Abstract
Although neoadjuvant chemotherapy (NACT) has been increasingly used to improve the outcome of advanced gastric cancer (GC) for decades, its precise efficacy has been difficult to evaluate yet. Abundant studies have investigated the predictive factors that represent the effect of NACT on advanced GC. In the present study, the intratumoral infiltration of regulatory T cells (Tregs) and dendritic cells (DCs) response to NACT in advanced GC and their correlation with prognosis were evaluated. Infiltration of Tregs (marked by Foxp3) and DCs (marked by S-100) in 102 advanced GC specimens with or without NACT was measured using immunohistochemical method. Intratumoral infiltration of Foxp3 Tregs was significantly lower and DC density was significantly higher in NACT group than that in nNACT group (P=0.007, P=0.002, respectively). Infiltration of Foxp3 Tregs was significantly associated with tumor invasion depth (P<0.001). The DC density was significantly correlated with histopathologic type (P=0.035), invasion depth (P=0.002), TNM stage (P=0.018), and lymph node metastasis (P<0.001). There was no significant difference of patient’s OS between NACT and nNACT groups (P=0.452); however, patients treated with NACT had longer OS with lower infiltration of Foxp3 Tregs (P<0.001) and higher infiltration of DCs (P=0.010). Univariate and multivariate analyses indicated that infiltration of Foxp3 Tregs and DCs were independent prognostic factors (P=0.002, P=0.003, respectively). The results demonstrated that NACT could decrease intratumoral Foxp3 Tregs infiltration and increase DCs density, and that infiltration of Foxp3 Tregs and DCs may serve as novel prognostic biomarkers of human GC.
Keywords: Neoadjuvant chemotherapy (NACT), advanced gastric cancer, regulatory T cells (Tregs), dendritic cells (DCs), FOXP3, S-100
Introduction
Although the incidence of gastric cancer (GC) is decreased, it is still the second most common cause of cancer-related death worldwide [1]. Survival rates of GC malignancy have been low because the moment most patients diagnosed with GC were at advanced stage, with local extension or lymph nodes metastasis, leading to the condition inoperable [2]. Neoadjuvant chemotherapy (NACT) has been increasingly used to improve the curative surgical resection, decrease the risk of metastasis, and prolong the survival time of patients with advanced GC over the past decades [3]. However, it is difficult to evaluate the efficacy of NACT because the clinical and histopathologic responses predicting the prognostic significance of NACT varies and results in divergent outcomes [4,5]. Numerous studies have investigated the predictive factors that affect clinical pathologic response to NACT.
Increasing evidences indicating that immune cells in tumor microenvironment such as innate immune cells [dendritic cells (DCs) and natural killer cells) and adaptive immune cells (T and B lymphocytes) play an important role in the progression of tumors [6]. Tumor-infiltrating lymphocytes (TILs) are deemed to host the immune response against malignant tumors, and tumor infiltrated T lymphocytes have a good prognosis in various human cancers, including melanoma [7], colorectal [8], ovarian [9], hepatocellular [10], cervical [11], and bladder [12] carcinoma. However, the prognostic role of TILs in GC is not widely investigated. A previous study results suggested that patients with high density of CD3+, CD8+, and C45RO+ TILs had a significantly longer survival time, indicating that the prognostic implications of TILs in GC depends on the density and type of TILs [13].
Regulatory T cells (Tregs), being called as immunosuppressive T lymphocytes, have a major role in promoting tumor growth and invasion by inducing immune escape and suppressing anti-tumor immune response [14,15]. Forkhead Box Protein 3 (Foxp3), a transcription factor which regulates the development of Tregs, is considered to be a marker of Tregs.
Effect of NACT on intratumoral Tregs has been reported in breast cancer with positive correlation between density of Tregs and prognosis [16]; however, there was no such report on GC.
The DCs are another type of immune cells that play an important role in antitumor immunity in tumor microenvironment [17]. The DCs, the most potent professional antigen-presenting cells (APCs), are majorly involved in primary antitumor immune response through recognition, acquisition, processing, and presentation of tumor-associated antigens (TAA) to naïve T cells, induce T cell proliferation by cytotoxic T lymphocytes or T helper cells, and thereby stimulate antitumor immunity. A previous study has demonstrated that NACT could improve survival time through increased DCs in breast cancer [18]; however, such study on GC has not been reported yet.
In the present study, intratumoral infiltration of Tregs and DCs response to NACT in advanced GC and their correlation with prognosis and overall survival (OS) of patients were evaluated.
Materials and methods
Patients
Patients with advanced GC (n=102) who received surgical treatment between 2008 and 2013 in Zhongnan Hospital of Wuhan University, China, were retrospectively evaluated. Fifty-six patients received NACT before surgery, whereas the remaining 46 patients received surgical treatment without NACT. The clinicopathologic data including time of cancer diagnosis, chemotherapy protocol, gender, age, clinical stage of carcinoma, histological type of tumor, follow-up procedures, and patient’s living condition were obtained from Department of Oncology, Zhongnan Hospital of Wuhan University, China. The histological type of tumor identified as intestinal or diffuse was according to Lauren’s classification [19] and TNM staging of GC was determined according to American Joint Committee on Cancer [20]. The written informed consent was obtained from all patients for the tissue ex vivo experimentation.
The detailed FOLFOX6 chemotherapy protocol was as follows: Patients received oxaliplatin at 85 mg/m2 (intravenous injection, iv>2 hours), calcium folinate at 400 mg/m2 (iv>2 hours), and 5-fluorouracil 500 mg, (iv by continuous infusion for 44 hours). Patients received two cycles of chemotherapy, with each cycle of 3 weeks. All patients also received adjuvant chemotherapy after the surgical resection of tumors.
Follow-up duration was defined as the time (months) from the diagnosis of GC till the final visit. The OS was defined as the time from the diagnosis of GC till the patient’s death (due to any cause) or the final visit.
Immunohistochemical staining
A conventional immunohistochemical (IHC) staining protocol was used in this study. Briefly, paraffin-embedded tumor tissue blocks were cut into species of 4 μm thickness, tissue sections were dried, deparaffinized, and dehydrated. Tissue sections were treated with 1% hydrogen peroxide for 10 min to block endogenous tissue peroxidase, followed by treatment with bovine serum for 30 min to reduce nonspecific binding. Then antigen retrieval using citrate buffer (pH 6.0) was accomplished as follows: high heat microwave processing for 5 min followed by low heat microwave processing for 20 min. All the slides were incubated with primary rabbit anti-human Foxp3 polyclonal antibody (BA2032-1, 1:200 dilution, marker for Tregs; Wuhan Boster Biological Technology, Ltd., Wuhan, China) and mouse anti-human S-100 monoclonal antibody (S1/61/69, 1:100 dilution, DCs marker; Zhongshan Golden Bridge Biotechnology, Beijing, China) for one hour at 37°C or 4°C overnight, respectively, followed by a 30-min incubation in Ultra-Sensitive S-P Kit (Maixin-Bio, Fuzhou, China). All slides were rinsed with phosphate-buffered saline, color developed using 3, 3’-diaminobenzidine substrate kit, and then counterstained with haematoxylin.
Intratumoral infiltration of Tregs (based on Foxp3 staining) and infiltration of DCs in tumor stroma (based on S-100 staining) were calculated in 10 representative high-power fields (hpf, ×400), respectively [21,22] for each slide. Two senior pathologists, Dr. Yang GF and Dr. Wang BC, were blinded to the clinicopathologic data. The average of 10 representative hpf areas was used to determine the infiltration of Foxp3 Tregs, and the sum of 10 representative hpf counting was regarded as density of DCs of tumor tissue. Then the median number of Tregs and DCs for each study group was separately determined. Values below and above the median level were defined as low and high, respectively. In case of discrepancy, a consensus was achieved using a multi-headed microscope.
Statistical analysis
Statistical analysis was performed using SPSS software, Version 17.0. The differences of Foxp3 Tregs, DCs, and clinicopathologic parameters between two groups were analyzed using unpaired Student’s t-test and chi-square test, which were expressed as mean±SD. Spearman correlation analysis was used to determine the correlation coefficients between clinicopathologic features and Foxp3 Tregs and DCs in NACT group. The OS after surgical treatment with or without NACT, Tregs density, and DCs density (both cutoff by median value) were analyzed using Kaplan-Meier method and Cox proportional hazards regression method. All two-sided P-values<0.05 were considered statistically significant.
Results
Patient characteristics and IHC features
The median follow-up duration was 19 months (range: 1-52 months). There were 69 male and 33 female patients; median age at surgery was 56 years (range: 32-78 years). The patients were divided into two groups: Patient’s received NACT before surgery (experimental or NACT group, n=56) and no NACT treatment before surgery (control or nNACT group, n=46). The patient’s characteristics are shown in Table 1. In NACT group, 40 (71.4%) patients were diagnosed with TNM stage III+IV cancer, while the remaining 16 (28.6%) were diagnosed with TNM stage I+II cancer; and at the time of diagnosis, 28 patients (50.0%) had lymph node metastasis. In nNACT group, 36 patients (78.3%) were diagnosed with TNM stage III+IV cancer, while the remaining 10 (21.7%) were diagnosed with TNM stage I+II cancer; and at the time of diagnosis, 30 patients (65.2%) had lymph node metastasis. Thirty-two (57.1%) deaths were observed during follow-up period in NACT group vs. 28 (60.9%) deaths in nNACT group, with median OS of 21 and 18 months, respectively (range: 6-46 and 1-52 months, respectively).
Table 1.
Foxp3 Tregs, DCs infiltration, and clinicopathologic parameters response to NACT
| NACT group | nNACT group | P value | |
|---|---|---|---|
| Number of patients | 56 | 46 | |
| Foxp3 Tregs (mean±SD) | 11.29±2.814 | 13.46±4.680 | 0.007 |
| Low, n (%) | 32 (57.1%) | 26 (56.5%) | |
| High, n (%) | 24 (42.9%) | 20 (43.5%) | |
| Dendritic cells (mean±SD) | 19.29±9.392 | 14.61±5.136 | 0.002 |
| High, n (%) | 28 (50.0%) | 20 (43.5%) | |
| Low, n (%) | 28 (50.0%) | 26 (56.5%) | |
| Status, n (%) | |||
| Event | 32 (57.1%) | 28 (60.9%) | |
| Censored | 24 (42.9%) | 18 (39.1%) | |
| Gender, n (%) | |||
| Male | 35 (62.5%) | 34 (73.9%) | 0.220 |
| Female | 21 (37.5%) | 12 (26.1%) | |
| Age, n (%) | |||
| <55 | 20 (35.7%) | 22 (47.8%) | 0.216 |
| ≥55 | 36 (64.3%) | 24 (52.2%) | |
| Lauren’s classification, n (%) | |||
| Intestinal | 15 (26.8%) | 10 (21.7%) | 0.555 |
| Diffuse | 41 (73.2%) | 36 (78.3%) | |
| Invasion depth, n (%) | |||
| T2 | 8 (14.3%) | 6 (13.0%) | 0.857 |
| T3/T4 | 48 (85.7%) | 40 (87.0%) | |
| TNM Stage, n (%) | |||
| I+II | 16 (28.6%) | 10 (21.7%) | 0.433 |
| III+IV | 40 (71.4%) | 36 (78.3%) | |
| Lymph node metastasis, n (%) | |||
| No | 27 (48.2%) | 16 (34.8%) | 0.172 |
| Yes | 29 (51.8%) | 30 (65.2%) |
T2, tumor invades the muscularis propria or subserosa; T3, tumor invasion extends to or beyond the serosa; T4, tumor invades adjacent structures. P and r values obtained using Spearman rank correlation.
Positive nuclear staining with Foxp3 antibody in intratumoral tissues were considered Foxp3 Tregs (Figure 1A) and positive nuclear and cytoplasm staining with S-100 antibody in tumor stroma were considered DCs (Figure 2A). The median infiltration of Foxp3 Tregs in NACT group was 10/hpf vs. 12/hpf in nNACT group. The median infiltration of DCs was 16.5/10hpf in NACT group vs. 15/10hpf in nNACT group. The number of Foxp3 Tregs in NACT group was less than that in nNACT group (Figure 1B, 1C), while DCs in NACT group was more than that in nNACT group (Figure 2B, 2C).
Figure 1.
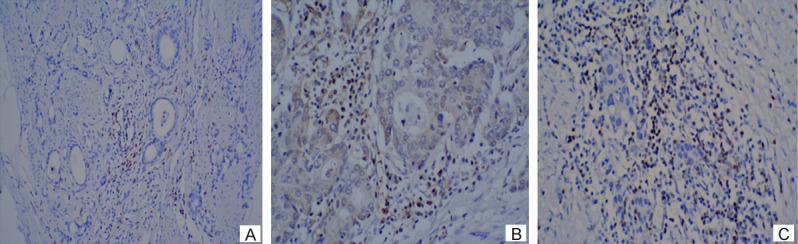
A. IHC analysis of Foxp3 expression with positive nuclear staining in intratumoral tissues (×100). B. Lower infiltration of Foxp3 Tregs in NACT group (×200). C. Higher infiltration of Foxp3 Tregs in nNACT group (×200).
Figure 2.
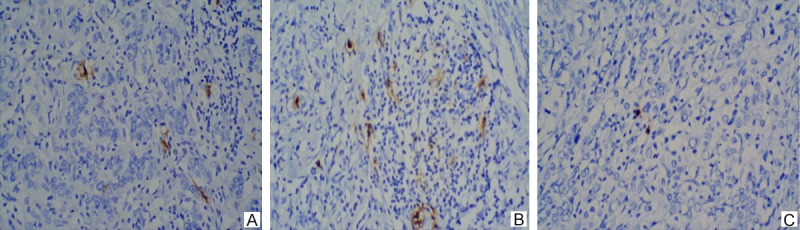
A. IHC analysis of S-100 expression with positive nuclear and cytoplasm staining in tumor stroma (×100). B. Higher DCs density in NACT group (×200). C. Lower DCs density in nNACT group (×200).
Association of intratumoral Foxp3 Tregs, DCs infiltration, and clinicopathologic features with NACT in advanced GC
The association of intratumoral Foxp3 Tregs and DCs infiltration with clinicopathological features of advanced GC with or without NACT was evaluated (Table 1). It was found that intratumoral Foxp3 Tregs and DCs infiltration in tumor stroma were associated with NACT. Intratumoral Foxp3 Tregs in NACT group (11.29±2.814) was lower than that in nNACT group (13.46±4.680; P=0.007). Density of DCs in tumor stroma in NACT group (19.29±9.392) was significantly higher than that in nNACT group (14.61±5.136; P=0.002) (Figure 3). There was no clinicopathological parameters associated with NACT, including gender (P=0.220), age (P=0.216), Lauren’s classification (P=0.555), TNM stage (P=0.433), invesion depth (P=0.857), and lymph node status (P=0.172).
Figure 3.
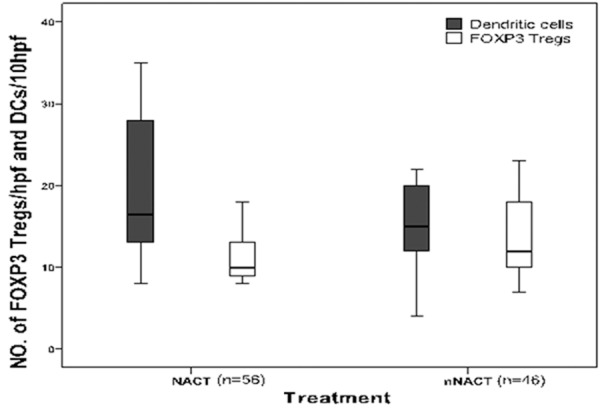
Intratumoral Foxp3 Tregs and DCs infiltration in NACT group vs. in nNACT group. Hpf, high-power field.
Association between intratumoral Foxp3 Tregs and DCs infiltration with clinicopathological parameters in NACT group
Spearman’s correlation analysis showed that Foxp3 Tregs infiltration in NACT group was not correlated with clinicopathologic features (all P>0.05) except invasion depth, where deeper invasion depth had more infiltrated Foxp3 Tregs (P<0.001, r=-0.471). However, DCs infiltration and various GC pathological features were correlated (Table 2): DCs infiltration in intestinal type was less than in diffuse type (P=0.035, r=-0.282), whereas higher DCs infiltration was observed with deeper invasion and higher TNM stage (P=0.018, r=0.316, and P=0.002, r=0.408, respectively). There were more DCs infiltration in cases without lymph node metastasis than cases with lymph node metastasis (P=0.000, r=0.465).
Table 2.
Correlation between Foxp3 Tregs and DCs density with clinicopathological parameters in NACT group
| Foxp3 Tregs (number of patients) | P value | r value | Dendritic cells (number of patients) | P value | r value | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Low | High | High | Low | |||||
| Lauren’s classification | ||||||||
| Intestinal | 7 | 8 | 0.347 | -0.128 | 4 | 11 | 0.035 | -0.282 |
| Diffuse | 25 | 16 | 24 | 17 | ||||
| Invasion depth | ||||||||
| T2 | 0 | 8 | 0.000 | -0.471 | 8 | 0 | 0.002 | 0.408 |
| T3/T4 | 32 | 16 | 20 | 28 | ||||
| TNM Stage | ||||||||
| I+II | 8 | 8 | 0.503 | -0.091 | 12 | 4 | 0.018 | 0.316 |
| III+IV | 24 | 16 | 16 | 24 | ||||
| Lymph node metastasis | ||||||||
| No | 15 | 12 | 0.821 | -0.031 | 20 | 7 | 0.000 | 0.465 |
| Yes | 17 | 12 | 8 | 21 | ||||
T2, tumor invades the muscularis propria or subserosa; T3, tumor invasion extends to or beyond the serosa; T4, tumor invades adjacent structures. P and r values obtained using Spearman rank correlation.
Association between OS and intratumoral Foxp3 Tregs as well as DCs infiltration response to NACT
The median survival time of patients with or without NACT was 21 months (range: 6-46 months) and 18 months (range: 1-52 months), respectively. Per univariate analysis, there was no significant difference between OS of both NACT and nNACT groups (log-rank test, P=0.452) (Figure 4). In NACT group, patients with a lower intratumoral Foxp3 Tregs infiltration had a longer OS than patients with a higher intratumoral Foxp3 Tregs infiltration (log-rank test, P<0.001) (Figure 5A). On the contrary, patients in NACT group with a higher infiltration of DCs in tumor stroma resulted in prolonged OS (log-rank test, P=0.010) (Figure 6A). On contrast, in nNACT group, there was no correlation between patient’s OS and intratumoral Foxp3 Tregs or DCs infiltration (log-rank test, P=0.191 and P=0.091, respectively) (Figures 5B, 6B).
Figure 4.
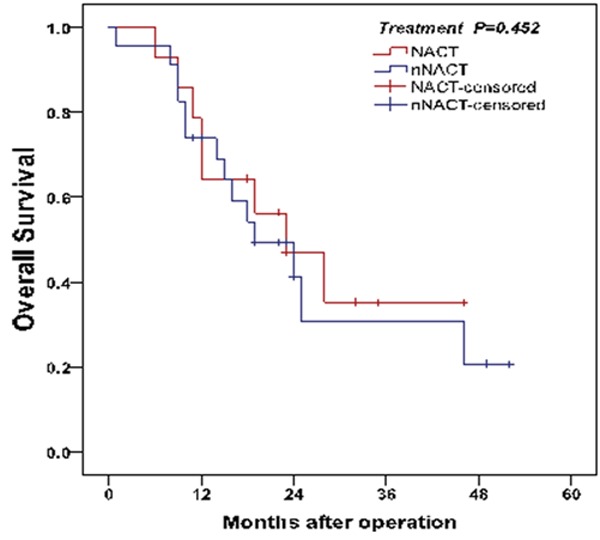
Analysis of OS between two groups. There was no significant difference between OS of patients treated with or without NACT (log-rank test, P=0.452).
Figure 5.
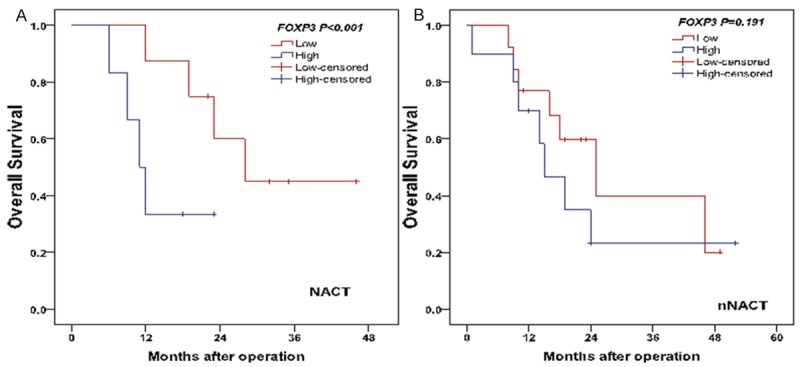
Comparison of OS with intratumoral Foxp3 Tregs level in NACTgroup (A) and nNACT group (B).
Figure 6.
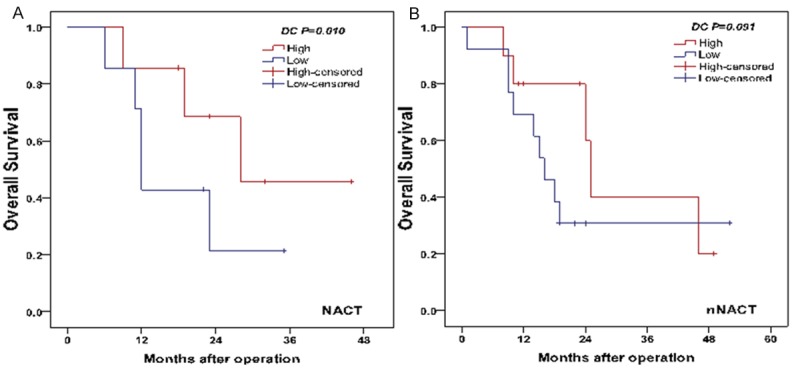
Comparison of OS with DC intensity in tumor stroma in NACT group (A) and nNACT group (B).
The factors found to have an effect on OS in NACT group were reanalyzed using Cox proportional hazards regression method (Table 3). Among the variables, number of Foxp3 Tregs and DCs were two effective independent factors for OS. Patients with GC with higher number of Foxp3 Tregs had a 7.6-fold increased risk of death [hazard ratio (HR) 7.599 (2.155-26.802); P=0.002]. Similarly, patients with GC with less DCs infiltration had a 4.6-fold increased risk of death [HR 4.617 (1.695-12.578); P=0.003].
Table 3.
Predictive factors of survival on univariate and multivariate analysis in NACT group
| n | b* | P value univariate | P value multivariate | Hazard Ratio 95% CI | |
|---|---|---|---|---|---|
| Foxp3 Tregs | |||||
| Low | 32 | - | 0.000 | 0.002 | 7.599 (2.155-26.802) |
| High | 24 | ||||
| Dendritic cells | |||||
| High | 28 | + | 0.010 | 0.003 | 4.617 (1.695-12.578) |
| Low | 28 | ||||
| Age | |||||
| <55 | 20 | + | 0.017 | 0.645 | 1.349 (0.378-4.812) |
| ≥55 | 36 | ||||
| Lauren’s classification | |||||
| Intestinal | 15 | + | 0.065 | 0.258 | 1.790 (0.652-4.917) |
| Diffuse | 41 | ||||
| Invasion depth | |||||
| T2 | 8 | + | 0.720 | 0.890 | 1.130 (0.201-6.361) |
| T3/T4 | 48 | ||||
| TNM Stage | |||||
| I+II | 16 | + | 0.191 | 0.084 | 0.352 (0.108-1.151) |
| III+IV | 40 |
regression coefficient.
Discussion
Diagnosis of GC in most patients occurs at an advanced disease stage, with local extension or lymph nodes metastasis. NACT has been increasingly used to improve the curative surgical resection, decrease the risk of metastasis, and prolong the survival time of patients with advanced GC over the past decades; however, its efficacy is difficult to evaluate because clinical and histopathologic response predicting the prognostic significance to NACT varies.In this study, effect of NACT on intratumoral Tregs and DCs infiltration as well as clinicopathological features of patients with advanced GC were investigated. Further correlation between changes on Tregs and DCs density with clinicopathological features and prognosis of patients with advanced GC were evaluated. The results demonstrated that there was no direct correlation between clinicopathological parameters of advanced GC and NACT. The OS of patients was not associated with NACT. However, it was found that NACT could decrease Foxp3 Tregs and increase DCs infiltration in tumor microenvironment, and the changes were positively correlated with clinicopathological parameters of advanced GC. Moreover, the OS was longer in patients with less intratumoral Foxp3 Tregs and more DCs infiltration. In addition, univariate and multivariate analyses indicated that infiltration of Foxp3 Tregs and DCs were independent prognostic factors.
Presently, the role of NACT in advanced GC is controversial because of diverse preoperative clinical and pathological response to NACT, results in varying predictive prognosis. A previous clinical trial have shown that NACT improved the OS of patients with advanced GC by means of down-staging the tumor, and thus improving the curative resectability of locally advanced tumors [23], while other studies indicated that NACT has no direct effect on the survival of patients with advanced GC, but could improve the factors that predicts the survival such as R0 resection status and tumor resection rate [24,25]. Accordingly, in the present study, figuring out the potential predictive factors that represent clinical and histopathologic response to NACT would be considered as the key. In the present study, it was found that NACT could decrease intratumoral Foxp3 Tregs and increase DCs infiltration in tumor stroma. The decreased infiltration of Foxp3 Tregs was inversely correlated with advanced GC invasion, whereas increased infiltration of DCs was positively correlated with advanced GC clinicopathological features, including invasion depth, TNM stage, and lymph node status. Furthermore, patients with lower number of Foxp3 Tregs and higher number of infiltrated DCs in tumor tissues resulted in prolonged OS. Thus, the authors of this study hypothesized that these clinical results may be partly associated with an enhanced tumor-specific immunity related to NACT, and the immunity of the host might be a predictive factor on clinicopathologic response to NACT.
It is well known that Foxp3 Tregs is a kind of immunosuppressive T lymphocytes. In preclinical studies of many cancers, it was demonstrated that the accumulation of Foxp3 Tregs around tumor tissues was activated by chemokines secreted from tumor cells and macrophages, which in turn promoted the tumor growth or distinct metastasis by means of its immunosuppressive function. Previous studies have indicated that Tregs are sensitive to chemotherapy, and the anti-tumor activity of chemotherapy is mediated by depletion and suppression of Tregs in breast cancer [26,27]. Liu et al [16]. demonstrated the favorable prognostic effect of lower number of intratumoral Foxp3 Tregs with NACT. In consistent with previous study results, a significant decrease in the infiltration of Foxp3 Tregs after NACT in GC was observed in this study. Consequently, the predictive role of Tregs to NACT was established.
The DCs are the most important kind of specialized APCs that acquire, process, and present TAA to naïve T cells for the induction of antigen-specific tumor immune responses, through which the body could play an important role in cancer immunosurveillance. However, during tumor progression, tumor cells may evade this immune response, which is called “Tumor Immune Escape”, by blocking the infiltration of DCs [28,29]. Pinedoa et al [18] have indicated that NACT enhanced the DC activation in tumor microenvironment and consequently improved the survival of patients with breast cancer. Tabachnyk et al [30] have demonstrated that NACT increased the DCs infiltration in tumor microenvironment and resulted in better outcome of patients with head and neck cancer. Similarly, in this study, it was found that DCs density was increased due to NACT and the patient’s OS prolonged with higher DCs infiltration in tumor stroma.
The present study had several limitations. First, the primary objective of this study was to investigate predictive factors that affect clinical pathologic response to NACT; hence, changes of Treg and DC infiltration to NACT was evaluated, but only in a small proportion of patients. Second, the heterogeneity of patients to NACT was not considered in the present study, which could affect the clinical outcomes. Third, the 5-year survival rates of the study group were not able to follow up. Hence, it would be appropriate to repeat the OS analyses after 5 years of follow-up.
In conclusion, the present study indicated that NACT may considered to have effects on anti-tumor immunity by decreasing intratumoral infiltration of Foxp3 Tregs and increasing DCs density in tumor stroma, and thus resulted in an improved prognosis of advanced GC. The intratumoral infiltration of Foxp3 Tregs and DCs could be independent prognostic predictors for advanced GC. However, the mechanisms leading to the decrease of intratumoral Foxp3 Tregs infiltration and increase of DCs density in tumor stroma by NACT and thus promoting anti-tumor immunity remain unclear. Therefore, the potential role of NACT in advanced GC treatment and their underlying mechanisms must be investigated further.
Acknowledgements
This work was supported by Natural Foundation of Hubei Province (NO. 2013CFB267) and 4693 Int J Clin Exp Pathol 2014;7(8):4685-4694 Wuhan Science and Technology Key Project (NO. 2013060602010248).
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Lochhead P, El-Omar EM. Gastric cancer. Br Med Bull. 2008;85:87–100. doi: 10.1093/bmb/ldn007. [DOI] [PubMed] [Google Scholar]
- 3.An JY, Kim HI, Cheong JH, Hyung WJ, Kim CB, Noh SH. Pathologic and oncologic outcomes in locally advanced gastric cancer with neoadjuvant chemotherapy or chemoradiotherapy. Yonsei Med J. 2013;54:888–894. doi: 10.3349/ymj.2013.54.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzen S, Blank S, Lordick F, Siewert JR, Ott K. Prediction of response and prognosis by a score including only pretherapeutic parameters in 410 neoadjuvant treated gastric cancer patients. Ann Surg Oncol. 2012;19:2119–2127. doi: 10.1245/s10434-012-2254-1. [DOI] [PubMed] [Google Scholar]
- 5.Liao Y, Yang ZL, Peng JS, Xiang J, Wang JP. Neoadjuvant chemotherapy for gastric cancer: a meta-analysis of randomized, controlled trials. J Gastroenterol Hepatol. 2013;28:777–782. doi: 10.1111/jgh.12152. [DOI] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oble DA, Loewe R, Yu P, Mihm MC Jr. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer Immun. 2009;9:3. [PMC free article] [PubMed] [Google Scholar]
- 8.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 10.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 11.Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, Melief CJ, Kenter GG, Fleuren GJ, Offringa R, van der Burg SH. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67:354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 12.Liakou CI, Narayanan S, Ng Tang D, Logothetis CJ, Sharma P. Focus on TILs: Prognostic significance of tumor infiltrating lymphocytes in human bladder cancer. Cancer Immun. 2007;7:10. [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704–1711. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol. 2012;22:327–334. doi: 10.1016/j.semcancer.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whiteside TL, Schuler P, Schilling B. Induced and natural regulatory T cells in human cancer. Expert Opin Biol Ther. 2012;12:1383–1397. doi: 10.1517/14712598.2012.707184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu FF, Li YQ, Ren MJ, Zhang XM, Guo XJ, Lang RG, Gu F, Fu L. Peritumoral FOXP3(+) regulatory T cell is sensitive to chemotherapy while intratumoral FOXP3(+) regulatory T cell is prognostic predictor of breast cancer patients. Breast Cancer Res Treat. 2012;135:459–467. doi: 10.1007/s10549-012-2132-3. [DOI] [PubMed] [Google Scholar]
- 17.Hargadon KM. Tumor-altered dendritic cell function: implications for anti-tumor immunity. Front Immunol. 2013;4:192. doi: 10.3389/fimmu.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinedo HM, Buter J, Luykx-de Bakker SA, Pohlmann PR, van Hensbergen Y, Heideman DA, van Diest PJ, de Gruijl TD, van der Wall E. Extended neoadjuvant chemotherapy in locally advanced breast cancer combined with GM-CSF: effect on tumour-draining lymph node dendritic cells. Eur J Cancer. 2003;39:1061–1067. doi: 10.1016/s0959-8049(03)00131-x. [DOI] [PubMed] [Google Scholar]
- 19.Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and so-called Intestina-type Carcinoma. An Attempt at a Histo-clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 20.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 21.Hilly O, Koren R, Raz R, Rath-Wolfson L, Mizrachi A, Hamzany Y, Bachar G, Shpitzer T. The role of s100-positive dendritic cells in the prognosis of papillary thyroid carcinoma. Am J Clin Pathol. 2013;139:87–92. doi: 10.1309/AJCPAKYDO56NKMYZ. [DOI] [PubMed] [Google Scholar]
- 22.Gai XD, Song Y, Li C, Lei YM, Yang B. Potential role of plasmacytoid dendritic cells for Foxp3 regulatory T cell development in human colorectal cancer and tumor draining lymph node. Pathol Res Pract. 2013;209:774–778. doi: 10.1016/j.prp.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Qin J, Sun YH, Liu TS. Neoadjuvant chemotherapy for advanced gastric cancer: a meta-analysis. World J Gastroenterol. 2010;16:5621–5628. doi: 10.3748/wjg.v16.i44.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basi A, Sohrabkhani S, Zamani F, Baghai-wadji M, Rabiei N, Razavi SM, Ajdarkosh H. Comparing Efficacy of Preoperative neo-Adjuvant Chemotherapy and Surgery versus Surgery Alone in Patients with Resectable Gastroesophageal Cancer. Int J Hematol Oncol Stem Cell Res. 2013;7:24–28. [PMC free article] [PubMed] [Google Scholar]
- 25.Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011;29:1715–1721. doi: 10.1200/JCO.2010.33.0597. [DOI] [PubMed] [Google Scholar]
- 26.Ladoire S, Mignot G, Dabakuyo S, Arnould L, Apetoh L, Rébé C, Coudert B, Martin F, Bizollon MH, Vanoli A, Coutant C, Fumoleau P, Bonnetain F, Ghiringhelli F. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol. 2011;224:389–400. doi: 10.1002/path.2866. [DOI] [PubMed] [Google Scholar]
- 27.Vicari AP, Luu R, Zhang NL, Patel S, Makinen SR, Hanson DC, Weeratna RD, Krieg AM. Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumor effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunol Immunother. 2009;58:615–628. doi: 10.1007/s00262-008-0586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wathelet N, Moser M. Role of dendritic cells in the regulation of antitumor immunity. Oncoimmunology. 2013;2:e23973. doi: 10.4161/onci.23973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orsini G, Legitimo A, Failli A, Ferrari P, Nicolini A, Spisni R, Miccoli P, Consolini R. Defective generation and maturation of dendritic cells from monocytes in colorectal cancer patients during the course of disease. Int J Mol Sci. 2013;14:22022–22041. doi: 10.3390/ijms141122022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabachnyk M, Distel LVR, Büttner M, Grabenbauer GG, Nkenke E, Fietkau R, Lubgan D. Radiochemotherapy induces a favourable tumour infiltrating inflammatory cell profile in head and neck cancer. Oral Oncol. 2012;48:594–601. doi: 10.1016/j.oraloncology.2012.01.024. [DOI] [PubMed] [Google Scholar]


