Abstract
This study aimed to evaluate the effect of methylprednisolone sodium succinate, dantrolene sodium, and their combination on experimental spinal cord injury. We used 25 rats (Rattus norvegicus) that were divided into five groups. The negative control group (NC) consisted of animals without spinal cord trauma. In the groups with spinal cord trauma, the positive control group (PC) was given no treatment, the MS group was treated with methylprednisolone, the MS/DS group was treated with methylprednisolone and dantrolene, and the DS group was treated with dantrolene alone. The animals’ motor function was evaluated daily, as measured with the open field test. Eight days after surgery, the animals were euthanized for spinal cord collection. Descriptive morphological evaluation, anti-NeuN immunohistochemistry, TUNEL, and anti-Bax immunofluorescence were performed. There was no significant difference between the PC, MS, MS/DS and DS groups with respect to BBB scores, neuronal and glial staining, or Bax expression (P < 0.05). Therefore, we conclude that methylprednisolone sodium succinate, dantrolene sodium, or the combination of these drugs did not reduce neuronal and glial loss, intrinsic pathway apoptosis, or promote functional recovery.
Keywords: Methylprednisolone, dantrolene, spinal cord injury, rats
Introduction
The treatment of spinal cord injury (SCI) is a major challenge for veterinarians and physicians due to the complex and progressive nature of this disease. The primary event, consisting of mechanical damage to the cell membranes and blood vessels of the spinal cord, triggers secondary events that are responsible for expanding and exacerbating the lesion. Among the significant secondary events are excitotoxicity, inflammation, and apoptosis [1,2].
Excitotoxicity is the process of cell death induced by an excess amount of glutamate released upon neuronal injury that activating nearby receptors causes a massive influx of calcium ions into those neurons [1,3]. The calcium influx activate ryanodine receptors (RyRs) present in the endoplasmic reticulum than induces the release of additional Ca2+ from intracellular stores [1,4,5]. This large increase of intracellular Ca2+ activates proteases such as calpains and caspases, which trigger apoptosis [6,7].
The Bax pro-apoptotic protein is a Bcl-2 protein family, which participate in the intrinsic pathway of apoptosis [8]. When subjected to certain stimuli such as excitotoxicity, this protein activates proteases that cause apoptosis [3,7,9,10].
Another important event results from inflammation. After spinal cord injury the blood brain barrier injury allows neutrophils and macrophages migration to the site of the injury. These cells phagocytose cellular debris and release proteases and oxygen-derived free radicals that damage membrane cells and cause the death of neurons and glia. Therefore, the use of drugs that reduce the inflammatory infiltrate may prevent the expansion of the lesion [2,11].
Methylprednisolone has been extensively studied in experimental spinal cord injury. This steroidal anti-inflammatory has been found to be capable of reducing the inflammatory cells at the site of injury [11-13], reducing oligodendrocyte apoptosis and promoting functional motor improvement [8,14,15].
However, because spinal cord injury involves a variety of secondary events, it is unlikely that the use of a single drug will be able to reduce lesions and promote functional recovery. Dantrolene sodium is a ryanodine receptor antagonist that blocks the intracellular release of Ca2+ [16]. Because Ca2+ reserves play a role in neuronal injury, it is thought that dantrolene can be used as a neuroprotective agent [6]. Studies have shown that the drug decreased neuronal cell death after ischemia, excitotoxicity [5], and reduce neurological deficits [17] and apoptosis [18].
Thus, the present study aimed to evaluate the effect of the combination of these drugs on rats subjected to experimental spinal cord injury.
Materials and methods
This study was approved by the Ethics Committee on Animal Experimentation of the Universidade Federal de Minas Gerais.
Animals and surgical procedure
Twenty-five male Wistar rats, aged 12 weeks and weighting 300 g, equally were used in this study. Rats were kept at room with controlled temperature (20°C) and a 12/12 hour light-dark cycle and given rodent chow and water ad libitum. Pre-anaesthetic medication was performed with tramadol (2 mg/kg, SC), and induction and maintenance was carried out with isoflurane administered by mask in a semi system. The animals were positioned in prone position, were prepared for aseptic surgery and received prophylactic antibiotic therapy with cephalothin (30 mg/kg, SC). Skin and subcutaneous tissue were incised in the dorsal midline extending from T10-L1, the paravertebral muscles dissected, and laminectomy of T12 performed with pneumatic drill. After visualization of the spinal cord covered by the intact dura, a compressive model of SCI was performed as previously described [19], using a weigh of 70 g/cm loading to the dorsal surface of the spinal cord. Afterwards the site was irrigated with saline, the muscles approximated and the reduced dead space and skin sutured using an unabsorbed suture. During anesthetic recovery, the animals received oxygen therapy and were held in a box heated to 37°C for recovery from anesthesia. Postoperatively, rats received tramadol for three consecutive days and cephalexin for seven consecutive days. The bladders of all animals were massaged to facilitate urination, twice daily for eight days.
Treatment
The therapeutic protocol consisted of 30 mg/kg of methylprednisolone sodium succinate, given in single dose, intravenously in the lateral coccygeal vein, 3 h after laminectomy, alone or in association with 10 mg/kg of dantrolene sodium, intraperitoneally 1 h after laminectomy. The control groups received only saline as placebo, intraperitoneally 1 h after laminectomy and/or intravenously 3 h after laminectomy.
Experimental groups
The animals were randomly divided into four groups according to the protocol of treatment. NC (negative control) underwent laminectomy and was treated with placebo; PC (positive control) underwent laminectomy followed by SCI and was treated with placebo; MS (methylprednisolone sodium succinate) underwent laminectomy followed by SCI and was treated with methylprednisolone alone; MS/DS (methylprednisolone sodium succinate and dantrolene sodium) underwent laminectomy followed by SCI and was treated with methylprednisolone and dantrolene; and DS (dantrolene sodium) underwent laminectomy followed by SCI and was treated with dantrolene alone.
Motor function (open field test)
Motor function was assessed in an open field using the BBB scoring system [20]. The animals were filmed for five minutes, and the videos were evaluated by two observers, who were blind to the treatment groups. The evaluations were performed 24 hours before surgery (day 0) and daily starting 24 hours after surgery, until the seventh day after surgery (day 7).
Histopathological evaluation of the spinal cord
On the eighth day, animals were euthanized with an overdose of sodium thiopental by intraperitoneal injection and transcardiac perfusion was performed with 200 ml of phosphate buffer (pH 7.4) and 4% buffered formaldehyde. The vertebral columns were removed and fixed in the same solution for seven days. Segments of the spinal cord between T8 and L3, were collected. A 4-mm-thick transverse section was made in the epicenter and 4 mm cranially and caudally.
Immunohistochemistry
Immunohistochemistry using the monoclonal antibody NeuN (Chemicon, USA) was performed to evaluate neuronal viability. Paraffin-embedded transversal spinal cord sections were deparaffinized and dehydrated according to the standard protocol. Antigen retrieval was performed by heating the slides in a solution of citric acid at 0.05%. Blocking endogenous peroxidase was accomplished using a methanol solution and hydrogen peroxide (3% H2O2) in a darkroom, and blocking of unspecific epitopes in protein block (DAKO, USA) was also performed in a humid dark chamber. Histological sections were incubated overnight in a humid dark chamber with the primary antibody anti-NeuN (1:1000, Chemicon, USA). The secondary antibody (DAKO, USA) was added, and the sections were incubated in the same manner for 45 minutes. Subsequently, we added a streptavidin biotin peroxidase complex (DAKO, USA). The reactions were developed with 3-3’ diaminobenzidine peroxidase (DAB, DAKO, USA). The sections were washed in water, counter-stained with Harris hematoxylin, dehydrated, and mounted on slides with coverslips and Canada balsam. Counting of the neuronal bodies was performed using a graticule of 121 points, with a 40x objective lens. Ten fields equally distributed throughout the gray matter of each court were counted, and the mean number of labeled cells/field was determined.
Quantification of cell death using the TUNEL technique
The DNA fragmentation was assessed by TUNEL (TdT mediated dUTP nick end labeling), using a commercial kit (Merck, Darmstadt, Germany). Histological sections were mounted on slides, dewaxed and dehydrated, and the standard protocol for the tunneling technique was used. The sections were permeabilized with 0.5% Triton X-100 in distilled water, and antigen retrieval was performed with proteinase K at room temperature. The inactivation of endogenous peroxidase was performed by immersion of the slides in a solution of H2O2 and 3% methanol. After being washed in TBS, slides were incubated with TdT Equilibration buffer at room temperature and subsequently incubated with TdT Labeling Reaction Mixture in a humid chamber at 37°C. The reaction was blocked with Stop Solution at 37°C. Blocking buffer was added, followed by 1× antibody conjugate in a humid chamber at room temperature. Finally, the slides were stained with DAB and counterstained with Methyl Green. For quantification of labeled cells, 15 fields equally distributed in the white matter were counted, using a graticule of 121 points with a 40x objective lens. Then, we determined the mean number of labeled cells/field.
Immunofluorescence
Immunofluorescence using the primary antibody anti-Bax (Chemicon, USA) was performed to evaluate the activation of the intrinsic apoptotic pathway. Histological sections were kept under ultraviolet light for two hours to reduce autofluorescence. They were then dewaxed in xylene and hydrated in a gradual sequence of alcohols. Permeabilization was carried out with 0.1% Triton X-100; antigen retrieval was performed in a citrate buffer (0.01 M, pH 6.0) at 98°C; and finally, blocking of endogenous peroxidase was performed with hydrogen peroxide, 3% methyl alcohol and goat serum in 1% bovine serum albumin (BSA). For autofluorescence, control sections were incubated in 0.01 Sudan Black B to 70% ethanol and then incubated overnight with the primary antibody anti-Bax (1:100, Chemicon, USA) at 4°C. The following day, incubation was performed with the secondary antibody, Alexa Fluor 488 (1:200, Chemicon, USA) and DAPI (4’, 6-diamidine-2-phenylindole). For the negative control, a solution of 1% BSA in 0.15 M PBS (pH 7.2) was used to replace the primary antibody. Slides were kept at -20°C and protected from light until the time of reading. The evaluation was conducted in an inverted fluorescence microscope equipped with an ApoTome system, using a 488 nm filter and a 20x objective lens. The exposure time was adjusted and standardized on the negative control slide and then used for all slices. The sections were photographed; three fields were selected in the gray matter, and three fields were selected in the white matter. The images were analyzed using ImageJ software®. For each image, the average integrated density of pixels expressing the primary anti-Bax in cells, minus the background, was obtained. Finally, we obtained the mean Bax expression.
Statistical analysis
The median motor evaluation scores were compared between the groups using the Kruskal-Wallis test. The average numbers of neurons labeled with anti-NeuN, anti-Bax and TUNEL were compared between the groups using the SNK test. Those variables that did not follow normality and homoscedasticity were transformed into log (x). Statistical analyses were performed in GraphPad InStat® 3.05 with a 95% significance level (P < 0.05).
Results
Motor function
The animals in the CN group showed normal gait (score 21) 24 hours after surgery. The animals in the PC, MS, DS/MS and DS groups showed no movement in the hindlimbs (score 0) or discrete movements of one or two joints (score 1). There was no significant difference between the mean scores obtained in the CP, SM, DS/SM, and DS groups at any of the time points (P > 0.05) (Figure 1).
Figure 1.
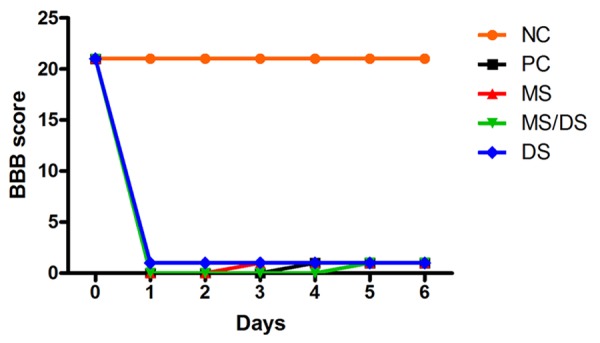
Median values obtained on measures of motor function (BBB) in rats subjected to spinal cord injury without treatment (PC), treated with methylprednisolone sodium succinate (MS), treated with the combination of methylprednisolone sodium succinate and dantrolene sodium (DS/MS), or treated with dantrolene sodium (DS), on different days of evaluation.
Histopathological evaluation of the spinal cord
Spinal cord from NC group animals showed normal architecture (Figure 2A, 2B). When examining the animals in the PC, MS, DS/MS, and DS groups, the histological patterns observed were similar in the epicenter region with malacia that was diffuse, affecting white and gray matter (Figure 2C). This region was filled with cellular debris and inflammatory infiltration of gitter cells. Around the lesion, the small strip of remaining white matter showed severe axonal degeneration and diffuse myelin swelling.
Figure 2.
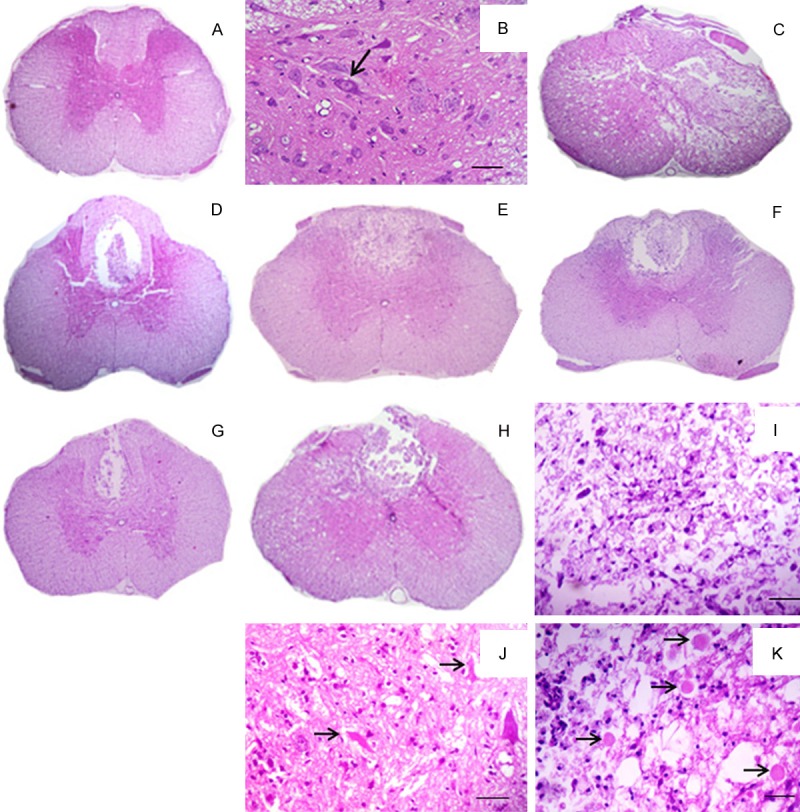
Photograph of rats spinal cord histological sections from an animal subjected to laminectomy (A); detailed view of the gray matter with neuronal integrity (arrow) (B); Photograph of epicenter histological section from an animal subjected to SCI (C); Photograph of cranial histological section from animals subjected to SCI and not treated (D); treated with methylprednisolone (E); methylprednisolone and dantrolene (F); and with dantrolene alone (G); Photograph of caudal histological section from an animal subjected to SCI, with malacia in the dorsal funiculus (H); detailed view of the region of malacia showing gitter cells, cellular debris and inflammatory cells (I); detailed view of the gray matter with degenerated neurons (arrows) (J); and white matter degeneration with axonal swelling and myelin sheaths (arrows) (K) (Figure 3A, C-H: bar=233 mm; Figure 3B, I-K: bar=23 μm.).
Histological sections from the cranial and caudal region of the PC, MS, DS/MS and DS groups exhibited focal malacia in the dorsal funiculus region, with cavitation filled with cellular debris and inflammatory infiltration of gitter cells (Figure 2H, 2I). The axonal and myelin degeneration was moderate and multifocal and affected the dorsal and ventral funiculi (Figure 2D, 2G, 2K). In the gray matter, neuronal degeneration was moderate, mainly occurring in the ventral horn (Figure 2J).
Immunohistochemistry
The average labeled neurons/ field was higher in the group that underwent only the laminectomy (NC) compared to the groups in which there was trauma (PC, MS, DS/MS, and DS) (P < 0.05). However, there was no difference between the mean of labeled neurons/field in PC, MS, DS/MS, and DS groups (p < 0.05, Figures 3 and 4).
Figure 3.
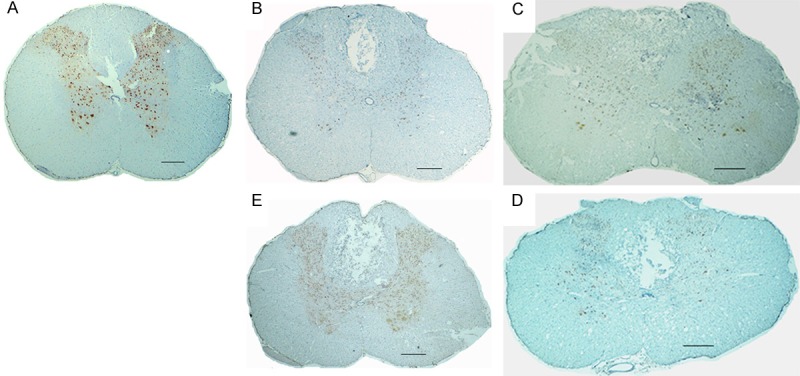
Photographs of rats spinal cords histologic sections subjected to immunohistochemistry with anti-NeuN, from rats subjected to laminectomy (A); spinal cord injury, not treated (B); treated with methylprednisolone (C); methylprednisolone and dantrolene (D); and dantrolene alone (E) (bar=233 μm).
Figure 4.
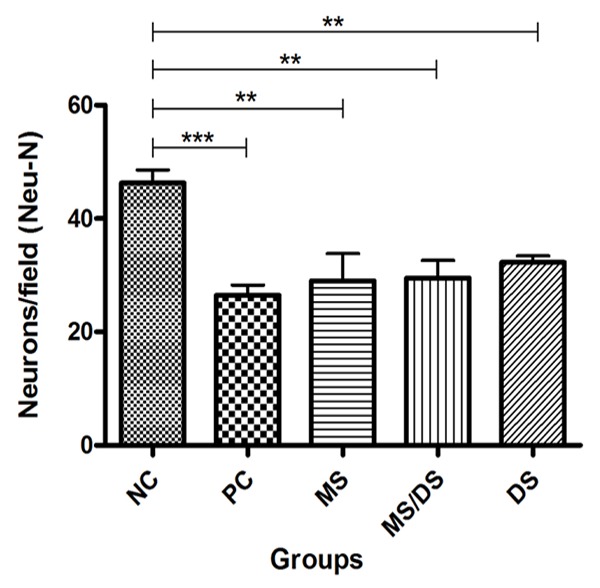
Means of labeled neurons/ field in histologic sections from the spinal cords of rats subjected to laminectomy (NC), spinal cord injury, not treated (PC) and treated with methylprednisolone (MS), a combination of methylprednisolone and dantrolene (DS/MS), or dantrolene (DS) alone, at 8 days after SCI.
Quantification of cell death using the TUNEL technique
The number of cells stained with the TUNEL was greater in the animals to the PC, MS, DS/MS and DS groups when compared to the NC group (P < 0.05, Figure 5). There was no significant difference between the PC, MS, DS/SM, and DS groups (P < 0.05).
Figure 5.
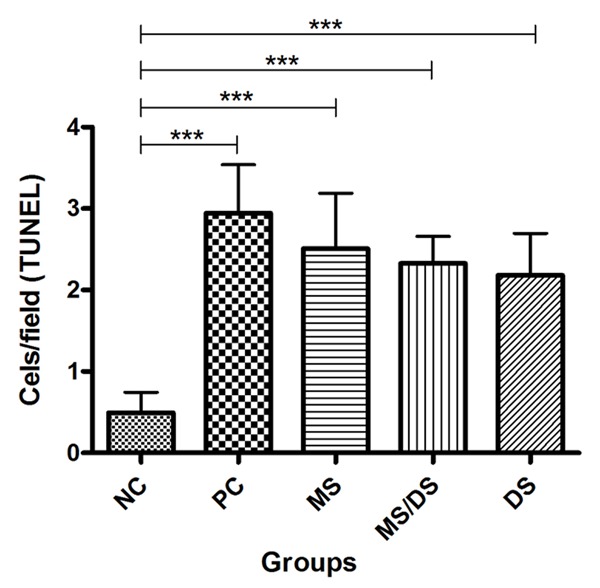
Means of TUNEL labeled cells/field in histologic sections from the spinal cords of rats subjected to laminectomy (NC), spinal cord injury, not treated (PC) and treated with methylprednisolone (MS), a combination of methylprednisolone and dantrolene (DS/MS), or dantrolene (DS) alone, at 8 days after SCI.
Quantification of the proapoptotic factor Bax by immunofluorescence
There were significant differences in the mean expression of primary anti-Bax between the groups without (NC) and with trauma (PC, MS, DS/MS, and DS) (P < 0.05). There was no significant difference between the PC, MS, DS/MS, and DS groups (P < 0.05, Figures 6 and 7).
Figure 6.
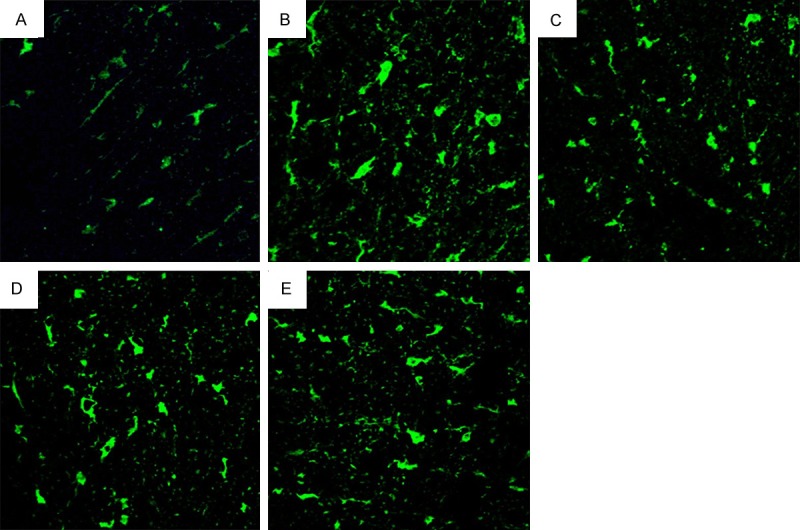
Photographs of rats spinal cord histological sections subjected to immunofluorescence with anti-Bax, from animals subjected to laminectomy (A), spinal cord injury, not treated (B) and treated with methylprednisolone (C), a combination of methylprednisolone and dantrolene (D), or dantrolene (E) alone, at 8 days after SCI (bar=233 μm.).
Figure 7.
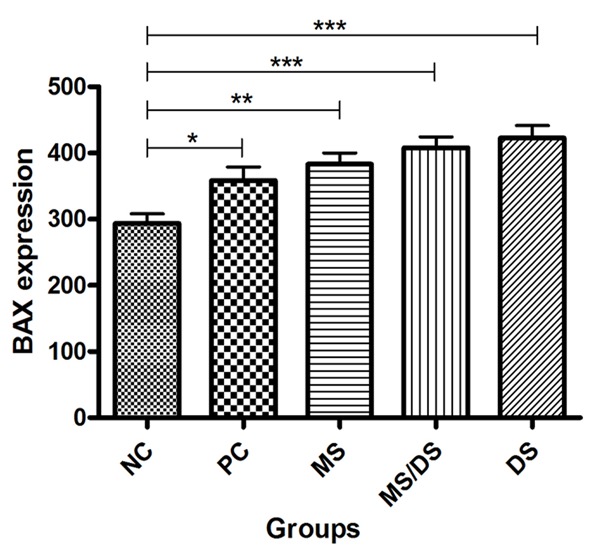
Means of average integrated density of pixels expressing by the primary anti-Bax antibody in histologic sections obtained from the spinal cords of rats subjected to laminectomy (NC), spinal cord injury not treated (PC), and treated with methylprednisolone (MS), a combination of methylprednisolone and dantrolene (DS/MS), and dantrolene alone (DS), at 8 days after SCI.
Discussion
In this study, it was observed rats eight days after spinal cord trauma to study the effect of MS, DS/MS and DS treatment on neuronal and glial loss, intrinsic pathway apoptosis, and functional recovery.
Regarding the appraisal of the histological patterns of injury, in our study they were very similar in all animals subjected to trauma, whether treated or untreated, and these patterns characterized trauma of severe intensity. In regions at the epicenter, we observed diffuse malacia (necrosis); the greatest amount of bleeding and inflammatory infiltration occurred in this region. The second most affected region was caudal to the epicenter, and in the cranial section the lesions were less intense, with a larger amount of preserved tissue.
The counting of viable neurons is an effective means to measure and evaluate treatments because the amount of preserved tissue is directly related to the potential for functional recovery [14]. In this study, there was a significant difference only between the group without trauma and those groups with trauma, confirming that spinal cord trauma caused neuronal death. Regarding the mean number of labeled neurons per field between the treated and untreated groups, there was no difference. Therefore, methylprednisolone, dantrolene, or the combination of the two drugs did not reduce neuronal death.
One key event that is well-defined in the pathophysiology of spinal cord injury is the second wave of apoptosis that occurs eight days after the trauma and primarily affects the oligodendrocytes in regions adjacent to the epicenter. Therefore, quantification of cells with DNA fragmentation was performed in the white matter of the segments cranial to the epicenter of the lesion to precisely quantify the death of these cells [21,22]. The number of labeled cells in this region was higher in animals subjected to trauma, showing that the spinal cord trauma likely induced apoptosis of these glial cells in the white matter. However, there was no difference between the treated and untreated (PC) groups. Thus methylprednisolone, dantrolene, and the combination of these drugs not decreased the death of glia in the white matter.
The mitochondrial apoptosis pathway is controlled by pro- and anti-apoptotic Bcl-2 family proteins. The expression of Bax was higher in the histological sections of the spinal cords of animals subjected to spinal cord injury, confirming that there was greater activation of the intrinsic apoptotic pathway in these animals. This result concurs with the results of other studies demonstrating that apoptosis of oligodendrocytes and neurons caused by trauma to the spinal cord is associated with increased expression of Bax [9,10].
Finally, the assessment of motor function in an open field using ranking scores enables observation and grading of functional recovery and comparison of the effectiveness of treatments [20,22]. In the present study, there was no functional improvement in the treated groups, similar to other studies in which no significant improvement of motor function after methylprednisolone treatment [8,11,19,23] or dantrolene [18]. By contrast, one study that evaluated the administration of dantrolene immediately after spinal cord injury in rabbits reported improvement in motor function 24 hours after injury; however, the trauma induced in that study was moderate: within the first 24 hours after injury, the animals maintained all movement of the hindlimb joints [17]. In addition, they used a different test [24] for the evaluation of motor function. This test was less precise and more subjective because it has only five scores, and thus, did not classify small variations [22].
The results obtained in our study, similar to many other studies, did not suggest neuroprotection associated with the use of methylprednisolone for treating experimental spinal cord injury [8,21]. These results provide a basis for questioning the use of methylprednisolone for the treatment of spinal cord trauma. Furthermore, although methylprednisolone reduces the inflammatory response, it was not associated with an increased survival of neurons or oligodendrocytes [12], most likely due to secondary events such as ischemia that occur due to impaired perfusion of the tissue by direct injury to the blood vessel, which is caused by the trauma. Furthermore, some authors [8,13,21] argue that methylprednisolone is not able to prevent axonal degeneration resulting from spinal cord trauma, which is caused by interrupting the propagation of synaptic impulses and the transport of substances produced in the neuronal bodies. The loss of these axons causes the death of neuronal cell bodies, and oligodendrocytes associated with these axons [9].
Our study did not achieve success with dantrolene treatment, which did not promote the recovery of motor function. In another study, dantrolene promoted only partial reduction of cell death [5] because the increase in intracellular Ca2+ occurs via different routes, for example, via influx through channels associated with glutamate receptors, by voltage dependent Ca2+ channels and by reversing the exchange of sodium for Ca2+. Additionally, Ca2+ is released from intracellular stores via others receptor-regulated channels like the IP3 receptors [1,4,21,25]. Therefore, because there are many pathways that lead to changes in Ca2+ homeostasis, dantrolene alone is not capable of preventing such change and thus not capable of preventing the apoptosis and death of neurons and glial cells or of promoting functional recovery. Perhaps the combination of different methods for blocking Ca2+ channels can achieve better results related to neuroprotection and functional recovery.
Therefore, we conclude that methylprednisolone, dantrolene or the combination of these drugs did not improve motor function, reduce the death of neurons and glial cells, or affect the activation of the intrinsic apoptotic pathway in rats subjected to experimental spinal cord injury, as assessed eight days after the trauma.
Acknowledgements
The authors thank the Fundaçao de Amparo a Pesquisa do Estado de Minas Gerais-(FAPEMIG) for funding the study, the Conselho Nacional de Desenvolvimento Cientlfico e Tecnologico (CNPq) for the masters grant, and the Veterinary School of Universidade Federal de Minas Gerais.
Disclosure of conflict of interest
None.
References
- 1.Sanchez-Gomez MV, Alberdi E, Ibarretxe G, Torre I, Matute C. Caspase-dependent and caspase-independent oligodendrocyte death mediated by AMPA and kainite receptors. J Neurosci. 2003;23:9519–28. doi: 10.1523/JNEUROSCI.23-29-09519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes-Leal W, Corkill DJ, Picanço-Diniz CW. Systematic analysis of axonal damage and inflammatory response in different white matter tracts of acutely injured rat spinal cord. Brain Res. 2005;1066:57–70. doi: 10.1016/j.brainres.2005.10.069. [DOI] [PubMed] [Google Scholar]
- 3.D’Orsi B, Bonner H, Tuffy LP, Dussman H, Woods I, Courtney MJ, Ward MW, Prehn JH. Calpains are downstream effectors of bax-dependent excitotoxic apoptosis. J Neurosci. 2012;32:1847–58. doi: 10.1523/JNEUROSCI.2345-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberdi E, Sanchez-Gomez MV, Marino A, Matute C. Ca2+ influx through AMPA or Kainate receptors alone is sufficient to iniciate excitotoxicity in cultured oligodendrocytes. Neurobiol Dis. 2002;9:234–43. doi: 10.1006/nbdi.2001.0457. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Nguyen HN, Maguire JL, Perry DC. Role of intracellular calcium stores in cell death from oxygen-glucose deprivation in neuronal cell line. J Cereb Blood Flow Metab. 2002;22:206–14. doi: 10.1097/00004647-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Thorell WE, Leibrock LG, Agrawal SK. Role of RyRs and IP3 receptors after traumatic injury to spinal cord white matter. J Neurotrauma. 2002;19:335–42. doi: 10.1089/089771502753594909. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Gomez MV, Alberdi E, Perez-Navarro E, Alberch J, Matute C. Bax and calpain mediate excitotoxic oligodendrocyte death induced by activation of both AMPA and kainate receptors. J Neurosci. 2011;31:2996–3006. doi: 10.1523/JNEUROSCI.5578-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JM, Yan P, Xiao Q, Chen S, Lee KY, Hsu CY, Xu J. Methylprednisolone protects oligodendrocytes but not neurons alter spinal cord injury. J Neurosci. 2008;28:3141–9. doi: 10.1523/JNEUROSCI.5547-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong H, Fazzaro A, Xiang C, Korsmeyer SJ, Jacquin MF, McDonald JW. Enhanced oligodendrocyte survival after spinal cord injury in Bax-deficient mice and mice with delayed Wallerian degeneration. J Neurosci. 2003;23:8682–91. doi: 10.1523/JNEUROSCI.23-25-08682.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotipatruni RR, Dasari VR, Veeravalli KK, Dinh DH, Fassett D, Rao JS. p53- and Bax- mediated apoptosis in injured rat spinal cord. Neurochem Res. 2011;36:2063–74. doi: 10.1007/s11064-011-0530-2. [DOI] [PubMed] [Google Scholar]
- 11.Weaver LC, Gris D, Saville LR, Oatway MA, Chen Y, Marsch DR, Hamilton EF, Dekaban GA. Methylprednisolone causes minimal improvement after spinal cord injury in rats, contrasting with benefits of an anti-integrin treatment. J Neurotrauma. 2005;22:1375–87. doi: 10.1089/neu.2005.22.1375. [DOI] [PubMed] [Google Scholar]
- 12.Bartholdi D, Schwab ME. Methylprednisolone inhibits early inflammatory process but not ischemic cell death after experimental spinal cord lesion in the rat. Brain Res. 1995;672:177–86. doi: 10.1016/0006-8993(94)01410-j. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Yang H, Qiu Z. Effect of combined treatment with methylprednisolone and Nogo-A monoclonal antibody alter rat spinal cord injury. J Int Med Res. 2010;38:570–82. doi: 10.1177/147323001003800219. [DOI] [PubMed] [Google Scholar]
- 14.Braughler JM, Hall ED, Means ED, Waters TR, Anderson DK. Evaluation of an intensive methylprednisolone sodium succinato dosing regimen in experimental spinal cord injury. J Neurosurg. 1987;67:102–5. doi: 10.3171/jns.1987.67.1.0102. [DOI] [PubMed] [Google Scholar]
- 15.Taoka Y, Okajima K, Uchiba M, Johno M. Methylprednisolone reduces spinal cord injury in rats without affecting tumor necrosis factor-alpha production. J Neurotrauma. 2001;18:533–43. doi: 10.1089/089771501300227332. [DOI] [PubMed] [Google Scholar]
- 16.Zhao F, Li P, Chen SR, Louis CF, Fruen BR. Dantrolene inhibition of ryanodine receptor Ca2+ release channels. Molecular mechanism and isoform selectivity. J Biol Chem. 2001;276:13810–6. doi: 10.1074/jbc.M006104200. [DOI] [PubMed] [Google Scholar]
- 17.Aslan A, Cemek M, Buyukokuroglu ME, Altunbas K, Bas O, Yurumez Y, Cosar M. Dantrolene can reduce secondary damage after spinal cord injury. Eur Spine J. 2009;18:1442–51. doi: 10.1007/s00586-009-1033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres BB, Caldeira FM, Gomes MG, Serakides R, de Marco VA, Bertagnolli AC, Fukushima FB, de Oliveira KM, Gomes MV, de Melo EG. Effects of dantrolene on apoptosis and immunohistochemical expression of NeuN in the spinal cord after traumatic injury in rats. Int J Exp Path. 2010;91:530–6. doi: 10.1111/j.1365-2613.2010.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres S, Salgado-Ceballos H, Torres JL, Orozco-Suarez S, Dlaz-Rulz A, Martinez A, Rivera-Cruz M, Rios C, Lara A, Collado C, Guizar-Sahagun G. Early metabolic reactivation versus antioxidant therapy after traumatic spinal cord injury in adult rats. Neuropathology. 2010;30:36–43. doi: 10.1111/j.1440-1789.2009.01037.x. [DOI] [PubMed] [Google Scholar]
- 20.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 21.Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basso DM. Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J Neurotrauma. 2004;21:395–404. doi: 10.1089/089771504323004548. [DOI] [PubMed] [Google Scholar]
- 23.Rabchevsky AG, Fugaccia I, Sullivan PG, Blades DA, Scheff SW. Efficay of methylprednisolone therapy for the injured rat spinal cord. J Neurosci Res. 2002;68:7–18. doi: 10.1002/jnr.10187. [DOI] [PubMed] [Google Scholar]
- 24.Tarlov IM, Klinger H. Spinal cord compression studies. II. Time limits for recovery after acute compression in dogs. AMA Arch Neurol Psychiatry. 1954;71:271–290. [PubMed] [Google Scholar]
- 25.Li S, Stys PK. Mechanisms of ionotropic glutamate receptor-mediated excitotoxicity in isolated spinal cord white matter. J Neurosci. 2000;20:1190–8. doi: 10.1523/JNEUROSCI.20-03-01190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


