Abstract
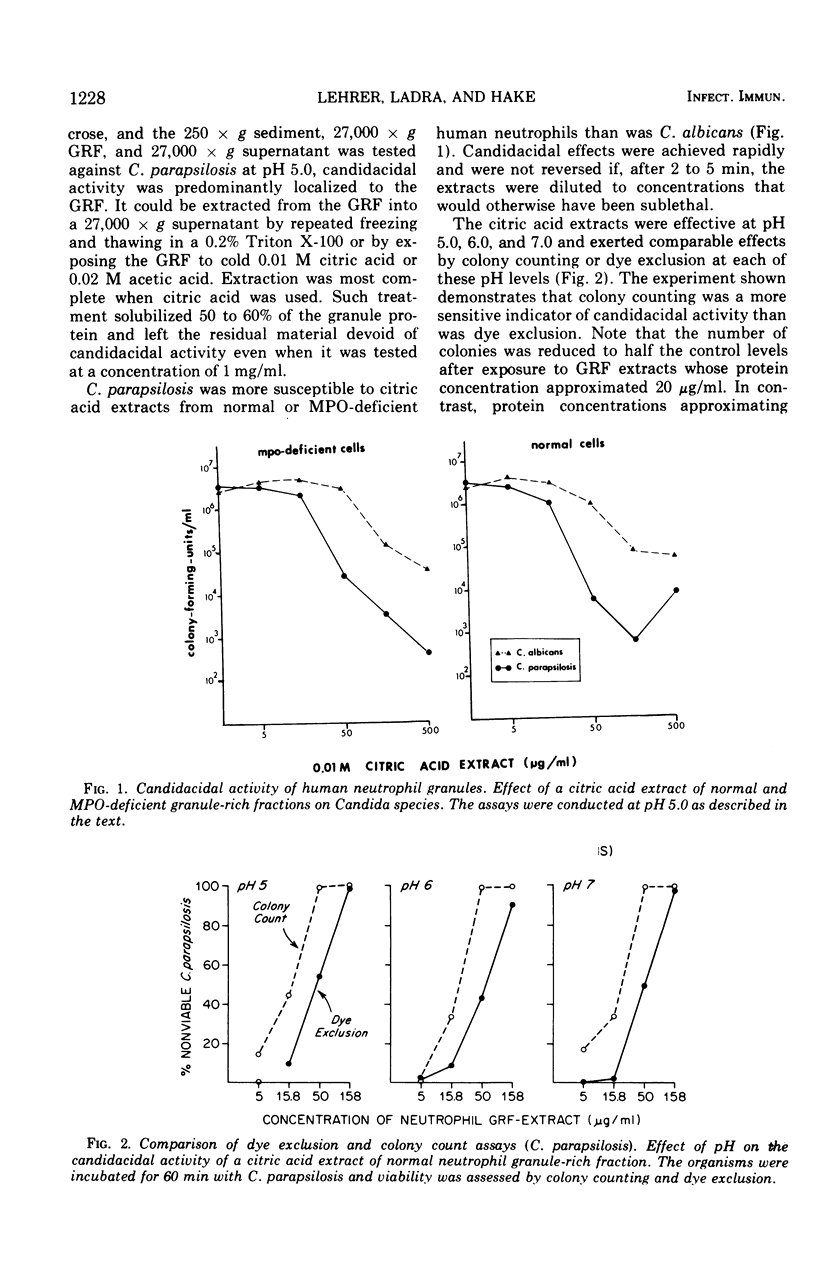
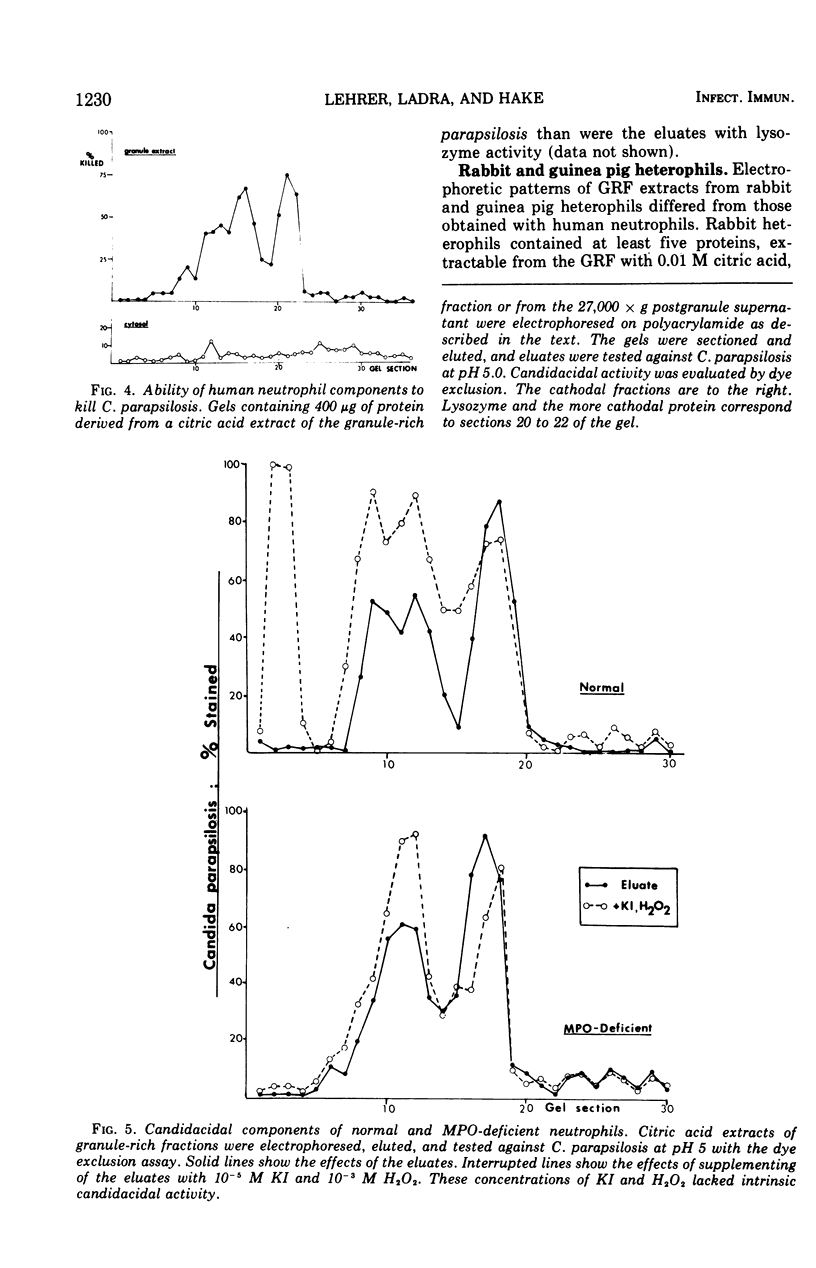
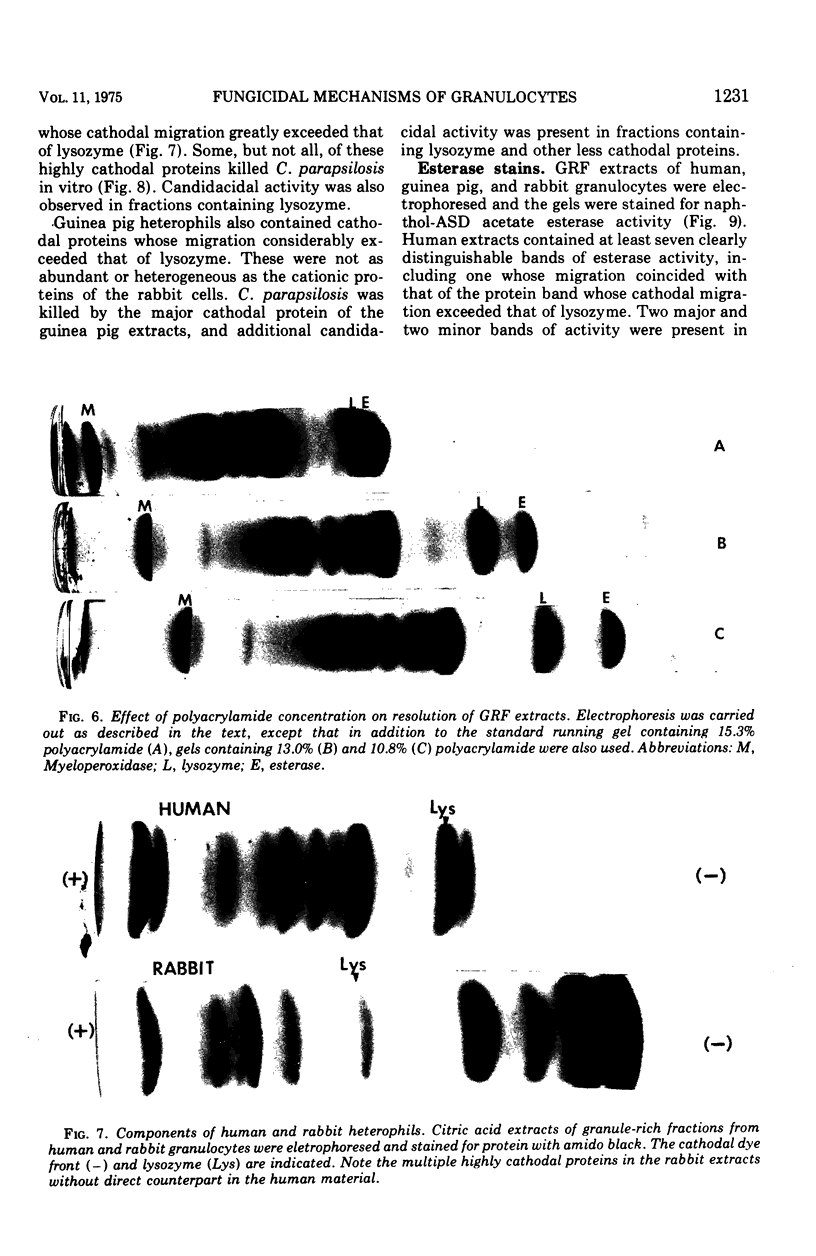
Granulocytes from the peripheral blood of normal subjects and a patient with hereditary myeloperoxidase deficiency were homogenized in 0.34 M sucrose. A granule-rich fraction, prepared by sedimentation at 27,000 × g for 20 min, contained components that killed C. parapsilosis in vitro. These were extractable with 0.01 M citric acid and were shown by micropreparative polyacrylamide electrophoresis to be multiple. The candidacidal activity of these neutrophil components was heat stable and they were somewhat more active at pH 5.0 than at pH 7.0. When rabbit or guinea pig heterophils were obtained from sterile peritoneal exudates and similarly fractionated, they also were found to contain components that killed C. parapsilosis in vitro. These were primarily associated with a group of lysosomal cationic proteins lacking direct counterpart in human neutrophils. Among the candidacidal components of the human neutrophil was a protein, more cationic than lysozyme, that exhibited naphthol-ASD acetate esterase activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brune K., Leffell M. S., Spitznagel J. K. Microbicidal activity of peroxidaseless chicken heterophile leukocytes. Infect Immun. 1972 Mar;5(3):283–287. doi: 10.1128/iai.5.3.283-287.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. S., Pappagianis D. Inhibition by lysozyme of growth of the spherule phase of Coccidioides immitis in vitro. Infect Immun. 1974 Sep;10(3):616–623. doi: 10.1128/iai.10.3.616-623.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Gadebusch H. H., Johnson A. G. Natural host resistance to infection with Cryptococcus neoformans. IV. The effect of some cationic proteins on the experimental disease. J Infect Dis. 1966 Dec;116(5):551–565. doi: 10.1093/infdis/116.5.551. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G. Further studies on preparation and properties of phagocytin. J Exp Med. 1960 Mar 1;111:323–337. doi: 10.1084/jem.111.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Role of the superoxide anion in the myeloperoxidase-mediated antimicrobial system. J Biol Chem. 1974 Jun 25;249(12):3724–3728. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. Functional aspects of a second mechanism of candidacidal activity by human neutrophils. J Clin Invest. 1972 Oct;51(10):2566–2572. doi: 10.1172/JCI107073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Goldberg L. S., Apple M. A., Rosenthal N. P. Refractory megaloblastic anemia with myeloperoxidase-deficient neutrophils. Ann Intern Med. 1972 Mar;76(3):447–453. doi: 10.7326/0003-4819-76-3-447. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I. Inhibition by sulfonamides of the candidacidal activity of human neutrophils. J Clin Invest. 1971 Dec;50(12):2498–2505. doi: 10.1172/JCI106750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Bactericidal activity of aerobic and anaerobic polymorphonuclear neutrophils. Infect Immun. 1974 Feb;9(2):337–341. doi: 10.1128/iai.9.2.337-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Venge P. Cationic proteins of human granulocytes. II. Separation of the cationic proteins of the granules of leukemic myeloid cells. Blood. 1974 Aug;44(2):235–246. [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Rindler R., Braunsteiner H. Soluble proteins from human leukocyte granules. I. Esterase activity of cationic proteins. Blut. 1973 Jul;27(1):26–32. doi: 10.1007/BF01631423. [DOI] [PubMed] [Google Scholar]
- Schill W. B., Schumacher G. F. Radial diffusion in gel for micro determination of enzymes. I. Muramidase, alpha-amylase, DNase 1, RNase A, acid phosphatase, and alkaline phosphatase. Anal Biochem. 1972 Apr;46(2):502–533. doi: 10.1016/0003-2697(72)90324-7. [DOI] [PubMed] [Google Scholar]
- Welsh I. R., Spitznagel J. K. Distribution of lysosomal enzymes, cationic proteins, and bactericidal substances in subcellular fractions of human polymorphonuclear leukocytes. Infect Immun. 1971 Aug;4(2):97–102. doi: 10.1128/iai.4.2.97-102.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Arginine-rich proteins of polymorphonuclear leukocyte lysosomes. Antimicrobial specificity and biochemical heterogeneity. J Exp Med. 1968 May 1;127(5):927–941. doi: 10.1084/jem.127.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. I. Resolution of antibacterial and enzymatic activities. J Bacteriol. 1966 Feb;91(2):750–754. doi: 10.1128/jb.91.2.750-754.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. II. Composition, properties, and mechanism of antibacterial action. J Bacteriol. 1966 Feb;91(2):755–762. doi: 10.1128/jb.91.2.755-762.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
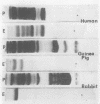
- Zeya H. I., Spitznagel J. K. Characterization of cationic protein-bearing granules of polymorphonuclear leukocytes. Lab Invest. 1971 Mar;24(3):229–236. [PubMed] [Google Scholar]