Abstract
Background: Pulmonary arterial hypertension (PAH) is a cardiovascular disorder associated with enhanced proliferation and suppressed apoptosis of pulmonary arterial smooth muscle cells (PASMCs). The sildenafil can regulate the Connexin (Cx) 43 in the PASMCs and thus inhibit the PASMCs proliferation and the remodeling of pulmonary arterial. However, how sildenafil exert regulation in the Cx40 in the PASMCs in PAH remains unclear. Methods and results: Using the rat PAH model induced by the monocrotoline, we demonstrated that the Cx40 in the PASMCs is down-regulated in the PAH. The sildenafil promotes the up-regulation of Cx40 in the PASMCs via bone morphogenetic protein (BMP) signaling, accompanied by an anti-proliferative response in PASMCs. Inhibition of the BMP axis reverses the up-regulation of Cx40 and anti-proliferation of the sildenafil in these cells. In monocrotaline-induced PAH rat models, which display reduced levels of BMP signaling, this study further indicates that the BMP-Cx40 axis is activated in lungs following the sildenafil treatment. Furthermore, we also find in vitro that sildenafil increases the Cx40 expression of PASMCs isolated from MCT-PAH rats and inhibit the proliferation of these cells. These phenomenon are reversed by LDN-193189, the antagonist of type II receptor for bone morphogenetic protein (BMPR2) treatment, providing strong evidence for the protect effect of sildenafil and the BMP-Cx40 axis involvement. Conclusions: Taken together, these data suggest the sildenafil activate BMP-Cx40 signaling in the PAH. This axis may be a potential therapeutic target in PAH.
Keywords: Sildenafil, connexin 40, hypertension, pulmonary, bone morphogenetic protein, PASMCs proliferation
Introduction
In the left to right shunt congenital heart disease, the large number of shunt resulted in pulmonary congestion. The pulmonary resistance increased, causing the sustained elevation of pulmonary arterial pressure that leads to the pulmonary arterial remodeling, muscularization, right ventricle failure and death [1]. The muscularization of pulmonary arterial is composed of the proliferation of the pulmonary arterial smooth muscle cells (PASMCs) which are interconnected by gap junction channels [2]. These channels are made of two connexons (also called hemi channels, Cx) whose association at intercellular junctions forms a functional pore enabling the diffusion of ions or intracellular messengers between adjacent cells [3]. The channels are clustered in specific regions named gap junctions providing a low resistance pathway for electronic transmission of vasomotor signals [4]. The Cx family is composed of 20 members named according to their molecular weight and Cx40, Cx43 are expressed in PASMCs [5]. The sildenafil can down-regulate the Cx43 in the PASMCs in the PAH and inhibit the pulmonary arterial remodeling [6]. However, the effect of sildenafil on Cx40 of PASMCs, which has a similar structure to Cx43, and the underlying mechanisms involved have not been well elucidated.
Nitric oxide (NO) is endogenously generated by nitric oxide synthases via the conversion of L-arginine into L-citrulline that in turn promotes cyclic guanosine monophosphate (cGMP) generation [7]. Phosphodiesterase type 5 (PDE5) is largely responsible for the hydrolysis of cGMP and is expressed at a high level in the pulmonary circulation compared with systemic vessels [8]. Sildenafil, a specific PDE5 inhibitor, increases intracellular cGMP levels and promotes vasodilatation. It is widely used as an effective treatment for clinical PAH [9]. Recently, Yang J et al. reported that sildenafil modulates bone morphogenetic protein (BMP) signaling in PASMCs. These authors found that Sildenafil enhances canonical BMP signaling via cyclic GMP and cyclic GMP-dependent protein kinase I in vitro and in vivo, and partly restores deficient BMP signaling in BMPR2 mutant PASMCs [10]. BMP plays an important role in the regulation of Cx [11].
In the present study, we demonstrate that sildenafil enhances the Cx40 expression via a BMP signaling-dependent mechanism. Moreover, the antagonist of type II receptor for bone morphogenetic protein (BMPR2), LDN-193189 reversed the sildenafil effect in the PAH. Furthermore, we also find in vitro that sildenafil increases the Cx40 expression of PASMCs isolated from MCT-PAH rats and inhibit the proliferation of these cells. These phenomena are reversed by LDN-193189, the antagonist of BMPR2 treatment. These studies provide further evidence for a central role of sildenafil via BMP signaling in the regulation of Cx40 in the PASMCs in PAH.
Methods
Animals
All experimental protocols and surgical procedures used in this study were approved by The Institutional Animal Care and Use Committee of Nanjing Medical University. Male Sprague-Dawley (SD) rats, 8 weeks of age, weighing 200-220 g, were purchased from Nanjing Medical University animal center. Rats were randomly assigned to one of seven experiment groups (n=6 per group). Rats were housed with free access to food and water under a natural 12/12 h day/night cycle.
Establishment of a PAH model
The Monocrotaline (MCT, Sigma, USA) was administered (60 mg/kg) to rats by subcutaneous injection into the back region. The animal’s lungs were harvested as described previously [12] at 28th day of the study after hemodynamic assessment. The sildenafil group received daily intragastric administration of sildenafil after the administration of MCT (60 mg/kg; Pfizer, USA). The LDN-193189 group received daily intragastric administration of sildenafil (50 mg/kg; Pfizer, USA) and intraperitoneal injection of LDN-193189 (10 mg/kg; Bi Yun Tian, China). In other groups, the same volume saline was given.
Hemodynamic and right ventricular hypertrophy measurements
Invasive hemodynamic measurements, including pulmonary arterial pressure (PAP) and right ventricular systolic pressure (RVSP) were performed as described previously [12]. After the anesthesia, the rats tracheas were orally intubated with a 16-gauge intravenous catheter and mechanical ventilation commenced using a rodent respirator (tidal volume: 8 mL/kg, respiratory rate: 60 minutes-1). The PAP and RVSP were measured as described previously. Briefly, a PE-50 catheter (Bi Yun Tian, China), angled to 90° over the distal 1 cm and curved slightly at the tip, was introduced into the right external jugular vein, with the angle directed interiorly, the catheter was inserted proximally, which placed the catheter in the right atrium. The catheter was rotated 90° counterclockwise and inserted further, which placed the catheter in the right ventricle, and then advanced approximately 1.5 cm, into the pulmonary artery. Placement at each stage was confirmed by standard right ventricle or pulmonary arterial pressure trace on the monitor screen connected to the pressure transducer of a BSM-1700 monitor (Nihon Kohden Company, Japan). The data of RVSP, mPAP and mean arterial blood pressure (mPAP) were recorded after 1 minute of stabilization. After the hemodynamic measurement, the RV free wall was dissected from the left ventricle plus septum (LV + S) and weighed separately; RV hypertrophy was expressed as RV weight/(LV + S weights).
Evaluation of pulmonary arterial remodeling
Lung tissue preparation, sectioning, staining with hematoxylin and pulmonary vascular morphometry were performed as described previously [13]. To determine the degree of muscularization of small pulmonary arteries, lung tissue sections were stained with anti-smooth muscle α-actin. At least 20 arteries with an external diameter smaller than 50 μm were identified per tissue section. Arteries were scored, as described previously [14].
Western blotting
Lung tissues were homogenized using tissue homogenizer (for tissues only) or lysed in RIPA buffer (Bi Yun-Tian, China) with addition of a protease inhibitor cocktail (Bi Yun-Tian, China) and PMSF. Tissue lysates were equalized with SDS 5 × sample buffer and electrophoretically separated on 10% polyacrylamide gels and transferred for 1 h on to nitrocellulose membranes. Subsequently membranes were blocked 1 h with 5% non-fat dry milk in Trisbuffered saline/0.1% Tween 20. After blocking, membranes were probed with primary antibodies diluted as follows: Cx40 (1:1000; Abcam, USA), phospho-Smad1/5 (1:1000; Cell Signaling Technology, USA), BMPR2 (1:500; Abcam, USA), β-actin (1:1000, Bi Yun-Tian, China). Samples were normalized to β-actin housekeeping gene and total protein of interest and further quantification was performed. After primary antibody incubation, membranes were incubated with secondary goat anti-rabbit (1:10000, Bi Yun-Tian, China) or rabbit anti-goat (1:10000, Bi Yun-Tian, China) HRP-conjugated antibodies (ZSGB-BIO). After washing, the membrane was visualized by a chemiluminescence reaction using an ECL-detection kit system (Amersham Pharmacia Biotechnology, CA, USA) and further quantified using specific software as Image Lab.
Real-time reverse-transcription polymerase chain reaction
Total RNA of the right lung was extracted with TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. The reverse transcription was then performed in 1 μg of total RNA with the Transcriptor First Strand cDNA Synthesis Kit (Roche, Germany). Realtime polymerase chain reaction (RT-PCR) was conducted using ABI PRISM® 7500 Real-time PCR System according to the manufacturer’s guidelines. Two-step real-time RT-PCR was used to perform relative quantification of mRNA. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was selected as an internal housekeeping gene control for the comparative Ct method for the relative quantification of target genes mRNA expressions. The fluorescent product was detected at the end of each cycle. Product specificity was confirmed by agarose gel electrophoresis and routinely by melting-curve analysis. The primers were designed as Paulo Renato A.V. Correa described using the software Primer3 based on the sequence deposited in the NCBI Nucleotide Bank. The fluorescent product was detected at the end of each cycle. Product specificity was confirmed by agarose gel electrophoresis and routinely by melting-curve analysis. Data were analyzed with the ABI Prism 7500 sequence detection system software (version 1.4) and GAPDH was used as an internal control for input RNA.
Proliferation assay
Rat PASMCs were isolated from small pulmonary arteries, as described previously [15]. Cells were made quiescent for 48 hours and then seeded in 96-well plate. Each well contained either 10% FBS (Invitrogen, USA)/DMEM (Invitrogen, USA) alone or with the sildenafil, or sildenafil + LDN-193189. The cells were incubated for 2 days at 37°C/5% CO2. At 4th day, 10 μL of CCK-8 (Dijindo) were added to each well for 4 hrs. The product was then quantified spectrophotometrically at a wavelength of 450 nM. The value of OD in each well was taken as the level of the PASMCs proliferation. Multiple wells were counted and averaged for each condition studied.
Statistical analysis
Data are presented as mean ± SEM. Statistical analyses were performed by using the IBM SPSS Statistics 19 statistical software. The means among groups were compared using one way ANOVA, followed by Student-Newman-Keuls’s post hoc test. Statistical significance was set at P < 0. 05.
Results
PAH model established
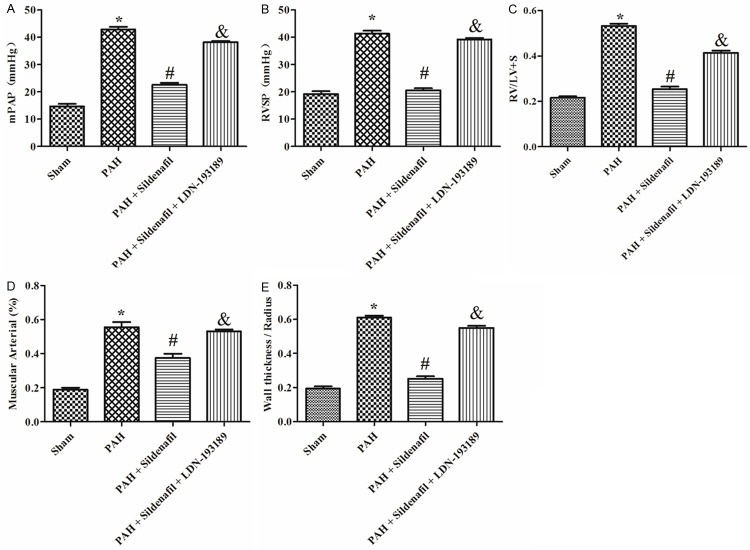
Elevations of RVSP and mPAP were observed in rats 28 days after a subcutaneous injection of MCT compared with the sham (Figure 1A, 1B). Likewise, the right ventricular hypertrophy assessed by RV/(LV + S) were significantly increase compared with the sham (Figure 1C). HE staining demonstrated a significant PASMCs proliferation and pulmonary vascular remodeling formation, in PAH models compared with the sham (Figure 3). Muscular Arterial (%) and Wall thickness/Radius (%) of muscular arteries was significantly increased in PAH groups than those in sham group, respectively (Figure 1D, 1E).
Figure 1.
Hemodynamic and pulmonary small arterial remodeling index in the Sham, PAH, PAH + Sildenafil and PAH + Sildenafil + LDN-193189 groups. A: mPAP in different groups, (n=6); B: RVSP in the Sham, PAH, PAH + Sildenafil and PAH + Sildenafil + LDN-193189 groups, (n=6); C: Right ventricular hypertrophy indexed by the ratio of wet weight of the right ventricle to the left ventricular wall plus septum [Rv/(Lv + S)] in the Sham, PAH, PAH + Sildenafil and PAH + Sildenafil + LDN-193189 groups (n=6); D, E: Pulmonary small arterial remodeling indexed by the ratio of muscular arterials and wall thickness/radius in the Sham, PAH, PAH + Sildenafil and PAH + Sildenafil + LDN-193189 groups (n=6).
Figure 3.
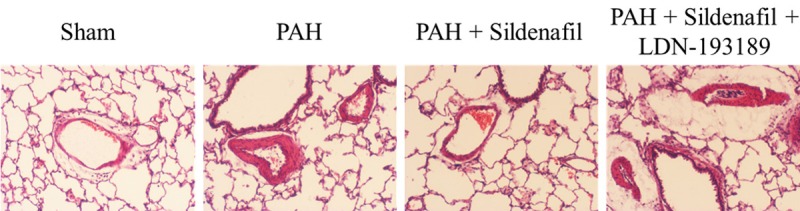
Representative images of HE stained lung sections from the Sham, PAH, PAH + Sildenafil and PAH + Sildenafil + LDN-193189 groups, respectively, showing small pulmonary arteries.
BMP signaling and Cx40 expression was altered in MCT-induced PAH
In the PAH group, the expression of BMPR2 and Id mRNA in rat lungs were significantly reduced by day 28 (Figure 2A, 2B) compared with the sham. The protein expression of BMPR2 and p-Smad1/5 was also significantly reduced in PAH models compared with the sham (Figure 4A, 4B). Furthermore, the gene and protein expression of Cx40 was significantly decreased by day 28 in the MCT rat lung compared with the sham (Figures 2C, 4C).
Figure 2.
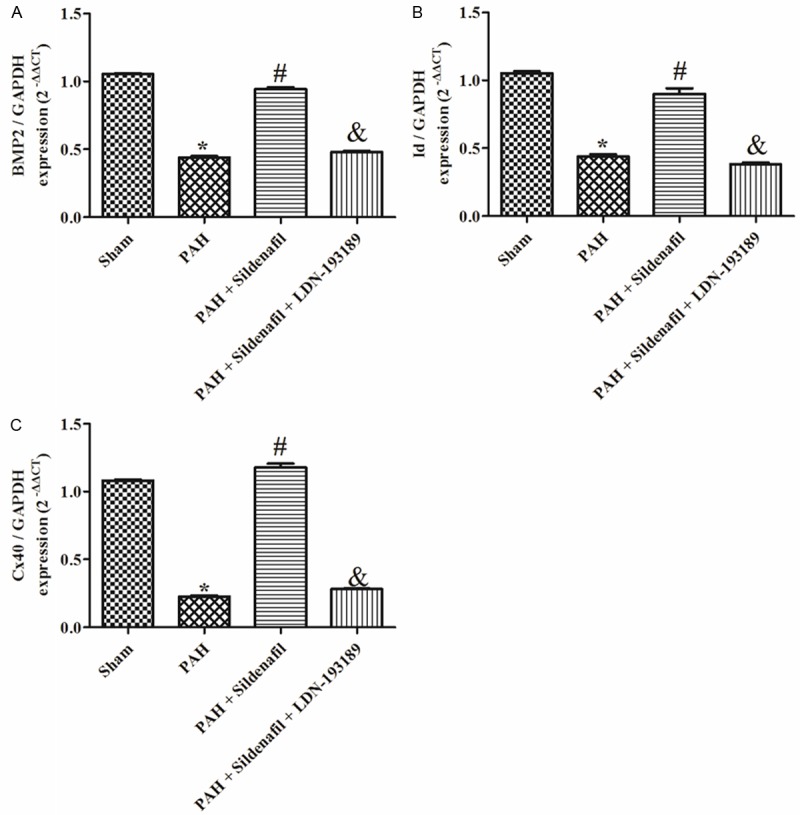
BMPR2, Id and Cx40 mRNA expression the Sham, PAH, PAH + Sildenafil and PAH + Sildenafil + LDN-193189 groups. A: BMPR2 mRNA expression in the Sham, PAH, PAH + Sildenafil and PAH + Sildenafil + LDN-193189 groups, (n=6); B: Id mRNA expression in the Sham, PAH, PAH + Sildenafil and PAH + Sildenafil + LDN-193189 groups, (n=6); C: Cx40 mRNA expression in the Sham, PAH, PAH + Sildenafil and PAH + Sildenafil + LDN-193189 groups (n=6).
Figure 4.
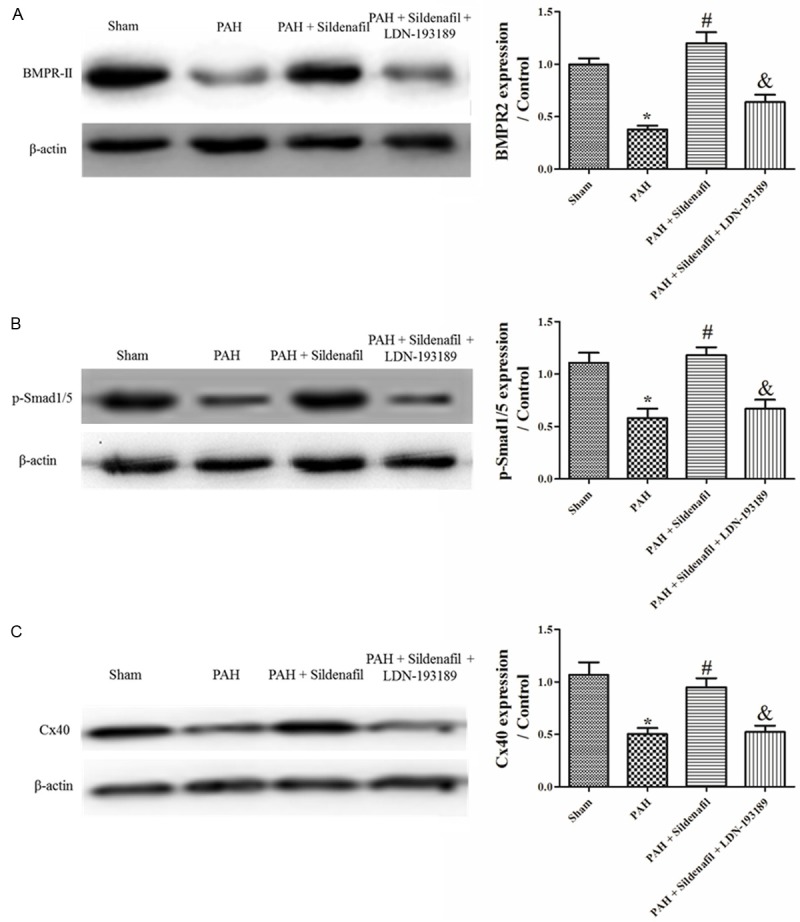
BMPR2, Id and Cx40 protein expression in the Sham, PAH, PAH + Sildenafil and PAH + Sildenafil + LDN-193189 groups. A: BMPR2 protein expression in the Sham, PAH, PAH + Sildenafil and PAH + Sildenafil + LDN-193189 groups, (n=3); B: p-Smad1/5 Protein expression in Sham, PAH, PAH + Sildenafil and PAH + Sildenafil + LDN-193189 Groups, (n=3); C: Cx40 protein expression in the Sham, PAH, PAH + Sildenafil and PAH + Sildenafil + LDN-193189 groups (n=3).
Sildenafil inhibits the hemodynamic deterioration and arterial remodeling in MCT-induced PAH
After 28 days administration of Sildenafil in the PAH model, it significantly inhibited the elevation of mPAP and RVSP elicited by the MCT compared with the PAH group (Figure 1A, 1B); it also significantly inhibited the hypertrophy and the severe vascular remodeling elicited by the MCT indexed by the ratio of muscular arterials and Wall thickness/Radius compared with the PAH group (Figure 1C-E). The H&E staining also founded that Sildenafil administration inhibited the PASMCs proliferation and remodeling in pulmonary arterial elicited by the MCT (Figure 3).
Sildenafil up-regulated the BMP signaling and the Cx40 expression in the MCT-induced PAH
Sildenafil used in the PAH model led to a significant increase in the BMPR2 and Id mRNA in rat lungs compared with the PAH by day 28 (Figure 2A, 2B). Western-blotting revealed that BMPR2 and phospho-Smad1/5 expression was also significantly increased at day 28 in the sildenafil group compared with the PAH group (Figure 4A, 4B). Furthermore, the expression of Cx40 was significantly increased in the Sildenafil rats compared with the PAH group (Figures 2C, 4C).
LDN-193189 reversed sildenafil-stimulated the BMP-Cx40 up-regulation in MCT-induced PAH
LDN-193189 used in the sildenafil rats led to a significant reduction in the BMPR2 and Id mRNA in rat lungs compared with the sildenafil group by day 28 (Figure 2A, 2B). Western-blotting revealed that the BMPR2 and phospho-Smad1/5 expression was also significantly decreased in the LDN-193189 + Sildenafil group compared with the sildenafil group at day 28 (Figure 4A, 4B). Furthermore, the expression of Cx40 was significantly decreased in the LDN-193189 + Sildenafil rats compared with the sildenafil group (Figures 2C, 4C).
LDN-193189 reversed sildenafil inhibition of the development and progression of pulmonary hypertension in MCT-induced PAH
After 28 days administration of LDN-193189 + Sildenafil in the PAH model, the LDN-193189 significantly reversed the sildenafil of the inhibition in the mPAP and RVSP compared with the Sildenafil group (Figure 1A, 1B); LDN-193189 also significantly inhibited the anti-hypertrophy and the anti-vascular remodeling effect elicited by the Sildenafil indexed by the ratio of muscular arterials and Wall thickness/Radius (Figure 1C-E). The H&E staining also founded that LDN-193189 administration inhibited the Sildenafil effect of inhibition of the PASMCs proliferation and remodeling in pulmonary arterial elicited by the MCT (Figure 3).
Sildenafil up-regulate the Cx40 expression of PASMCs isolated from MCT-PAH rats and this effect was reversed by the LDN-193189
Rat PASMCs were provided by small peripheral pulmonary arteries from the PAH rats. The Sildenafil significantly inhibits the proliferation and significantly up-regulate the BMPR2 and Cx40 expression in the PASMCs isolated from the PAH (Figures 5, 6). Co-incubation of PASMCs from the PAH rats with sildenafil and LDN-193189 completely inhibited the anti-proliferation and up-regulation of the BMPR2 and Cx40 expression by the sildenafil (Figures 5, 6).
Figure 5.
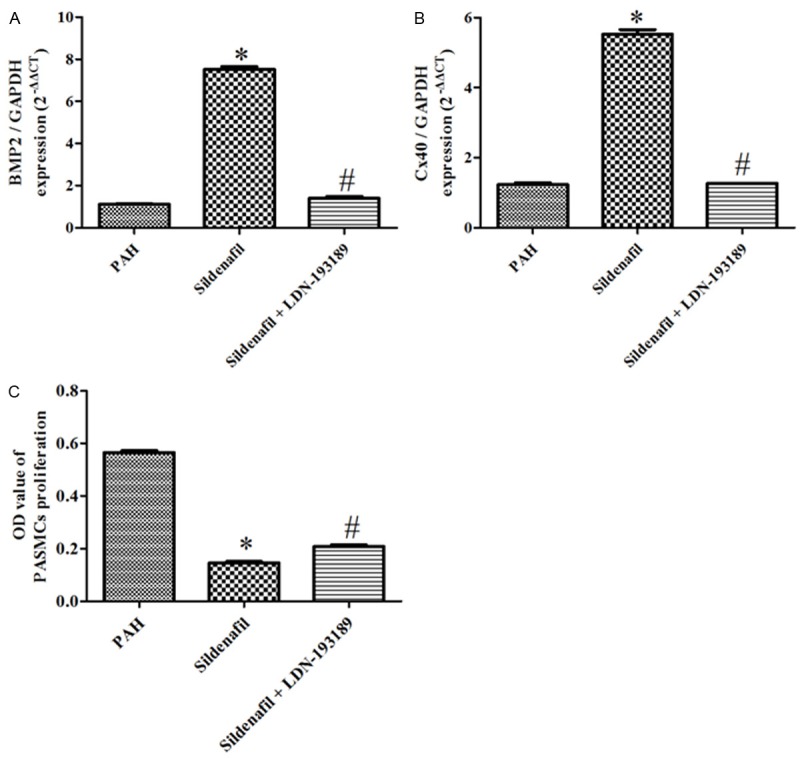
BMPR2 and Cx40 Gene expression and the CCK8 experiment in the PAH, Sildenafil and Sildenafil + LDN-193189 groups. A: BMPR2 mRNA expression in the PAH, Sildenafil and Sildenafil + LDN-193189 groups, (n=6); B: Cx40 mRNA expression in the PAH, Sildenafil and Sildenafil + LDN-193189 groups, (n=6); C: the CCK8 experiment in the Sham, PAH, Sildenafil and Sildenafil + LDN-193189 groups (n=6).
Figure 6.
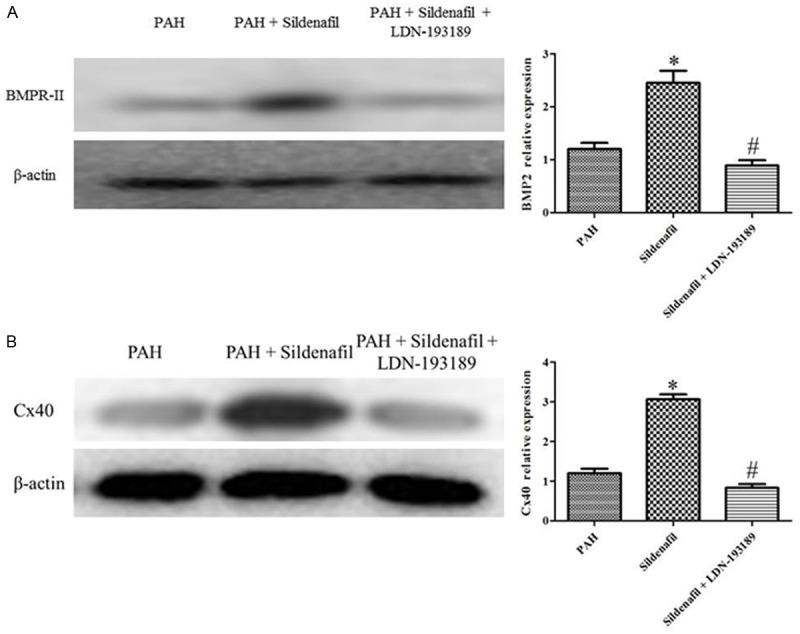
BMPR2 and Cx40 protein expression in the PAH, Sildenafil and Sildenafil + LDN-193189 groups. A: BMPR2 protein expression in the PAH, Sildenafil and Sildenafil + LDN-193189 groups, (n=6); B: Cx40 protein expression in the PAH, Sildenafil and Sildenafil + LDN-193189 groups, (n=6).
Discussion
In this study, we put forward new concepts pertaining to the involvement of cGMP-BMP signaling in regulating the Cx40 expression of PAH. We now report that the PASMCs express the Cx40; the MCT models were associated with significant down-regulations in the expression of BMPR2, Id, p-Smad1/5 and Cx40 mRNA and protein by the end of the 28 day; then, we used the cGMP-specific phosphodiesterase 5 (PDE5) inhibitor, sildenafil, to determine whether the activation of cGMP would show efficacy in the Cx40 expression in PASMCs. We find that the administration of sildenafil significantly up-regulated the BMPR2, Id, p-Smad1/5 and Cx40 mRNA and protein expression, inhibit the pulmonary arterial muscularization and improve the hemodynamics in lung. Having established that the activation of cGMP could inhibit the up regulation of the Cx40, we went on to confirm that BMP signaling inhibition could reverse the sildenafil effect in the PAH. Our experiment showed that the BMPR2 inhibitor, LDN-193189 could significantly inhibit the sildenafil-induced up-regulation of Cx40, so we confirmed that the cGMP-BMP signaling way lies in the Cx40 expression of PASMCs in PAH. Moreover, we confirm that sildenafil up-regulate the cx40 expression of PASMCs isolated from MCT-PAH rats, and LDN-193189 could prevent the sildenafil-induced of Cx40 up-regulation in vitro.
MCT-induced PAH in the rat has been used extensively to research the pathophysiological changes of PAH. In this animal model, the MCT provokes the pulmonary hypertension and a vascular masculinization of pulmonary arterial in 3-4 weeks [16]. It is demonstrated the pulmonary vasoconstriction severity (pulmonary arteriolar wall thickness, decreased alveolar-sac numbers) were significantly increased in the MCT-treated animals compared to sham. Furthermore, the mPAP, RVSP and RV weight showed an identical pattern compared to that of vascular remodeling [17]. It is also found that in the MCT model, the increase of mPAP, right ventricular hypertrophy, and small vessel remodeling is seen [18]. In our study, the mPAP, gold standard of the PAH diagnosis, are significantly elevated in the MCT models in 28 days compared to sham, along with the elevation of RVSP and the right ventricle hypertrophy. And there was a significant increase of the vascular remodeling of pulmonary arteries in the MCT model. Moreover, the HE staining showed that the proliferation of PASMCs significantly increased in the MCT model compared to sham. Taken together, the MCT models, better in mimicking pulmonary hemodynamics and vascular muscularization in human PAH, are the ideal models for the study of PAH.
The Cx40 is crucial in vascular physiology. For example, introduction of a mutant form of Cx40 in the endogenous endothelial Cx40 population prevents endothelium-derived hyperpolarization activation during myogenic constriction, enhancing sensitivity to intraluminal pressure and increasing arterial smooth muscle cell proliferation [19-21]. Furthermore, it is suggested that Cx40 (-/-) mice are at significantly greater risk for poor recovery from ischemic insult due to compromised regulation of vascular remodeling, and prolonged inflammatory response [22]. In our research, we found that in PAH, the Cx40 expression level is down-regulated in the pulmonary aerial in PAH. Furthermore, the sildenafil can up-regulate the Cx40 expression in PASMCs of PAH, and this may contribute to the effect of anti-muscularization of sildenafil. Furthermore, we also find in vitro that in the PASMCs isolated from the PAH rats, the BMPR2 and Cx40 expression of PASMCs decreased, and this phenomenon is reversed by the sildenafil treatment, providing strong evidence for the protect effect of sildenafil.
Heterozygous non-sense mutations in the gene encoding the BMPR2, a member of the TGFb receptor family, underlie the majority of inherited and familial forms of PAH [23]. BMPR2 inhibit the proliferation of human and rat smooth muscle cells via the small mothers against SMAD1/5 pathway [24]. In PASMCs isolated from PAH subjects, the anti-proliferative effect of BMPs is greatly reduced [25]. Furthermore, BMP-promoted apoptosis in PAH-PASMCs with reduced BMPR2 protein levels is attenuated. Sildenafil up-regulate the BMP signaling, for example, in PAH, sildenafil enhanced BMP-induced phosphorylation of Smad1/5, Smad nuclear localization, and Inhibitor of DNA binding protein 1 gene and protein expression. Furthermore, sildenafil restored the anti-proliferative response to BMP in PASMCs harboring mutations in BMPR2 [26]. Because the mechanism of action of sildenafil include enhancement of BMP signaling, here, we questioned whether the mechanism of up-regulation of Cx40 with sildenafil in PAH could include it. We demonstrate that in the PAH, the BMPR2 and Id gene is down-regulated; sildenafil increased the level of BMPR2, and enhances BMP-stimulated Id gene expression. We provide evidence that BMP signaling is central to the regulation of Cx40 with sildenafil, because the pharmacological inhibition of BMPR2 with LDN-193189 could abolish the up-regulation of cx40 induced by sildenafil in PASMCs of PAH. To our knowledge, this is the first report that sildenafil regulate the expression of the Cx40 in PASMCs of PAH. Our findings also reveal the critical involvement of BMP signaling during this progress.
In summary, we have provided evidence to confirm that the sildenafil trigger the Cx40 in the PASMCs via the BMP signaling in the MCT models of PAH. Inhibition of BMP signaling reversed the improvement of sildenafil on the Cx40 expression, the hemodynamics and vessel remodeling in the PAH and in vitro. These studies provide proof-of-concept for approaches that manipulate BMP/Cx40 signaling in clinical pulmonary hypertension.
Acknowledgements
The work was supported by The National Nature Science Foundation of China (81370277), The Research and Innovation Project for College Graduates of Jiangsu Province (CXLX-0560), and sponsored by The Science and Technology Development Project of Nanjing Medical University (2011NJMU140).
Disclosure of conflict of interest
None.
References
- 1.Petersen C, Helvind M, Jensen T, Andersen HØ. Potts shunt in a child with end-stage pulmonary hypertension after late repair of ventricular septal defect. World J Pediatr Congenit Heart Surg. 2013;4:286–9. doi: 10.1177/2150135113482739. [DOI] [PubMed] [Google Scholar]
- 2.Maeda AH, Nishi S, Hatada Y, Ozeki Y, Kanaly RA. Biotransformation of the high-molecular weight polycyclic aromatic hydrocarbon (PAH) benzo[k] fluoranthene by Sphingobium sp. strain KK22 and identification of new products of non-alternant PAH biodegradation by liquid chromatography electrospray ionization tandem mass spectrometry. Microb Biotechnol. 2014;7:114–29. doi: 10.1111/1751-7915.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Short KW, Head WS, Piston DW. Connexin 36 mediates blood cell flow in mouse pancreatic islets. Am J Physiol Endocrinol Metab. 2014;306:E324–31. doi: 10.1152/ajpendo.00523.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagashima F, Suzuki IK, Shitamukai A, Sakaguchi H, Iwashita M, Kobayashi T, Tone S, Toida K, Vanderhaeghen P, Kosodo Y. Novel and Robust Transplantation Reveals the Acquisition of Polarized Processes by Cortical Cells Derived from Mouse and Human Pluripotent Stem Cells. Stem Cells Dev. 2014 doi: 10.1089/scd.2013.0251. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le HT, Sin WC, Lozinsky S, Bechberger J, Vega JL, Guo XQ, Saez JC, Naus CC. Gap junction intercellular communication mediated by connexin43 in astrocytes is essential for their resistance to oxidative stress. J Biol Chem. 2014;289:1345–54. doi: 10.1074/jbc.M113.508390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin YC, Chiang CH, Chang LT, Sun CK, Leu S, Shao PL, Hsieh MC, Tsai TH, Chua S, Chung SY, Kao YH, Yip HK. Simvastatin attenuates the additive effects of TNF-α and IL-18 on the connexin 43 up-regulation and over-proliferation of cultured aortic smooth muscle cells. Cytokine. 2013;62:341–51. doi: 10.1016/j.cyto.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Guo C, Gao R, Ge M, Zhu Y, Zhang Z. The Protective Role of Resveratrol against Arsenic Trioxide-Induced Cardiotoxicity. Evid Based Complement Alternat Med. 2013;2013:407839. doi: 10.1155/2013/407839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhuang T, Chelluboina B, Ponnala S, Velpula KK, Rehman AA, Chetty C, Zakharian E, Rao JS, Veeravalli KK. Involvement of nitric oxide synthase in matrix metalloproteinase-9- and/or urokinase plasminogen activator receptor-mediated glioma cell migration. BMC Cancer. 2013;13:590. doi: 10.1186/1471-2407-13-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokoyama Y, Tomatsuri M, Hayashi H, Hirai K, Ono Y, Yamada Y, Todoroki K, Toyo’oka T, Yamada H, Itoh K. Simultaneous microdetermination of bosentan, ambrisentan, sildenafil, and tadalafil in plasma using liquid chromatography/tandem mass spectrometry for pediatric patients with pulmonary arterial hypertension. J Pharm Biomed Anal. 2013;89:227–232. doi: 10.1016/j.jpba.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Li X, Al-Lamki RS, Wu C, Weiss A, Berk J, Schermuly RT, Morrell NW. Sildenafil potentiates bone morphogenetic protein signaling in pulmonary arterial smooth muscle cells and in experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2013;33:34–42. doi: 10.1161/ATVBAHA.112.300121. [DOI] [PubMed] [Google Scholar]
- 11.Chang HM, Cheng JC, Leung PC. Theca-derived BMP4 and BMP7 down-regulate connexin43 expression and decrease gap junction intercellular communication activity in immortalized human granulosa cells. J Clin Endocrinol Metab. 2013;98:E437–45. doi: 10.1210/jc.2012-3851. [DOI] [PubMed] [Google Scholar]
- 12.Na S, Kim OS, Ryoo S, Kweon TD, Choi YS, Shim HS, Oh YJ. Cervical ganglion block attenuates the progression of pulmonary hypertension via nitric oxide and arginase pathways. Hypertension. 2014;63:309–15. doi: 10.1161/HYPERTENSIONAHA.113.01979. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Lu W, Cai WW, Wang PJ, Zhang N, Yu CP, Wang DL, Liu BC, Sun W. Telmisartan attenuates monocrotaline-induced pulmonary artery endothelial dysfunction through a PPAR gamma-dependent PI3K/Akt/eNOS pathway. Pulm Pharmacol Ther. 2014;28:17–24. doi: 10.1016/j.pupt.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Zeng GQ, Liu R, Liao HX, Zhang XF, Qian YX, Liu BH, Wu QH, Zhao J, Gu WW, Li HT. Single intraperitoneal injection of monocrotaline as a novel large animal model of chronic pulmonary hypertension in tibet minipigs. PLoS One. 2013;8:e78965. doi: 10.1371/journal.pone.0078965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Wang ZS, Luan Y, Lin M, Zhu XB, Ma Y, Zhang ZH, Wang YB. The effect of PS-341 on pulmonary vascular remodeling in high blood flow-induced pulmonary hypertension. Int J Mol Med. 2014;33:105–10. doi: 10.3892/ijmm.2013.1562. [DOI] [PubMed] [Google Scholar]
- 16.Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, Delisser H, Krymskaya VP, Tuder RM, Kawut SM, Goncharova EA. Mammalian target of rapamycin complex (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation and survival in pulmonary arterial hypertension. Circulation. 2014;129:864–74. doi: 10.1161/CIRCULATIONAHA.113.004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang DM, Han J, Zhu JH, Fu GS, Zhou BQ. Paracrine effects of bone marrow-derived endothelial progenitor cells: cyclooxygenase-2/prostacyclin pathway in pulmonary arterial hypertension. PLoS One. 2013;8:e79215. doi: 10.1371/journal.pone.0079215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee FY, Lu HI, Zhen YY, Leu S, Chen YL, Tsai TH, Chung SY, Chua S, Sheu JJ, Hsu SY, Chang HW, Sun CK, Yip HK. Benefit of combined therapy with nicorandil and colchicine in preventing monocrotaline-induced rat pulmonary arterial hypertension. Eur J Pharm Sci. 2013;50:372–84. doi: 10.1016/j.ejps.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Pankey EA, Thammasiboon S, Lasker GF, Baber S, Lasky JA, Kadowitz PJ. Imatinib attenuates monocrotaline pulmonary hypertension and has potent vasodilator activity in pulmonary and systemic vascular beds in the rat. Am J Physiol Heart Circ Physiol. 2013;305:H1288–96. doi: 10.1152/ajpheart.00329.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gairhe S, Bauer NN, Gebb SA, McMurtry IF. Serotonin passes through myoendothelial gap junctions to promote pulmonary arterial smooth muscle cell differentiation. Am J Physiol Lung Cell Mol Physiol. 2012;303:L767–77. doi: 10.1152/ajplung.00183.2012. [DOI] [PubMed] [Google Scholar]
- 21.Yao QP, Qi YX, Zhang P, Cheng BB, Yan ZQ, Jiang ZL. SIRT1 and Connexin40 Mediate the normal shear stress-induced inhibition of the proliferation of endothelial cells co-cultured with vascular smooth muscle cells. Cell Physiol Biochem. 2013;31:389–99. doi: 10.1159/000343376. [DOI] [PubMed] [Google Scholar]
- 22.Chaston DJ, Baillie BK, Grayson TH, Courjaret RJ, Heisler JM, Lau KA, Machaca K, Nicholson BJ, Ashton A, Matthaei KI, Hill CE. Polymorphism in endothelial connexin40 enhances sensitivity to intraluminal pressure and increases arterial stiffness. Arterioscler Thromb Vasc Biol. 2013;33:962–70. doi: 10.1161/ATVBAHA.112.300957. [DOI] [PubMed] [Google Scholar]
- 23.Fang JS, Angelov SN, Simon AM, Burt JM. Compromised regulation of tissue perfusion and arteriogenesis limit, in an AT1R-independent fashion, recovery of ischemic tissue in Cx40(-/-) mice. Am J Physiol Heart Circ Physiol. 2013;304:H816–27. doi: 10.1152/ajpheart.00719.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu XH, Tang ZB, Liu LJ, Qian H, Tang SL, Zhang DW, Tian GP, Tang CK. Apelin and its receptor APJ in cardiovascular diseases. Clin Chim Acta. 2013;428:1–8. doi: 10.1016/j.cca.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, Haghighat L, de Jesus Perez V, Wang L, Reddy S, Zhao M, Bernstein D, Solow-Cordero DE, Beachy PA, Wandless TJ, Ten Dijke P, Rabinovitch M. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123:3600–13. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Upton PD, Davies RJ, Tajsic T, Morrell NW. Transforming growth factor-β1 represses bone morphogenetic protein-mediated Smad signaling in pulmonary artery smooth muscle cells via Smad3. Am J Respir Cell Mol Biol. 2013;49:1135–45. doi: 10.1165/rcmb.2012-0470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]