Abstract
To determine whether Hyperbaric oxygen preconditioning (HBO-PC) promotes neovascularization by increasing Stromal cell derived factor-1 (SDF-1) and CXC chemokine receptor 4 (CXCR4) in transplanted skin flaps of rats. The epigastric pedicle skin flap was established in a rat model. Rats were randomly assigned to the following five groups: 1) sham-operated group (SH); 2) ischemia followed by reperfusion 3 days postoperatively group (IR3d); 3) ischemia followed by reperfusion 5 days postoperatively group (IR5d); 4) hyperbaric oxygen preconditioning and ischemia followed by reperfusion 3 days postoperatively group (HBO-PC3d); and 5) hyperbaric oxygen preconditioning and ischemia followed by reperfusion 5 days postoperatively group(HBO-PC5d). For the groups receiving HBO-PC, animals underwent 1 hour of HBO at 2.0 ATA in 100% O2 twice per day for 3 days consecutively prior to surgery. After perfusion, Laser Doppler perfusion imaging (LDPI) was performed, and skin flap tissue samples were harvested for histological evaluation and western blot analysis. Perfusion was significantly improved in the HBO-PC groups compared with the IR groups on postoperative 3 and 5. Microvessel density (MVD) was significantly increased by HBO-PC compared with IR groups postoperatively. Western blot analysis revealed that SDF-1 and CXCR4 expression in the HBO-PC groups was significantly increased compared with IR groups. HBO-PC promoted neovascularization via increasing expression levels of SDF-1 and CXCR4 in transplanted skin flaps of rats.
Keywords: Hyperbaric oxygen preconditioning, neovascularization, skin flaps, stromal cell derived factor-1, CXC chemokine receptor 4
Introduction
Partial necrosis of skin flaps remains a significant problem in plastic and reconstructive surgery. Several surgical and non-surgical techniques have been tried to enhance blood supply and tissue perfusion, thereby reducing the risk of flap ischemia. For instance, surgical delay [1], and ischemic [2], pharmacological [3], and cytokines preconditioning [4,5] have all been attempted; however, safety concerns and practical feasibility have limited their application in clinical practice.
Hyperbaric oxygen preconditioning (HBO-PC) has been tested to as a way of inducing ischemic tolerance in heart models [6] and in organs such as the spinal cord [7], brain [8], and liver [9]. It suggests that HBO-PC produces a wide-scale protective effect, and may be a safer preconditioning stimulus compared with other stimuli involved, for example, ischemia. In addition, HBO-PC has been shown to promote angiogenesis in the rat liver after partial hepatectomy [9]. HBO-PC may alleviate myocardial ischemia through promotion of neovascularization [10]. However, to the best of our knowledge, the effect of HBO-PC on neovascularization of skin flaps has not been studied before in the English literature, the underlying precise mechanism remains unclear.
Stromal cell-derived factor-1 (SDF-1), also known as CXCL12, is a member of the CXC chemokine subfamily and the only known ligand for CXC chemokine receptor 4 (CXCR4). Both SDF-1 and CXCR4 are constitutively expressed in a variety of tissues and cell types, such as the liver, skin, lung, and in endothelial cells [11,12]. Mounting evidence has suggested that the SDF-1/CXCR4 axis plays a role in vascular growth and development [13,14]. Therefore, we designed and conducted the present study, in which HBO-PC is hypothesized to increase chemokine SDF-1and CXCR4 levels in flap tissue, resulting in neovascularization of transplanted skin flaps in rats.
Materials and methods
Animal care
The experiment protocol was approved by the Committee on the Ethics of Animal Experiments of Capital Medical University (Permit Number: 2010-D-013). All surgery was performed under chloral hydrate anesthesia, and all efforts were made to minimize suffering. Healthy adult male Sprague-Dawley rats weighing between 250 and 300 grams were used in all experiments. Animals were housed in individual cages in a temperature-controlled setting with 12 hour light and dark cycles and with free access to food and distilled water.
Experimental groups
Forty rats were randomly assigned to one of the following five groups: 1) sham-operated (SH; 21% O2 at 1.0 ATA; n=8); 2) ischemia followed by reperfusion 3 days postoperatively (IR3d; 21% O2 at 1.0 ATA; n=8); 3) ischemia followed by reperfusion 5 days postoperatively (IR5d; 21% O2 at 1.0 ATA; n=8); 4) hyperbaric oxygen preconditioning and ischemia followed by reperfusion 3 days postoperatively (HBO-PC3d; 100% O2 at 2.0 ATA; n=8); and 5) hyperbaric oxygen preconditioning and ischemia followed by reperfusion 5 days postoperatively (HBO-PC5d; 100% O2 at 2.0 ATA; n=8).
Hyperbaric oxygen preconditioning
Rats in HBO-PC3d and HBO-PC5d groups were exposed to 100% O2 at 2.0 ATA for 1 hour in a hyperbaric chamber twice daily, at 8 hour intervals for three consecutive days. Compression and decompression were carried out at a rate of 0.2 kg/cm2/minute. During HBO exposure, oxygen and carbon dioxide contents were continuously monitored and maintained at ≥98% and ≤0.03%, respectively. The temperature of the chamber was maintained at a range of 22-25°C. After exposure to HBO, rats were maintained in a normoxic environment until skin flap or sham surgery. In groups SH, IR3d, and IR5d, rats were treated with normobaric air at 1.0 ATA in 21% O2 at an ambient temperature of 22°C to 25°C postoperatively.
The epigastric pedicle skin flap model
The epigastric pedicle skin flap model used in this study has been previously described, with a modification in flap design [15]. All procedures were performed aseptically under anesthesia using intraperitoneal injections of 10% chloral hydrate at a dose of 350 mg/kg. After shaving and sterilizing the abdomen, single inferior epigastric vessel pedicle skin fl aps were obtained (9 cm×6 cm). Skeletonization of the right inferior epigastric artery and vein pedicle was performed, while the contralateral inferior epigastric vessel was suture ligated. The feeding vessel was clamped using a microvascular clamp to achieve ischemia. For reperfusion, the microvascular clamp was removed 3 hours later and flow was restored. The fl aps were repositioned above a silicone sheet (the same area as the flap) with continuous 5-0 monofilament nylon sutures to prevent vascular supply other than the pedicle. The sham-operated group underwent the same operation but was not exposed to ischemia. All rats received a single dose of intramuscular penicillin sodium (0.8 mg/gram) postoperatively.
Laser Doppler perfusion imaging
Blood flow in pedicled skin flaps in the epigastric zone was mapped with a high resolution PIM-2 Laser Doppler perfusion imager (Perimed Inc., NorthRoyalton, OH). The parameter settings during measurement were as following: scanning area, 70 mm×80 mm; high resolution; distance between the scanner head and wound, 42.3 cm. The measurement of the image and perfusion value was carried out with LDISOFT software.
Histologic analysis
Flaps were evaluated 3 and 5 days postoperatively. For each fl ap, 3-4 mm sectioned tissue blocks from viable regions were fixed in 10% formalin and embedded in paraffin for hematoxylin-eosin staining. Microvessel density (MVD) was measured by two double-blinded pathologists, using the “hot spot” method. Briefly, five areas with the highest accumulation of small vessels were identified at low magnification (100×) in each specimen. These areas were then examined at high (400×) magnification and microvessels were counted in five high-power fields. Blood vessels with muscular layers were excluded.
Immunohistochemical staining
Monoclonal antibody analysis was performed 3 and 5 days postoperatively. For each fl ap, 3-4 mm sectioned tissue blocks taken from the viable portion of the fl ap were preserved in 10% formalin. Sections (4 μm thick) were obtained and embedded in paraffin for immunohistologic analysis. The monoclonal antibodies anti-SDF-1 (1:100, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and anti-CXCR4 (1:500, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) were used for immunohistochemical staining. The proportion of positive cells in at least 10 areas in each section at high (400×) magnification was analyzed by two double-blinded pathologists.
Protein preparation
Flap tissues were frozen in liquid nitrogen and then stored at -80°C until analysis. The tissues were homogenized in ice-cold isolation solution containing 250 mmol/L sucrose, 10 mmol/L triethanolamine, 1 μg/mL leupeptin, and 0.1 mg/mL phenylmethylsulfonyl fl uoride. Homogenates were centrifuged at 12,000 rpm for 10 minutes at 4°C to separate incompletely homogenized tissue. The supernatants were obtained and protein concentrations were measured using a protein assay kit (Sunbio, Beijing, China). For deglycosylation of proteins, an N-glycosidase F deglycosylation Kit (Roche, Mannheim, Germany) was used.
Western blotting
Total proteins (50 μg/sample) were diluted in 5×loading buffer (0.25 mol/L Tris HCl, pH 6.8; 10% sodium dodecyl sulfate; 0.5% bromophenol blue; 50% glycerol; 0.5 mol/L dithiothreitol) and then boiled for 5 minutes. Sodium dodecyl sulfate polyacrylamide gel electrophoresis was carried out on 12% gradient gels. The proteins were electrophoretically transferred to polyvinylidene difl uoride membranes previously treated with methanol and blocked for 1 hour at room temperature in TBS-T (Tris-buffered saline containing 0.1% Tween 20) containing 5% nonfat dry milk and incubated overnight at 4°C with anti-SDF-1 (1:100, Santa Cruz Biotechnology) and anti-CXCR4 (1:500, Santa Cruz Biotechnology) in TBS-T containing 5% nonfat dry milk. After washing in TBS-T, the membranes were incubated with horseradish peroxidase-labeled anti-rabbit antibody (1:3,000, Santa Cruz Biotechnology) for 2-3 hours at room temperature. Blots were developed with enhanced chemiluminescence agents (ECL Plus, Sunbio) before exposure to x-rays. To confirm equivalent loading of samples, the same membranes were incubated with anti-β-actin antibody (1:300, Santa Cruz Biotechnology) and visualized via enhanced chemiluminescence as mentioned earlier. For quantification, films of western blots were scanned using a Minolta scanner and Adobe Photoshop software. The labeling density was quantitated using LabWorks software (UVP, Upland, CA, USA). The value of the relative density of SDF-1 and CXCR4 bands was normalized to the density of actin to represent the amount of SDF-1 and CXCR4 protein.
Statistical analysis
Statistical analysis was performed using SPSS version 15.0 (SPSS, Chicago, IL, USA) software. All quantitative data were expressed as the mean ± SD. One-way analysis of variance procedures were used to test the differences in blood perfusion, MVD, SDF-1, and CXCR4. A p-value of less than 0.05 was considered statistically significant.
Results
HBO-PC increased blood perfusion in epigastric skin flaps
Laser Doppler perfusion imaging (LDPI) is a noninvasive and relatively new technique for measuring skin blood perfusion [16]. LDPI was performed 3 and 5 days postoperatively to detect the blood perfusion of the skin flaps. As shown in Figure 1, compared to the SH group, perfusion was significantly decreased in the IR and HBO-PC groups (p<0.01). However, HBO-PC increased perfusion when compared with the IR group (p<0.01). Importantly, LDPI data were consistent with histological data, which implied that HBO-PC increased neovascularization in the transplanted skin flaps of rats.
Figure 1.
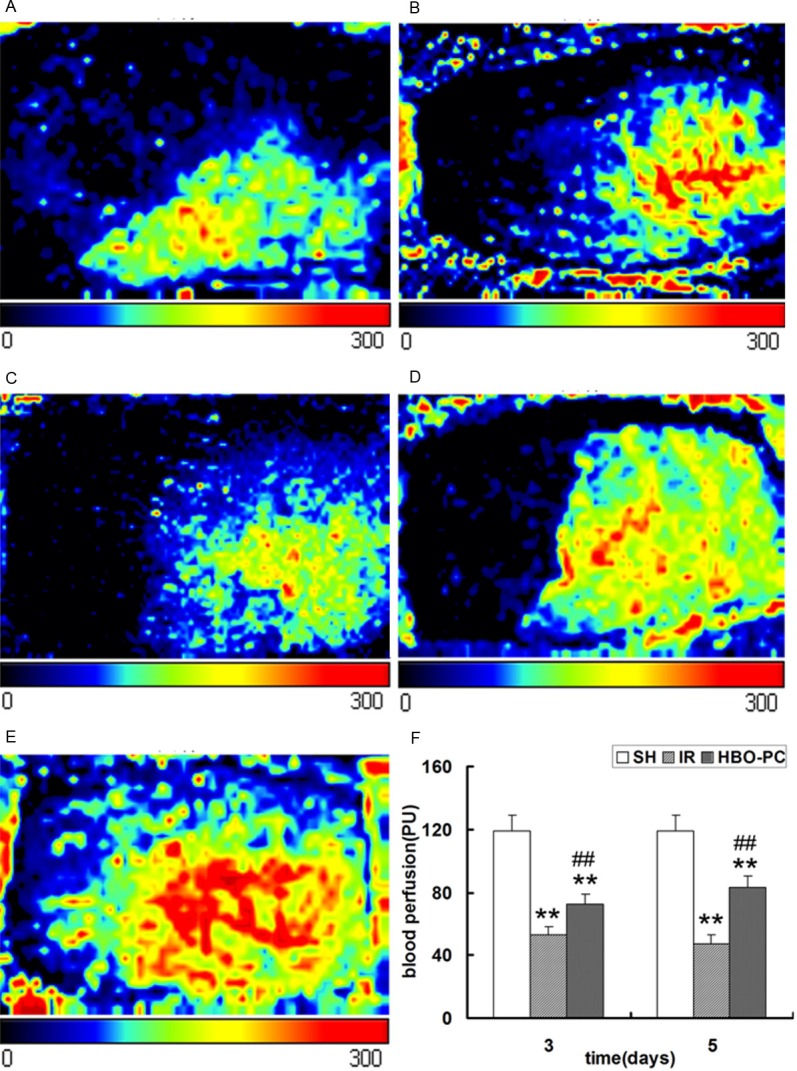
Perfusion images of skin flaps at 3 and 5 days postoperatively. The color scale of all images was defined by setting the lowest perfusion value to 0 and the highest perfusion value to 300. (A) IR3d, (B) HBO-PC3d, (C) IR5d, (D) HBO-PC5d and (E) SH groups. (F) Perfusion in the IR and HBO-PC groups was significantly lower than that in the SH group (**P<0.01). Compared with the same day IR groups, perfusion was significantly increased in the HBO-PC groups (##P<0.01). IR, ischemia reperfusion; HBO-PC, hyperbaric oxygen preconditioning; SH, sham-operated.
HBO-PC promotes neovascularization of transplanted skin flaps
To evaluate whether neovascularization varied among the different groups, we measured MVD in the skin flaps. Compared with the SH group, the MVD were significantly reduced in the IR and HBO-PC groups (p<0.05; p<0.01); but compared to the same day IR groups, HBO-PC remarkably increased the MVD postoperatively (p<0.05; p<0.01) (Figure 2). These results indicated that HBO-PC significantly promoted neovascularization in transplanted skin flaps of rats.
Figure 2.
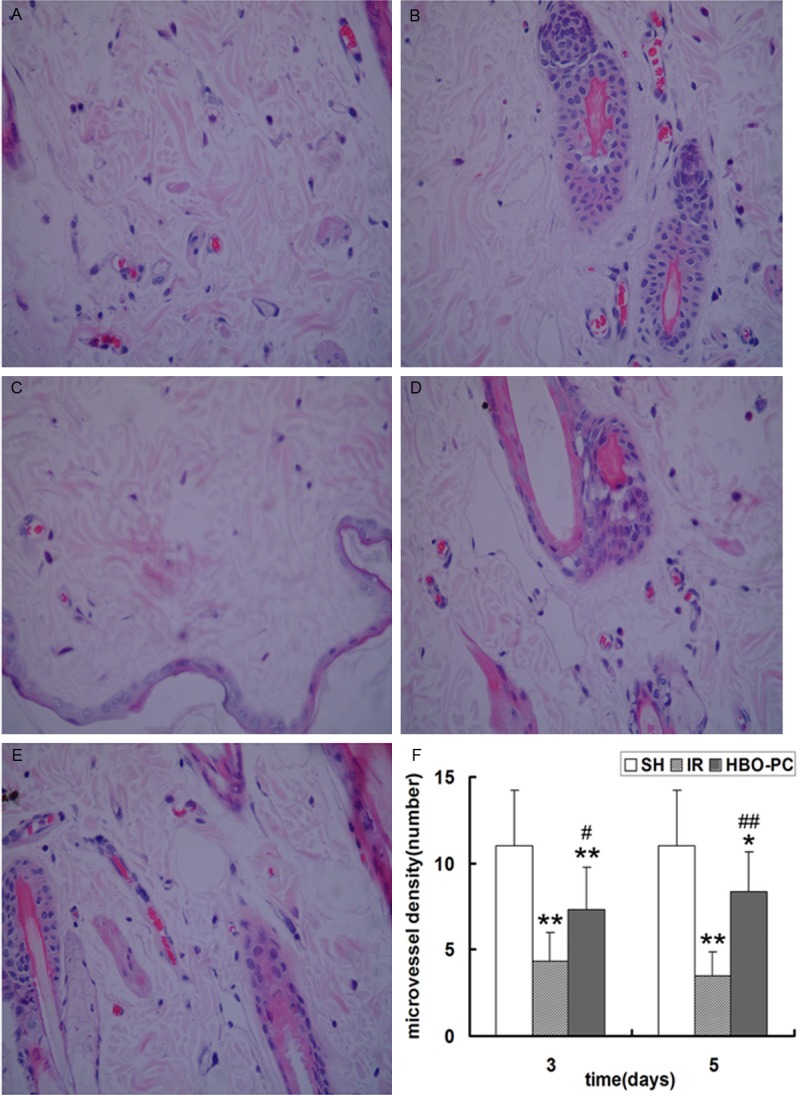
Vascularization in the skin flaps of rats. MVD was defined as the total number of microvessels divided by the number of microvessels observed under a high-power field microscope (magnification, ×400) in the (A) IR3d, (B) HBO-PC3d, (C) IR5d, (D) HBO-PC5d and (E) SH groups. (F) MVD in the IR and HBO-PC groups was significantly lower than that in the SH group (**P<0.01; *P<0.05). Compared with the same day IR groups, MVD was significantly increased in the HBO-PC groups (##P<0.01; #P<0.05). MVD, microvessel density; IR, ischemia reperfusion; HBO, hyperbaric oxygen; SH, sham-operated.
HBO-PC improves the expression of SDF-1 and CXCR4 of transplanted skin flaps
To study how HBO-PC promoted neovascularization in transplanted skin flaps of rats, we evaluated the expression of SDF-1 and CXCR4 in skin flap tissue by immunohistochemical staining and western blot. Immunohistochemical staining showed that cells were positive for brown particles in the cell membrane, cytoplasm and nucleus. Compared to SH group, the SDF-1 and CXCR4 positive cells were significantly increased in IR and HBO-PC groups (p<0.05; p<0.01); HBO-PC remarkably increased the number of SDF-1 and CXCR4 positive cells compared with the same day IR groups (p<0.01) (Figures 3 and 4).
Figure 3.
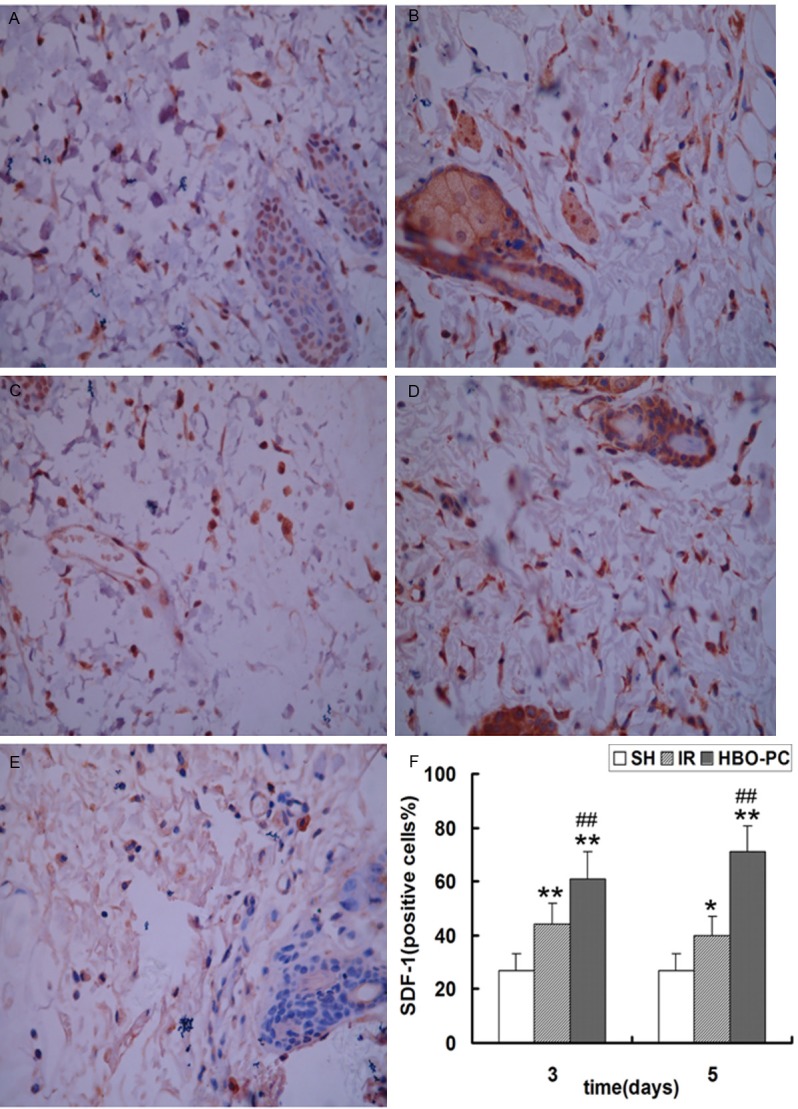
Immunohistochemical staining of SDF-1 in each group. The proportion of positive cells was analyzed under a high magnification (×400) in the (A) IR3d, (B) HBO-PC3d, (C) IR5d, (D) HBO-PC5d and (E) SH groups. (F) SDF-1-positive cells in the IR and HBO-PC groups were significantly higher than those in the SH group (*P<0.05; **P<0.01). Compared with the same day IR groups, SDF-1-positive cells were significantly increased in the HBO-PC groups (##P<0.01). IR, ischemia reperfusion; HBO-PC, hyperbaric oxygen preconditioning; SH, sham-operated.
Figure 4.
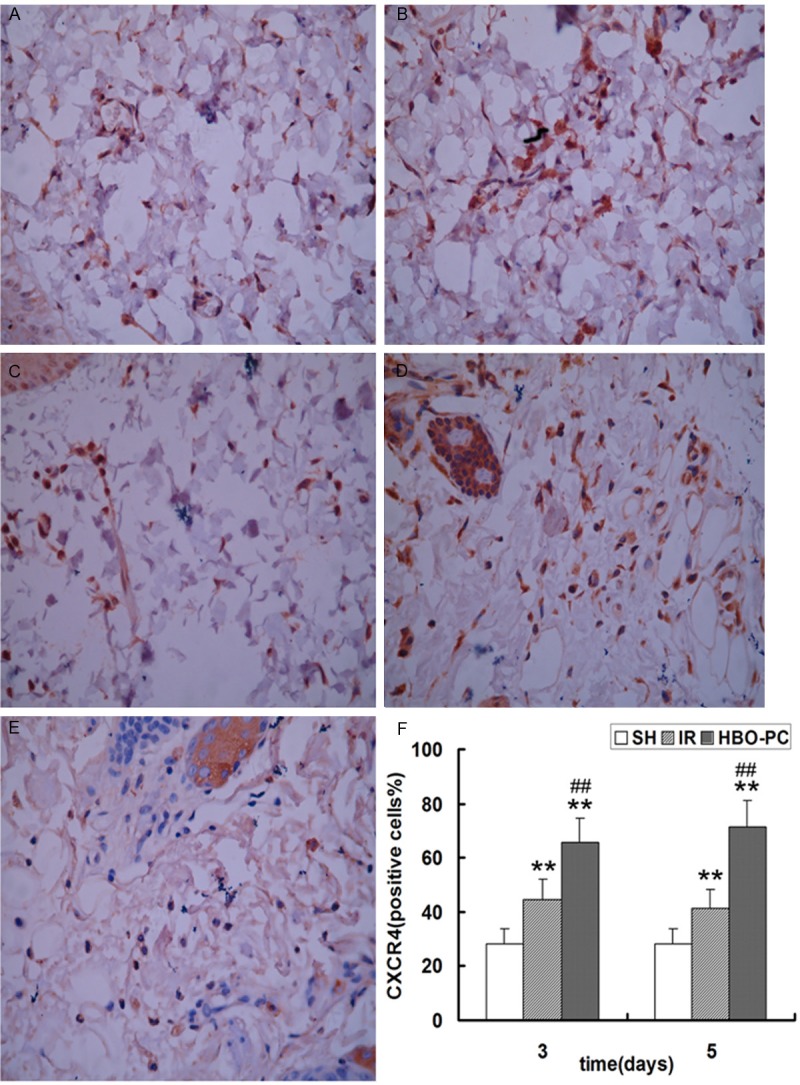
Immunohistochemical staining of CXCR4 in each group. The proportion of positive cells was analyzed under a high magnification (×400) in the (A) IR3d, (B) HBO-PC3d, (C) IR5d, (D) HBO-PC5d and (E) SH groups. (F) CXCR4-positive cells in the IR and HBO-PC groups were significantly higher than those in the SH group (**P<0.01). Compared with the same day IR groups, CXCR4-positive cells were significantly increased in the HBO-PC groups (##P<0.01). IR, ischemia reperfusion; HBO-PC, hyperbaric oxygen preconditioning; SH, sham-operated.
In agreement with the result of immunohistochemical staining, western blot analysis identified that HBO-PC apparently increased the expression of SDF-1 and CXCR4 proteins compared with the same day IR groups (p<0.01); IR showed a remarkable tendency to up-regulate the expression of SDF-1 and CXCR4 proteins postoperatively, but the difference was not statistically significant (Figure 5). These data suggest that HBO-PC accelerated neovascularization of skin flaps may be associated with the upregulation of SDF-1 and CXCR4 after transplantation.
Figure 5.
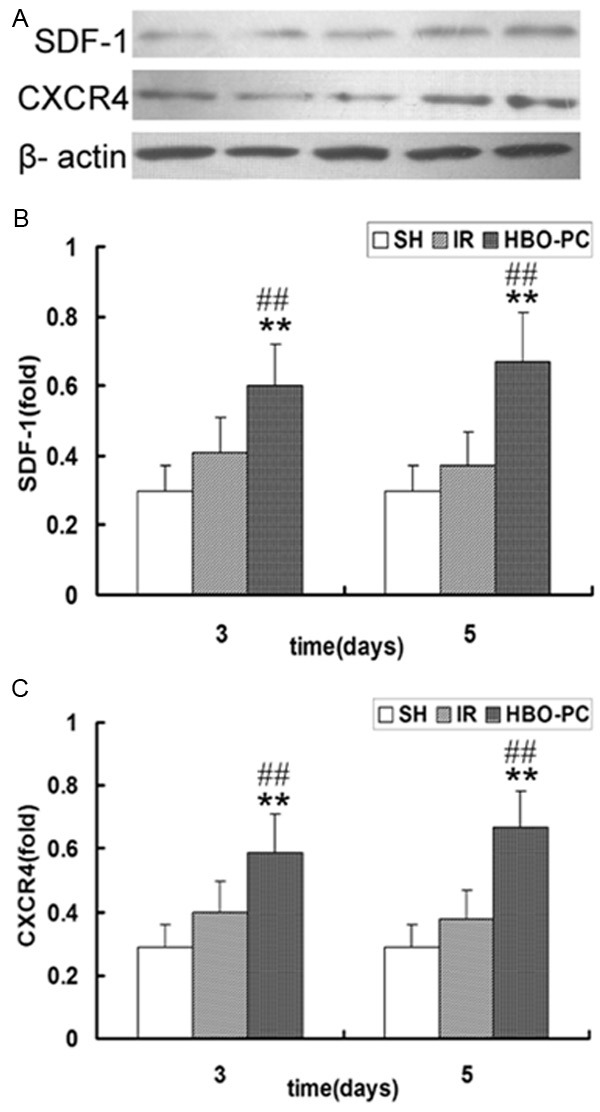
(A) Immunoblot analysis of SDF-1 and CXCR4 in each group. Western blot analysis of (B) SDF-1 and (C) CXCR4 in the skin flap tissue of each group. SDF-1 and CXCR4 expression in the HBO-PC groups were significantly higher than those in the SH group (**P<0.01). Compared with the same day IR groups, SDF-1 and CXCR4 expression were significantly increased in the HBO-PC groups (##P<0.01). IR, ischemia reperfusion; HBO-PC, hyperbaric oxygen preconditioning; SH, sham-operated.
Discussion
We demonstrated that preconditioning with hyperbaric oxygen prior to skin flap transplantation significantly improves perfusion and increases the MVD of skin flaps in the rat model. SDF-1 and CXCR4 expression and activity were increased by skin flap transplantation and by HBO-PC. This beneficial effect of HBO-PC in promoting neovascularization may result from high SDF-1 and CXCR4 expression in the skin flap tissues of rats.
Since the preconditioning phenomenon was first identified in the heart by Murry et al [17], the same effect could be induced by several other stimuli, such as hyperbaric oxygen, chemical agents, sleep deprivation, dietary restriction, and both hyperthermia and hypothermia. Among these stimuli, hyperbaric oxygen is an outstanding candidate for clinical application because of its minimal side effects under proper management. Previous findings have shown that HBO-PC promotes angiogenesis in the rat liver after partial hepatectomy [9]. Other studies also suggested that HBO-PC accelerates angiogenesis and alleviates myocardial ischemic injury [10]. Ju reported that administration of HBO during rapid expansion resulted in increased perfusion in the expanded skin [18]. However, we found no published studies on the effect of HBO-PC on perfusion of skin flaps. In the current study, we found that both perfusion and the MVD were increased in the HBO-PC group compared to the IR group postoperatively (p<0.05; p<0.01). After HBO-PC, as perfusion improves, skin flaps acquires more oxygen and nutrients to stimulate angiogenesis. To our knowledge, this is the first experimental study on the effect of HBO-PC on neovascularization in transplanted skin flaps. Moreover, at the molecular level, we are the first to have explored the mechanisms of HBO-PC on neovascularization by measuring SDF-1 and CXCR4 expression in skin flaps.
Various studies indicate that SDF-1 and its receptor CXCR4 are beneficial for neovascularization in ischemic cardiac disease or stroke [19,20]. Blocking the SDF-1/CXCR4 axis resulted in reduced homing of cells to lesions and decreased MVD after ischemic heart disease [21]. SDF-1 and its receptor CXCR4 are upregulated in ischemic or hypoxic tissues, such as the retina [22] and spinal cord [23]. We demonstrated that SDF-1 and CXCR4 expression in the IR groups was higher than that in the SH group, in accordance with previous studies. In addition, compared with IR groups, SDF-1 and CXCR4 expression were significantly increased by HBO-PC in our experiments, consistent with the increase of MVD.
How HBO-PC upregulated SDF-1 and CXCR4 expression remains unclear. Endothelial progenitor cells (EPC) appear to play a role in the HBO effect. Bone marrow (BM) derived EPC has been shown to act as a key cell involved in neovascularization and homes to peripheral tissue in response to ischemia [12]. Induction of hyperoxia via HBO treatment has been shown to increase NO levels in perivascular tissues and BM via a nitric oxide synthase (NOS)-mediated mechanism, resulting in EPC release from the BM and homing to ischemia tissues [24-26]. EPCs themselves express and secrete SDF-1 and CXCR4 [27], thus HBO treatment could increase SDF-1 and CXCR4 expression. Salvucci et al demonstrated endothelial expression of SDF-1 and CXCR4 may be reduced by inflammatory cytokines, such as tumor necrosis factor-α and interferon-γ [28]. As HBO treatment inhibits these inflammatory cytokines, SDF-1 and CXCR4 expression may be upregulated by HBO treatment. Since HBO stimulates SDF-1 and CXCR4 expression, it is reasonable to deduce that HBO-PC increases SDF-1 and CXCR4 expression. In addition, Grunewald considered that high levels of VEGF-A in the liver induces SDF-1 and CXCR4 expression [29]. While HBO-PC treatment increased VEGF expression after partial hepatectomy [9], HBO-PC may induce SDF-1 and CXCR4 expression via upregulation at the VEGF level.
There are many other mechanisms, in addition to SDF-1/CXCR4 (such as VEGF and HIF-1) [9] that are responsible for neovascularization in skin flap transplantation. The increase of SDF-1 and CXCR4 may possibly be one of potential mechanisms for the HBO-PC induced increase of neovascularization in transplanted skin flaps. The involvement of other related mechanisms requires further study.
Conclusion
In conclusion, we demonstrated that HBO-PC (2.0 ATA, 100% O2, 1 hour per session, twice daily for 3 days) increased SDF-1 and CXCR4 expression in skin flap tissue, thereby promoting neovascularization and perfusion of transplanted skin flaps in rats. Our results provide a theoretical basis for broadening the clinical application of HBO-PC treatment. HBO-PC may hold therapeutic value for patients with skin flap transplantation.
Acknowledgements
This research has no funding supports. We wish to thank professor Youbin Wang, Department of Plastic and Reconstructive Surgery, Peking Union Medical College Hospital for his help in animal model preparation and tissue sample collection.
Disclosure of conflict of interest
None.
References
- 1.Ulkür E, Karagoz H, Ergun O, Celikoz B, Yildiz S, Yildirim S. The effect of hyperbaric oxygen therapy on the delay procedure. Plast Reconstr Surg. 2007;119:86–94. doi: 10.1097/01.prs.0000244829.68008.21. [DOI] [PubMed] [Google Scholar]
- 2.De Souza Filho MV, Loiola RT, Rocha EL, Simão AFL, Ribeiro RA. Remote is chemic preconditioning improves the survival of rat random-pattern skin flaps. Eur J Plast Surg. 2010;33:147–152. [Google Scholar]
- 3.Matsumura H, Yashizawa A, Vedder NB, Watanabe K. Preconditioning of the distal portion of a rat random-pattern skin flap. Br J Plast Surg. 2001;54:58–61. doi: 10.1054/bjps.2000.3470. [DOI] [PubMed] [Google Scholar]
- 4.Simman R, Craft C, McKinney B. Improved survival of ischemic random skin flaps through the use of bone marrow nonhematopoietic stem cells and angiogenic growth factors. Ann Plast Surg. 2005;54:546–552. doi: 10.1097/01.sap.0000158068.86576.73. [DOI] [PubMed] [Google Scholar]
- 5.Hom DB, Unger GM, Pernell KJ, Manivel JC. Improving surgical wound healing with basic fibroblast growth factor after radiation. Laryngoscope. 2005;115:412–422. doi: 10.1097/01.mlg.0000157852.01402.12. [DOI] [PubMed] [Google Scholar]
- 6.Sun Q, Sun Q, Liu Y, Sun X, Tao H. Anti-Apoptotic Effect of Hyperbaric Oxygen Preconditioning on a Rat Model of Myocardial Infarction. J Surg Res. 2011;171:41–46. doi: 10.1016/j.jss.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Li W, Kang Z, Liu Y, Deng X, Tao H, Xu W, Li R, Sun X, Zhang JH. Hyperbaric oxygen preconditioning attenuates early apoptosis after spinal cord is chemia in rats. J Neurotrauma. 2009;26:55–66. doi: 10.1089/neu.2008.0538. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Liu W, Ding S, Xu W, Guan Y, Zhang JH, Sun X. Hyperbaric oxygen preconditioning induces tolerance against brain ischemia-reperfusion injury by up-regulation of antioxidant enzymes in rats. Brain Res. 2008;1210:223–229. doi: 10.1016/j.brainres.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Ren P, Kang Z, Gu G, Liu Y, Xu W, Tao H, Zhang JH, Sun X, Ji H. Hyperbaric oxygen preconditioning promotes angiogenesis in rat liver after partial hepatectomy. Life Sci. 2008;83:236–241. doi: 10.1016/j.lfs.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Han C, Lin L, Zhang W, Zhang L, Lv S, Sun Q, Tao H, Zhang JH, Sun X. Hyperbaric oxygen preconditioning alleviates myocardial ischemic injury in rats. Exp Biol Med (Maywood) 2008;233:1448–1453. doi: 10.3181/0801-RM-8. [DOI] [PubMed] [Google Scholar]
- 11.Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 13.Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, Sata M, Ichiki T, Takeshita A, Egashira K. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Wei M, Zhou Z, Wang B, Zhao X, Zhang J. SDF-1α induces angiogenesisafter traumatic brain injury. Brain Res. 2012;1444:76–86. doi: 10.1016/j.brainres.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 15.Padubidri AN, Browne E Jr. Modification in flap design of the epigastric artery flap in rats: A new experimental flap model. Ann Plast Surg. 1997;39:500–504. doi: 10.1097/00000637-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Rosato E, Borghese F, Pisarri S, Salsano F. Laser Doppler perfusion imaging is useful in the study of Raynaud’s phenomenon and improves the capillaroscopic diagnosis. J Rheumatol. 2009;36:2257–2263. doi: 10.3899/jrheum.090187. [DOI] [PubMed] [Google Scholar]
- 17.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 18.Ju Z, Wei J, Guan H, Zhang J, Liu Y, Feng X. Effects of hyperbaric oxygen therapy on rapid tissue expansion in rabbits. J Plast Reconstr Aesthet Surg. 2012;65:1252–1258. doi: 10.1016/j.bjps.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Ghadge SK, Muhlstedt S, Ozcelik C, Bader M. SDF-1a as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2011;129:97–108. doi: 10.1016/j.pharmthera.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Rosenkranz K, Kumbruch S, Lebermann K, Marschner K, Jensen A, Dermietzel R, Meier C. The chemokine SDF-1/CXCL12 contributes to the ‘homing’ of umbilical cord blood cells to a hypoxic-ischemic lesion in the rat brain. J Neurosci Res. 2010;88:1223–1233. doi: 10.1002/jnr.22292. [DOI] [PubMed] [Google Scholar]
- 21.Dai S, Yuan F, Mu J, Li C, Chen N, Guo S, Kingery J, Prabhu SD, Bolli R, Rokosh G. Chronic AMD3100 antagonism of SDF-1alpha-CX CR4 exacerbates cardiac dysfunction and remodeling after myocardial infarction. J Mol Cell Cardiol. 2010;49:587–597. doi: 10.1016/j.yjmcc.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lima e Silva R, Shen J, Hackett SF, Kachi S, Akiyama H, Yokoi K, Hatara MC, Lauer T, Aslam S, Gong YY, Xiao WH, Khu NH, Thut C, Campochiaro PA. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21:3219–3230. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- 23.Tysseling VM, Mithal D, Sahni V, Birch D, Jung H, Belmadani A, Miller RJ, Kessler JA. SDF1 in the dorsal corticospinal tract promotes CXCR4+ cell migration after spinal cord injury. J Neuroinflammation. 2011;8:16. doi: 10.1186/1742-2094-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thom SR, Bhopale V, Fisher D, Manevich Y, Huang PL, Buerk DG. Stimulation of nitric oxide synthase in cerebral cortex due to elevated partial pressures of oxygen: an oxidative stress response. J Neurobiol. 2002;51:85–100. doi: 10.1002/neu.10044. [DOI] [PubMed] [Google Scholar]
- 25.Thom SR, Fisher D, Zhang J, Bhopale VM, Ohnishi ST, Kotake Y, Ohnishi T, Buerk DG. Stimulation of perivascular nitric oxide synthesis by oxygen. Am J Physiol Heart Circ Physiol. 2003;284:H1230–1239. doi: 10.1152/ajpheart.01043.2002. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein LJ, Gallagher KA, Bauer SM, Bauer RJ, Baireddy V, Liu ZJ, Buerk DG, Thom SR, Velazquez OC. Endothelial progenitor cell release into circulation is triggered by hyperoxia-induced increases in bone marrow nitric oxide. Stem Cells. 2006;24:2309–2318. doi: 10.1634/stemcells.2006-0010. [DOI] [PubMed] [Google Scholar]
- 27.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Salvucci O, Basik M, Yao L, Bianchi R, Tosato G. Evidence for the involvement of SDF-1 and CXCR4 in the disruption of endothelial cell-branching morphogenesis and angiogenesis by TNF-alpha and IFN-gamma. J Leukoc Biol. 2004;76:217–226. doi: 10.1189/jlb.1203609. [DOI] [PubMed] [Google Scholar]
- 29.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]


