Abstract
Background/purpose: SEMA3A (semaphorin-3A), is a secreted protein that belongs to the semaphorin family and can function as both a chemoattractive agent or a chemorepulsive agent. SEMA3A has been shown to be a tumor suppressor in various cancers. This study investigated the expression of SEMA3A in gastric cancer and its prognostic value for gastric cancer patients. Methods: We examined the expression of SEMA3A in paired cancerous and matched adjacent noncancerous gastric mucosa tissues by real-time quantitative RT-PCR (qRT-PCR) and western blotting. In vitro, we evaluate the effects of SEMA3A on gastric cancer cell proliferation and migration by MTT, transwell and wound-healing assays. Furthermore, we analyzed SEMA3A expression in 128 patients who underwent resection procedures using immunohistochemistry. The relationships between the SEMA3A expression levels, the clinicopathological factors, and patient survival were investigated. Results: Our results revealed decreased SEMA3A mRNA (P = 0.0037) and protein (P = 0.033) expression in tumor tissue samples compared with matched adjacent non-tumorous tissue samples. Overexpression of SEMA3A inhibits gastric cancer cell proliferation and migration in vitro. Immunohistochemical staining data showed that SEMA3A expression was significantly decreased in 54.68% of gastric cancer cases. In addition, the chi-square test revealed that low SEMA3A expression was significantly correlated with poor differentiation (P = 0.015), Vascular invasion (P = 0.001), depth of invasion (P < 0.001), lymph node metastasis (P = 0.029), distant metastasis (P = 0.002) and advanced TNM stage (P = 0.003). SEMA3A expression was positively correlated with clinical TNM stage, that suggested the more advanced clinical TNM stage corresponding to the lower expression level of SEMA3A (rs = -0.322, P < 0.001) by Spearman rank correlation analysis. Kaplan-Meier survival analysis demonstrated that low expression of SEMA3A was significantly correlated with a poor prognosis for gastric cancer patients (P < 0.001). The multivariate analysis revealed that SEMA3A expression was an independent prognostic factor of the overall survival rate of patients with gastric cancer. Conclusion: SEMA3A expression decreased significantly as gastric cancer progressed and metastasized, suggesting that SEMA3A might serve as a candidate tumor suppressor and a potential prognostic biomarker in gastric carcinogenesis.
Keywords: SEMA3A, gastric cancer, prognosis
Introduction
Gastric cancer is the second leading cause of cancer-related death in the world and tumor metastasis is the biggest obstacle to its successful treatment and the major cause of patient mortality [1,2]. In China, gastric cancer is regarded as the second most frequently diagnosed cause of cancer death [3]. Despite great advancements in diagnosis and treatment modalities for this disease, especially surgery, chemotherapy, and radiotherapy, its survival rate remains very low [4]. Accordingly, there is great demand to further find new clinically applicable molecular targets for the diagnosis and treatment of this disease. The incidence, development, invasion, and metastasis of GC are a multi-step and multi-factor complex process. It may be regulated by many genes and involves a variety of gene activation, regulated disorder, or inactivation [5]. Therefore novel well-characterized biomarkers would be helpful for clinicians to predict metastatic progression and prognosis of gastric cancer patients for facilitation of therapeutic intervention.
Semaphorins, also known as collapsins, were first identified as a family of genes encoding guidance molecules for the embryologic development of the nervous system and were described as negative mediators of axonal guidance in the central nervous system [6]. The semaphorin family comprises soluble and membrane bound proteins that function during neuronal development, organogenesis, angiogenesis, and cancer progression [7,8]. Over the past decade, the role of SEMA3s in the pathogenesis of multiple malignancies has also been investigated in preclinical studies. SEMA3s comprise one of five vertebrate families of semaphorins and are known to play an important role in tumor biology [9]. The SEMA3 class consists of seven soluble proteins of ~100 kDa (designated by the letters A-G), which are secreted by cells of multiple lineages, including epithelial cells, neurons, and specific tumor cells. SEMA3s act in a paracrine fashion by binding to neuropilins via a highly conserved amino-terminal 500-amino acid region in the SEMA3 protein called the Sema domain [10]. What’s more, semaphorin 3 (Sema3) family are involved in suppression of tumor progression and have been considered as potent tumor suppressors [11]. For example, SEMA3B and SEMA3F have been shown to regulate tumor angiogenesis, growth and metastasis in different manners [12,13]. It is becoming increasingly evident that sema3A as well as other class 3 semaphorins play an important role in cancer [14]. It was shown that tumor-derived SEMA3A negatively modulates T-cell functions by inhibiting T-cell receptor (TCR)-mediated proliferation and cytokine production [15], and SEMA3A inhibits platelet aggregation, allowing speculation that, by keeping platelets in the resting state, endothelial-derived SEMA3A may contribute to maintaining blood flow in newly synthesized vessels [16]. Also, SEMA3A suppresses the adhesion and migration of endothelial cells [17], and induces the collapse of the actin cytoskeleton, promotes apoptosis and inhibits angiogenesis in vitro [18]. Furthermore, SEMA3A can inhibit angiogenesis in vivo and induce microvascular permeability [19].
Beyond all that, previous studies showed that SEMA3A has been implicated in the inhibition of tumor cell migration and chemotaxis in breast cancer cells [10]. In prostate cancer, SEMA3A-transfected cells differentially regulate adhesion of cells together and have been shown to exhibit decreased invasion and adhesion [20]. While high expression of SEMA3A seems to correlate with poor clinical outcome in pancreatic cancer [21]. However, to our knowledge, the role of sema3A in gastric cancer has not been studied extensively and their effects in gastric cancer are not known. Therefore, in the present study, we aimed to analyze the SEMA3A expression level in gastric cancer using real-time quantitative PCR, western blotting and immunohistochemistry. Furthermore, we identified the relationship between SEMA3A expression and the clinicopathological features of the disease and evaluated its prognostic value for survival of gastric cancer patients. Our study might be useful to develop more rational SEMA3A-mediated therapeutic strategy for the next generation of cancer management.
Materials and methods
Patients and tumor tissue samples
From January 2006 to December 2008, clinicopathological data from 128 gastric cancer patients who underwent surgical resection at the Second Affiliated Hospital of Nantong University were retrospectively analyzed. Fresh gastric cancer and surrounding non-tumor tissue samples were randomly obtained from 40 gastric cancer patients who underwent surgical resection at the Second Affiliated Hospital of Nantong University between 2012 and 2013. Both the tumor tissues and the surrounding non-tumor tissues, which were located more than 3 cm away from the gastric cancer, were sampled and then verified by pathological examination. After surgical resection, fresh samples were frozen in liquid nitrogen immediately and divided into two parts, one was maintained at -80°C until use for real-time PCR, another use for Western blot analysis. Paraffin-embedded samples were obtained from 128 gastric cancer patients who underwent surgical resection at Nantong First People’s Hospital. None of these patients had received radiotherapy or chemotherapy prior to surgery. The clinical information related to the 128 gastric cancer patients, including gender, age, tumor size, TNM stage, lymph node involvement etc. was also collected. The histopathological type and stage of the gastric cancer were determined according to the criteria of the World Health Organization classification and the TNM stage set out by the Union for International Cancer Control. The presence or absence of distant metastasis was determined through radiological examination. An additional 8 normal gastric mucosal tissues were obtained from individuals who underwent endoscopy for asymptomatic Barrett’s esophagus surveillance and exhibited no abnormalities in the stomach. The follow-up data from the gastric cancer patients in this study were available and complete. Overall survival, which was defined as the time from the operation to the time of patient death or the last follow-up, was used as a measure of prognosis. The research was conducted with the approval of the institutional ethics board of our institute, and written informed consent was obtained from each patient involved in the study.
Cell culture and transfection
Human gastric cancer cell lines SGC7901, AGS and MKN45 were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). All cell lines were maintained in RPMI-1640 medium (GIBCO, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA) in a humidified incubator with a mixture of 5% CO2 at 37°C. For overexpression of endogenous SEMA3A, the coding sequence of SEMA3A was amplified and subcloned into the pcDNA3.1 (+) vector (Invitrogen, Carlsbad, CA, USA) according to the manufacturer instructions. AGS cells were then transfected with a negative control vector or a SEMA3A expressing plasmid using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
Real-time quantitative PCR
Total RNA was extracted from tissues lysate using a Trizol kit (Invitrogen, Carlsbad, CA), and cDNA was subsequently synthesized from total RNA using an Omniscript RT kit (Qiagen, Valencia, CA) following the supplier’s instructions. For detecting the mRNA level of SEMA3A, quantitative real-time RT-PCR was conducted on the Mastercycler Ep Realplex (Eppendorf 2 S, Hamburg, Germany). A 25 μl reaction mixture contained 1 μl of cDNA from samples, 12.5 μl of 2× Fast EvaGreenTM qPCR Master Mix, 1 μl primers (10 mM), and 10.5 μl of RNase/DNase-free water. PCR procedures: incubation at 96°C for 2 min, 40 cycles at 96°C for 15 s and 60°C for 1 min. The Ct value was defined as the cycle number at which the fluorescence intensity reached a certain threshold where amplification of each target gene was within the linear region of the reaction amplification curves. Relative expression level for each target gene was normalized by the Ct value of GAPDH (internal control) using a 2-ΔΔCt relative quantification method. The sequences of the primers for SEMA3A as follows: Sema3A forward: 5’-CAG CCA TGT ACA ACC CAG TG-3’; Sema3A reverse: 5’-ACG GTT CCA ACA TCT GTT CC-3’. The glyceraldehyde-3’phosphate dehydrogenase (GAPDH) gene served as an internal control.
Western blot analysis
Paired tumor tissues and the surrounding non-tumor tissues were treated with lysis buffer containing protease inhibitors (Promega, Madison, WI). After centrifugation at 12,000 rpm for 20 min, the supernatant was collected for determination of total protein concentration by DC-protein assay method (Bio-Rad) to maintain the same loads. Protein samples were electrophoretically separated on a 10% SDS-polyacrilamide gel (PAGE), and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with 5% non-fat dry milk in TBST buffer (50 mm Tris-HCl, 100 mm NaCl, and 0.1% Tween-20, pH 7.4) and incubated with a polyclonal goat anti-human SEMA3A antibody (1:500, Santa Cruz Biotechnology, Inc, USA) at 4°C overnight. The membranes were washed three times with TBST buffer for 5 minutes, and further incubated with secondary antibody, anti-goat IgG conjugated IRDye800 (1:5000, Rockland Gilbertsville, CA) at room temperature for 2 h, followed by scanning with an Odyssey infrared imaging system (LI-COR, Lincoln, NE), and analyzed with PDQuest 7.2.0 software (Bio-Rad).
Immunohistochemistry
Tissues were de-waxed in xylene, rehydrated in alcohol. After three washes in PBS (phosphate-buffered saline), the slides were boiled in antigen retrieval buffer containing 0.01 M sodium citrate-hydrochloric acid (pH = 6.0) for 15 min in a microwave oven. After rinsing with PBS, the tissue sections were incubated with polyclonal goat anti-human SEMA3A antibody (1:100, Santa Cruz Biotechnology, Inc, USA) and the slides were then rinsed in 3% hydrogen peroxide to block endogenous peroxidase. The sections were then incubated with a donkey anti-goat second antibody conjugated horseradish peroxidase (1:5000; Abcam, Cambridge, UK) at 4°C overnightand. After washing in PBS, the visualization signal was developed with 3, 3’-diaminobenzidine (DAB) solution, and all of the slides were counterstained with hematoxylin. As negative controls, adjacent sections were processed as described above except that they were incubated overnight at 4°C in blocking solution without the primary antibody.
Semiquantitative estimation was made using a composite score obtained by multiplying the values of staining intensity and relative abundance of positive cells. Intensity was graded as 0 (no staining), 1 (weak staining), 2 (moderate staining), or 3 (strong staining). The abundance of positive cells was graded from 0 to 4 (0, < 5% positive cells; 1, 5-25%; 2, 26-50%; 3, 51-75%; 4, > 75%).
The total immunohistochemical staining score was ranged from 0 to 12. The expression level of SEMA3A was defined as following: “-” (negative, score 0), “+” (weakly positive, score 1-4), “++” (positive, score 5-8), “+++” (strong positive, score 9-12). Results from the immunohistochemical (IHC) staining were independently evaluated by two pathologists who had no prior knowledge of the clinical features and outcomes of the patients. Discrepancies between the pathologists were resolved by consensus after discussion.
Cell migration assay
The cell migratory capacity was determined using transwell chambers (BD Biosciences). Briefly, cells (1×105/well) were suspended in 100 μl serum-free medium and then added to the upper chamber of the inserts, RPMI 1640 medium (GIBCO) containing 10% FBS (500 μl) was added to the lower chamber as the chemotactic factor. After culture for 48 hours, non-migrated cells on the upper surface were removed gently with a cotton swab and cells that migrated to the lower side of the department were fixed and dyed with 0.1% crystal violet. The numbers of migrated cells were calculated by counting five different views under the microscopy. The experiment was performed in triplicate and repeated for three times.
Cell viability assay
To determine the effect of SEMA3A on cellular proliferation, an MTT assay was performed. A total of 1×103 cells were plated in each well of a 96-well plate containing 200 ml RPMI-1640 supplemented with 10% FBS. After 1, 2, 3 days of incubation, 20 ml MTT (5 mg/ml; Sigma-Aldrich, St. Louis, MO USA) was added, followed by a 4 h incubation at 37°C in a 5% CO2 incubator. The supernatant was removed and 150 ml dimethyl sulfoxide was added. Culture plates were shaken for 10 min at room temperature to dissolve the MTT crystals. The absorbance values of each sample were read at 490 nm using a Microplate Reader (Model 550, BIO-RAD, Shanghai, China). Each experiment was repeated at least three times.
Wound healing assay
In vitro wound healing assay was performed to examine the migration of SGC7901 cells transfected with either a control vector or pcDNA3.1 (+)-SEMA3A. Transfected cells were grown on 6-well plates with their respective culture media. After the growing cell layers had reached confluence, wounds were prepared by a single scratch on the monolayer using a yellow pipette tip and washed the wounded layers with PBS to remove cell debris. We measured the closure or filling of the wounds at 0, 12, 24, 48 and 72 h using an Olympus I×71 fluorescence microscope with a TH4-200 camera. All experiments were performed in triplicate.
Follow-up
The postoperative follow-up was conducted at our outpatient department and included clinical and laboratory examinations every 3 months for the first 2 years, every 6 months during the third to fifth years, and annually for an additional 5 years or until patient death. The cause of death was registered and classified as mortality due to gastric cancer, other causes or unknown causes. Overall survival was used as a measure of prognosis, which was defined as the time from the operation to the patient’s death or the last follow-up (at five years). Death of a patient was ascertained by reporting from the family and verified by a review of public records.
Statistical analysis
Differences in mRNA and protein expression between tumor samples and the paired adjacent non-tumor tissue samples were evaluated with the paired-samples t-test. The χ 2 test for proportion was used to analyze the relationship between the SEMA3A expression level and various clinicopathological characteristics. Spearman rank correlation analysis was used to analyze the relationship between SEMA3A expression in gastric cancer and TNM stage. Overall survival curves were calculated with the Kaplan-Meier method and were analyzed with the log-rank test. A multivariate analysis of several prognostic factors was carried out using the Cox proportional hazards regression model. P < 0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed with the Statistical Package for the Social Sciences, version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
SEMA3A mRNA expression analyzed by real-time quantitative RT-PCR
The mRNA level of SEMA3A was measured by real-time quantitative PCR in 40 paired cancerous and the matched adjacent normal gastric mucosa tissues from primary gastric cancer patients. The SEMA3A mRNA expression level was significantly lower in 29 of 40 (72.5%) gastric cancer tissues compared with the adjacent non-tumor tissues (Figure 1A). Furthermore, the average relative expression of SEMA3A in all 40 cases of gastric cancer tissues is lower than that of SEMA3A in adjacent normal tissues. There is significant difference in SEMA3A mRNA expression between cancer tissues and adjacent normal tissues (P = 0.0037) (Figure 1C).
Figure 1.
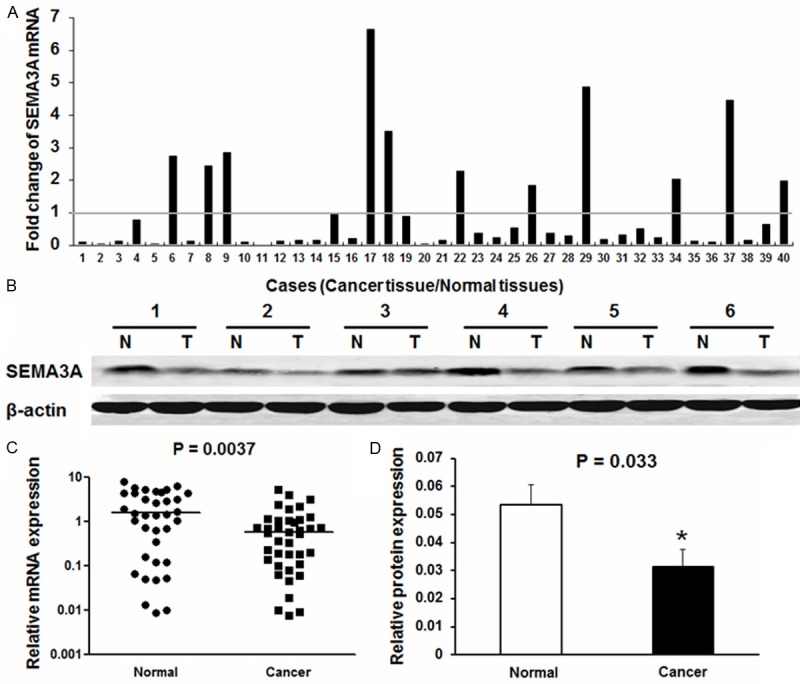
SEMA3A expression in gastric cancer and adjacent normal tissues. A. The fold change of SEMA3A expression in gastric cancer tumor tissues compared to paired adjacent normal tissues (n = 40) evaluated by qRT-PCR and normalized to GAPDH. B. Western blot analysis of SEMA3A proteins expressed in six paired representative gastric cancer (GC) tissues and their matched adjacent nontumor tissues. β-actin was used as a control for equal loading. Abbreviations: T tumor tissues, N nontumor tissues. C. The average relative expression of mRNA level of SEMA3A in gastric cancer tumor tissues compared to paired adjacent normal tissues (P = 0.0037). D. Relative SEMA3A protein expression levels was remarkably decreased in 18 of 24 (75%) gastric tumor tissues compared with the corresponding adjacent non-tumor tissues (P = 0.033).
SEMA3A protein expression analyzed by western blotting
Western blotting was performed on 24 gastric cancer specimens and corresponding adjacent non-cancerous gastric mucosa tissues to evaluate SEMA3A protein expression. The representative western blotting results in six cases were shown in (Figure 1B). The relative quantity of SEMA3A protein expression was normalized to the β-actin of the same samples. We found that SEMA3A expression was remarkably decreased in 18 of 24 (75%) gastric tumor tissues compared with the corresponding adjacent non-tumorous tissues, which was consistent with the quantitative real-time PCR results. The average SEMA3A protein level in 24 gastric cancer tissues was significantly lower than that of SEMA3A in adjacent normal tissues (P = 0.033, Figure 1D).
Immunohistochemical analysis of SEMA3A expression in gastric cancer tissue samples
To further investigate the clinicopathological and prognostic roles of SEMA3A expression, we performed immunohistochemical analyses of the 128 paraffin-embedded gastric cancer tissue blocks. Overall, 70 of 128 (54.68%) cases showed low SEMA3A expression (SEMA3A - or SEMA3A +) in gastric cancer tissues, whereas the remaining 58 (45.31%) cases displayed high SEMA3A expression (SEMA3A ++ or SEMA3A +++) (Figure 2). Most of the surrounding non-tumor tissues and all the normal gastric mucosa tissues showed the strongest SEMA3A positive staining (Figure 2A and 2B). Overall, 70 of 128 (54.68%) GC tissues showed low SEMA3A expression and compared with SNTs, normal gastric mucosa tissues, and the difference had statistically significant (P < 0.05; Table 1).
Figure 2.
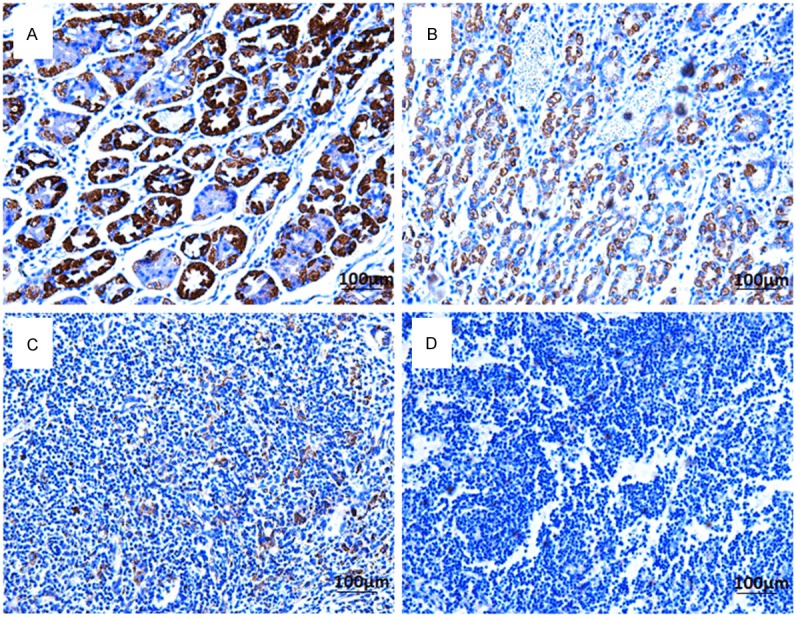
Immunohistochemical detection of the SEMA3A protein expression in gastric cancer tissues, their corresponding adjacent non-tumor tissues and normal gastric mucosa tissues. A. Normal gastric tissues, scored as SEMA3A (+++); B. Well-differentiated gastric cancer, scored as SEMA3A (++); C. Moderately differentiated gastric cancer, scored as SEMA3A (+); D. poorly differentiated gastric cancer, scored as SEMA3A (-). Original magnification: 200×.
Table 1.
Sema3a expression compared in gastric cancer (GC), surrounding nontumor tissues (SNTs) and gastric normal tissues
| Clinical parameters | Number | Sema3a expression | |||
|---|---|---|---|---|---|
|
|
|||||
| (-) | (+) | (+ +) | (+ + +) | ||
| GC a | 128 | 40 | 30 | 31 | 27 |
| SNT b | 66 | 4 | 7 | 24 | 31 |
| Normal tissue c | 8 | 0 | 0 | 3 | 5 |
P-value: a/b: P < 0.05 (χ 2 = 27.960, P < 0.001); a/c: P < 0.05 (χ 2 = 9.153, P = 0.008); b/c: P > 0.05 (χ 2 = 2.847, P = 0.416); * P-value < 0.05 is considered statistically significant.
Correlations between the expression of SEMA3A and various clinicopathological parameters
The Chi square analysis showed that the expression level of SEMA3A in tumor tissues was significantly correlated with various clinicopathological parameters, such as TNM stage (P = 0.003), differentiation (P = 0.015), depth of invasion (P < 0.001), lymph node metastasis (P = 0.029), Vascular invasion (P = 0.001) and distance metastasis (P = 0.002), but not with age (P = 0.990), gender (P = 0.976), tumor size (P = 0.394), location (P = 0.924), growth pattern (P = 0.403) and H. pylori infection (P = 0.142) (Table 2).
Table 2.
Clinico-pathological correlation of SEMA3A protein expression in gastric cancer tissues
| Clinical parameters | Total | Sema3a expression | P-value | ||
|---|---|---|---|---|---|
|
|
|||||
| Low (-~+) | Middle (++) | High (+++) | |||
|
|
|||||
| (n = 70) | (n = 31) | (n = 27) | |||
| Gender | 0.976 | ||||
| Male | 88 | 48 | 21 | 19 | |
| Female | 40 | 22 | 10 | 8 | |
| Age (years) | 0.990 | ||||
| < 50 | 20 | 11 | 5 | 4 | |
| ≥ 50 | 108 | 59 | 26 | 23 | |
| Tumor size (cm) | 0.394 | ||||
| < 4 | 53 | 26 | 16 | 11 | |
| ≥ 4 | 75 | 44 | 15 | 16 | |
| Location | 0.924 | ||||
| Cardia | 37 | 21 | 9 | 7 | |
| Body/Antrum | 91 | 49 | 22 | 20 | |
| Growth pattern | 0.403 | ||||
| Expanding type | 65 | 33 | 19 | 13 | |
| Infiltration type | 63 | 37 | 12 | 14 | |
| TNM stage | 0.003* | ||||
| Stage I/II | 85 | 38 | 23 | 24 | |
| Stage III/IV | 43 | 32 | 8 | 3 | |
| Differentiation | 0.015* | ||||
| Well/moderate | 54 | 25 | 11 | 18 | |
| Poor | 74 | 45 | 20 | 9 | |
| Depth of invasion | < 0.001* | ||||
| T1/T2 | 80 | 32 | 23 | 25 | |
| T3/T4 | 48 | 38 | 8 | 2 | |
| H.pylori infection | 0.142 | ||||
| Negative | 46 | 23 | 9 | 14 | |
| Positive | 82 | 47 | 22 | 13 | |
| Lymphnode metastasis | 0.029* | ||||
| Negative | 61 | 29 | 13 | 19 | |
| Positive | 67 | 41 | 18 | 8 | |
| Vascular invasion | 0.001* | ||||
| Negative | 58 | 27 | 10 | 21 | |
| Positive | 70 | 43 | 21 | 6 | |
| Distant metastasis | 0.002* | ||||
| M0 | 120 | 69 | 25 | 26 | |
| M1 | 8 | 1 | 6 | 1 | |
aStatistical analyses were performed by the Pearson χ 2 test.
P-value < 0.05 was considered statistically significant.
Spearman rank correlation analysis was used to analyzed the relationship between SEMA3A expression in GC and TNM stage, and it showed that SEMA3A expression in GC was negative correlation with TNM stage, that suggested the more advanced clinical TNM stage corresponding to the lower expression level of SEMA3A in GC (rs = -0.322, P < 0.001; Table 3).
Table 3.
Correlation analysis SEMA3A expression in gastric cancer (GC) and TNM stage
| TNM stage | Sema3A expression | Total | rs | P value | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| - | + | + + | + + + | ||||
| I | 5 | 9 | 8 | 10 | 32 | ||
| II | 14 | 10 | 15 | 14 | 53 | ||
| III | 16 | 9 | 7 | 3 | 35 | -0.322 | < 0.001 |
| IV | 5 | 2 | 1 | 0 | 8 | ||
| Total | 40 | 30 | 31 | 27 | 128 | ||
P-value < 0.05 was considered statistically significant.
Overexpression of SEMA3A inhibits gastric cancer cell proliferation and migration in vitro
Given that SEMA3A is significantly decreased in GC tissues, it may function as a tumor suppressor. Therefore, we examined whether overexpression of SEMA3A in gastric cancer cells affected cell growth and migration. SGC-7901 cell line, whose expression of SEMA3A was the lowest in the three tested GC cell lines, was chosen for the subsequent experiments. As SEMA3A expression is associated with distant metastasis in gastric cancer patients, we evaluated the potential role of SEMA3A on cellular migration by transwell assays. SGC-7901 cells were transfected with SEMA3A overexpressing or control plasmid and seeded in the chamber, and their migratory abilities were determined 24 hours later. The results showed overexpression of SEMA3A significantly decreased the migratory capacity of SGC-7901 cells (Figure 3A and 3B, P < 0.001). Then the effects of SEMA3A on cell growth and proliferation were then evaluated by MTT assay and the results showed that overexpression of SEMA3A significantly inhibited the viability of SGC-7901 cells (Figure 3C, P = 0.027).
Figure 3.
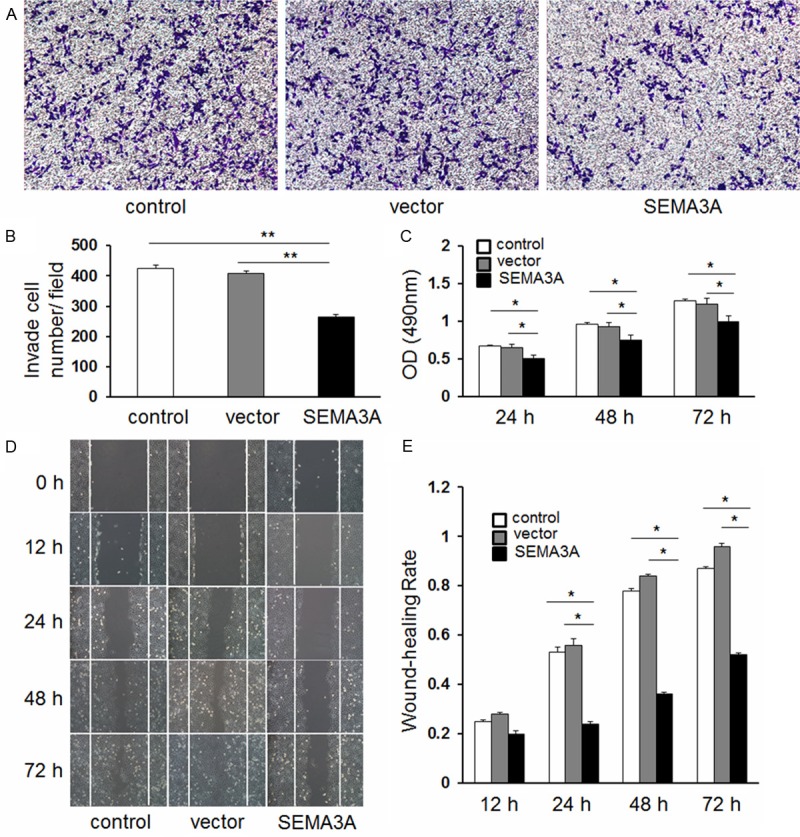
A and B. Abnormal expression of SEMA3A promotes cell migration in SGC-7901 cells as demonstrated by transwell assays (**P < 0.001). Representative photos of stained cells are shown with the original magnification of 100×. C. Abnormal expression of SEMA3A inhibits cell proliferation in SGC-7901 cells (P = 0.027). Cell numbers were evaluated with the MTT assay using absorbance readings at 490 nm. The values shown are the mean of three determinations. D and E. SGC-7901 cells were transfected with pcDNA3.1 (+)-SEMA3A or empty vector for 12, 24, 48 and 72 h, respectively. (*P < 0.05 compared to empty vector).
These findings were further confirmed by the wound healing assay. The overexpression of SEMA3A significantly inhibited the migration of SGC-7901 cells at 48 h after transfection (Figure 3D and 3E, P < 0.05).
Reduction of SEMA3A expression predicts poor survival in gastric cancer
To investigate the prognostic value of SEMA3A expression in gastric cancer patients, overall survival (OS) analysis was performed in these 128 gastric cancer cases, and the five-year OS rate was 47.6% for these patients (Figure 4A). The five-year OS rate was 32.8% for patients with low SEMA3A expression, and 65.5% for patients with high SEMA3A expression, which was a significant difference (χ 2 = 16.338, P < 0.001, Figure 4B). It was found that OS of the high level expression group was significantly longer than that of the low level expression group. For the purpose of seeing the true affect of SEMA3A, we analyzed survival based on SEMA3A expression by separating stage I-II and stage III-IV. In early stage (stages I and II) gastric cancer, the patients with the low levels of SEMA3A expression (n = 38) had a poorer prognosis than the patients with high levels of SEMA3A expression (n = 47) (χ 2 = 5.435, P = 0.020, Figure 4C). Meanwhile, in late stage (stages III and IV) gastric cancer, the patients with the low levels of SEMA3A expression (n = 32) also had a poorer prognosis than the patients with high levels of SEMA3A expression (n = 11) (χ 2 = 4.648, P = 0.031, Figure 4D).
Figure 4.
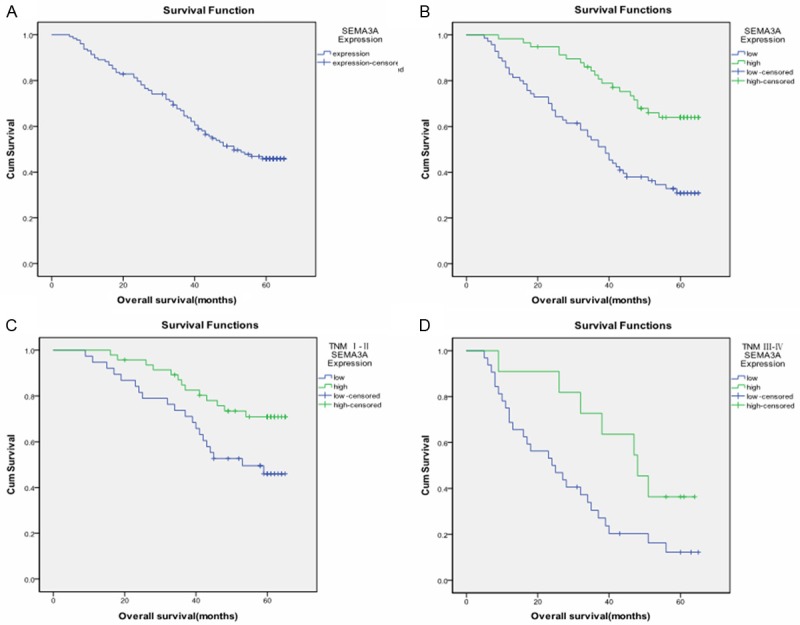
Comparison of different overall survival cumulative Kaplan-Meier curves for patients grouped by immunohistochemical levels of SEMA3A. A. Kaplan-Meier curves for overall survival (OS) of the 128 gastric cancer patients; B. Kaplan-Meier curves for OS in gastric cancer patients with low level and high level SEMA3A expression (χ 2 = 16.338, P < 0.001); C. Kaplan-Meier curves for OS in early stage (stage I and II) gastric cancer patients with low level and high level SEMA3A expression(χ 2 = 5.435, P = 0.020 ); D. Kaplan-Meier curves for OS in advanced stage (stage III and IV) gastric cancer patients with low level and high level SEMA3A expression (χ 2 = 4.648, P = 0.031).
Multivariate analysis using the Cox proportional hazards model for all of the significant covariates in the univariate analysis showed that SEMA3A expression (P = 0.021), TNM stage (P = 0.001), depth of invasion (P = 0.004), lymph node metastasis (P < 0.001), distant metastasis (P < 0.001), vascular invasion (P = 0.001), differentiation (P = 0.001) were independent prognostic factors gastric cancer patients (Table 4), which were performed to compare the impact of SEMA3A expression and other clinicopathological parameters on prognosis.
Table 4.
Cox proportional hazards model analysis of prognostic factors
| Variables | Multivariate analysis | ||
|---|---|---|---|
|
|
|||
| HR | 95% CI | P value | |
| Sema3a expression (High vs. low) | 0.570 | 0.353-0.919 | 0.021* |
| TNM stage (III-IV vs. I-II) | 0.453 | 0.288-0.713 | 0.001* |
| Depth of invasion (T3 + T4 vs. T1 + T2) | 0.513 | 0.324-0.810 | 0.004* |
| Lymph node metastasis (positive vs. negative) | 0.423 | 0.262-0.685 | < 0.001* |
| Distant metastasis (M1 vs. M0) | 0.350 | 0.224-0.545 | < 0.001* |
| Gender (Male vs. Female) | 0.824 | 0.530-1.282 | 0.390 |
| Age (years) (≥ 50 vs. < 50) | 1.358 | 0.876-2.107 | 0.171 |
| Location (Cardia vs. Body/Antrum) | 1.384 | 0.845-2.267 | 0.197 |
| Vascular invasion (positive vs. negative) | 0.439 | 0.273-0.704 | 0.001* |
| Differentiation (Well/moderate vs. poor) | 0.449 | 0.284-0.709 | 0.001* |
HR: Hazard ratio; CI: Confidence interval; TNM: Tumor node metastasis;
P < 0.05 was considered significant.
Discussion
Gastric cancer is one of the most deadly human carcinomas, and it has a dismal prognosis despite improved diagnosis and composite therapy [2,22]. For most Cases, despite progress in diagnostic tumor imaging, combination chemotherapy, and radiotherapy, little improvement has been achieved in terms of the advanced stage when diagnosed, and surgery is the only curative procedure for localized gastric cancer. Previous evidences indicate that gastric cancer is the result of various genetic and epigenetic alterations of oncogenes, tumor suppressor genes, DNA repair genes, cell cycle regulating proteins and cell adhesion molecules [23-26]. Defining molecular subgroups may identify patients who could benefit from targeted therapies and personalized treatment is regarded as the best option to reduce gastric cancer mortality rates [2,20]. Therefore, it is urgently needed to find a sensitive biomarker for the detection of gastric cancer at the curative stage.
Over the past decade, some semaphoring-mediated signals might inhibit rather than promote tumor growth and invasion, as many studies have shown that their expression is often lost during advanced cancer [27]. A further mechanism through which class-3 semaphorins can regulate cell migration is by interfering with VEGF-mediated signaling. In human tumor cells, it was shown that cell migration is finely regulated by a balance between autocrine loops of SEMA3A and VEGF expression [28]. Moreover, the axon repulsion factor Semaphorin3A (SEMA3A) promotes growth cone collapse by binding to its receptor, Neuropilin-1 (NP-1) [29,30]. Interestingly, SEMA3A and NP-1 are also expressed in endothelial cells, and serve as endogenous suppressors of integrin activity [31,32]. In cancer, experimental systems have shown that SEMA3A may alter tumor cell behavior directly by influencing migration and growth or indirectly by interfering with tumor angiogenesis or immune response [33,34]. Our finding that Sema3A inhibits breast tumor cell migration in part by stimulating RhoA expression/activity has important clinical implications [35], and possibly by stimulating the expression of α2β1 integrin. In fact, SEMA3A has been implicated as a tumor suppressor in other types of cancer, such as prostate cancer, mesothelioma, myeloma, melanoma, tongue cancer [36-40], there has been no report on the expression profile of SEMA3A in gastric cancer.
In this study, we evaluated the expression of SEMA3A and its prognostic role in human gastric cancer for the first time. We found, using qRT-PCR and western blotting analysis, that SEMA3A expression was decreased at the mRNA and protein levels, respectively, in most tumor tissues compared to their adjacent non-tumorous tissues. Immunohistochemical staining in analysis also exhibited that SEMA3A expression was significantly lower in the tumor tissues than other gastric tissues. Also we show that overexpression of SEMA3A in AGS cell significantly inhibits cell proliferation and migration both in vitro. Furthermore, a decreased expression of SEMA3A was significantly associated with advanced TNM stage, poor differentiation, depth of invasion, lymph node metastasis, vascular invasion and distance metastasis, suggesting that abnormal SEMA3A expression might be involved in gastric cancer tumor progression and metastasis and that SEMA3A could also play a tumor suppressor role in gastric cancer. Moreover, it is well known that a high prevalence of H. pylori is always accompanied by a high incidence of gastric cancer [41]. One study demonstrates that H. pylori positivity is a beneficial prognostic indicator in patients with gastric cancer [42]. However, in the present study, no significance discrepancy in SEMA3A expression was observed in the patients with and without H. pylori infection.
A Kaplan-Meier survival analysis showed low SEMA3A expression significantly correlated with shorter survival time of gastric cancer patients. Spearman rank correlation analysis suggested the more advanced clinical TNM stage corresponding to the lower expression level of SEMA3A in GC. Cox hazard ratio regression analyses further demonstrated that the SEMA3A expression level was an independent risk factor for survival, suggesting that it may serve as a valuable prognostic biomarker for gastric cancer patients after surgery and a potential target for gene therapy in the treatment of gastric cancer. These results are consistent with other reports indicating that sema3A upregulation decreases adhesion of endothelial cells and that increased sema3A expression inhibits breast cancer migration [10,32]. Some studies also unveil what we believe to be a new role of Sema3A as an endogenous antiangiogenic inhibitor that impairs angiogenesis and reduces late-stage tumor volume without inducing enduring hypoxia or interfering with normal vessels. Since reexpression of exogenous Sema3A in tumors induces stable disease and normalizes the vasculature, this molecule holds promise as a target to be considered in designing new and more efficient antiangiogenic and antitumor therapies [43]. Finally, because our knowledge of SEMA3A is still far from complete, further studies on mechanism that are involved in the effect of SEMA3A will be necessary. The mechanisms that contribute to the down-regulated expression of SEMA3A in gastric cancer require further investigation.
To the best of our knowledge, this is the first report that demonstrates the involvement of SEMA3A in the carcinogenesis of GC. In the current study, we have demonstrated the loss of SEMA3A expression in gastric cancer and its correlation with poor differentiation, vascular invasion, deep invasion level, distant metastasis, advanced tumor stage, the presence of lymph node metastasis, and poor outcome in patients who underwent gastrectomy. Taken together, these results strongly demonstrated that the decreased SEMA3A expression in GC should be a factor contributing to the development rather than being affected as a consequence of GC and we confirmed that SEMA3A might serve as a candidate tumor suppressor and prognostic biomarker in gastric carcinogenesis. Moreover, we expect that SEMA3A may function as a useful target for new therapeutic interventions against gastric cancer.
Disclosure of conflict of interest
None.
References
- 1.Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14(Suppl 2):ii31–6. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 2.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–90. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin Y, Ueda J, Kikuchi S, Totsuka Y, Wei WQ, Qiao YL, Inoue M. Comparative epidemiology of gastric cancer between Japan and China. World J Gastroenterol. 2011;17:4421. doi: 10.3748/wjg.v17.i39.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao JJ, Pan K, Wang W, Chen JG, Wu YH, Lv L, Li JJ, Chen YB, Wang DD, Pan QZ, Li XD, Xia JC. The prognostic value of tumor-infiltrating neutrophils in gastric adenocarcinoma after resection. PLoS One. 2012;7:e33655. doi: 10.1371/journal.pone.0033655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howlett M, Menheniott TR, Judd LM, Giraud AS. Cytokine signalling via gp130 in gastric cancer. Biochim Biophys Acta. 2009;1793:1623–33. doi: 10.1016/j.bbamcr.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–27. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- 7.Tamagnone L, Comoglio PM. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol. 2000;10:377–83. doi: 10.1016/s0962-8924(00)01816-x. [DOI] [PubMed] [Google Scholar]
- 8.Comoglio PM, Tamagnone L, Giordano S. Invasive growth: a two-way street for semaphorin signalling. Nat Cell Biol. 2004;6:1155–7. doi: 10.1038/ncb1204-1155. [DOI] [PubMed] [Google Scholar]
- 9.Raper JA. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol. 2000;10:88–94. doi: 10.1016/s0959-4388(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 10.Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, Mercurio AM. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–3. [PubMed] [Google Scholar]
- 11.Bielenberg DR, Klagsbrun M. Targeting endothelial and tumor cells with semaphorins. Cancer Metastasis Rev. 2007;26:421–31. doi: 10.1007/s10555-007-9097-4. [DOI] [PubMed] [Google Scholar]
- 12.Rolny C, Capparuccia L, Casazza A, Mazzone M, Vallario A, Cignetti A, Medico E, Carmeliet P, Comoglio PM, Tamagnone L. The tumor suppressor semaphorin 3B triggers a prometastatic program mediated by interleukin 8 and the tumor microenvironment. J Exp Med. 2008;205:1155–71. doi: 10.1084/jem.20072509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng C, Zhou Q, Wu F, Peng Q, Tang A, Liang H, Zeng Y. Semaphorin3F down-regulates the expression of integrin alpha(v)beta3 and sensitizes multicellular tumor spheroids to chemotherapy via the neuropilin-2 receptor in vitro. Chemotherapy. 2009;55:344–52. doi: 10.1159/000232449. [DOI] [PubMed] [Google Scholar]
- 14.Neufeld G, Shraga-Heled N, Lange T, Guttmann-Raviv N, Herzog Y, Kessler O. Semaphorins in cancer. Front Biosci. 2005;10:751–60. doi: 10.2741/1569. [DOI] [PubMed] [Google Scholar]
- 15.Catalano A, Caprari P, Moretti S, Faronato M, Tamagnone L, Procopio A. Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood. 2006;107:3321–9. doi: 10.1182/blood-2005-06-2445. [DOI] [PubMed] [Google Scholar]
- 16.Kashiwagi H, Shiraga M, Kato H, Kamae T, Yamamoto N, Tadokoro S, Kurata Y, Tomiyama Y, Kanakura Y. Negative regulation of platelet function by a secreted cell repulsive protein, semaphorin 3A. Blood. 2005;106:913–21. doi: 10.1182/blood-2004-10-4092. [DOI] [PubMed] [Google Scholar]
- 17.Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, Tessier-Lavigne M, Taniguchi M, Püschel AW, Bussolino F. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–7. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 18.Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem. 2007;282:26294–305. doi: 10.1074/jbc.M609711200. [DOI] [PubMed] [Google Scholar]
- 19.Acevedo LM, Barillas S, Weis SM, Göthert JR, Cheresh DA. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood. 2008;111:2674–80. doi: 10.1182/blood-2007-08-110205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herman JG, Meadows GG. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. Int J Oncol. 2007;30:1231–8. [PubMed] [Google Scholar]
- 21.Capparuccia L, Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment-two sides of a coin. J Cell Sci. 2009;122:1723–36. doi: 10.1242/jcs.030197. [DOI] [PubMed] [Google Scholar]
- 22.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 23.Piazuelo MB, Epplein M, Correa P. Gastric cancer: an infectious disease. Infect Dis Clin North Am. 2010;24:853. doi: 10.1016/j.idc.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu WK, Cho CH, Lee CW, Fan D, Wu K, Yu J, Sung JJ. Dysregulation of cellular signaling in gastric cancer. Cancer Lett. 2010;295:144–53. doi: 10.1016/j.canlet.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Oguma K, Oshima H, Oshima M. Inflammation, tumor necrosis factor and Wnt promotion in gastric cancer development. Future Oncol. 2010;6:515–26. doi: 10.2217/fon.10.13. [DOI] [PubMed] [Google Scholar]
- 26.Saikawa Y, Fukuda K, Takahashi T, Nakamura R, Takeuchi H, Kitagawa Y. Gastric carcinogenesis and the cancer stem cell hypothesis. Gastric Cancer. 2010;13:11–24. doi: 10.1007/s10120-009-0537-4. [DOI] [PubMed] [Google Scholar]
- 27.Capparuccia L, Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment--two sides of a coin. J Cell Sci. 2009;122:1723–36. doi: 10.1242/jcs.030197. [DOI] [PubMed] [Google Scholar]
- 28.Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, Mercurio AM. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–3. [PubMed] [Google Scholar]
- 29.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–51. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 30.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–62. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 31.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–42. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, Tessier-Lavigne M, Taniguchi M, Püschel AW, Bussolino F. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–7. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 33.Tamagnone L, Comoglio PM. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol. 2000;10:377–83. doi: 10.1016/s0962-8924(00)01816-x. [DOI] [PubMed] [Google Scholar]
- 34.Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 35.Pan H, Bachelder RE. Autocrine Semaphorin3A stimulates eukaryotic initiation factor 4E-dependent RhoA translation in breast tumor cells. Exp Cell Res. 2010;316:2825–32. doi: 10.1016/j.yexcr.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herman JG, Meadows GG. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. Int J Oncol. 2007;30:1231–8. [PubMed] [Google Scholar]
- 37.Catalano A, Caprari P, Rodilossi S, Betta P, Castellucci M, Casazza A, Tamagnone L, Procopio A. Cross-talk between vascular endothelial growth factor and semaphorin-3A pathway in the regulation of normal and malignant mesothelial cell proliferation. FASEB J. 2004;18:358–60. doi: 10.1096/fj.03-0513fje. [DOI] [PubMed] [Google Scholar]
- 38.Vacca A, Ribatti D. Bone marrow angiogenesis in multiple myeloma. Leukemia. 2006;20:193–9. doi: 10.1038/sj.leu.2404067. [DOI] [PubMed] [Google Scholar]
- 39.Chakraborty G, Kumar S, Mishra R, Patil TV, Kundu GC. Semaphorin 3A suppresses tumor growth and metastasis in mice melanoma model. PLoS One. 2012;7:e33633. doi: 10.1371/journal.pone.0033633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song X, Zhang W, Zhang Y, Zhang H, Fu Z, Ye J, Liu L, Song X, Wu Y. Expression of semaphorin 3A and neuropilin 1 with clinicopathological features and survival in human tongue cancer. Med Oral Patol Oral Cir Bucal. 2012;17:e962–8. doi: 10.4317/medoral.18168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med. 2008;47:1077–83. doi: 10.2169/internalmedicine.47.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang F, Sun GP, Zou YF, Zhong F, Ma T, Li XQ, Wu D. Helicobacter pylori infection predicts favorable outcome in patients with gastric cancer. Curr Oncol. 2013;20:e388. doi: 10.3747/co.20.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maione F, Molla F, Meda C, Latini R, Zentilin L, Giacca M, Seano G, Serini G, Bussolino F, Giraudo E. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J Clin Invest. 2009;119:3356–72. doi: 10.1172/JCI36308. [DOI] [PMC free article] [PubMed] [Google Scholar]


