Abstract
Objective: To study the effect of venlafaxine on the expression of brain-derived neurotrophic factor (BDNF) in rat hippocampal neurons, as well as its inhibitory effect on apoptosis of hippocampal neurons. Methods: Differences in behavioral ability between the depression model group and the Venlafaxine treatment group were observed using behavioral, sucrose-water and open field tests. The rat hippocampal tissue was sliced, stained and observed for BDNF distribution by immunohistochemistry. Apoptosis of hippocampal neurons was detected by TUNEL. BDNF expression in the hippocampal tissue was detected by Western blot. Injury and apoptosis of the hippocampal tissue were observed by electron microscopy. Results: Behavioral test showed that venlafaxine effectively improved the behavioral abilities of depressed rats. Immunohistochemistry showed that venlafaxine markedly increased the BDNF expression in the rat hippocampus. TUNEL showed that venlafaxine markedly inhibited apoptosis of hippocampal neurons, which was also confirmed by electron microscopic observation of the pathologic sections. Conclusion: Venlafaxine improved the expression of BDNF through working on PI3k/PKB/eNOS pathway and repressed the apoptosis of hippocampal neurons.
Keywords: Venlafaxine, brain-derived neurotrophic factor (BDNF), hippocampal neuron, apoptosis
Introduction
Major depression is a common affective psychotic disorder characterized by an extremely low mood, sluggish thinking and reduced activity tolerance. Somatic manifestations include anorexia, weight loss, sleeping disturbance and hyposexuality. Extreme pessimism and world-weariness are present in severe cases, and 10~15% depressive patients have the tendency of suicide. According to the statistics of the World Health Organization (WHO), by 2020 depression will have become the second disease that affects the life expectancy and increases spiritual distress. There are not enough types and numbers of antidepressants currently available for clinical use, and their therapeutic efficacy is not satisfactory on the whole. The main reason is that the unique anatomic structures of the brain prevent them from entering the brain tissue [1].
In recent years, the association between depression and re-generation of neurons, especially those in the hippocampal dentate gyrus, has aroused more attention. Studies [2,3] suggest that antidepressants can promote the re-generation of hippocampal neurons, knowing that the level of hippocampal neuron re-generation is decreased. Many studies [2,3] have demonstrated that neuronal nitric oxide synthase (nNOS) is an inhibitory molecule of neuron re-generation, and is expressed in the hippocampus. The hippocampus is one of the important structures of the cerebral limbic system related to the pathogenesis and treatment of affective psychotic disorders.
Related clinical data have shown that serotonin (5-HT)-norepinephrine (NE) reuptake inhibitors (SNRIs) can effectively treat affective psychotic disorders such as depression. Currently published evidence-based treatment guidelines recommend the use of SNRIs such as venlafaxine HCL for the treatment of acute major depression and as the first-line medication for the treatment of refractory depression, which probably implies that the pathogenesis of depression is more than the hypothesis that depression is simply associated with the monoamine neurotransmitter; rather it may be associated with multiple transmitters and other neurophysiological mechanisms [4-11].
Venlafaxine is a 5-serotonin/norepinephrine dual reuptake inhibitor and can increase the activity of some CNS neurotransmitters, especially the activity of some neurotrophic factors (NTF). Research in recent years indicates that the etiologic association between hippocampal neuron re-generation and neural plasticity impairment has become one of the most concerned topics in the neuropsychiatric filed, and the brain-derived neurotrophic factor (BDNF) supports this hypothesis in many aspects [12-17].
Numerous studies have demonstrated that acute and chronic stress would reduce the expression of BDNF in the rat hippocampus, while administration of anti-suppressants couldup-regulate the expression of BDNF. Studies have demonstrated that nitric oxide (NO), as a cellular signal molecule, can affect the expression of BDNF [18-21].
Then how NO affects the expression of BDNF? Which signal pathway NO take effect through? We will find the answer in the following results.
Materials and methods
Animals
Thirty healthy adult male Sprague-Dawley (SD) rats weighing 200±20 g (Shanghai Research Center for Model Organisms, License No. SCXK (HU) 2009-0023) were group-housed in 450×240×200 mm clear plastic containers under 12:12 h light/dark cycles, with food and water ad libitum, and accustomed at 23°C and relative humidity 45%~52% for a week before the experiments were begun. Animal studies were approved by the Animal Care and Use Committee of Fudan University.
Experimental design
The animals were equally assigned to a normal control (NC) group, a depression modeled group and a venlafaxine-treatment group. Animals in NC group did not receive any stimulation, and the remaining animals received any one of the following stimulations randomly daily for 28 days: swimming in ice cold water (4°C) for 5 min, swimming in hot water (48°C) for 5 min, starvation for 48 h, restraint from food and water for 24 h, Day-Night reversal for 24 h, clamping the tail and shaking for 2 min, and electric shock of the sole at 1 mA for 10 s at a 1-min interval x 30. The same stimulation would not be used consecutively. Behaviors of the animals were evaluated at the end of stimulation every day.
Drug administration
Venlafaxine (2 mg) was dissolved in 1 ml normal saline (NS). After successful establishment of the depression model as described [18-21], venlafaxine (1 mg/100 g, 2 mg/ml) was injected intraperitoneally (i.p.) to animals in venlafaxine group 30 min before initiation of the stimulation at 7:30. The same amount of NS was injected i.p. to animals in depression group, both for 28 days.
Material preparation and perfusion
The rats were anesthetized by i.p. injection of 10% chloral hydrate (0.2 ml/100 g), and fixed on the operating table to expose the thoracic cavity and heart. The left ventricle was punctured and perfused quickly with 150 ml NS. At the same time, the right atrial appendage was broken with the operation forceps to drain out the perfusion fluid. When the perfusion fluid from the right atrial appendage became clear and transparent, the NS was replaced with 4% paraformaldehyde for continuous perfusion for 1 h. Soon after completion of the perfusion procedure, the head of the animal was isolated and the brain tissue was dissected using the operation forceps, which was fixed in 4% paraformaldehyde for 24 h, and dehydrated by gradient ethanol [21-24].
Sucrose-water test
After starvation for food and water for 24, 1% sucrose-water was administered orally to rats before modeling and at day 7, 14, 21 and 28 after modeling to observe the amount of 1-h water intake and calculate the amount of water intake per 100 g body weight. The observation parameters included consumption of sucrose-water: the sucrose-water preference value = sucrose-water consumption/total fluid consumption and sucrose-water relative consumption value = sucrose-water consumption/body weight (to eliminate the influence of weight change on sucrose-water consumption).
Open field test (OFT)
In a quiet room with soft light, the rat was placed in the central compartment of an open field (100 cm ×100 cm ×60 cm), the bottom of which was equally divided into 25 compartments of equal area. The rat was observed for 5 min for the duration of staying in the central compartment of the open box, the frequency of standing up, the frequency of tidying up the hair, the number of compartments that the animal passed through, and the number of the fecal particles that the animal eliminated. OFT was conducted before and after modeling.
Immunohistochemistry assay
Rats were sacrificed by overdose of sodium pentobarbitone for immunohistochemical analysis, and then intracardially perfused with PBS, followed by chilled 4% PFA in PBS. The brain was prepared as described in Method 2.3, sliced into 15 mm sections on a cryostat, blocked in PBS containing 1% goat serum and 0.1% Triton-X 100, and incubated at 4°C overnight with anti-BNDF (rat IgG, 1:500; Chemicon). After washing, a streptavidin horseradish peroxidase (HRP) complex (1:1000; Dako) was applied for 1 h. Color development was performed with a diaminobenzidine peroxidase substrate kit (Vector Labs, Burlingame, CA). Sections were counterstained with hematoxylin or eosin.
Western blotting analysis
Equal amounts of whole-tissue lysates were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (Pall Corp., Port Washington, NY). This was followed by incubation with primary mouse monoclonal antibodies against rat PI3K (1:100 dilution; Abnova), mouse monoclonal antibodies against rat p-AKT and t-AKT (1:100 dilution; Abnova), goat polyclonal antibodies against rat p-eNOS and t-NOS (1:200 dilution; Abnova), rabbit polyclonal antibodies against rat BAX and Bcl-2 (1:200 dilution; Abnova), mouse monoclonal antibodies against rat b-actin (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), respectively. Immunoreactive proteins were detected with enhanced chemiluminescence detection reagents (Amersham Biosciences, Uppsala, Sweden) according to the manufacturer’s instructions.
Transmission electron microscopy (TEM)
Sections for TEM were post-fixed for 1 h in 2% glutaraldehyde in PB, treated in 1% osmium tetroxide, dehydrated, and flat-embedded in Araldite resin. Plastic-embedded sections were studied by correlative optical microscopy and TEM as previously described [22]. Briefly, sections were photographed under the light microscope and then serially cut into 2-μm semi-thin sections with a Leica ultramicrotome (EM UC6; Leica Microsystems, Wetzlar, Germany). The semi-thin sections were stained with 1% toluidine blue in 1% borax, and examined under the light microscope. Selected semi-thin sections were sliced into serial ultrathin sections with a silver-gray interference color, corresponding to a thickness of approximately 60-70 nm. The ultrathin sections were collected on formvar-coated, single-slot grids stained with uranyl acetate and lead citrate, and examined using a JEOL TEM (JEM-1011; JEOL Ltd., Tokyo, Japan).
Nitric oxide (NO) test
Rat hippocampus was Isolated in the ice tray. The tissue blood was removed with normal saline, and right hippocampus was dried with filter paper and weighed. The tissue homogenates was prepared in 10% of normal saline (1:9) at 4°C and centrifuged at 3000 r/min for 10 min, NO in this supernatant was detected by commercial kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacture’s specifications.
Statistical analysis
Data are presented as mean ± SD calculated from each experimental group (n=10). Statistical significance was determined by one-way ANOVA, followed by Bonferroni’s post hoc test for comparison. P values less than 0.05 were considered statistically significant.
Results
Changes in the weight gain and amount of sucrose-water consumption in venlafaxine-treated rats
Anorexia is one of the clinical manifestations of depression, leading to weight reduction. Sucrose-water test showed that water consumption in depression group was decreased significantly as compared with NC group, while the amount of food intake in venlafaxine-treatment group improved significantly (Figure 1A). Then we compared the sucrose-water consumption after establishment of the depression model. When compared with depression group, the values improved significantly in venlafaxine-treatment group (Figure 1B, p<0.001).
Figure 1.

A: Venlafaxine effectively improves depression-induced anorexia and activity intolerance. Sucrose-water test showed that water consumption in depression group was decreased significantly as compared with NC group, while the amount of food intake in venlafaxine-treatment group improved significantly. B: Comparisons in weight gain and the amount of sucrose-water consumption after establishment of the depression model. *compared with depression group, the values improved significantly in venlafaxine-treatment group (p<0.001).
Behavioral changes during OFT
As seen in Figure 2, when compared with NC group, the duration of staying in the central compartment was prolonged significantly in depression group, and the frequencies of standing up, tidying up the hair and passing through the compartments were decreased significantly, while there was no significant difference in the number of fecal particles that the animals eliminated. After venlafaxine treatment, the time of staying in the central compartment was shortened, and the frequencies of standing up, tidying up the hair and passing through the compartments were increased to some extent.
Figure 2.
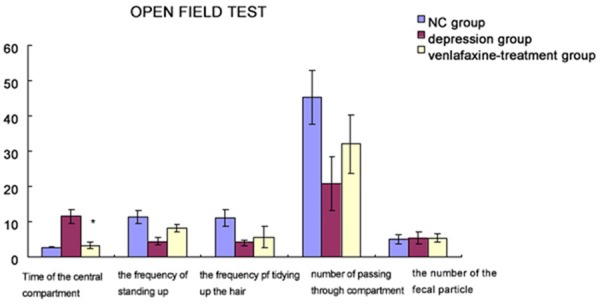
OFT before and after depression modeling shows behavioral changes during OFT. Compared with NC group, the duration of staying in the central compartment was prolonged significantly in depression group, and the frequencies of standing up, tidying up the hair and passing through the compartments were decreased significantly, while there was no significant difference in the number of fecal particles that the animals eliminated. After venlafaxine treatment, the time of staying in the central compartment was shortened, and the frequencies of standing up, tidying up the hair and passing through the compartments were increased to some extent. *compared with depression group, the values improved significantly in venlafaxine-treatment group (p<0.001).
HE staining of rat hippocampal pyramidal cells
Figure 3A revealed that in NC group, the hippocampal pyramidal cell layer is thick; cells are densely, closely and regularly arranged; the cellular structure is complete with a clear edge. (Figure 3B) showed that in depression group, the hippocampal pyramidal cell layer is thin; intercellular spaces are widened; cells are irregularly and loosely arranged; the cellular structure is incomplete, even with loss of large amounts of cells, indicating that the hippocampal tissue was damaged and cell apoptosis occurred in depression group. In venlafaxine group, the hippocampal pyramidal cell layer is restored and intercellular spaces are narrowed to some extent, indicating that venlafaxine has some rehabilitative effect against damage to the hippocampus (Figure 3C).
Figure 3.
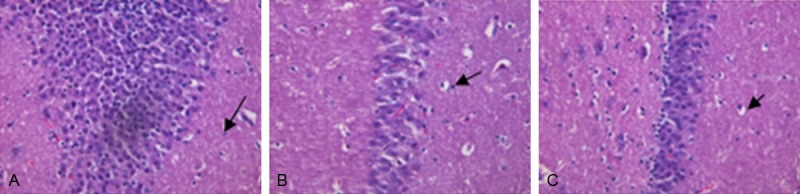
A: Brain of NC group (×100). B: Brain of depression group (×100). C: Brain of venlafaxine-treatment group (×100). A: In NC group, the hippocampal pyramidal cell layer is thick; cells are densely, closely and regularly arranged; the cellular structure is complete with a clear edge. B: In depression group, the hippocampal pyramidal cell layer is thin; intercellular spaces are widened; cells are irregularly and loosely arranged; the cellular structure is incomplete, even with loss of large amounts of cells, indicating that the hippocampal tissue was damaged and cell apoptosis occurred in depression group. C: In venlafaxine group, the hippocampal pyramidal cell layer is restored and intercellular spaces are narrowed to some extent, indicating that venlafaxine has some rehabilitative effect against damage to the hippocampus.
Immunohistochemistry analysis of BNDF protein in the rat hippocampus
BDNF is an important neurotrophic factor expressing highly in the hippocampus of normal rats. As shown in Figure 4A-C, integrated optical density (IOD) of BDNF (brown particles) in depression group was significantly lower than that in NC group (P<0.05). The number of positive hippocampal neurons and IOD in venlafaxine group were significantly higher than those in depression group (P<0.05), indicating that venlafaxine up-regulated the BDNF expression in hippocampal neurons.
Figure 4.
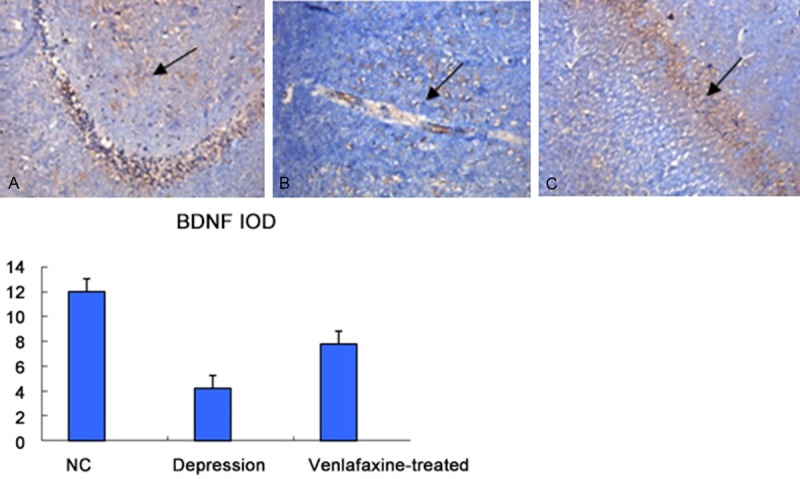
A: Brain of NC group (×100). B: Brian of depression group (×100). C: Brain of venlafaxine-treatment group (×100). Immunohistochemistry of BNDF protein in the rat hippocampus, IOD of BDNF (brown particles) in depression group was significantly lower than that in NC group (P<0.05). The number of positive hippocampal neurons and IOD in venlafaxine group were significantly higher than those in depression group (P<0.05), indicating that venlafaxine up-regulated the BDNF expression in hippocampal neurons. *compared with NC group, P<0.05. Data are expressed as mean ± SE.
Detection of hippocampal cell apoptosis by TUNEL
TUNEL assay (Figure 5) showed a significant inhibitory effect of venlafaxine on hippocampal cell apoptosis. The number of positive hippocampal cells (brown particles) and IOD in depression group were significantly lower than those in NC group (P<0.05). The number of positive hippocampal cells and IOD in venlafaxine group were significantly higher than those in depression group (P<0.05). * compared with NC group, P<0.05. Data are expressed as mean ± SE.
Figure 5.
A: Brain of NC group (×100). B: Brian of depression group (×100). C: Brain of venlafaxine-treatment group (×100). TUNEL shows a significant inhibitory effect of venlafaxine on hippocampal cell apoptosis. The number of positive hippocampal cells (brown particles) and IOD in depression group were significantly lower than those in NC group (P<0.05). The number of positive hippocampal cells and IOD in venlafaxine group were significantly higher than those in depression group (P<0.05). *compared with NC group, P<0.05. Data are expressed as mean ± SE.
Effects of venlafaxine-treatment on PI3K/Akt/eNOS pathway in depression rats
The levels of PI3K protein expression (Figure 6A), Akt phosphorylation (Figure 6B) and eNOS phosphorylation (Figure 6C) in cerebral tissues were determined by western blot analysis. β-actin was used as the internal control. Data are representative of 5 individual rats in each group.
Figure 6.

Effect of Venlafaxine-treatment on PI3K/Akt/eNOS pathway in depression rats. The levels of PI3K protein expression, Akt phosphorylation and eNOS phosphorylation in cerebral tissues were determined by western blot analysis. β-actin was used as the internal control. Data are representative of 5 individual rats in each group. The symbols “*”and “**” indicate that means are significantly different from that of the normal controls (*: P<0.05, **: P<0.001, respectively).
Effects of electroacupuncture on the expression of Bcl-2 and Bax in depression rats
The protein expression levels of Bcl-2 and Bax were analyzed by western blotting. Figure 7 indicated that means are significantly different from that of the normal controls.
Figure 7.
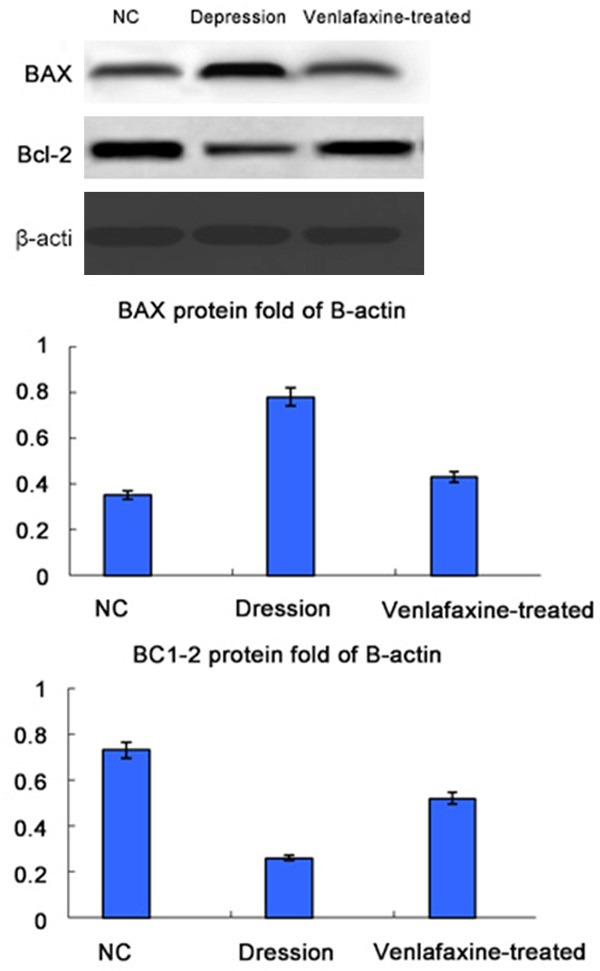
Effect of electroacupuncture on the expression of Bcl-2 and Bax in depression rats. The protein expression levels of Bcl-2 and Bax were analyzed by western blotting. β-actin was used as the internal controls for western blot analyses. Data are representative of five individual rats in each group. The symbols “*”and “**” indicate that means are significantly different from that of the normal controls (*: P<0.05, **: P<0.001, respectively).
Pathologic changes in the ultrastructure of the rat hippocampus by TEM
In NC group, the nuclear membrane is complete, without evidence of abnormality in mitochondria, endoplasmic reticula and other organelles (Figure 8A). In depression group, the ultrastructure of the hippocampus undergoes pathologic changes, as represented by shrinkage of the nuclei of hippocampal cells; the presence of irregular, uneven and unclear nuclear membranes, with local ruptures; swelling/degeneration of mitochondria in the cytoplasm and disappearance of the crests; mild dilation and degranulation of the rough endoplasmic reticulum lumen. (Figure 8B) In venlafaxine group, shrinkage of the nuclei of hippocampal cells and swelling/degeneration of mitochondria in the cytoplasm are improved.
Figure 8.

A: Ultrastructure of the hippocampus in NC group. B: Pathologic presentation of the ultrastructure of the hippocampus in depression group. C: Pathologic presentation of the ultrastructure of the hippocampus in venlafaxine group. (×10000). Pathologic changes in the ultrastructure of the rat hippocampus by TEM. In NC group, the nuclear membrane is complete, without evidence of abnormality in mitochondria, endoplasmic reticula and other organelles. A: In depression group, the ultrastructure of the hippocampus undergoes pathologic changes, as represented by shrinkage of the nuclei of hippocampal cells; the presence of irregular, uneven and unclear nuclear membranes, with local ruptures; swelling/degeneration of mitochondria in the cytoplasm and disappearance of the crests; mild dilation and degranulation of the rough endoplasmic reticulum lumen. B: In venlafaxine group, shrinkage of the nuclei of hippocampal cells and swelling/degeneration of mitochondria in the cytoplasm are improved.
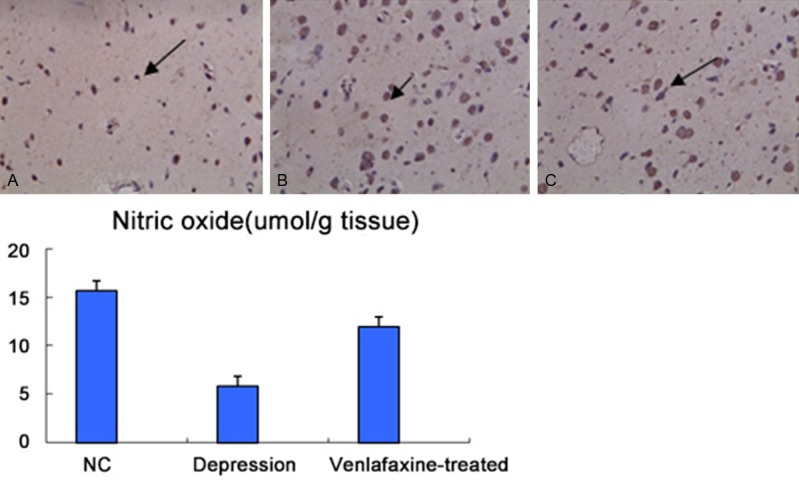
Effects of venlafaxine-treatment on nitric oxide content of hippocampal tissues in rats
As seen in Figure 9, Venlafaxine could reduce the nitric oxide content of hippocampal tissues in rats.
Figure 9.

Effect of Venlafaxine-treatment on nitric oxide content of hippocampal tissues in rats. Data are presented as mean ± SEM. (n=5). The symbols “*”and “**” indicate that means are significantly different from that of the normal controls (*: P<0.05, **: P<0.001, respectively).
Discussion
Animals in depression group exhibited loss of interest, decreased activity tolerance, sleeping pattern change and weight loss, which are similar to those seen in clinical depressive patients. The reduction in sugar intake can be regarded as a manifestation of anhedonia. Most studies use the amount of sucrose-water intake and the value of sucrose-water preference as effective parameters for evaluating anhedonia in depression models.
The result of the present study showed that the amount of sucrose-water intake and the value of sucrose-water preference decreased markedly 7, 14, 21 and 28 days after application of chronic mild stress in depression group, which is consistent with the finding of previous studies in stress-induced depression models. After i.p. administration of venlafaxine, these parameters increased as compared with depression group, suggesting that venlafaxine could relieve the symptoms of depression.
OFT is a common method used to evaluate depression and anxiety in animal models. It can also be used to observe activity tolerance, the ability of exploration, and the emotional state, among which the distance of horizontal movement can reflect the ability of activity, and vertical movement and the distance of the central movement route can reflect the ability of exploration in a new environment. Some researchers believe that the central movement route can not only reflect the ability of exploration in rats but the state of anxiety. In the present experiment, we applied chronic mild stress to the animals for 7, 14, 21 and 28 days, and then compared them with the normal control animals. It was found that the duration of staying in the central zone was prolonged, and the frequencies of standing up, tidying up the hair and passing through the compartments were decreased to some extent in animals of depression group. There was no significant difference in the number of fecal particles that the animals eliminated. After venlafaxine treatment, the duration of staying in the central zone was shortened, and the frequencies of standing up, tidying up the hair and passing through the compartments were increased to some extent. Most OFT studies demonstrated that the abilities of activity and exploration were decreased in rats when they were subjected to chronic mild stress. However, some other studies reported that the activity of stressed rats was increased during OFT, or there was no significant change in the overall activity ability. These conflicting findings suggest that behavioral changes are not always the same in stressed rats during OFT, which is consistent with the clinical finding that spontaneous activities were decreased in some depressive patients and increased in some other depressive patients.
Many studies have demonstrated that the hippocampus became atrophic in both depressive patients and depression-modeled animals. Anti-depressants can improve the symptoms by increasing neural re-generation and restoring the volume of the damaged hippocampus. For this reason, most researchers believe that the occurrence of depression may be associated with abnormal re-generation of hippocampal neurons, which also indicates that the phenomenon of hippocampal neural re-generation exists in various life periods. Current studies have demonstrated that nerve re-generation occurs continuous in two brain zones: the subventricular zone (SVZ) and the subgranular zone (SGZ). With the progression of proliferation and differentiation, newly generated neurons migrate gradually to CA3, where they conjunct with pyramidal neurons in the hippocampal cortical circuit to adjust emotions and cognitive function. Studies have shown that re-generation of hippocampal neurons is affected by multiple factors, including neurotransmitters, hormones, nerve growth factors, and external factors such as stress, substance dependence and activity. It was found in the present study that BDNF was up-regulated after venlafaxine administration, which effectively inhibited apoptosis of hippocampal neurons. The TUNEL and TEM results showed that apoptosis of hippocampal neurons in venlafaxine group was improved significantly as compared with depression group.
Venlafaxine is a common and widely used anti-depressant at present, and its therapeutic effect of improving the symptoms of depression is evident. Despite the short half-life, a better understanding about its action mechanism would be of great help to the research and development of new anti-depressants of like structures.
Acknowledgements
Dr. Ji and Dr. Huang were financially supported by an educational grant from Zhongshan Hospital, Fudan University.
Disclosure of conflict of interest
The authors have no potential conflict of interests.
References
- 1.Xu H, Steven Richardson J, Li XM. Dose related effects of chronic antidepressants on neuroprotective proteins BDNF, Bc-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology. 2003;28:53–62. doi: 10.1038/sj.npp.1300009. [DOI] [PubMed] [Google Scholar]
- 2.Maes M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuro Endocrinol Lett. 2008;29:287–291. [PubMed] [Google Scholar]
- 3.D’Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4:183–194. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- 4.Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 5.Mössner R, Mikova O, Koutsilieri E, Saoud M, Ehlis AC, Müller N, Fallgatter AJ, Riederer P. Consensus paper of the WFSBP Task Force on Biological Markers: Biological markers in depression. World J Biol Psychiatry. 2007;8:141–174. doi: 10.1080/15622970701263303. [DOI] [PubMed] [Google Scholar]
- 6.Miller AH, Maletic V, Raison CL. Inflammation and its discontents; the role of cytokines in the pathophysiology of Major Depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of major depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 11.Neumeister A. Tryptophan depletion, serotonin, and depression: where do we stand? Psychopharmacol Bull. 2003;37:99–115. [PubMed] [Google Scholar]
- 12.Hashimoto K, Shimizu E, Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res Brain Res Rev. 2004;45:104–114. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Seidah NG, Benjannet S, Pareek S, Chrétien M, Murphy RA. Physiology of the neurotrophins. Lewin GR, Barde YA. Seidah NG, Benjannet S, Pareek S, Chrétien M, Murphy RA. Cellular processing of the neurotrophin precursors of NT3 and BDNF by the mammalian proprotein convertases. FEBS Lett. 1996;379:247–250. doi: 10.1016/0014-5793(95)01520-5. [DOI] [PubMed] [Google Scholar]
- 14.Zhou XF, Song XY, Zhong JH, Barati S, Zhou FH, Johnson SM. Distribution and localization of pro-brain-derived neurotrophic factor-like immunoreactivity in the peripheral and central nervous system of the adult rat. J Neurochem. 2004;91:704–715. doi: 10.1111/j.1471-4159.2004.02775.x. [DOI] [PubMed] [Google Scholar]
- 15.Gray K, Ellis V. Activation of pro-BDNF by the pericellular serine protease plasmin. FEBS Lett. 2008;582:907–910. doi: 10.1016/j.febslet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canossa M, Giordano E, Cappello S, Guarnieri C, Ferri S. Nitric oxide down-regulates brain-derived neurotrophic factor secretion in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99:3282–3287. doi: 10.1073/pnas.042504299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kume T, Kouchiyama H, Kaneko S, Maeda T, Kaneko S, Akaike A, Shimohama S, Kihara T, Kimura J, Wada K, Koizumi S. BDNF prevents NO mediated glutamate cytotoxicity in cultured cortical neurons. Brain Res. 1997;756:200–204. doi: 10.1016/s0006-8993(97)00195-9. [DOI] [PubMed] [Google Scholar]
- 21.Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G, Halberg F. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen CK, Arnt J, Sánchez C. Intracranial self-stimulation and sucrose intake differ as hedonic measures following chronic mild stress: interstrain and interindivial differences. Behav Brain Res. 2000;107:21–33. doi: 10.1016/s0166-4328(99)00110-2. [DOI] [PubMed] [Google Scholar]
- 24.Bielajew C, Konkle AT, Merali Z. The effects of chronic mild stress on male Sprague·Dawley and Long Evans rats: I, Biochemical and physiological analyses. Behav Brain Res. 2002;136:583–592. doi: 10.1016/s0166-4328(02)00222-x. [DOI] [PubMed] [Google Scholar]
- 25.DeFelipe J, Faire′n A. A simple and reliable method for correlative light and electron microscopic studies. J Histochem Cytochem. 1993;41:769–772. doi: 10.1177/41.5.8468459. [DOI] [PubMed] [Google Scholar]


