (F [1, 134] = 1.81, p = .18). The optimism scales were averaged across waves to obtain a reliable measure of dispositional optimism.
Sociodemographic and health-related covariates were included into the study to minimize the presence of spurious associations. Age and sex was assessed by self-report. Socioeconomic status was measured using three baseline variables (highest education, yearly family income, and perceived social status, α = .69) and averaged to obtain a reliable indicator of socioeconomic status. We coded participants as smokers if they smoked at any time during the study (average stability: r = .67, p < .01). Chronic illness was measured by assessing the presence of 17 different health problems (e.g., coronary heart disease, arthritis, or cancer) and averaged across waves (average stability: r = .75, p < .01). Self-reported body-mass-index (BMI in kg/m2) was calculated and averaged across waves (average stability: r = .79, p < .01). Finally, we calculated change scores of participants’ chronic health problems and BMI across waves by predicting in regression analyses the wave 4 levels by the baseline levels and saving the standardized residuals for further analyses.
Data Analysis
Preliminary analyses were conducted in order to describe the sample (by calculating means and frequencies) and the zero-order associations among main study variables (by calculating correlations). In addition, we examined whether indicators of cortisol secretion and perceptions of stress would vary as a function of assessment day and/or wave (by using ANOVAs) to assess whether these factors need to be included as covariates in subsequent analyses.
Next, we tested the study’s hypotheses (see Figure 1) by performing two sets of hierarchical linear models, using HLM 7.0. In the Level-1 models of the first set of analyses, variability in the four different cortisol indicators (i.e., AUC, awakening level, afternoon/evening level, and CAR) across assessments was estimated as a function of person-centered scores of daily stress perceptions, person-centered time-related factors (that proved to be significant in the prelimary analyses), and a residual term. In these models, the intercepts represented participants’ average cortisol levels across daily assessments, while the slopes for stress perceptions indicated whether deviations from a person’s average level of stress perceptions were reliable predictors of variability in cortisol output. In the Level-2 models, we predicted all coefficients obtained in the Level-1 models by between-person differences in dispositional optimism and the covariates to examine the presence of significant cross-level interaction effects between optimism and intra-individual variability of stress perceptions in predicting particpants’ cortisol secretion.
The second set of hierarchical models examined between-person associations among optimism, stress perceptions, and cortisol (see Figure 1). In contrast to the first set of analyses, the Level-1 models only included person-centered time-related factors and a residual term as predictors of variability in daily cortisol volume (and excluded person-centered stress perceptions). The coefficients of interest in these analyses were the intercepts, which represented participants’ average cortisol levels across daily assessments. In the first step of the Level-2 models, we estimated the obtained variability in average cortisol output (and in the associations between time-related factors and cortisol) as a function of between-person differences in optimism, perceptions of stress, and the covariates. In a second step we tested whether the interaction between optimism and inter-individual variability of stress perceptions would significantly predict variability in participants’ cortisol secretion. Both sets of hierarchical models were based on using restricted maximum likelihood estimation and robust standard errors. Level-2 predictors were standardized prior to conducting the analyses. Specifications of the models are reported in Tables 1 and 2 of the “Online Supplemental Materials” [OSM].
Table 1.
Means, Standard Deviations and Frequencies of Main Study Variables (N = 135)
| Constructs | Mean (SD) or Percentage a |
|---|---|
| Average cortisol AUC (in log nmol/L×h) | 12.00 (1.65) |
| Average cortisol awakening level (in log nmol/L) | 1.05 (.14) |
| Average cortisol afternoon/evening level (in log nmol/L) | .66 (.10) |
| Average cortisol awakening response (in log nmol/L) | .11 (.12) |
| Average perceptions of stress | .79 (.71) |
| Dispositional optimism | 16.65 (3.43) |
| Age | 71.54 (5.20) |
| Female (%) | 53% |
| Average number of chronic health problems | 2.38 (1.59) |
| Average body-mass-index | 25.75 (3.59) |
| Smoking (%) | 7.4% |
| Education (%) | |
| None | 3.8% |
| High School | 29.2% |
| College/Trade | 23.0% |
| Bachelor | 24.6% |
| Masters/PhD | 12.3% |
| Income | |
| Less than $17,000 | 21.4% |
| $17,001 – $34,000 | 37.3% |
| $34,001 – $51,000 | 19.0% |
| $51,001 – $68,000 | 15.1% |
| > $68,000 | 3.2% |
| Subjective social status | 6.22 (1.85) |
Note.
Mean (SD) are presented for continuous variables. For more specific cortisol and stress values across study waves and assessment days, see SOM Table 2 and 3.
Results
Preliminary Analyses
As reported in Table 1, approximately half of the sample was female and participants were on average 72 years old. They experienced an average of 2–3 chronic health problems and had an average BMI that was located at the cusp between normal weight and overweight. Less than 10% of the sample smoked and 37% of participants had obtained a graduate degree. Participants’ income was quite heterogeneous and approximately half of the sample had an annual income between $17,000 and $51,000. The sample average for perceived social status was slightly above the midrange of the scale. The socio-demographic and health related characteristics of this sample were representative of community-dwelling older adults (National Advisory Council on Aging, 2006).
Results from correlational analyses among the main between-person variables showed that optimism was significantly associated with lower perceptions of stress, r = −.37, p < .01 (for correlations among other study variables, including covariates, see OSM Table 3). Average AUC, awakening levels, and afternoon/evening levels of cortisol were positively correlated, rs > .31, ps < .01. CAR was not significantly associated with AUC or afternoon/evening levels of cortisol, but correlated with lower awakening levels, r = −.33, p < .01. There were no significant correlations between the averaged four indicators of cortisol secretion with dispositional optimism or averaged levels of stress.
To explore time-related changes in the four indicators of cortisol secretion and perceptions of stress, five separate repeated measurement ANOVAs were conducted including the within-subject factors Wave (4 levels) and Day (3 levels). These analyses are presented in Table OSM 4 to Table OSM 6. The results showed significant linear effects of Wave for stress perceptions and all cortisol indicators, except CAR (see Table OSM 4). In addition, they indicated linear effects of Day for awakening and afternoon/evening levels of cortisol. Finally, Table OSM 4 shows quadratic Wave and Day effects for some indicators of cortisol secretion, and a quadratic Wave effect for stress perceptions. Overall, the pattern of findings indicated that cortisol levels (except CAR) mostly increased over the first three waves and declined in the last wave. Stress perceptions, however, were higher in the last three waves, as compared to baseline (see Table OSM 5). With respect to Day, afternoon/evening levels of cortisol increased across days, and AUC and awakening levels peaked during the second assessment day, while CAR levels were comparably low during the second day (see Table OSM 6). These findings indicate that further hypotheses-related analyses should control for linear and quadratic effects of time since study entry and assessment day.
Predicting Within-Person Variation in Diurnal Cortisol Secretion
The analyses examining the within-person associations between perceptions of stress and the four indicators of cortisol predicted in separate Level-1 models variability in participants’ cortisol secretion across 12 daily measures by person-centered scores of stress peceptions, linear and quadratic effects of years since study entry and assessment day, and a residual term. (for dfs, see Tables OSM 1 and 2). The results of the analyses showed that average levels (i.e., intercepts) of AUC, β = 12.00, SE = .14, p < .01, awakening cortisol, β = 1.05, SE = .01, p < .01, afternoon/evening cortisol, β = .66, SE = .01, p < .01, and CAR, β = .11, SE = .01, p < .01, were significantly different from zero. In addition, person-centered stress perceptions (i.e., slope) significantly predicted variability of AUC, β = .17, SE = .05, p < .01, awakening level, β = .02, SE = .01, p < .01, and afternoon/evening level of cortisol secretion, β = .01, SE = .00, p = .03, but not CAR, β = −.01, SE = .01, p = .17. The latter findings indicate that participants secreted higher AUC, awakening, and afternoon/evening levels of cortisol on days during which they perceived high levels of stress as compared to days that involved comparatively lower stress levels. Finally, the results from the Level-1 models showed that there was considerable variability in the average levels of all cortisol indicators, χ2s = 1027.86 to 335.80, ps < .01, as well as in the within-person association between perceptions of stress and AUC, awakening levels, and afternoon/evening levels of cortisol, χ2s = 160.74 to 143.30, ps = .02 to .15. There was less variability in the associations between stress perceptions and CAR, χ2 = 119.77, p > .50.
In the Level-2 models, we attempted to explain the observed variability in participants’ cortisol secretion and in their within-person associations between stress perceptions and cortisol by predicting all Level-1 coefficients by dispositional optimism and the covariates. The obtained results indicate that of the covariates only sex, average chronic illness, and increases in chronic illness exerted significant effects on participants’ average (i.e., intercept) AUC of cortisol. In addition, sex and age significantly predicted participants’ average afternoon/evening levels of cortisol. Women had lower AUC, β = −.46, SE = .14, p < .01, and afternoon/evening levels of cortisol, β = −.03, SE = .01, p < .01, than men. Moreover, older, as compared with younger, participants had higher afternoon/evening levels of cortisol, β = .02, SE = .01, p < .01. Finally, while increases in chronic health problems were associated with a higher AUC of cortisol, β = .30, SE = .14, p = .04, high average levels of chronic health problems were associated with a lower AUC of cortisol, β = −.39, SE = .15, p < .01.4 None of the remaining covariates or dispositional optimism predicted AUC or afternoon/evening levels of cortisol, and there were no significant effects on awakening levels or CAR.
With respect to the within-person associations between stress perceptions and the four indicators of cortisol, none of the covariates explained significant proportions of variance in these associations. However, dispositional optimism showed significant cross-level interaction effects on the associations between within-person perceptions of stress and AUC, β = −.19, SE = .04, p < .01, awakening levels, β = −.01, SE = .00, p < .01, and afternoon/evening levels, β = −.01, SE = .00, p = .04, of cortisol (but not CAR, β = .01, SE = .01, p = .07).5
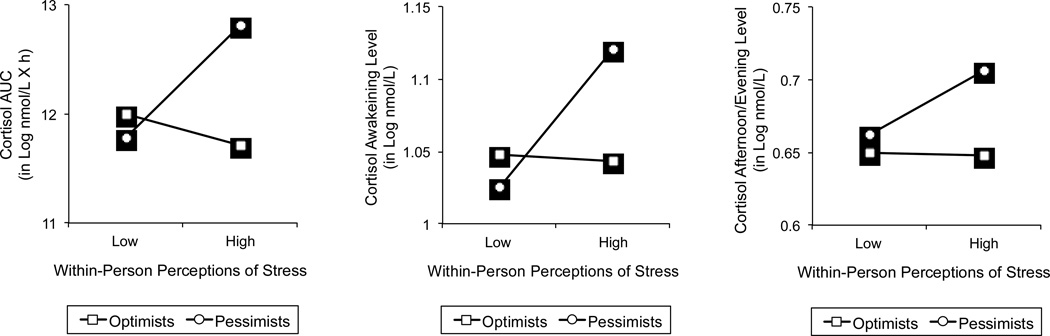
To interpret the significant interactions, we plotted in Figure 2 the within-person associations between perceptions of stress and AUC (left panel), awakening levels (middle panel), and afternoon/evening levels (right panel) of cortisol, separately for optimists and pessimists, using the averaged upper and lower quartiles of the distributions of dispositional optimism and daily stress perceptions as reference points (Preacher, Curran, & Bauer, 2006). In support of our hypotheses, analyses of the simple slopes demonstrated that within-person perceptions of stress were significantly associated with elevated AUC, β = .40, SE = .08, p < .01, awakening levels, β = .04, SE = .01, p < .01, and afternoon/evening levels of cortisol, β = .02, SE = .01, p < .01, among pessimists, but not among optimists, |βs| < .10, SEs < .07, ps > .05. In addition, optimism was significantly associated with lower AUC, β = −.42, SE = .17, p = .01, awakening levels, β = −.03, SE = .02, p = .05, and afternoon/evening levels of cortisol, β = −.02, SE = .01, p = .04, on days when participants perceived higher-than-normal stress, but not on days that involved lower-than-normal level of stress, |βs| < .08, SEs < .13, ps > .50.
Figure 2.
Within-person associations between stress perceptions and AUC (left panel), awakening level (middle panel), and afternoon/evening level (right panel) of cortisol secretion, separately for pessimists and optimists. Associations were plotted for the averaged upper and lower quartiles of the predictor variables.
Predicting Between-Person Variation in Diurnal Cortisol Secretion
To examine whether between-person differences in levels of stress perceptions and dispositional optimism would also be associated with participants’ diurnal cortisol secretion, we repeated the previously reported Level-1 models by predicting the four indicators of cortisol secretion (and excluding person-centered scores of stress perceptions as a predictor from the Level-1 analyses). In the Level-2 models, we included between-person differences in perceptions of stress (averaged across 12 days), dispositional optimism, and the covariates as predictors of the Level-1 coefficients. In a final step, we tested the interaction term between perceptions of stress and dispositional optimism for significance.
The significance and direction of effects for the Level-1 models, and the Level-2 effects of the covariates and dispositional optimism on the Level-1 intercept were identical to the previously reported analyses, and are therefore not reported again. However, and in contrast to the reported within-person analyses, the Level-2 main effect of between-person differences in perceptions of stress did not significantly predict the average levels of any of the four indicators of cortisol secretion, |βs| < .03, SEs < .12, ps > .32. In addition, the subsequently tested interaction between perceptions of stress and dispositional optimism did not significantly predict AUC, awakening, or afternoon/evening levels of cortisol, |βs| < .01, SEs < .10, ps > .26 but did significantly predict CAR, β = −.03, SE = .01, p < .01.
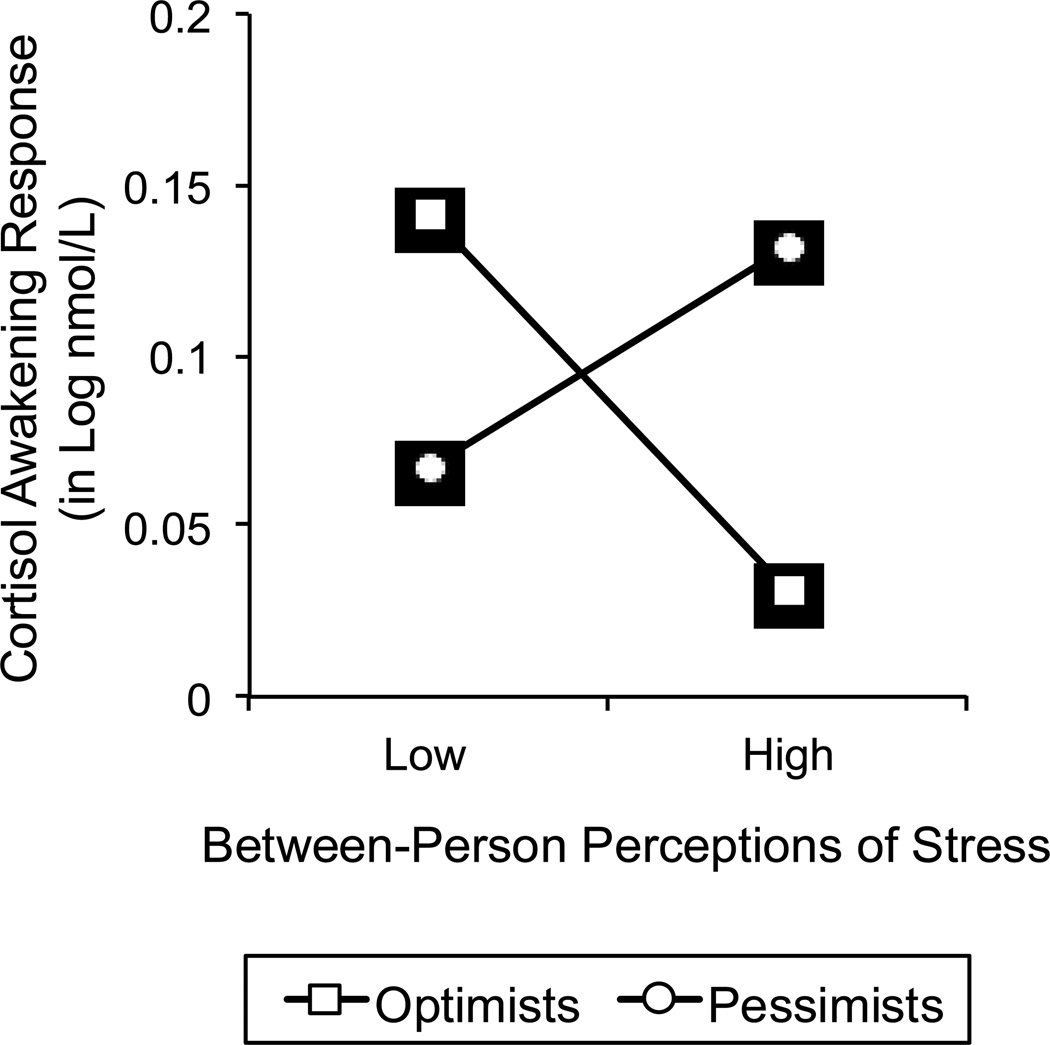
The interaction involving between-person associations of stress perceptions and dispositional optimism in predicting average levels of CAR is illustrated in Figure 3 for the averaged upper and lower quartiles of the predictor variables. Follow-up analyses of the simple slopes suggested that similar to the previously reported interactions, pessimists who perceived high levels of stress across the study exhibited a larger CAR than their counterparts who generally perceived lower levels of stress, β = .03, SE = .01, p < .01. In addition, among participants who perceived high levels of stress, optimism was significantly associated with lower CAR, β = −.04, SE = .01, p < .01. However, the obtained interaction was also somewhat different from the within-person results, in that optimists who perceived low levels of stress secreted relatively elevated levels of CAR, which were higher than CAR of pessimists who perceived low levels of stress, β = .03, SE = .01, p < .01, as well as higher than CAR of optimists who reported high levels of stress perceptions, β = −.04, SE = .01, p < .01.
Figure 3.
Between-person associations between stress perceptions and cortisol awakening response, separately for pessimists and optimists. Associations were plotted for the averaged upper and lower quartiles of the predictor variables.
Discussion
This study showed that dispositional optimism can moderate the associations between psychological perceptions of stress and increased cortisol secretion in a community sample of older adults. However, this association was obtained for most indicators of cortisol (except for CAR) only when stress perceptions were evaluated within each individual in comparison to each person’s average level of stress (i.e., within-person associations) and not when participants’ absolute stress perceptions were compared to the sample mean (i.e., between-person associations). In particular, higher-than-normal perceptions of stress were reliably associated with elevated AUC, awakening, and afternoon/evening levels of cortisol secretion among pessimists. Optimists, by contrast, were protected from exhibiting higher levels of cortisol secretion on days they perceived stress levels that were higher than their normal average.
We did not find the same associations for participants’ absolute levels of stress perceptions. Here, pessimists who perceived relatively high levels of stress across the entire study period did not differ from optimists in their levels of AUC, awakening, or afternoon/evening levels of cortisol. To provide an explanation for this finding, we suggest that even though pessimists’ absolute stress levels were higher than their optimistic counterparts’, associations between inter-individual differences in stress perceptions and pessimists’ cortisol secretion may not have been observed because pessimists had become physiologically habituated to their typical and high levels of stress (cf. Miller et al., 2007; Wüst et al., 2005). This conclusion is supported by the reported within-person analyses, which demonstrated that pessimists exhibited stress-related elevations of cortisol if habituation effects of typical levels of stress were controlled for.
The study’s findings further showed that a general association between stress perceptions and AUC, awakening, and afternoon/evening levels of cortisol was observed only if relative stress perceptions were compared within individuals. By contrast, and consistent with some other research (e.g., van Eck, Berkhof, Nicolson & Sulon, 1996), we did not obtain a significant association between inter-individual differences in stress perceptions and cortisol secretion. In this regard, we suspect that a similar habituation mechanism could underlie this pattern of findings. Consistent with this notion, perceptions of stress were significantly correlated across waves of assessment. Thus, there was some stability in stress ratings across time. This could set the stage for cortisol secretion to habituate to chronically high levels of stress. If so, links between stress and cortisol might emerge only when stress perceptions are higher than individuals’ normal levels.
Of interest, our analyses did not confirm significant within-person associations between optimism, stress perceptions, and CAR. However, the interaction between optimism and averaged perceptions of stress significantly predicted CAR in the reported between-person analyses. The shape of this interaction suggests that dispositional optimism can buffer the association between absolute levels of stress perceptions and CAR. Absolute levels of stress perceptions were positively associated with a larger CAR among pessimists, and this association became increasingly negative among optimists. While we did not postulate a-priori that CAR would be associated with the interaction of stress and optimism in between-person analyses, previous research may provide an explanation for this effect. In this regard, it has been shown that CAR can be relatively independent from other aspects of the diurnal rhythm of cortisol (e.g., cortisol across day, Schmidt-Reinwald et al., 1999). In addition, a recent meta analysis indicated that CAR in particular is reliably associated with high levels of chronic life stress (Chida & Steptoe, 2009). Thus, it is possible that CAR is less suceptible to stress habituation than other aspects of cortisol functioning, which could explain the significant effects of absolute levels of stress perceptions on CAR.
We acknowledge that the shape of the interaction effect for predicting CAR was not completely identical with the interactions obtained in the within-person analyses. In particular, optimists who generally perceived low stress exhibited a relatively elevated CAR (see Figures 2 and 3). In this regard, we note that other factors have been implicated in enhanced CAR, such as low depression or fatigue (Chida & Steptoe, 2009). Post-hoc analyses of our data showed that the significant association between optimism and CAR among participants who generally perceived low stress was rendered non-significant, β = .02, SE = .01, p = .12, if we additionally controlled the analysis for average levels of depressive symptoms.6 Follow-up regression analyses further indicated that this reduction was based on associations between low depressive symptoms and high CAR, particularly so among optimists, β = −.53, p < .01 (versus pessimists: β = .09, p = .51), and participants who perceived low stress, β = −.44, p < .01 (versus high stress: β = −.02, p = .87). Given that depressive symptoms were also associated with low optimism, β = −.37, p < .01, and high stress, β = .41, p < .01, an implication of these findings is that optimism may prevent low mood and foster engagement with desired activities among individuals who generally perceive low stress, which could contribute to optimists’ somewhat higher CAR in such circumstances.
Overall, the study’s results may help reconcile some of the mixed findings in the literature on dispositional optimism, stress, and cortisol secretion. While a large body of research has documented stress-related benefits of dispositional optimism on effective coping, subjective well-being, and physical health (Carver et al., 2010; Rasmussen et al., 2009), several studies examining inter-individual differences in stress levels failed to support that dispositional optimism also facilitates cortisol regulation (Ebrecht et al., 2004; Endrighi et al., 2011; Taylor et al., 2008). Contrary to these previous studies, our findings support the idea that optimism can ameliorate the association between stress perceptions and cortisol. However, for most indictators of cortisol secretion this association only emerged when participants perceived stress levels that were higher than their normal average, and not if stress levels were compared to the sample mean. As a consequence, our study suggests that it is advantageous to examine the associations between optimism, perceived stress, and cortisol using within-person designs. In particular, a within-person approach controls for habituation effects and is thus well-suited to uncover protective psychological factors that buffer the association between perceived stress and cortisol.
We note that this conclusion does not imply that stress-related effects of optimism (for habituation-susceptible indicators of cortisol) cannot be observed in between-person research. From our perspective, the occurrence of such effects may depend on the levels of stress that are perceived. Thus, if absolute stress levels are higher than individuals’ typical levels in any particular study, between-person research may document a pattern of results that is similar to the within-person results reported here. However, given that such a discrepancy may not always occur and that it is difficult to determine whether inter-individual differences in actual stress deviate from individuals’ typical stress levels, between-person studies may just be less likely to detect buffering effects of optimism on the stress-cortisol link.7
Further Issues and Future Research
There are some issues that need to be addressed in future research. First, we examined a sample of older adults, and future studies should extend this approach to studying younger indiviudals. Such a life-span approach may be important because it could illuminate age-related processes in the physiological habituation to psychological stress. Given that there is considerable time-related stability in stress perceptions, and that physiological habituations requires repeated exposure to stress over an extended period of time, with advancing age individuals may be more likely to show cortisol habituation to the perception of stress.
Second, our study focused on perceptions of stress, and some of the covariates (e.g., health problems or BMI) were assessed with bias-prone self-reports. While our hypotheses are based on theories that emphasize the psychological perception of stress (Lazarus & Folkman, 1984), we suggest that future studies should additionally assess actual stressors to examine the conditions under which the experience of stressors results in the perception of stress and influences cortisol secretion. In addition, such studies should include objective measures of physical health and BMI.
Third, mean levels differences in cortisol and perceived stress could have contributed to the obtained pattern of findings. In our study, stress perceptions increased over the first two years of study and remained stable in subsequent waves, while AUC, awakening, and afternoon/evening levels of cortisol mostly increased over the first four years and declined in the last wave. This divergence of cortisol and stress levels could imply that some participants’ cortisol response habituated over time to increasing and high levels of stress. Alternatively, we note that cortisol assays of each wave of our study were performed in different batches, which could have attenuated the mean levels of cortisol across waves. Regardless of the reasons, we think that the observed mean levels differences across time are unlikely to compromise the overall interpretation of findings as our analyses controlled for linear and quadratic variation in the sampling of cortisol and stress perceptions over time. In support of this conclusion, we note that we would have observed a highly similar pattern of findings if time-related covariates were excluced from the analyses and if measures of cortisol secretion and stress were standardized for each day of assessment.
Fourth, although our study operationalized optimism on a continum from high pessimism to high optimism (Scheier & Carver, 1985), some research has analyzed the subscales of optimism and pessimism separately (Marshall, Wortman, Kusulas et al., 1992). To address this issue empricially, we performed separate follow-up analyses of the subscales of optimism and pessimism (see Tables OSM 7 and 8). The analyses showed that except for afternoon/evening levels of cortisol, all significant effects of dispositional optimism reported in this manuscript remained significant if the optimism and pessimism items of the LOT were analyzed separately. Nonetheless, the analyses also documented a trend in that the effects of optimism on the within-person associations of stress perceptions and cortisol secretion were somewhat stronger than the effects of pessimism. Thus, future research may examine whether the presence of optimism or the absence of pessimism can affect cortisol functioning among individuals who perceive high levels of stress.
Finally, the study’s results may reveal a novel pathway through which dispositional optimism could protect individuals’ immune function and physical health (Rasmussen et al., 2009; Segerstrom et al., 1998). In particular, an accumulation of cortisol volume across circumstances that involve higher-than-normal perceptions of stress could compromise pessimists’ immune function and increase their susceptibility for developing physical illness. However, given that cortisol can also serve adaptive anti-inflammatory function, it may require repeated exposure to elevated cortisol over a sustained period of time to render immune cells partially resistant to glucocorticoid inhibition and trigger mild, chronic inflammation (Segerstrom & Miller, 2004). In addition, we note that there could also be health-related consequences of increased levels of CAR among pessimists who generally perceive high levels of stress. We therefore suggest that future studies should examine whether effects of optimism on cortisol regulation can mediate subsequent biological and health-related outcomes. Research along these lines may uncover psychological factors that ameliorate the effects of psychological stress on biological and physical health problems.
Supplementary Material
Footnotes
Some studies have found reversed associations among individuals who suffer from chronic or uncontrollable stressors, in that optimism was associated with decrements in immune function (Cohen et al., 1999; Segerstrom, 2005).
Psychological theories emphasize that appraisals of life circumstances, rather than the circumstances per se, influence the biological consequences of stress (Lazarus & Folkman, 1984).
The pattern of significant effects in the associations between perceived stress, optimism, and cortisol did not change if missing data were not replaced and addressed in the HLM analyses.
Note that the health effects were based on some suppression associated with the remaining covariates. If only measures of chronic illness were included into the Level-2 model, increases in chronic illness, β = .37, SE = .16, p = .02, but not averaged levels of chronic illness, β = −.24, SE = .14, p > .05, were associated with higher AUC.
The effects of optimism were also significant if Level-2 covariates were not included into the models, and therefore are not based on potential suppression effects.
All reported within-person interactions remained significant if the analyses were additionally controlled for depressive symptoms.
Post-hoc analyses conducted for each wave separately showed that differences between pessimists and optimists arose in only one wave of data collection (i.e., wave 3). In that wave, and not in other waves, absolute levels of stress perceptions were associated with higher AUC among pessimists, β = .19, p = .05, but not among optimists, β = −.12, p = .45; F(1, 121) = 4.18, p = .04. Further, in this wave, pessimists had significantly higher stress levels than optimists, after controlling for the stress levels of the other waves, F(1, 120) = 4.17, β = −.21, p =.04.
References
- Bjoerntorp P, Rosmond R. Hypothalamic origin of the metabolic Syndrome X. Annals of the New York Academy of Science. 1999;892:297–307. doi: 10.1111/j.1749-6632.1999.tb07803.x. [DOI] [PubMed] [Google Scholar]
- Boehm JK, Kubzansky LD. The heart’s content: The association between positive psychological well-being and cardiovascular health. Psychological Bulletin, Advance online publication. 2012 Apr 16; doi: 10.1037/a0027448. [DOI] [PubMed] [Google Scholar]
- Brydon L, Walker C, Wawrzyniak AJ, Chart H, Steptoe A. Dispositional optimisim and stress-induced changes in immunity and negative mood. Brain, Behavior, and Immunity. 2009;23:810–816. doi: 10.1016/j.bbi.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Scheier MF, Segerstrom SC. Optimism. Clinical Psychology Review. 2010;30:879–889. doi: 10.1016/j.cpr.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Cohen F, Kearney KA, Zegans LS, Kemeny ME, Neuhaus JM, Stites DP. Differential immune system changes with acute and persistent stressors for optimists vs pessimists. Brain, Behavior, and Immunity. 1999;13:155–174. doi: 10.1006/brbi.1998.0531. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. Journal of the American Medical Association. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Ebrecht M, Hextall J, Kirtley L-G, Taylor A, Dyson M, Weinman J. Perceived stress and cortisol levels predict speed of wound in healthy male adults. Psychoneuroendocrinology. 2004;29:798–809. doi: 10.1016/S0306-4530(03)00144-6. [DOI] [PubMed] [Google Scholar]
- Endrighi R, Hamer M, Steptoe A. Associations of trait optimism with diurnal neuroendocrine activity, cortisol responses to mental stress, and subjective stress in healthy men and women. Psychosomatic Medicine. 2011;73:672–678. doi: 10.1097/PSY.0b013e31822f9cd7. [DOI] [PubMed] [Google Scholar]
- Ironson G, Balbin E, Stuetzle R, Fletcher MA, O’Cleirigh C, Laurenceau JP, Schneiderman N, Solomon G. Dispositional optimism and the mechanisms by which it predicts slower disease progression in HIV: proactive behavior, avoidant coping, and depression. International Journal of Behavioral Medicine. 2005;12:86–97. doi: 10.1207/s15327558ijbm1202_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JC, Evans PD, Ng SH, Chong AML, Siu OT, Chan CLW, Chan CC. Optimism, positive affectivity, and salivary cortisol. British Journal of Health Psychology. 2005;10:467–484. doi: 10.1348/135910705X26083. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer Publications; 1984. [Google Scholar]
- Marshall GN, Wortman CB, Kusulas JW, Hervig LK, Vickers RR. Distinguishing optimism form pessimism: Relations to fundamental dimensions of mood and personality. Journal of Personality and Social Psychology. 1992;62:1067–1074. [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Archives of Internal Medicine. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Minton ME, Hertzhog M, Barron CR, French JA, Reiter-Palmon R. The first anniversary: Stress, well-being, and optimism in older widows. Western Journal of Nursing Research. 2009;31:1035–1056. doi: 10.1177/0193945909339497. [DOI] [PubMed] [Google Scholar]
- National Advisory Council on Aging (NACA) Seniors in Canada 2006: A report card. Ottawa: NACA; 2006. [Google Scholar]
- Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational & Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Rasmussen HN, Scheier MF, Greenhouse JB. Optimism and physical health: A meta-analytic review. Annals of Behavioral Medicine. 2009;37:239–256. doi: 10.1007/s12160-009-9111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier MF, Carver CS. Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychology. 1985;4:219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self mastery, and self-esteem): A re-evaluation of the life orientation test. Journal of Personality and Social Psychology. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schurmeyer TH, Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Science. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC. Optimismandimmunity:Dopositivethoughtsalwaysleadtopositiveeffects? Brain, Behavior, and Immunity. 2005;19:195–200. doi: 10.1016/j.bbi.2004.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Taylor SE, Kemeny ME, Fahey JL. Optimism is associated with mood, coping, and immune changes in response to stress. Journal of Personality and Social Psychology. 1998;74:1646–1655. doi: 10.1037//0022-3514.74.6.1646. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Burklund LJ, Eisenberger NI, Lehman BJ, Hilmert CJ, Lieberman MD. Neural bases of moderation of cortisol stress responses by psychological resources. Journal of Personality and Social Psychology. 2008;95:197–211. doi: 10.1037/0022-3514.95.1.197. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-Befriend, not Fight-or-Flight. Psychological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Turek FW. Endocrine and other biological rhythms. In: DeGroot LJ, editor. Endocrinology. Philadelphia: W.B. Saunders; 1994. pp. 2487–2548. [Google Scholar]
- Van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosomatic Medicine. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Wrosch C, Miller GE, Schulz R. Cortisol secretion and functional disabilities in old age: Importance of using adaptive control strategies. Psychosomatic Medicine. 2009;71:996–1003. doi: 10.1097/PSY.0b013e3181ba6cd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrosch C, Scheier MF. Personality and quality of life: The importance of optimism and goal adjustment. Quality of Life Research. 2003;12:59–73. doi: 10.1023/a:1023529606137. [DOI] [PubMed] [Google Scholar]
- Wrosch C, Schulz R. Health engagement control strategies and 2-year changes in older adults’ physical health. Psychological Science. 2008;19:537–541. doi: 10.1111/j.1467-9280.2008.02120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrosch C, Schulz R, Miller GE, Lupien S, Dunne E. Physical health problems, depressive mood, and cortisol secretion in old age: Buffer effects of health engagement control strategies. Health Psychology. 2007;26:341–349. doi: 10.1037/0278-6133.26.3.341. [DOI] [PubMed] [Google Scholar]
- Wüst S, Fedorenko IS, vanRossum EFC, Koper JW, Hellhammer DH. Habituation of cortisol responses to repeated psychological stress – further characterization and impact of genetic factors. Psychoneuroendocrinology. 2005;30:199–211. doi: 10.1016/j.psyneuen.2004.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.