Abstract
Thioredoxin-1 (Trx-1), an important redox regulatory factor, plays a significant role in drug-induced apoptosis. Here we investigated the effects of the Trx-1 inhibitor 1-methylpropyl 2-imidazolyl disulfide (PX-12) on human acute myeloid leukemia cells (AML) and the sensitivity of cells to arsenic trioxide (As2O3, ATO). Treatment of cells with a different concentration of PX-12 for 48 h resulted in growth inhibition, the induction of apoptosis and increased the levels of activated caspase-3 expression in AML cell lines HL-60, NB4, U937 and primary AML cells in a dose-dependent manner. In addition, PX-12 enhanced the sensitivity of U937 cells to ATO. These results suggest the effects of Trx-1 inhibitor PX-12 to induce apoptosis in AML cells and therapeutic potential in AML by enhancing the sensitivity of cells to ATO.
Keywords: PX-12, acute myeloid leukemia, apoptosis, arsenic trioxide
Introduction
Acute myeloid leukemia (AML) is a clonal disorder characterized by a disruption in normal hematopoietic differentiation and the accumulation of abnormal, immature myeloid cells in the bone marrow, resulting in hematopoietic failure [1]. AML is the most common acute leukemia affecting adults and its incidence rises dramatically with age. Despite recent advancements, treatment of AML remains unsatisfactory [2]. Arsenic trioxide (As2O3, ATO), an ancient traditional Chinese medicine, has been reported to be an effective therapeutic agent for acute promyelocytic leukemia (APL) [3,4]. ATO induces apoptosis and partial differentiation in a variety of leukemia cells, suggesting that it may be effective against other hematologic malignancies [5]. However, ATO used as a single agent at higher concentrations causes many side effects, cytotoxicity and drug resistance are major concern [6,7]. More recently it has been shown to be effective, particularly in combination with other drugs in the treatment of leukemia [7-9].
The thioredoxin (Trx) system is one of the major cellular antioxidant system integral to maintaining the intracellular redox state [10]. Trx-1 which is principally localized in the cytoplasm and Trx-2 which is mainly localized in the mitochondria, suggests their specific effects in different cellular compartments [11]. Trx-1, a 12-kDa ubiquitous protein that has disulfide-reducing activity and protects cells against oxidant damage, is one member of the Trx system. Accumulating evidence indicates that Trx-1 plays an important role in tumor progression and metastasis. Elevation of Trx-1 expression increases cancer cell proliferation, inhibites apoptosis and aggressive tumor growth. Therefore, Trx-1 inhibitors have been regarded as potential anticancer drugs. PX-12 which has antitumor effects is an irreversible inhibitor of Trx-1 [12]. PX-12 particularly reduces the activity of Trx-1 by means of thio-alkylating critical cysteine residue (Cys73) which is located in the outside the conserved redox catalytic site of Trx-1 [13,14]. Currently, PX-12 is considered as an effective antitumor agent in clinical development. PX-12 has been tested in a phase I trial in patients with terminal malignant tumor [15]. PX-12 also reduces vascular endothelial growth factor (VEGF) and hypoxiainducible factor-1α transactivation (HIF-1α) which may be conducive to its antitumor activity [16,17]. However, few investigations have been done to evaluate the effects of PX-12 on human AML cells.
In the present study, we used human acute myeloid leukemia (AML) cell lines (NB4, HL-60 and U937) and primary AML cells to investigate the effect of the Trx-1 inhibitor PX-12 on human AML cells and the combined effect of PX-12 and ATO against AML. Our study may offer a new strategy for treating AML and combinative drug therapy.
Materials and methods
Cell lines and primary leukemic cells
Human AML cell lines (NB4, HL-60 and U937) and primary AML cells were employed for the present study. All cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (Invitrogen, Groud Island, USA) in humidified 37°C incubator with 5% CO2. Primary AML cells were obtained from 5 untreated patients with AML (1 M2, 2 M3, 1 M4 and 1 M5, the diagnosis was established according to the French-America-British classification) after informed consent. Mononuclear cells were isolated from bone marrow aspirates by Ficoll density gradient centrifugation (GE Healthcare, Uppsala, Sweden) [18].
Cytotoxicity assay
To investigate the cell viability, AML cells (HL-60, NB4, U937 and Primary AML cells) were seeded in 96-well plates at 5 × 103 cells per well. After 24 h, the cells were then treated with various concentrations of PX-12, a selective inhibitor of Trx-1 (SigmaAldrich, St Louis, MO, USA) for 48 h, and cell viabilities were assessed by MethylthiazolTetrazolium (MTT) test. Briefly, 20 μL MTT solution (5 mg/mL) was added to the culture plates and incubated for 4 h. At the end of the incubation period, the culture supernatant was moved and 100 μL DMSO was used to completely dissolve the crystals. The absorbance (OD) of each well was measured on an ELISA reader (ELx800; Bio-Tek Instruments, Winooski, VT, USA) at a wavelength of 490 nm. Growth inhibitory rate (%) = (OD value in the control group - OD value in the treatment group)/OD value in the control group × 100%.
Apoptosis assay
The cells were seeded in 6-well plates at density of 4 × 105 cells per well and treated with various concentrations of PX-12 for 48 h. Apoptosis was assessed by Annexin V-FITC Apoptosis Detection Kit II (BD Pharmingen™, San Diego, CA, USA) according to the manufacturer’s protocols. CellQuest software was used for data acquisition and analysis. Cells that were Annexin V negative and PI negative are considered viable, Annexin V positive and PI negative cells are considered early apoptosis, and Annexin V positive and PI positive cells are considered end stage apoptosis and death.
Detection of activated caspase-3 by flow cytometer
For detection of activated caspase-3, cells were treated with various concentration of PX-12 for 48 h, and then permeabilized, fixed, and stained for active caspase-3 PE (BD Pharmingen™) according the staining protocol. Cells were then analyzed by flow cytometer.
Western blotting
Western blot analysis was used for the detection of Trx-1 and actin proteins according to previously published protocols [19]. Briefly, Cells were harvested and washed twice with PBS. Cells lysates were prepared, separated by 15% SDS-PAGE and transferred to PVDF membranes. The membranes were block in 5% non-fat milk solution for 1 h, and probed with primary antibodies overnight at 4°C. Then, the membranes were incubated 1 h at room temperature with the horseradish peroxidase-conjugated secondary antibodies, and visualized using the enhanced chemiluminescence substrate kit (Amersham Biosciences, Inc.) according to the manufacturer’s instructions.
Statistical analysis
Data were presented as mean ± SD and statistical analysis was performed using student’s t-test (SPSS, USA). A P value of less than 0.05 was considered statistically significant.
Results
PX-12 inhibited growth of human AML cells
To evaluate the effect of PX-12 on AML cell growth, AML cell lines HL-60, NB4, U937 and primary AML cells were treated with PX-12 at the concentration of 0, 1, 3, 5, 7, 9 μM for 48 h. As shown in Figure 1, PX-12, a Trx-1 inhibitor, inhibited the proliferation of AML cell lines HL-60, NB4, and U937 in a dose-dependent manner. Also, PX-12 inhibited the proliferation of primary AML cells.
Figure 1.

The Trx-1 inhibitor PX-12 inhibits growth of human AML cells. AML cell lines (NB4, HL-60 and U937) and primary AML cells were treated with various concentrations of PX-12. Inhibition rates were assessed by MTT assay at 48 h. All experiments were repeated three times with similar results.
PX-12 induced apoptosis in AML cells
AML cell lines HL-60, NB4, U937 and primary AML cells were treated with various concentrations PX-12 (0, 1, 3, 5, 7, 9 μM) for 48 h, and cell apoptosis was assessed by Annexin V-FITC/PI double staining. PX-12 induced cell apoptosis in AML cell lines as well as primary AML cells in a dose-dependent manner (Figure 2). And the maximal apoptosis rates were 79.26% in NB4 cells, 42.03% in HL-60 cells, 76.58% in U937 and 50.3% in primary AML cells at the concentration of 9 μM PX-12, respectively (Figure 2B).
Figure 2.
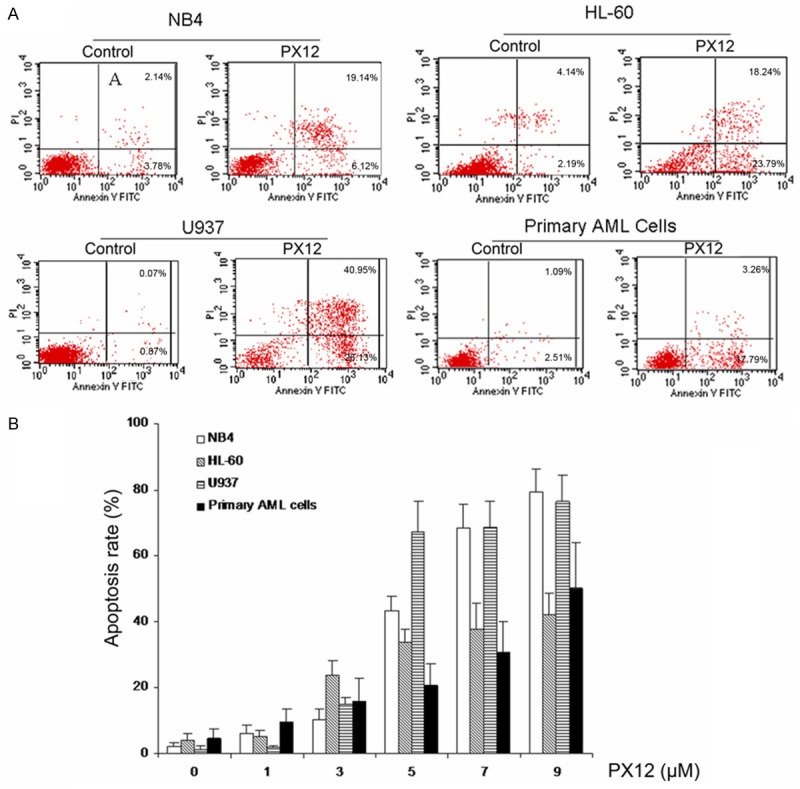
PX-12 induces apoptosis in AML cells. AML cells were treated for 48 h with various concentrations of PX-12. Cells apoptosis was detected using the Annexin V-FITC apoptosis detection kit, the percentages of annexin V positive apoptotic cells were determined by flow cytometer. A. Representatives were shown in AML cells treated by 5 μM PX-12 for 48 h. B. After treatment with indicated concentrations of PX-12 for 48 h, cells apoptosis was detected using the Annexin V-FITC apoptosis detection kit. Each value represented the mean ± SD of three independent experiments.
PX-12 induced activation of caspase-3 in AML cells
Activation of caspase-3 is a key event in apoptosis. We investigated the effect of PX-12 on the expression of activated caspase-3 by flow cytometer. PX-12 strongly increased the levels of activated caspase-3 expression in AML cell lines (HL-60, NB4 and U937) and primary AML cells at the concentration of 5 μM for 48 h (Figure 3A and 3B).
Figure 3.
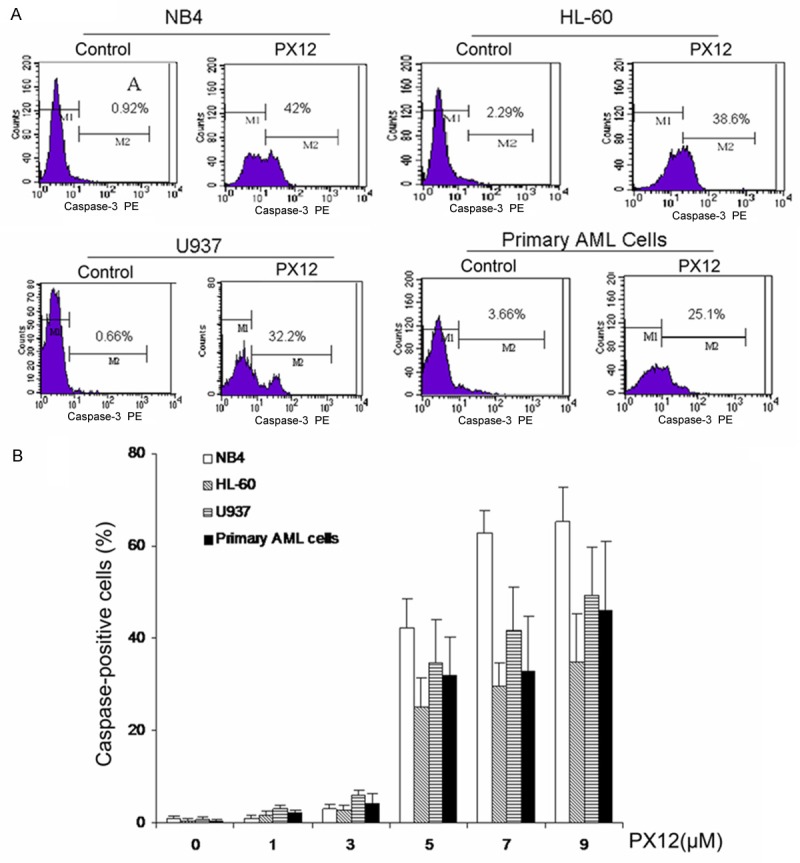
Effects of PX-12 on level of caspase-3 expression in AML cells. A. Representatives were shown in AML cells treated by 5 μM PX-12 for 48 h. B. AML cells were treated with indicated concentration of PX-12 for 48 h, the level of caspase-3 expression was detected by flow cytometer.
Thioredoxin-1 inhibitor PX-12 enhances the sensitivity of cells to arsenic trioxide
To investigate whether the Trx-1 involved in the sensitivity of cells to ATO, NB4 and U937 cells were treated with 5 μM ATO for 48 h, the inhibition was determined by MTT assay and the level of Trx-1 expression was detected by western blotting. Our results showed that NB4 cells were more sensitive than U937 cells (Figure 4A), this result was consistent with previously report [20]. ATO inhibited Trx-1 protein expression in NB4 cells but not in U937 cells (Figure 4B). PX-12, a Trx-1 inhibitor, enhanced growth inhibitory and apoptotic effects of ATO in U937 cells (Figure 5A-C). These results indicated that the Trx-1 inhibitor PX-12 could enhance the sensitivity of cells to ATO.
Figure 4.

Comparison of cell growth inhibition and Trx-1 expression induction by ATO in NB4 versus U937 cells. A. NB4 and U937 cells were treated with 5 μM ATO for 48 h, and the inhibition was determined by MTT assay. B. The level of Trx-1 expression was detected by western blotting. Cells were treated with ATO for 48 h.
Figure 5.

PX-12 enhances the sensitivity of U937 cells to ATO. A. Combination treatment with ATO and PX-12 enhances U937 cell growth inhibition. U937 cells were treated with 5 μM ATO together with 1 μM PX-12, a selective inhibitor of Trx-1, cell growth inhibition was detected by MTT assay. B. PX-12 enhances the apoptosis of U937 cell induced by ATO. C. Quantitation of apoptosis in U937 cell. U937 cells were treated with 5 μM ATO together with 1 μM PX-12, a selective inhibitor of Trx-1, the percentage of cells in apoptosis were measured using the Annexin V-FITC apoptosis detection kit by flow cytometer. *P < 0.05.
Discussion
Leukemia, a kind of malignant clones that originates in hematopoietic stem cell disorder, is often characterized by clinical symptoms such as anemia, bleeding, infection and infiltration, which is seriously threatening human survival and health. AML accounts for 70% of all acute leukemia, its morbidity increases with age [21]. In recent years, the diagnostic and operative technology is improving. However, AML is still one of the most difficult human malignant tumor treatments, which is prevailingly due to the advanced cancer and new resistance behavior to cytotoxic chemotherapy drugs and radiotherapy. In addition, apoptosis deficiency is regarded as the most common cause in the chemotherapy resistance, because a lot of chemotherapy agents act by means of the induction of apoptosis [22]. Despite a lot of the efforts in the treatment and detection, many patients with AML still died in this cancer, suggesting that further researches are urgently needed to develop new strategies to improve the therapeutic effect of AML [23-25].
Trx-1 is over-expressed in a number of human cancer, such as gastric, lung, cervical, colon, and pancreatic cancer [26]. Clinically, overexpression of Trx-1 causes aggressive tumor growth, inhibits apoptosis, and decreases patient survival [27]. PX-12, the first Trx-1 inhibitor, reduces Trx-1 and VEGF levels in the plasma of cancer patients [14]. Several studies showed that PX-12 inhibited the growth of cancer, such as lung, pancreatic cancer and induce HeLa cell death [28-30]. PX-12 also has conducted clinical practice in solid cancer patients and has completed a Phase I clinical trial [15]. On the basis of pharmacokinetic and pharmacodynamic data, a series of long-term infusion schedule of PX-12 has been started [31]. However, the effects of PX-12 on acute myeloid leukemia (AML) are still unknown. In this study, we used human AML cell lines and primary AML cells to investigate the effect of the Trx-1 inhibitor PX-12 on AML cells. Cytotoxicity assay revealed that PX-12, a Trx-1 inhibitor, inhibited the proliferation of AML cell lines and primary AML cells. PX-12 also induced cell apoptosis and increased the levels of activated caspase-3 expression in AML cell lines as well as primary AML cells in a dose-dependent manner. It is well-known that apoptosis is modulated through the extrinsic and intrinsic pathways, and both result in the activation of caspases [32] among which caspase-3 is the crucial executioners of apoptosis [33], as it takes the partial or total responsibility for cleaving many key protein including the nuclear enzyme (ADP ribose) and polymerase (PARP).
ATO, a traditional Chinese medicine, is the main effective components of arsenic. Currently, ATO has shown strong antitumor effects on APL cells in vitro and in vivo [34], and has been assessed in clinical studies for the treatment of AML [35]. More interestingly, accumulating evidence suggests that ATO has a fine therapeutic effect in a certain variety of solid tumors [36-38]. Although the definite mechanisms of antitumor effect are not fully understood, ATO has been regarded as a strong inducer of oxidative stress in cancer cells [39,40]. At present, apoptosis inhibition is one of the most principal mechanisms of leukemic cells drug resistance. Research has shown that ATO induced apoptosis of leukemia with multidrug resistant cell and regulated the expression of apoptosis related genes with multiple resistance [41,42]. However, ATO which is highly poisonous to the cells of our bodies can emerge toxicity of cardiovascular system and cause hyperleukocy. Tian C et al reported that inhibition of TRX1 expression by RNAi sensitized HepG2 cells to ATO-induced apoptosis and over-expression of Trx-1 in HepG2 cells resulted in the inhibition of ATO-induced cytochrome c (cyto c) release, caspase activation and apoptosis [43]. In this study, we found that the expression level of Trx-1 in AML cells was important for ATO sensitivity. ATO treatment decreased Trx-1 protein expression in sensitive NB4 cells but not in U937 cells. Inhibition of Trx-1 with PX-12 sensitizes U937 cells towards ATO induced growth inhibitory and apoptosis. These results demonstrated that PX-12 could enhance growth inhibitory and apoptotic effects of ATO in U937 cells.
In summary, our results showed that the Trx-1 inhibitor PX-12 could inhibit AML cell growth and induce cell apoptosis. Furthermore, Trx-1 inhibitor PX-12 enhances the sensitivity of cells to ATO. PX-12 may be a possible potential adjuvant drug in treatment of human AML. The current study provides some theoretical basis for its clinical application value of further study. However, additional preclinical research is needed to further determine the antitumor activity of PX-12 in AML in vivo.
Acknowledgements
This work was supported by The National Natural Science Foundation of China (81101823), Medicine and Health Technology Program of Zhejiang Province (2013KYA129) and Wenzhou Science and Technology Bureau Program (Y20120181).
Disclosure of conflict of interest
None.
References
- 1.Pollyea DA, Kohrt HE, Medeiros BC. Acute myeloid leukaemia in the elderly: a review. Br J Haematol. 2011;152:524–542. doi: 10.1111/j.1365-2141.2010.08470.x. [DOI] [PubMed] [Google Scholar]
- 2.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Lowenberg B, Bloomfield CD, European L. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 3.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 4.Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP Jr. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 5.Slack JL, Waxman S, Tricot G, Tallman MS, Bloomfield CD. Advances in the management of acute promyelocytic leukemia and other hematologic malignancies with arsenic trioxide. Oncologist. 2002;7(Suppl 1):1–13. doi: 10.1634/theoncologist.7-suppl_1-1. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Liu YF, Wu CF, Xu F, Shen ZX, Zhu YM, Li JM, Tang W, Zhao WL, Wu W, Sun HP, Chen QS, Chen B, Zhou GB, Zelent A, Waxman S, Wang ZY, Chen SJ, Chen Z. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2009;106:3342–3347. doi: 10.1073/pnas.0813280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 8.Li Y, Zhu X, Gu J, Dong D, Yao J, Lin C, Huang K, Fei J. Anti-miR-21 oligonucleotide sensitizes leukemic K562 cells to arsenic trioxide by inducing apoptosis. Cancer Sci. 2010;101:948–954. doi: 10.1111/j.1349-7006.2010.01489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao SM, Chen C, Wu J, Tan Y, Yu K, Xing CY, Ye A, Yin L, Jiang L. Synergistic apoptosis induction in leukemic cells by miR-15a/16-1 and arsenic trioxide. Biochem Biophys Res Commun. 2010;403:203–208. doi: 10.1016/j.bbrc.2010.10.137. [DOI] [PubMed] [Google Scholar]
- 10.Tonissen KF, Di Trapani G. Thioredoxin system inhibitors as mediators of apoptosis for cancer therapy. Mol Nutr Food Res. 2009;53:87–103. doi: 10.1002/mnfr.200700492. [DOI] [PubMed] [Google Scholar]
- 11.Yoshihara E, Masaki S, Matsuo Y, Chen Z, Tian H, Yodoi J. Thioredoxin/Txnip: Redoxisome, as a Redox Switch for the Pathogenesis of Diseases. Front Immunol. 2014;4:514. doi: 10.3389/fimmu.2013.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wondrak GT. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxid Redox Signal. 2009;11:3013–3069. doi: 10.1089/ars.2009.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan BF, Runquist M, Raghunand N, Gillies RJ, Tate WR, Powis G, Baker AF. The thioredoxin-1 inhibitor 1-methylpropyl 2-imidazolyl disulfide (PX-12) decreases vascular permeability in tumor xenografts monitored by dynamic contrast enhanced magnetic resonance imaging. Clin Cancer Res. 2005;11:529–536. [PubMed] [Google Scholar]
- 14.Baker AF, Dragovich T, Tate WR, Ramanathan RK, Roe D, Hsu CH, Kirkpatrick DL, Powis G. The antitumor thioredoxin-1 inhibitor PX-12 (1-methylpropyl 2-imidazolyl disulfide) decreases thioredoxin-1 and VEGF levels in cancer patient plasma. J Lab Clin Med. 2006;147:83–90. doi: 10.1016/j.lab.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkpatrick L, Dragovich T, Ramanathan R, Sharlow E, Chow S, Williams D, Himler R, Baker A, Egorin M. Results from Phase I study of PX-12, a thioredoxin inhibitor in patients with advanced solid malignancies. J. Clin. Oncol. 2004;22:3089. [Google Scholar]
- 16.Welsh SJ, Williams RR, Birmingham A, Newman DJ, Kirkpatrick DL, Powis G. The Thioredoxin Redox Inhibitors 1-Methylpropyl 2-Imidazolyl Disulfide and Pleurotin Inhibit Hypoxia-induced Factor 1α and Vascular Endothelial Growth Factor Formation 1. Mol Cancer Ther. 2003;2:235–243. [PubMed] [Google Scholar]
- 17.Mukherjee A, Martin SG. The thioredoxin system: a key target in tumour and endothelial cells. Br J Radiol. 2008;81:S57–68. doi: 10.1259/bjr/34180435. [DOI] [PubMed] [Google Scholar]
- 18.Gao SM, Yang J, Chen C, Zhang S, Xing CY, Li H, Wu J, Jiang L. miR-15a/16-1 enhances retinoic acid-mediated differentiation of leukemic cells and is up-regulated by retinoic acid. Leuk Lymphoma. 2011;52:2365–2371. doi: 10.3109/10428194.2011.601476. [DOI] [PubMed] [Google Scholar]
- 19.Jiang L, Chen Y, Chan CY, Wang X, Lin L, He ML, Lin MC, Yew DT, Sung JJ, Li JC, Kung HF. Down-regulation of stathmin is required for TGF-beta inducible early gene 1 induced growth inhibition of pancreatic cancer cells. Cancer Lett. 2009;274:101–108. doi: 10.1016/j.canlet.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- 21.Thein MS, Ershler WB, Jemal A, Yates JW, Baer MR. Outcome of older patients with acute myeloid leukemia. Cancer. 2013;119:2720–2727. doi: 10.1002/cncr.28129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 23.Smits EL, Berneman ZN, Van Tendeloo VF. Immunotherapy of acute myeloid leukemia: current approaches. Oncologist. 2009;14:240–252. doi: 10.1634/theoncologist.2008-0165. [DOI] [PubMed] [Google Scholar]
- 24.Szer J. The prevalent predicament of relapsed acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2012;2012:43–48. doi: 10.1182/asheducation-2012.1.43. [DOI] [PubMed] [Google Scholar]
- 25.Kupsa T, Horacek JM, Jebavy L. The role of cytokines in acute myeloid leukemia: a systematic review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2012;156:291–301. doi: 10.5507/bp.2012.108. [DOI] [PubMed] [Google Scholar]
- 26.Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Biophys Biomol Struct. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- 27.Raffel J, Bhattacharyya AK, Gallegos A, Cui H, Einspahr JG, Alberts DS, Powis G. Increased expression of thioredoxin-1 in human colorectal cancer is associated with decreased patient survival. J Lab Clin Med. 2003;142:46–51. doi: 10.1016/S0022-2143(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 28.You BR, Shin HR, Park WH. PX-12 inhibits the growth of A549 lung cancer cells via G2/M phase arrest and ROS-dependent apoptosis. Int J Oncol. 2014;44:301–308. doi: 10.3892/ijo.2013.2152. [DOI] [PubMed] [Google Scholar]
- 29.Ramanathan RK, Abbruzzese J, Dragovich T, Kirkpatrick L, Guillen JM, Baker AF, Pestano LA, Green S, Von Hoff DD. A randomized phase II study of PX-12, an inhibitor of thioredoxin in patients with advanced cancer of the pancreas following progression after a gemcitabine-containing combination. Cancer Chemother Pharmacol. 2011;67:503–509. doi: 10.1007/s00280-010-1343-8. [DOI] [PubMed] [Google Scholar]
- 30.Shin HR, You BR, Park WH. PX-12-induced HeLa cell death is associated with oxidative stress and GSH depletion. Oncol Lett. 2013;6:1804–1810. doi: 10.3892/ol.2013.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramanathan RK, Kirkpatrick DL, Belani CP, Friedland D, Green SB, Chow HS, Cordova CA, Stratton SP, Sharlow ER, Baker A. A Phase I pharmacokinetic and pharmacodynamic study of PX-12, a novel inhibitor of thioredoxin-1, in patients with advanced solid tumors. Clin Cancer Res. 2007;13:2109–2114. doi: 10.1158/1078-0432.CCR-06-2250. [DOI] [PubMed] [Google Scholar]
- 32.Lin TH, Lu FJ, Yin YF, Tseng TH. Enhancement of esculetin on arsenic trioxide-provoked apoptosis in human leukemia U937 cells. Chem Biol Interact. 2009;180:61–68. doi: 10.1016/j.cbi.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Cohen G. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachleitner-Hofmann T, Kees M, Gisslinger H. Arsenic trioxide: acute promyelocytic leukemia and beyond. Leuk Lymphoma. 2002;43:1535–1540. doi: 10.1080/1042819021000002857. [DOI] [PubMed] [Google Scholar]
- 35.Berenson JR, Yeh HS. Arsenic compounds in the treatment of multiple myeloma: a new role for a historical remedy. Clin Lymphoma Myeloma. 2006;7:192–198. doi: 10.3816/CLM.2006.n.058. [DOI] [PubMed] [Google Scholar]
- 36.Nakagawa Y, Akao Y, Morikawa H, Hirata I, Katsu K, Naoe T, Ohishi N, Yagi K. Arsenic trioxide-induced apoptosis through oxidative stress in cells of colon cancer cell lines. Life Sci. 2002;70:2253–2269. doi: 10.1016/s0024-3205(01)01545-4. [DOI] [PubMed] [Google Scholar]
- 37.Oketani M, Kohara K, Tuvdendorj D, Ishitsuka K, Komorizono Y, Ishibashi K, Arima T. Inhibition by arsenic trioxide of human hepatoma cell growth. Cancer Lett. 2002;183:147–153. doi: 10.1016/s0304-3835(01)00800-x. [DOI] [PubMed] [Google Scholar]
- 38.Shao QS, Ye ZY, Ling ZQ, Ke JJ. Cell cycle arrest and apoptotic cell death in cultured human gastric carcinoma cells mediated by arsenic trioxide. World J Gastroenterol. 2005;11:3451–6. doi: 10.3748/wjg.v11.i22.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 40.Miller WH, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- 41.Wang DH, Wei HL, Zhao HS, Hao CY, Min ZH, Liu JM. Arsenic trioxide overcomes apoptosis inhibition in K562/ADM cells by regulating vital components in apoptotic pathway. Pharmacol Res. 2005;52:376–385. doi: 10.1016/j.phrs.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Wei H, Su H, Bai D, Zhao H, Ge J, Wang B, Yao X, Ma L. Arsenic trioxide inhibits p-glycoprotein expression in multidrug-resistant human leukemia cells that overexpress the MDR1 gene. Chin Med J (Engl) 2003;116:1644–1648. [PubMed] [Google Scholar]
- 43.Tian C, Gao P, Zheng Y, Yue W, Wang X, Jin H, Chen Q. Redox status of thioredoxin-1 (TRX1) determines the sensitivity of human liver carcinoma cells (HepG2) to arsenic trioxide-induced cell death. Cell Res. 2008;18:458–471. doi: 10.1038/cr.2007.112. [DOI] [PubMed] [Google Scholar]


