Abstract
Human embryonic stem cells (hESCs) are pluripotent cells which can give rise to almost all adult cell lineages. Culture system of hESCs is complex, requiring exogenous b-FGF and feeder cell layer. Human mesenchymal stem cells (MSCs) not only synthesize soluble cytokines or factors such as b-FGF, but also provide other mechanism which might play positive role on sustaining hESCs propagation and pluripotency. Human amniotic fluid stem (AFS) cells, which share characteristics of both embryonic and adult stem cells, have been regarded as promising cells for regenerative medicine. Taking advantage by AFS cells, we studied the ability of AFS cells in supporting undifferentiated propagation and pluripotency of Chinese population derived X-01 hESCs. Human AF-type amniotic fluid stem cells (hAF-AFSCs) transcribed genes including Activin A, TGF-β1, Noggin and b-FGF, which involved in maintaining pluripotency and self-renewal of hESCs. Compared to mouse embryonic fibroblasts (MEFs), hAF-AFSCs secreted higher concentration of b-FGF which was important in hESCs culture (P < 0.05). The hESCs were propagated more than 30 passages on hAF-AFSCs layer with exogenous b-FGF supplementation, keeping undifferentiated status. While exogenous b-FGF was obviated, propagation of hESCs with undifferentiated status was dependent on density of hAF-AFSC feeder layer. Lower density of hAF-AFSCs resulted in rapid decline in undifferentiated clone number, while higher ones hindered the growth of colonies. The most appropriate hAF-AFSCs feeder density to maintain the X-01 hESC line without exogenous b-FGF was 15-20×104/well. To the best of our knowledge, this is the first study demonstrating that hAF-AFSCs could support undifferentiated propagation and pluripotency of Chinese population derived hESCs without exogenous b-FGF supplementation.
Keywords: Human embryonic stem cells, b-FGF, feeder cell, pluripotency
Introduction
Human embryonic stem cells (hESCs) are pluripotent cells which can give rise to almost all adult cell lineages by appropriate induction. Since hESCs possess many kinds of advantages such as self-renewal, unlimited proliferation and multipotent differentiation potential, they herald tremendous promise with progress of regenerative medicine both in basic research (e.g. developmental studies) and clinical application (e.g. drug screening) in future. The first human embryonic stem cell line was established in 1998 by Thomson [1]. Mouse embryonic fibroblasts (MEFs) served as feeder layer to support the undifferentiated growth of hESCs. However, it is unsafe to use MEFs as feeder layer from bench to bedside since existence of latent zoonotic pathogens. Thus, varieties of researchers have attempted to establish human tissue derived feeder cells or feeder-free culture system to avoid pathogens contamination. Human foreskin fibroblasts, fetal muscle fibroblast [2], umbilical cord mesenchymal stem cells [3], bone marrow stromal cells [4], placental cells [5] were reported to support the growth of hESCs with supplementation of exogenous b-FGF.
Despite the type and origin of feeder cell, b-FGF is indispensable to sustain the self-renewal potential and pluripotency of hESCs during the expansion culture period. Recently, researchers have demonstrated that hESCs could expand on some human feeder cells without exogenous b-FGF [5,6]. Instead of supplementation of exogenous b-FGF, some human feeder cell could synthesize and secret endogenous b-FGF. This is the unique characteristic of human feeder cells, while MEFs does not express b-FGF [6]. Obviation of continuous b-FGF supplementation could dramatically reduce the expense.
Human mesenchymal stem cells (MSCs) seem to possess definite superiority than somatic cell in maintaining undifferentiated state of hESCs. MSCs synthesize not only b-FGF, but also other soluble cytokines or factors which might play positive role on sustaining hESCs propagation and pluripotency. Apart from higher level b-FGF secretion and some undefined soluble factors [7], the other mechanism of MSCs supporting hESCs propagation and pluripotency may be the feeder cell-hESCs interaction.
Human amniotic fluid is enriched by large number of stem cells. Human amniotic fluid stem (AFS) cells are easily obtained through amniocentesis, a protocol that is harmless to pregnant women and fetuses [8]. These cells can expand extensively and possess some intermediate characteristics between pluripotent ESCs and adult stem cells [9]. Human AFS cells and bone marrow MSCs (BM-MSCs) share some characteristics (CD105, CD90, CD29 etc), but differ from BM-MSCs in Oct4, Nanog, SSEA4, Tra-1-81 expression. Further more, protein array of cell-free supernatants revealed that human AFS cells secreted multiple factors distinguishing from BM-MSCs [10]. Thus, we aim to utilize human AFS cells as feeder cell to support hESCs propagation.
The culture condition of hESCs is rigorous and complex. One of key parameter in hESCs culture is feeder cell density [11]. However, no relevant investigation has been implemented concerning about the density parameter of human AFS cells during hESCs cultivation. In previous study, we have isolated human AF-type AFS cells (hAF-AFSCs) from discarded amniotic fluid and identify their biological characteristics [12]. We also proved multi-lineage differentiation potential by osteogenic and adipogenic differentiation [13]. In the present study, we aim to utilize hAF-AFSCs as feeder cell to support hESCs propagation and make it clear whether exogenous b-FGF can be omitted from medium.
Materials and methods
Ethics statement
Animal experiments were performed in accordance with a protocol approved by Shanghai Jiaotong University School of Medicine Animal Care and Use Committee [Animals use license: SYXK (Shanghai) 2003-0031]. All mice were housed in a sterile environment and could access to food and water commodiously as outlined in our institutional guidelines. The protocol to use discarded human amniotic fluid for subsequent cell isolation and culture was approved by ethics committee of Shanghai Jiaotong University School of Medicine.
Isolation and culture of human AF-type amniotic fluid stem cells
HAF-AFSCs were isolated from second-trimester amniotic fluid according to our previous published method [12,13]. HAF-AFSCs within passage 5 were used in further experiments.
Flow cytometry analysis of hAF-AFSCs
HAF-AFSCs were characterized by flow-cytometry analysis, using phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated mouse anti-human monoclonal antibodies CD10, CD14, CD34, CD44, CD45, CD90, CD105, CD117 or appropriate isotype controls [all purchased from Becton Dickinson (BD)]. Cells were incubated at 4°C for 30-60 min with varying concentrations of the primary or isotype control antibodies and analysis was performed on a BD FACScan flow cytometer.
Feeder cell preparation and hESCs culture
HAF-AFSCs were seeded on Matrigel-coated well of 6-well plate at the density of 18.7×104 cells/well as recommended by WiCell Research Institute (with supplement of exogenous b-FGF) or at gradient density (5, 10, 15, 20 and 25×104/well, abbreviated as D5, D10, D15, D20 and D25, without supplement of exogenous b-FGF) 2 days before hESCs passage.
MEFs isolated from 13-days-old C57BL/6 mouse embryos were seed at a density of 18.7×104 cells/well as recommended previously. Cells were mitotically inactivated by mitomycin C (10 μg/ml) for 3 hrs one day before hESCs passage. The feeder cell culture medium consisted of DMEM (Invitrogen, USA), 10% FBS (Hyclone, USA) and 2 mM l-glutamine. Discarded the medium and refreshed with hESCs growth medium [Knockout DMEM (Invitrogen), 20% Knock-out SR (Invitrogen), 2 mmol/L L-glutamine (Invitrogen), 1% NEAA (Invitrogen), 0.1 mmol/L β-mercaptoethanol (Sigma), 1% penicillin/streptomycin (Invitrogen)]. HESCs lines (termed X-01, kindly provided by Professor L Xiao, Stem Cell Bank, Shanghai Institutes for Biological Sciences). HESC colonies were mechanically dissociated into clumps with appropriate size and transferred onto newly prepared feeder cells. HESCs were incubated in growth medium at 37°C in a 5% CO2 and 95% humidity chamber with or without exogenous b-FGF (4 ng/ml) as described above. Passage of hESCs was performed about once a week.
Immunostaining
Akaline phosphatase (AKP) detection kit (Millipore, USA) was used to verify AKP activity of hESCs according to manufacturer’s instructions. Expression of six stem cell markers (Oct-4, Nanog, SSEA-3, SSEA-4, Tra-1-60 and Tra-1-81) and three somatic cell markers (βIII-tublin, Desmin and AFP) was measured using immunofluorescence staining. Briefly, cells cultured on cover slips were washed with PBS and fixed with 4% paraformaldehyde for 20 min, followed by three washes with PBS. The samples were blocked with 3% bovine serum albumin (BSA; Sigma-Aldrich) for 30 min at room temperature and then washed again in PBS. For Oct-4, Nanog staining, the cells were permeabilized with 0.3% Triton X-100 (Sigma-Aldrich) for 20 min prior to blocking. Then incubated the cells with primary antibodies [mouse anti-human Oct-4 (1:100; Chemicon); rabbit anti-human Nanog (1:100; Abcam); mouse anti-human SSEA-4 (1:100; RD); mouse anti-human SSEA-3 (1:100; RD); mouse anti-human Tra-1-81 and Tra-1-60 (1:100; Millipore); rabbit anti-human Desmin (1:200; Abcam), rabbit anti-human α-fetoprotein (AFP 1:200; Abcam), rabbit anti-human β-tublin III (1:200; Abcam)] overnight at 4°C. After three washes, the samples were incubated with Cy3- or FITC-conjugated anti-mouse or rabbit IgG antibody (1:100; Jackson Immuno Research) for 2 hrs at room temperature. The nuclei were counterstained with DAPI (5 mg/ml; Roche). For all staining procedures, the primary antibody was omitted as the control. Samples were observed using a Fluoview scanning confocal microscope (Olympus). Images were acquired and stored using FV1000 Viewer software.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from cells with Trizol (Invitrogen). The first strand of cDNA was synthesized from 6 μg total RNA, using Reverse Transcriptase M-MLV, and PCR was performed with Ex Taq polymerase (all from Takara), according to the manufacturer’s instructions. For each reaction, 1.5 μl of the first strand cDNA was used as a template, and PCR was carried out using the following cycles: initial denaturation at 94°C for 4 min, denaturation at 94°C for 30 s, annealing at the primer-specific temperature for 30 s and extension at 72°C for 30 s. The final extension was performed at 72°C for 10 min. The products were held at 4°C until use. The level of GAPDH mRNA was quantified as an internal control. The forward and reverse primer sequences are listed in Table 1.
Table 1.
RT-PCR primer sequences
| Gene (Accession No.) | Primer sequences (5’-3’) | ProductSize (bp) | Annealingtemperature (°C) |
|---|---|---|---|
| bFGF | Forward: AGAGCGACCCTCACATCAAG | 234 | 55 |
| (NM_002006.4) | Reverse: ACTGCCCAGTTCGTTTCAGT | ||
| TGF-β1 | Forward: GTCACCCGCGTGCTAATG | 644 | 56 |
| (NM_011577.1) | Reverse: CAGAAGTTGGCATGGTAGCC | ||
| Activin A | Forward: AGAAGAGACCCGATGTCACC | 237 | 55 |
| (NM_002192.2) | Reverse: ACAGGTCACTGCCTTCCTTG | ||
| Noggin | Forward: TGTGCAAGCCGTCCAAGT | 121 | 56 |
| (NM_005450.4) | Reverse: GAGCACTTGCACTCGGAAAT | ||
| Desmin | Forward: CCAACAAGAACAACGACG | 288 | 55 |
| NM_001927.3 | Reverse: CAATCTCCACATCCAGGG | ||
| GAPDH | Forward: AGCCACATCGCTCAGACACC | 302 | 56 |
| (NM_002046.3) | Reverse: GTACTCAGCGCCAGCATCG |
Karyotype analysis
The cells were incubated in medium supplemented with 0.2 mg/ml colchicine for 2 hrs at 37°C, 5% CO2. After washing, the cells were disaggregated with trypsin-EDTA solution for 2 min, and resuspended in 75 mM KCl. The cells were fixed in a series of cool methanol:acetic acid (3:1, 2:1, and 3:1) and drops of the cell suspension were spread on clean microscope slides. The chromosomes were stained with 5% Giemsa for 40 minutes and examined at 1000×. At least 20 photographs of 6 metaphase spreads were counted.
Enzyme-linked immunosorbent assay of b-FGF
HAF-AFSCs were planted at gradient density in DMEM with 10% FBS and 2 mM l-glutamine. Twenty-four hours after incubation, supernatant were collected to measure the concentration of bFGF with b-FGF ELISA kit (R&D) according to manufacturer’s instructions.
Differentiation in vitro and in vivo
In vitro differentiation potential of hESCs was evaluated by embryoid body (EB) formation. HESCs were suspended to form embryoid body (EB) formation. Round shaped EBs formed after 7 days. The culture medium was switched to DMEM, 20% FBS, 1% l-glutamine, 0.1 mM NEAA, 0.1 mM β-mercaptoethnol. The EBs gradually attached onto the Matrigel coated dishes. After 14 days of spontaneous differentiation, EBs were fixed with 4% paraformaldehyde and used for further immunofluorescence staining of three germ layer cell markers. Teratoma formation was performed to test the in vivo differentiation potential. 5×106 hESCs combined with matrigel were injected intramuscularly into NOD-SCID mice. Teratomas were harvested after 8-10 weeks and prepared for hematoxylin and eosin staining.
Statistical analysis
SAS 6.12 statistics software was used for statistical analysis. Dates were expressed as averagestandard deviation. The differences were evaluated using Student’s t-tests. A statistical threshold of P < 0.05 was used to detect whether there were statistical significances among different groups.
Results
Biological characteristics of hAF-AFSCs
Ten discarded second-trimester amniotic fluid samples were collected for the present study. Five days after primary cultivation, the cell colonies with distinguishing shape and heterogeneous cell morphology gradually emerged. The colonies enlarged and some colonies with different cell morphology became fused with each other. In regard to the classification of human amniotic fluid cells, there is unanimous consensus classification of these cells into three types: amniotic fluid-specific (AF-type), fibroblastic-type (F-type) and epitheloid-type (E-type) cells, although some other cells could also been observed. On the 10th day of primary cultivation, we calculated the total three type colonies and picked the AF-type ones according to our previous established method. Totally, 116 (80.5%) AF-type (Figure 1A), 23 (16%) F-type and 5 (3.5%) E-type colonies presented while we picked 85 AF-type colonies without contamination of other type cells. Flow cytometry analysis of revealed the biomarkers of hAF-AFSCs (Passage 4) including CD44, CD90, CD117, CD105, CD10, CD14, CD34 and CD45 (Table 2).
Figure 1.
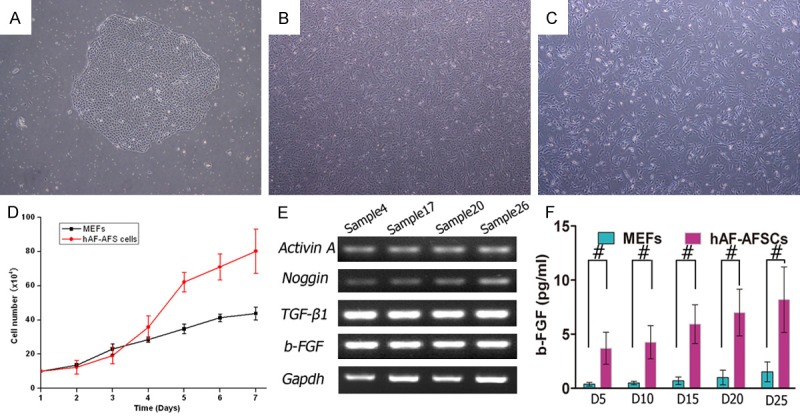
Comparision of hAF-AFSCs with MEFs. A. A single hAF-AFS cell clone (P0) on the 10th day of primary cultivation. B. Morphology of hAF-AFS cells (P4). C. Morphology of MEFs (P4). D. Growth curve showed significant higher proliferation rate of hAF-AFS cells than that of MEFs. E. RT-PCR analysis of mRNA transcript profile revealed hAF-AFSCs transcribed genes engaged in maintaining the pluripotency and self-renewal of human ESCs (b-FGF, Activin A, TGF-β1 and Noggin) (n = 4). F. Concentration of b-FGF secreted to culture medium by hAF-AFSCs and MEFs at different planting density after 24 hours’ culture. (#P < 0.05).
Table 2.
Phenotype of hAF-AFSCs
| Antigen | Posstive rate (%) |
|---|---|
| CD44 | 97.7 |
| CD90 | 96.65 |
| CD117 | 12.92 |
| CD105 | 2.88 |
| CD10 | 0.97 |
| CD14 | 0.93 |
| CD34 | 0.93 |
| CD45 | 0.65 |
The morphology of hAF-AFSCs and MEFs were showed in Figure 1B. Growth curve analysis showed the proliferation rate was faster than that of MEFs (Figure 1C). mRNA transcription profile by RT-PCR analysis demonstrated that hAF-AFSCs transcribed some genes (Activin A, TGF-β1, Noggin and b-FGF, Figure 1E) which involved in maintaining pluripotency and self-renewal of hESCs [14-19].
HAF-AFSCs secreted b-FGF
HAF-AFSCs were seeded into a well of 6-well plate at gradient density of 5, 10, 15, 20 and 25×104/well. After 24 hours’ culture, the b-FGF from supernatant was detectable and the concentration was related to the cell density (Figure 1F).
HAF-AFSCs supported hESCs growth
We selected the hAF-AFSCs at density of 18.7×104/well to serve as feeder cell to support hESCs growth. The hESCs proliferated and were subcultured, maintained undifferentiated state (Figure 2A). We successfully propagate the hESCs more than 30 passages, and the differentiated colonies were less than 10 percent. Immunofluorescence staining demonstrated intensive expression of Oct4, Nanog, SSEA-3, SSEA-4, Tra-1-81 and Tra-1-60 (Figure 2B-G).
Figure 2.
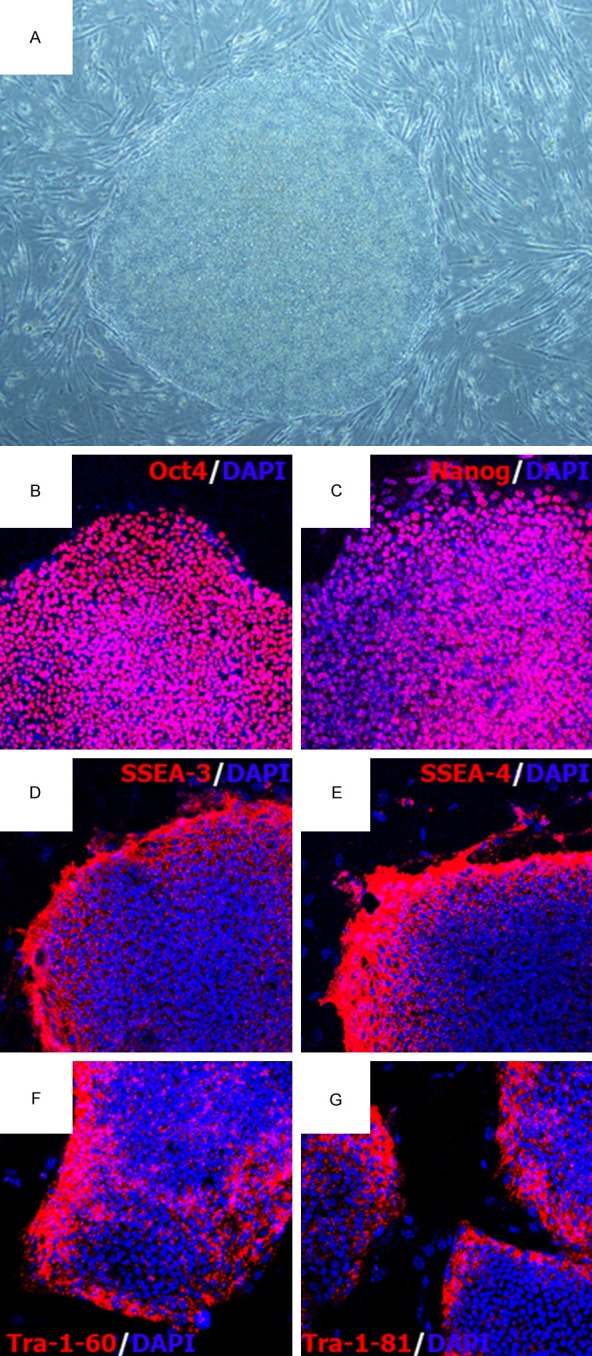
Culture hESCs on hAF-AFSCs feeder layer with exogenous b-FGF supplementation. A. Morphology of hESCs cultured on hAF-AFSCs feeder layer. B-G. Immunofluorescence staining of hESCs cultured on hAF-AFSCs feeder layer with Oct4, Nanog, SSES-3/4, Tra-1-60 and Tra-1-81.
HAF-AFSCs supported hESCs growth without exogenous b-FGF supplementation in a density-dependent manner
HAF-AFSCs were platted at gradient density (5, 10, 15, 20 and 25×104/well) as feeder layer. Morphologic changes of some colonies occurred. The D5 and D10 hESCs colonies became scattered and dispersed with increasing passages, simultaneously. The border of colonies lost its sharp morphology. The number of undifferentiated colonies of D5 declined sharply during the first 10 passages, while it was more moderate in D10 (Figure 3A, 3B and 3F). For D15 and D20 colonies, no obvious change was observed during more than 30 passages (Figure 3C, 3D). The colonies kept undifferentiated status with sharp margin. Colonies of D25 were somewhat smaller compared to those of D15 and D20 (Figure 3E). This might due to the feeder cells physically hinder the growth of colonies. For further passaging, the feeder cells which hindered the colonies must be stripped.
Figure 3.
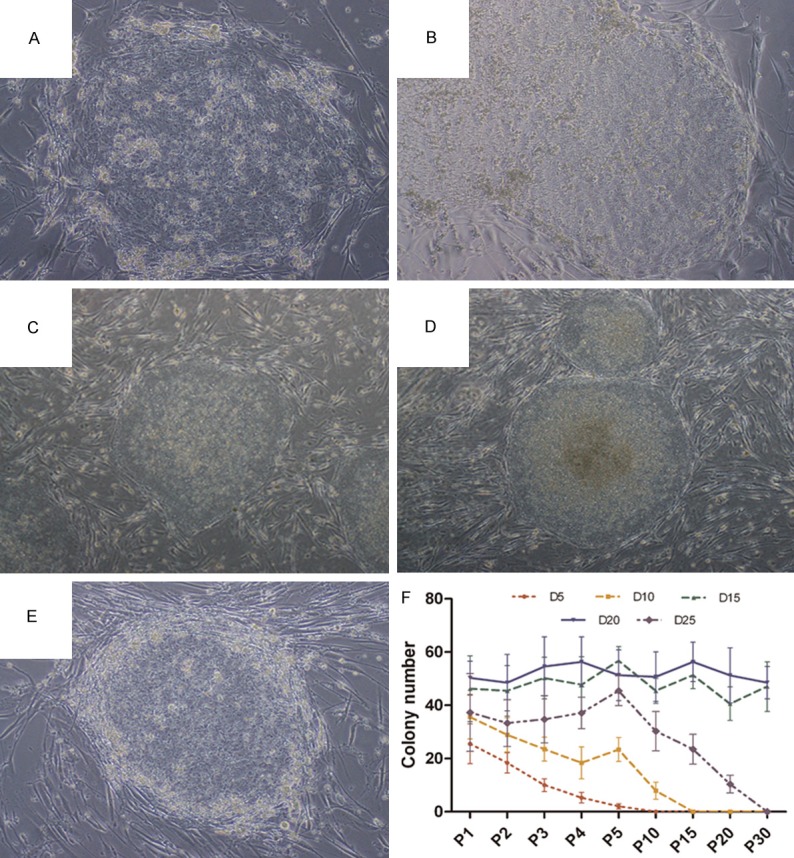
Culture hESCs on hAF-AFSCs feeder layer without exogenous b-FGF supplementation. (A-E) Morphology of hESCs cultured on hAF-AFSCs feeder layer. The morphology of hESCs cultured on D5 and D10 became scatted and flatted during propagation. The colonies lost undifferentiated status and pluripotency (A, B). The colonies planted on D15 and D20 displayed typical morphology of hESCs, including compact colonies with high nuclear to cytoplasmic ratios and well-defined colony borders (C, D). The colonies planted on D25 were encompassed by the feeder cells and became differentiated. (F) The number of undifferentiated colonies in wells with different feeder cell density.
HAF-AFSCs with appropriate density maintained pluripotency of hESCs without b-FGF supplementation
Since D15 and D20 colonies kept undifferentiated status during the propagation for more than 30 passages, we thought D15 and D20 should be the appropriate density for hESCs growth. The stem cell markers were analyzed every 10 passages. The hESC colonies intensively expressed Oct4, Nanog, SSEA-3, SSEA-4, Tra-1-81 and Tra-1-60 (Figure 4). AKP staining also revealed the pluripotency of these colonies (Figure 5A, 5B). Karyotype analysis showed normal karyotype of hESCs after 30 passages (Figure 5C, 5D).
Figure 4.
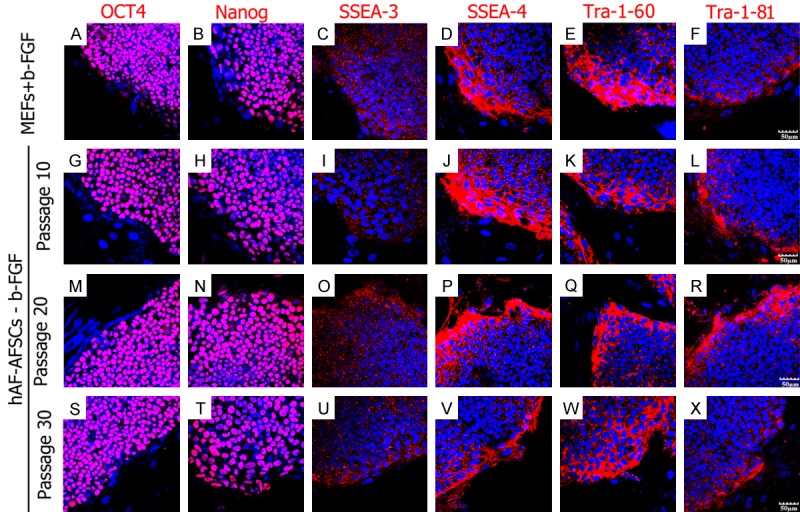
hESCs cultured on hAF-AFSCs feeder layer without exogenous b-FGF supplementation expressed stemness markers. The up line: hESCs colonies cultured on MEFs with b-FGF supplementation (30th passage). The second to the bottom line: hESCs colonies cultured on hAF-AFSCs without b-FGF supplementation (the 10th passage to 30th passage). The stemness markers included Oct4, Nanog, SSES-3, SSEA-4, Tra-1-60 and Tra-1-81.
Figure 5.
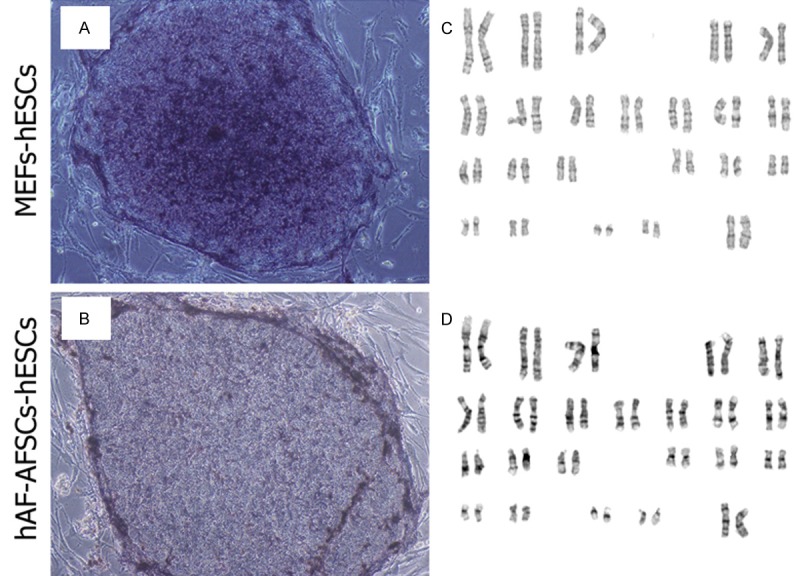
AKP staining and karyotype of hESCs. A, C. AKP staining and normal karyotype of hESCs cultured on MEFs feeder layer with b-FGF supplementation. B, D. AKP staining and normal karyotype of hESCs cultured on hAF-AFSCs feeder layer without exogenous b-FGF supplementation after 30 passages.
HESCs cultured without b-FGF supplementation retained differentiation potency
Round shaped EB formed after 7 days (Figure 6A). After 14 days of spontaneous differentiation on the Matrigel coated dishes, activation of all three embryonic germ layer markers was confirmed (Figure 6B). As shown in (Figure 6C-E), immunostaining illustrated expression of β-III Tubulin (ectoderm), AFP (endoderm) and Desmin (mesoderm) at protein level of differentiated hESCs. To exclude the expression of these markers from contamination of hAF-AFSCs, we stained the feeder cells by the three antibodies. It was affirmative that no positive staining could be observed (Date not shown).
Figure 6.
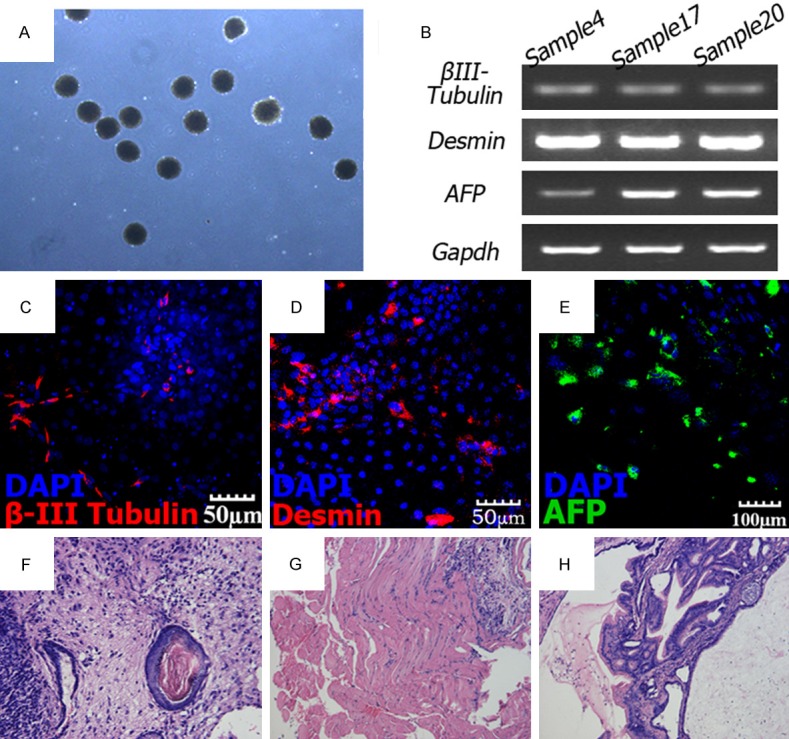
Differentiation potential of hESCs. (A) EB formation of hESCs. (B) RT–PCR analysis of various germ layer markers from EB samples. Representative results of EB samples derived from hESCs cultured on three hAF-AFSCs lines are shown. (C) β-III Tubulin (ectoderm), (D) Desmin (mesoderm) and (E) AFP (endoderm). (F) Skin keratin pearl (ectoderm); (G) muscle (mesoderm) and (H) intestinal epithelia (endoderm) tissues.
Teratoma formation was performed to verify in vivo differentiation potential of hESCs cultured by the present method. Teratomas formed 6-8 weeks after intramuscular injection. Hematoxylin and eosin staining analysis found the three germ layer tissues including skin keratin pearl (ectoderm), muscle (mesoderm) and intestinal epithelia (endoderm) tissues (Figure 6F-H).
Discussion
The first hESCs line was cultured on MEFs feeder layer [1]. In recent years, it has been unquestionable that animal derived cells are unsuitable for feeder layer since they might result in pathogens contamination, which could be a biohazard. Beside this, it exists possibility that sialic acids would trigger an immune response upon clinical application [20]. Varity of human feeder cells and feeder-free culture systems have been intensively developed in the past decade. For feeder-free culture systems, the widely used alternative layer included Matrigel, fibronectin, vitronectin, laminin and gelatin. Commercial artificially synthesized media such TeSR or serum replacement were also recognized as effective substance in supporting hESCs growth and proliferation. Even the risk of animal pathogen contamination declined, hESCs maintained in feeder-free systems could become karyotypically unstable [21,22].
b-FGF is a key factor in maintenance of undifferentiated growth of hESCs. b-FGF activates the mitogen-activated protein kinase (MAPK) pathway directly and modulates Activin A and TGF-β1 signaling indirectly. b-FGF induces the production of TGF-β1 and insulin-like growth factor-II (IGF-II) from feeder cells. The hESCs also secreted endogenous b-FGF during culture. The functions of exogenous and endogenous b-FGF on hESCs should be disparate and complex. The main effect of exogenous b-FGF is promoting cell adhesion and survival, but has no impact on maintenance of pluripotency of hESCs. Absence of exogenous does not affect proliferation of hESCs. b-FGF secreted by hESCs maintains pluripotency gene expression. In previous studies, human feeder cells including MSCs, human foreskin fibroblasts (HFFs) and placental cells (HPCs) transcribed b-FGF mRNA or released b-FGF to medium, with a higher concentration from MSCs than other twos. MSCs were thought to be rich sources of cytokines, including VEGF, b-FGF, IGF, EGF, TGF-β1 and HGF. The property of multiple cytokines synthesis and other features such as ability to regulate of the extracellular matrix and adhesion or modulate immune reaction inspired researchers to harness MSCs to sustain hESCs culture. The superiority of MSCs served as feeder layer with obviation of b-FGF has also been proved. Human AFS cells are adult MSCs, but different from adult MSCs [9,23,24]. Thus, we planed to establish the human AFS cell layer culture system. There is unanimous consensus regarding the classification of amniotic fluid cells into three types: AF-type, F-type and E-type cells. AF-type cell colonies are more abundant (> 60%) than F-type cell colonies (5-6%). F-type cells usually occur late during cultivation and cannot be cloned from every sample. Taking the advantage of AF-type cell colonies, we selected this type cells for further investigation. In previous study, we have identified their characteristics and proved their stemness [13]. The results of present study also demonstrated the feasibility of hAF-AFSCs supporting hESCs self-renewal and proliferation in the presence of exogenous b-FGF.
Compared to other human somatic or stem cell types, hAF-AFSCs offer potential advantages for supporting undifferentiated propagation and pluripotency of hESCs. Amniocytes can be easily isolated from discarded amniotic fluid through amniocentesis and expand extensively in vitro. Researchers can obtain adequate cells after several sub-cultures. Although banking of amniocytes has not launched, but it seem promising in future if this work once launches in the way of banking umbilical cord blood cells. Reprogramming amniocytes into patient and disease specifical induced pluripotent stem (iPS) cells and propagation them upon the autogenous feeder cells by employing xeno-free culture medium can circumvent immune reaction, ethical issues and resultant zoonosis.
Human AFS cells secrete variety of soluble factors, including b-FGF, EGF, Activin A and TGF-β [23], which have direct roles in hESCs prolonged propagation. In a proteomic analysis between AFS cells and BM-MSCs, AFS cells displayed a number of unique proteins related to proliferation and primitive phenotype [24]. These proteins might also play role in supporting hESCs growth. Further more, several researches reported human amniocytes are more efficiently reprogrammed to generated iPS cells [25-27]. Generation of iPS cells from human amniocytes occurs in 5-7 days with 0.5% efficiency or twice as fast and yielded nearly a two-hundred percent increase in clone number, compared to cultured adult skin cells. Human AFS cells are embryonic cells derived from the developing fetus. The embryonic cells more closely resemble the pluripotent status in terms of transcriptional and chromatin states than other somatic cell types. We thought the pluripotent state of hAF-AFSCs should benefit maintaining pluripotency of hESCs.
The undoubted mechanism of supporting hESCs growth is the soluble factors secreted by feeder cells. The routine concentration of b-FGF supplemented is 4 ng/ml. While in our present study and others, the concentration of endogenous b-FGF secreted by feeder cells was lower than 4 ng/ml. The underlying mechanism why hESCs could maintain undifferentiated status without exogenous b-FGF has also been explored. Feeder cells did not only secreted soluble factors, but also provided extracellular matrix and cellular contact to the hESCs. Unfortunately, the messages transmitted between the hAF-AFSCs and hESCs remains unknown. The problem we raise here is that once the hESCs clone settled onto the feeder layer, feeder cells would be shoved. Thus, the cells in the center of clone would lose contact with feeder cells. The fact that lost of contact combined with insufficient b-FGF still did not hamper undifferentiation of hESCs encouraged us to reveal the deep-seated mechanism.
Epigenetic modification is another possible mechanism in regulation hESCs propagation. Liu et al. reported that human amniotic epithelial cells (HuAECs) feeder layers altered mouse ESC gene expression via epigenetic modification of c-Myc, Nanog and Oct4. HuAECs also inhibited endogenous microRNA-145 or DNA methyltransferase 1 and increased Sox2 expression to maintain human iPS cell pluripotency [28-30]. Since some human amniocytes derive from amnion membrane, we think parts of hAF-AFSCs possess properties of HuAECs. Thus, whether hAF-AFSCs have the ability to modify hESCs’ gene epigenetically is our next work to do.
The culture condition of hESCs is rigorous and complex. Feeder cell density which is largely ignored is a key parameter in hESCs culture [11]. High variability in feeder cell density may result in different outcome in culturing hESCs. Too low density would result in insufficient levels of extracellular matrix, secreted factors and intra-cellular contacts provided by feeder cells. Improper high density (30,000 cells/cm2 and above) may result in rapid depletion of nutrients and oxygen, as well as physically hinder the attachment and growth of hESC colonies. Empiric evidence suggested that feeder cell density affect differentiation with both higher and lower quantities of MEF feeders. Different hESCs lines demand inequable density of different feeder cell lines [11]. WiCell recommends the standard planting density for freshly or frozen inactivated CF1 strain MEFs is 18.7×104 and 22.5×104 cells/well per well in a 6 well plate, respectively. Reubinoff et al. recommended the density of MEF feeder cells from 129/Sv strain or F1 Crosses of 129/Sv strain with C57/BL6 mice at 75,000 cells/cm2 might support HES-1 and HES-2 cell lines [31]. Concerning about human feeder cells, Richards et al. used human fetal fibroblasts and adult skin fibroblasts at the density of 60,000-75,000 cells/cm2 [32]. Human adult bone marrow cells at a density of 20,000 cells/cm2 were described by Cheng et al. in culturing the H1 ES cell lines from WiCell Research Institute [33]. Ozolek et al. observed that approximately 20×104 feeders per well of a 6-well culture plate might be appropriate for maintaining ideal pluripotent colony morphology. H1 hESCs required higher feeder density and H9 hESCs required lower density for ideal colony morphology [34]. Reduced feeder density not only provided insufficient ECM or factors to maintain hESCs’ pluripotency, but also promoted rapid progression to neural phenotypes. However, no relevant investigation has been implemented concerning about the density parameter of human AFS cells during a Chinese population derived ESCs line cultivation, especially when b-FGF is obviated. In the previous study, CF1 feeder cells at a density of 30,000 cells/cm2 were used to culture the X-01 hESC lines [35]. Human amniotic mesenchymal cells (AMCs) at confluent density were also used to support H1 ES cells with b-FGF supplement [36]. We also succeeded in culturing X-01 hESCs on hAF-AFSCs (18.7×104 cells/well) more than 30 passages with b-FGF supplement. In the view of evidence in present study, we firstly elucidated the most appropriate hAF-AFSCs feeder density for culturing the Chinese population derived X-01 hESC line in absence of exogenous b-FGF. The other problem is that the most popular culture condition of hAFS cells needed fetal bovine serum (FBS) which also contained ingredients of animal origin. Nest step should develop a culture system free of animal origin for hAFS cells.
Acknowledgements
The authors thank Professor Lei Xiao for his kindness of providing X-01 hESC line. This research was supported by the Bureau Level Research Project of Shanghai Municipal Health Bureau (20114020).
Disclosure of conflict of interest
None.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 3.Ding DC, Shyu WC, Lin SZ, Liu HW, Chiou SH, Chu TY. Human umbilical cord mesenchymal stem cells support nontumorigenic expansion of human embryonic stem cells. Cell Transplant. 2012;21:1515–1527. doi: 10.3727/096368912X647199. [DOI] [PubMed] [Google Scholar]
- 4.Havasi P, Nabioni M, Soleimani M, Bakhshandeh B, Parivar K. Mesenchymal stem cells as an appropriate feeder layer for prolonged in vitro culture of human induced pluripotent stem cells. Mol Biol Rep. 2013;40:3023–3031. doi: 10.1007/s11033-012-2376-3. [DOI] [PubMed] [Google Scholar]
- 5.Park Y, Kim JH, Lee SJ, Choi IY, Park SJ, Lee SR, Sung HJ, Yoo YD, Geum DH, Choi CW, Kim SH, Kim BS. Human feeder cells can support the undifferentiated growth of human and mouse embryonic stem cells using their own basic fibroblast growth factors. Stem Cells Dev. 2011;20:1901–1910. doi: 10.1089/scd.2010.0496. [DOI] [PubMed] [Google Scholar]
- 6.Park Y, Choi IY, Lee SJ, Lee SR, Sung HJ, Kim JH, Yoo YD, Geum DH, Kim SH, Kim BS. Undifferentiated propagation of the human embryonic stem cell lines, H1 and HSF6, on human placenta-derived feeder cells without basic fibroblast growth factor supplementation. Stem Cells Dev. 2010;19:1713–1722. doi: 10.1089/scd.2010.0014. [DOI] [PubMed] [Google Scholar]
- 7.Chin AC, Fong WJ, Goh LT, Philp R, Oh SK, Choo AB. Identification of proteins from feeder conditioned medium that support human embryonic stem cells. J Biotechnol. 2007;130:320–328. doi: 10.1016/j.jbiotec.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Eddleman KA, Malone FD, Sullivan L, Dukes K, Berkowitz RL, Kharbutli Y, Porter TF, Luthy DA, Comstock CH, Saade GR, Klugman S, Dugoff L, Craigo SD, Timor-Tritsch IE, Carr SR, Wolfe HM, D’Alton ME. Pregnancy loss rates after midtrimester amniocentesis. Obstet Gynecol. 2006;108:1067–1072. doi: 10.1097/01.AOG.0000240135.13594.07. [DOI] [PubMed] [Google Scholar]
- 9.De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 10.Moorefield EC, McKee EE, Solchaga L, Orlando G, Yoo JJ, Walker S, Furth ME, Bishop CE. Cloned, CD117 selected human amniotic fluid stem cells are capable of modulating the immune response. PLoS One. 2011;6:e26535. doi: 10.1371/journal.pone.0026535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heng BC, Liu H, Cao T. Feeder cell density--a key parameter in human embryonic stem cell culture. In Vitro Cell Dev Biol Anim. 2004;40:255–257. doi: 10.1290/0407052.1. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Geng H, Xie H, Wu Q, Ma X, Zhou J, Chen F. The heterogeneity of cell subtypes from a primary culture of human amniotic fluid. Cell Mol Biol Lett. 2010;15:424–439. doi: 10.2478/s11658-010-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma X, Zhang S, Zhou J, Chen B, Shang Y, Gao T, Wang X, Xie H, Chen F. Clone-derived human AF-amniotic fluid stem cells are capable of skeletal myogenic differentiation in vitro and in vivo. J Tissue Eng Regen Med. 2012;6:598–613. doi: 10.1002/term.462. [DOI] [PubMed] [Google Scholar]
- 14.Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC, Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- 15.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 16.Saha S, Ji L, de Pablo JJ, Palecek SP. TGFbeta/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys J. 2008;94:4123–4133. doi: 10.1529/biophysj.107.119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 18.Avery S, Inniss K, Moore H. The regulation of self-renewal in human embryonic stem cells. Stem Cells Dev. 2006;15:729–740. doi: 10.1089/scd.2006.15.729. [DOI] [PubMed] [Google Scholar]
- 19.Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24:1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- 20.Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 21.Draper JS, Smith K, Gokhale P, Moore HD, Maltby E, Johnson J, Meisner L, Zwaka TP, Thomson JA, Andrews PW. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22:53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- 22.Catalina P, Montes R, Ligero G, Sanchez L, de la Cueva T, Bueno C, Leone PE, Menendez P. Human ESCs predisposition to karyotypic instability: Is a matter of culture adaptation or differential vulnerability among hESC lines due to inherent properties? Mol Cancer. 2008;7:76. doi: 10.1186/1476-4598-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon BS, Moon JH, Jun EK, Kim J, Maeng I, Kim JS, Lee JH, Baik CS, Kim A, Cho KS, Lee HH, Whang KY, You S. Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev. 2010;19:887–902. doi: 10.1089/scd.2009.0138. [DOI] [PubMed] [Google Scholar]
- 24.Roubelakis MG, Pappa KI, Bitsika V, Zagoura D, Vlahou A, Papadaki HA, Antsaklis A, Anagnou NP. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16:931–952. doi: 10.1089/scd.2007.0036. [DOI] [PubMed] [Google Scholar]
- 25.Galende E, Karakikes I, Edelmann L, Desnick RJ, Kerenyi T, Khoueiry G, Lafferty J, McGinn JT, Brodman M, Fuster V, Hajjar RJ, Polgar K. Amniotic fluid cells are more efficiently reprogrammed to pluripotency than adult cells. Cell Reprogram. 2010;12:117–125. doi: 10.1089/cell.2009.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anchan RM, Quaas P, Gerami-Naini B, Bartake H, Griffin A, Zhou Y, Day D, Eaton JL, George LL, Naber C, Turbe-Doan A, Park PJ, Hornstein MD, Maas RL. Amniocytes can serve a dual function as a source of iPS cells and feeder layers. Hum Mol Genet. 2011;20:962–974. doi: 10.1093/hmg/ddq542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C, Zhou J, Shi G, Ma Y, Yang Y, Gu J, Yu H, Jin S, Wei Z, Chen F, Jin Y. Pluripotency can be rapidly and efficiently induced in human amniotic fluid-derived cells. Hum Mol Genet. 2009;18:4340–4349. doi: 10.1093/hmg/ddp386. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q, Qiu C, Huang Y, Jiang L, Huang Q, Guo L, Liu T. Human amniotic epithelial cell feeder layers maintain iPS cell pluripotency by inhibiting endogenous DNA methyltransferase 1. Exp Ther Med. 2013;6:1145–1154. doi: 10.3892/etm.2013.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu T, Cheng W, Guo L, Huang Q, Jiang L, Du X, Xu F, Liu Z, Lai D. Human amniotic epithelial cell feeder layers maintain mouse embryonic stem cell pluripotency via epigenetic regulation of the c-Myc promoter. Acta Biochim Biophys Sin (Shanghai) 2010;42:109–115. doi: 10.1093/abbs/gmp115. [DOI] [PubMed] [Google Scholar]
- 30.Liu T, Cheng W, Huang Y, Huang Q, Jiang L, Guo L. Human amniotic epithelial cell feeder layers maintain human iPS cell pluripotency via inhibited endogenous microRNA-145 and increased Sox2 expression. Exp Cell Res. 2012;318:424–434. doi: 10.1016/j.yexcr.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 32.Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546–556. doi: 10.1634/stemcells.21-5-546. [DOI] [PubMed] [Google Scholar]
- 33.Cheng L, Hammond H, Ye Z, Zhan X, Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21:131–142. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- 34.Ozolek JA, Jane EP, Esplen JE, Petrosko P, Wehn AK, Erb TM, Mucko SE, Cote LC, Sammak PJ. In vitro neural differentiation of human embryonic stem cells using a low-density mouse embryonic fibroblast feeder protocol. Methods Mol Biol. 2010;584:71–95. doi: 10.1007/978-1-60761-369-5_4. [DOI] [PubMed] [Google Scholar]
- 35.Wu Z, Li H, Rao L, He L, Bao L, Liao J, Cui C, Zuo Z, Li Q, Dai H, Qian L, Tian Q, Xiao L, Tan X. Derivation and characterization of human embryonic stem cell lines from the Chinese population. J Genet Genomics. 2011;38:13–20. doi: 10.1016/j.jcg.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Zhang K, Cai Z, Li Y, Shu J, Pan L, Wan F, Li H, Huang X, He C, Liu Y, Cui X, Xu Y, Gao Y, Wu L, Cao S, Li L. Utilization of human amniotic mesenchymal cells as feeder layers to sustain propagation of human embryonic stem cells in the undifferentiated state. Cell Reprogram. 2011;13:281–288. doi: 10.1089/cell.2010.0103. [DOI] [PubMed] [Google Scholar]


