Abstract
High intraocular pressure (IOP) is a risk factor for primary open-angle glaucoma (POAG). The trabecular meshwork (TM), a reticular tissue in the outflow passage of the aqueous humor (AH), is a major contributor to intraocular outflow resistance. High levels of myocilin (MYOC), which is expressed in the TM, are associated with high IOP. Furthermore, transforming growth factor-β2 (TGF-β2) concentrations in human AH are significantly elevated in POAG patients. This study was designed to investigate the effects of TGF-β2 on MYOC expression and secretion in human primary cultured TM cells. Primary cultured human TM cells were treated with 0 (control group), 1, 10, and 100 ng/mL TGF-β2 for 12, 24, or 48 h. MYOC mRNA and protein expressions in TM cells and protein secretion in conditioned media were analyzed by semi-quantitative RT-PCR, Western blotting, and enzyme-linked immunosorbent assays (ELISA), respectively. TM cells treated with 1, 10, and, 100 ng/mL TGF-β2 for 48 h showed higher MYOC mRNA and protein expressions than those in the control group (0 ng/mL TGF-β2) (all P < 0.05). Treatment with TGF-β2 for 48 h also induced MYOC secretion in conditioned media in a dose-dependent manner (0 ng/mL: 7.107±1.163 pg/ml; 1 ng/mL: 7.879±1.894 pg/ml; 10 ng/mL: 8.063±1.181 pg/ml; 100 ng/mL: 8.902±0.699 pg/ml; all P < 0.05). In Conclusion, TGF-β2 induced MYOC expression and secretion in human primary cultured TM cells. Further investigations are required to confirm the involvement of these two factors in POAG pathogenesis.
Keywords: Primary cell culture, trabecular meshwork cells, glaucoma, transforming growth factor-beta 2, myocilin
Introduction
Glaucoma is the second most prevalent cause of blindness in the world [1-5]. It is characterized by abnormally elevated intraocular pressure (IOP), chronic, progressive optic nerve injury, and visual field defects. Recent epidemiological surveys indicate that the incidence of primary open-angle glaucoma (POAG), a common type of glaucoma, is increasing in China [6,7]. Although the pathogenesis of POAG remains unclear, it is commonly held that genetic and local environmental factors play important roles [8,9]. High IOP is an important risk factor for POAG [10-12]; therefore, controlling IOP is the principal therapeutic approach. The trabecular meshwork (TM), a reticular tissue in the outflow passage of the aqueous humor (AH), is a major contributor to outflow resistance and is therefore a key focus of glaucoma research. Changes in TM structure and function may affect IOP [13,14]. In patients with POAG, changes such as collagen and elastic fiber degeneration, increased extracellular matrix (ECM) deposition, cell shedding, and mesh narrowing or occlusion have been reported [15]. Such structural changes ultimately lead to increased AH outflow resistance and increased IOP.
Alterations in the concentration of a variety of cytokines have been reported in the AH of patients with POAG, and these changes in expression partially account for structural changes in the TM [16]. Notably, high concentrations of transforming growth factor-β2 (TGF-β2) in the AH of POAG patients have been reported [17]. Altered expression of TGF-β2 is thought to underlie many of the changes in the TM that contribute to the development and progression of glaucoma [18,19]. These changes occur both intracellularly, within the actin cytoskeleton, and extracellularly, within the ECM of the TM. The result of elevated TGF-β2 is an increase in the outflow resistance of the TM brought about by modulation of the contractile properties of TM cells and of the composition and quantity of the TM ECM.
The myocilin gene (MYOC), which is also known as the TM glucocorticoid-induced response protein (TIGR) gene [20], is the first gene to be linked to POAG. To date, almost 100 disease-causing mutations have been characterized in the MYOC gene (see www.myocilin.com) [21]. The protein encoded by MYOC, which is highly expressed in the TM [22], contains domains that exhibit significant homology to the myosin and olfactomedin proteins [23,24]. Various studies have demonstrated that MYOC protein localizes to intracellular compartments and to the extracellular matrix and that it exists in glycosylated and non-glycosylated forms [24,25]. Given this complex expression pattern, its biological functions in normal and glaucomatous eyes have been difficult to elucidate. Findings published by a number of labs indicate that MYOC binds Ca++ in the olfactomedin domain, displays molecular chaperone activity, participates in receptor-mediated endocytosis of a G-protein coupled receptor, and stimulates cell migration in a manner similar to that of Wnt3a [26-29]. MYOC interacts with itself and with a diverse array of proteins. These include extracellular matrix components such as collagens, laminin, fibronectin, and members of the secreted protein acidic and rich in cysteine (SPARC) family as well as with glyceraldehyde 3-phosphate dehydrogenase, alpha1-syntrophin, gamma-synuclein, and myosin regulatory light chain [30-34]. The majority of disease-causing mutations in MYOC lie within the olfactomedin domain and are known to display altered intracellular sequestration, altered levels of secretion, or increased aggregation [35,36]. Collectively, these and other data indicate that POAG caused by mutations in MYOC may result primarily from protein misfolding or improper protein trafficking.
Patients with mutations in MYOC account for only a small percentage of individuals with POAG. Howell and colleagues detected elevated levels of MYOC in approximately 70% of AH samples from 29 patients with POAG [37]. This finding indicates that MYOC expression may be associated with disease pathology in patients with POAG who lack MYOC mutations. Moreover, recent research suggests that MYOC protein can influence the organization and function of the TM and lead to elevated IOP [38]. Therefore, wild-type MYOC, like TGF-β2, may participate in the pathogenesis of some forms of POAG [39,40]. However, the mechanisms underlying the effects of these two proteins remain to be elucidated, as does their regulatory interactions. In this study, TM cells from the eyes of non-glaucomatous donors were cultured in vitro to investigate the effects of TGF-β2 on MYOC expression and secretion. The results from the present study could provide new knowledge on the pathogenesis of POAG.
Materials and methods
Cell culture
TM tissues were obtained from four human eye donors (all of whom died in accidents; 2 women, aged 38 and 43 years, whose eyes were processed within 10 and 12 h, respectively, after their deaths; and 2 men, aged 35 and 40 years, whose eyes were processed within 12 and 15 h, respectively, after their deaths) who did not have any diagnosed eye disorder. Ethical approval was obtained from the Ethics Committee of Fujian Medical University, Quanzhou, China, and informed consent was obtained from the family members of the donors. The eyes were carefully dissected and divided into 3-5-mm fragments. The fragments were explanted into 25-cm2 disposable plastic tissue culture flasks and cultured in Dulbecco’s modified Eagle’s medium (Hyclone, Logan, UT, USA) containing 20% fetal bovine serum (Invitrogen-Gibco, Carlsbad, CA, USA) in an incubator set at 37°C and 5% CO2. This procedure resulted in the establishment of four cell lines, with one line being generated from one eye of one donor. Cell characterization was performed as described previously [41]. Cell growth was monitored regularly with an inverted microscope (Olympus, Japan). Prior to reaching to 80% confluence, cells were subcultured by digestion with 0.25% trypsin (Hyclone, Logan, UT, USA) in culture medium for 10 minutes, followed by centrifugation (800 rpm) for 5 minutes. For subculture, the cells were used at a concentration of 5×105 cells/mL. All of the experiments were conducted in accordance with the tenets of the Declaration of Helsinki.
Cell identification
Trabecular meshwork cells (third passage) were identified by transmission electron microscopy and immunocytochemical analysis. After centrifugation (800 rpm, 5 min), the cells were fixed with a mixture of 2.5% glutaraldehyde and 2% paraformaldehyde in preparation for transmission electron microscopy (Hitachi H-7650, Tokyo, Japan) observation or grown on 25x25 mm cover slips in 6-well plates. At confluence, the cells were fixed in 4% paraformaldehyde for 5 min and a mixture of 30% H2O2 and methyl was added to annihilate endogenous peroxidase. Then, after the addition of 5 % BSA, the cells were incubated with mouse monoclonal anti-human laminin (LN), fibronectin (FN), neuron-specific enolase (NSE), Factor VIII-associated antigen, and vimentin antibodies for 1 hr and goat anti-mouse IgG (all from Boster Company, Wuhan, China). Immunocytochemical staining (with a kit purchased from Boster) of LN, FN, NSE, Factor VIII-associated antigen, and vimentin were visualized with a Nikon Eclipse TE2000-U inverted fluorescent microscope (Tokyo, Japan).
TM cell treatment with TGF-β2
Trabecular meshwork cells (third passage) were grown to a density of 5×106 cells/mm2 in 6-well plates and incubated with serum-free DMEM for 24 h before drug treatments. The cells were then randomly divided into four groups and treated with recombinant human TGF-β2 (Peprotech, USA), which was dissolved in sterile distilled water to concentrations of 1, 10, and 100 ng/mL. Distilled water was used as the 0 ng/mL control solution. After incubation for 12, 24, or 48 h, culture media were collected and centrifuged for 20 min (2,000 rpm), and the supernatants were carefully extracted for analysis by enzyme-linked immunosorbent assay (ELISA). Adherent cells were prepared for RT-PCR and Western blot analysis of MYOC mRNA and protein expression, respectively.
ELISA
To determine whether TGF-β2 influences the secretion of MYOC by TM cells, ELISAs were performed according to the kit instructions (R&D Systems, Minneapolis, MN, USA). The standard solution was diluted (concentration gradient: 60 pg/ml, 40 pg/ml, 20 pg/ml, 10 pg/ml, 5 pg/ml, respectively) and 50 μL was added to each well. At the same time, 40 μL sample diluent was added to 10 μL culture supernatant from the TM cells treated with or without TGF-β2 or an equal volume of culture medium for blank samples. These samples were added and the plate was incubated at 37°C for 30 min before being washed and then dried. Then, 50 μL ELISA reagents were added to all wells and the plate was incubated at 37°C, washed, and dried again. Chromogenic reagents A and B (50 μL of each) were mixed gently and added to each well. The plate was incubated in the dark at 37°C for 15 min before 50 μL stop solution was added to each well to terminate the color reaction. The absorbance of each well was measured at 450 nm using a microplate reader (KHB ST-360, Shanghai, China).
RT-PCR
Total RNA was isolated with TRIzol according to manufacturer’s instructions (Beyotime Corporation, Jiangsu, China). RNA purity was determined by measuring the absorbance at 260 and 280 nm (A260/280), and the integrity of the RNA was verified by electrophoresis on formaldehyde gels. Reverse transcription was performed to obtain first-strand cDNAs by using an RT kit (Beyotime) according to the protocol. β-actin was used as an internal reference. Primer pairs for amplification of MYOC and β-actin were designed and synthesized by Sangon Co. Ltd. (Shanghai, China) as follows: MYOC: sense 5’-CCATTCAAGAACCGCTAT-3’ and antisense 5’-GAAATTGTCTACGCCCTC-3’; β-actin sense 5’-CGGCTACAGCTTCACCAC-3’ and antisense 5’-GTACTTGCGCTCAGGAGG-3’. PCR amplification was performed with pre-denaturation for 3 min at 94°C, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 1 min, with a final extension for 10 min at 72°C. The PCR products were visualized by 1.5% agarose gel electrophoresis and photographed with a gel electrophoresis image analyzer (Bio-Rad, Hercules, CA, USA). The relative gray values (ODMYOC/ODβ-actin) represented relative expression levels of MYOC mRNA.
Western blot analysis
TM cells were rinsed with cold phosphate-buffered saline (PBS) solution, and RIPA lysis buffer (Beyotime) containing 100 mM phenylmethyl sulfonylfluoride (Beyotime) was added to extract the total proteins. All steps were carried out on ice when possible. Then, the lysates were centrifuged (12,000 rpm) for 20 min at 4°C. The total protein in the supernatant of each group was determined with a bicinchoninic acid assay (BCA) kit (Beyotime). A total of 30 μg protein from each group was used for 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the proteins were transferred to 0.2-mm pore nitrocellulose (NC) membranes (Millipore, MA, USA) that were then immersed in 5% skimmed milk dissolved in Tris-buffered saline containing 0.02% Tween-20 (TBST) overnight at 4°C. Then, the membranes were washed in TBST, incubated overnight at 4°C in blocking buffer containing 200 μg/mL mouse polyclonal anti-myocilin (R&D Systems, Minneapolis, MN, USA) and mouse monoclonal anti-β-actin (Biomiga, USA) antibodies. After washing in TBST, the membranes were incubated for 3 h at room temperature with 0.8 mg/mL horseradish peroxide-conjugated goat anti-mouse IgG (Zhongshan, Beijing, China) diluted in blocking buffer, and then washed in TBST. The blots were visualized with an ECL kit (Zhongshan, Beijing, China). After exposure for 2 min, the images were collected and analyzed by using a gel electrophoresis image analysis system. Relative MYOC protein expression was normalized to that of β-actin.
Statistical analysis
All statistical evaluations were conducted by using a statistical software package (SPSS11.5, Chicago, IL, USA). Data are presented as the means±standard error of the mean (SEM) from the four cell lines with one cell line conducted in triplicate and were evaluated for normality and variance homogeneity. One-way analysis of variance (ANOVA) was employed to test differences among multiple groups, followed by the Student-Newman-Keuls (SNK) post-hoc test. If the tests indicated deviations from the parametric assumptions, the data were analyzed with the Kruskal-Wallis H test. P < 0.05 was considered to be statistically significant.
Results
TM cell identification
Donor eyes were processed as described in Materials and Methods. Four cell lines were established, with one cell line being generated from one eye of one donor. The cultured cells had various shapes that were stelliform or irregular. Each had processes [3-5] and obvious cell protrusions (Figure 1A). The nuclei were oval with clear membranes, and two or three prominent nucleoli were also visible. The cells were also hypertrophic with abundant transparent cytoplasm and a large number of phagocytosed black particles. Transmission electron microscopy showed that the cells were oval or round, with cell surface microvilli. Gap and tight junctions were the major connections between cells, and abundant organelles including lysosomes, endoplasmic reticulum, mitochondria, phagocytic vesicles, and ribosomes were visible in the cytoplasm (Figure 1B). Immunocytochemistry revealed that the cells expressed LN, FN, vimentin, and NSE, but did not express factor VIII-associated antigen (Figure 1C-G). These morphological and immunocytochemical results confirm that the cultured cells were TM cells [2-5].
Figure 1.
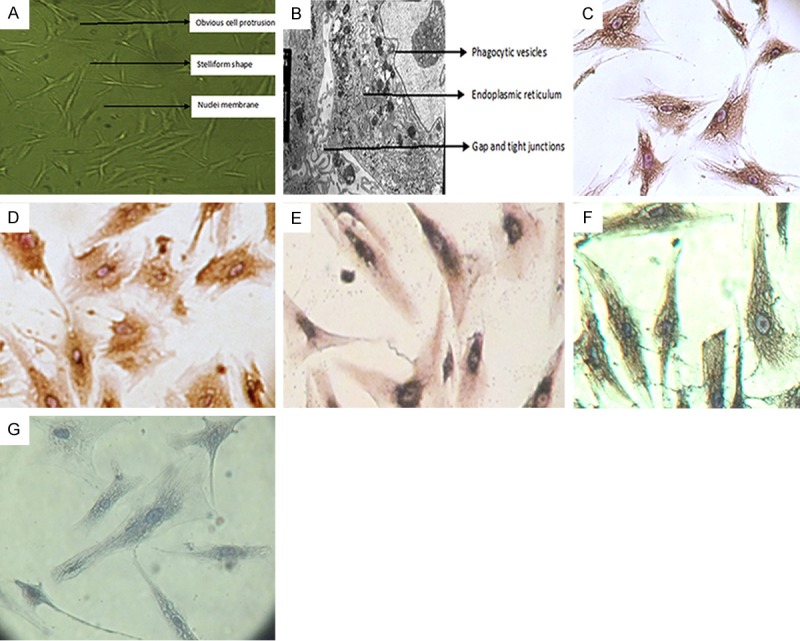
Identification of trabecular meshwork (TM) cells. (A-G) Representative images of TM cells from one of four cell lines established from the eyes of four donors. Cells from all four lines exhibited similar morphological and immunocytochemical features. The cells were observed by light microscopy (×40) (A) and transmission electron microscopy (×8,000) (B). Immunocytochemical staining of laminin (C) (×400), fibronectin (D) (×400), vimentin (E) (×400), neuron-specific enolase (F) (×400), and Factor VIII-associated antigen (G) (×400).
Effect of TGF-β2 on MYOC mRNA expression in TM cells
We used RT-PCR to analyze the expression of MYOC mRNA in TM cells after treatment with different concentrations of TGF-β2. The expression levels, described as the ratio of the gray values of MYOC and β-actin, were 0.532±0.058, 0.625±0.035, 0.720±0.018, and 0.828±0.024 after treatment with 0, 1, 10 and 100 ng/ml TGF-β2, respectively, for 48 h (Figure 2). The relative values of MYOC mRNA in all of the experimental groups were significantly higher than that in the control group (0 ng/mL TGF-β2; P < 0.05). The expression of MYOC mRNA following TGF-β2 treatment increased in a dose-dependent manner. MYOC expression in cells exposed to TGF-β2 for 12 or 24 h was not significantly different from that of control cells (data not shown).
Figure 2.
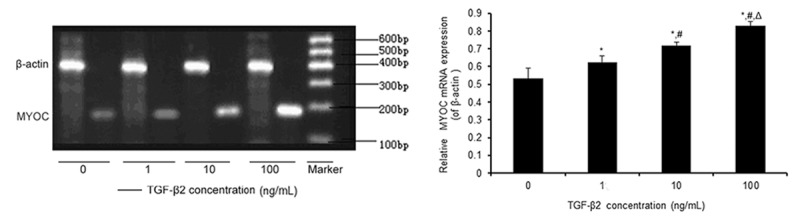
Effect of TGF-β2 on the expression of myocilin (MYOC) mRNA in human primary cultured TM cells. TM cells were treated with 0, 1, 10, and 100 ng/ml TGF-β2 (Lanes 1-4, respectively) for 48 h. Relative MYOC mRNA expression was determined by semi-quantitative RT-PCR. β-actin was used as an internal reference. The data are shown as the means±standard error of the mean (SEM) from the four cell lines with one cell line conducted in triplicate. *P < 0.05 vs. 0 ng/mL; #P < 0.05 vs. 1 ng/mL; ΔP < 0.05 vs. 10 ng/mL.
Effect of TGF-β2 on MYOC protein expression in TM cells
The ratios of the gray values of MYOC and β-actin in each group were 0.759±0.039, 0.809±0.015, 0.939±0.028, and 1.069±0.011 after treatment with 0, 1, 10, and 100 ng/ml TGF-β2, respectively, for 48 h (Figure 3). The relative values of MYOC protein in TM cells treated with 10 and 100 ng/ml TGF-β were both significantly higher than that in the control group (P < 0.05). The expression of MYOC protein following TGF-β2 treatment increased in a dose-dependent manner. MYOC expression in cells exposed to TGF-β2 for 12 or 24 h was not significantly different from that of control cells (data not shown).
Figure 3.
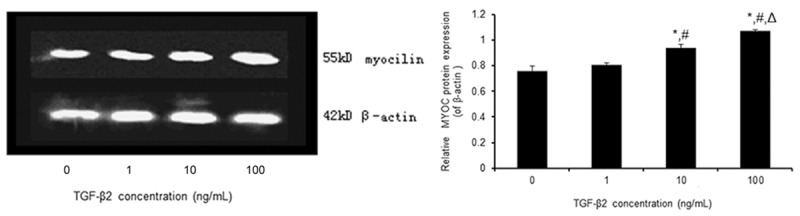
Effect of TGF-β2 on the expression of MYOC protein in human primary cultured TM cells. TM cells were treated with 0, 1, 10, and 100 ng/ml TGF-β2, respectively, for 48 h. Relative MYOC protein expression was determined by Western blot. β-actin was used as an internal reference. The data are shown as the means±SEM from the four cell lines with one cell line conducted in triplicate. *P < 0.05 vs. 0 ng/ml; #P < 0.05 vs. 1 ng/ml; ΔP < 0.05 vs. 10 ng/ml.
Effect of TGF-β2 on MYOC protein secretion in TM cells
The results of analysis with ELISA showed that the concentrations of MYOC protein in the culture media were 7.107±1.163 pg/ml, 7.879±1.894 pg/ml, 8.063±1.181 pg/ml, and 8.902±0.699 pg/ml after treatment with 0, 1, 10, and 100 ng/ml of TGF-β2, respectively, for 48 h (Figure 4). The concentrations MYOC protein were significantly higher in all of the experimental groups compared to the concentration in cells not exposed to TGF-β2 (0 ng/ml, P < 0.05). MYOC protein secretion following TGF-β2 treatment increased in a dose-dependent manner. MYOC secretion in cells exposed to TGF-β2 for 12 or 24 h was not significantly different from that of control cells (data not shown).
Figure 4.
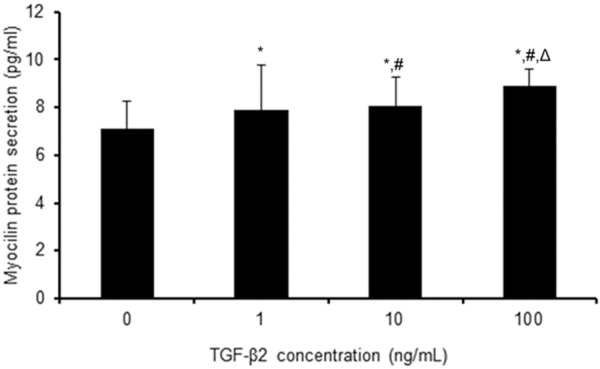
Effect of TGF-β2 on the secretion of MYOC protein in human primary cultured TM cells. TM cells were treated with 0, 1, 10, and 100 ng/ml TGF-β2, respectively, for 48h. The secretion of MYOC protein was determined by ELISA. The data are shown as the means±SEM from the four cell lines with one cell line conducted in triplicate. *P < 0.05 vs. 0 ng/ml; #P < 0.05 vs. 1 ng/ml; ΔP < 0.05 vs. 10 ng/ml.
Discussion
Although increased levels of MYOC and TGF-β2 can be detected in the AH of some patients with POAG [17,37], a clear relationship between these two factors and a precise understanding of their role in the disease etiology have yet to be fully established [39,40]. In this study, we showed that MYOC mRNA and protein expression in primary cultured TM cells was increased by TGF-β2 treatment in a dose-dependent manner. Furthermore, our results of an analysis with ELISA indicate that all of the concentrations of TGF-β2 that were tested increased MYOC secretion.
In this study, we showed that TGF-β2 facilitates transcription of the MYOC gene in TM cells in a dose-dependent manner. This is in accordance with the results of the RT-PCR analysis reported by Tamm et al. following an in vitro investigation of the factors regulating TM MYOC/TIRG mRNA expression in fresh TM, perfusion-cultured TM, and primary TM cells [42]. With a significantly increased expression in the AH of patients with POAG, it can be hypothesized that TGF-β2 plays a much more important role than does TGF-β1 in the pathogenesis of this disease. We also found that the expression of MYOC protein increased in response to TGF-β2 treatment in a dose-dependent manner. This finding is in accordance with the RT-PCR results. These data indicate that TGF-β2 promotes the expression of MYOC protein in TM cells in a concentration-dependent manner.
The concentration of TGF-β2 in human AH is significantly elevated in patients with POAG [17,43]. The results of our study provide further evidence in support of the speculation that TGF-β2 may be involved in the pathogenesis of POAG. In the human TM, TGF-β2 enhances the expression of the matrix metalloproteinase-2 (MMP-2) precursor but reduces the activity of MMP-2 by enhancing the activity of plasminogen activator inhibitor-1 (PAI-1) [44]. As a result, exposure to TGF-β2 decreases degradation of the TM ECM, and this effect contributes to greater AH outflow resistance and increased IOP. These and other data indicate that TGF-β2 can trigger a complex process of remodeling within the TM ECM. How might MYOC contribute to this event? MYOC interacts with itself and with a number of ECM proteins, such as collagen, fibronectin, and laminin [33]. Additionally, analysis in mice that overexpress MYOC revealed altered expression of two proteins involved in cell adhesion and signaling: the carcinoembryonic-antigen-related celladhesion molecule (CEACAM) and mindin/spondin 2 [45]. Taken together, the available evidence indicate that MYOC functions downstream of TGF-β2 and may regulate the ECM of the TM.
TGF-β2 expression in the AH can regulate the contractile properties of TM cells in part through regulation of the expression of FN and α-smooth muscle actin and the formation of actin stress fibers and αvβ3 integrin-containing cross-linked actin networks (CLANS) in the TM [46-48]. These results suggest a potential molecular interaction between the cytoskeleton and the synthesis or gathering of ECM components, and this interaction may have a substantial effect on drainage of AH. Notably, CLANS are induced in TM cells by not only exposure to TGF-β2, but also by treatment with dexamethasone [46,49], a potent inducer of MYOC expression. However, data exist that indicate MYOC can disrupt actin stress fibers and focal adhesions [50]. Therefore, TGF-β2-induced rearrangements of the cytoskeletal network of TM cells appear to be mediated by opposing forces, one of which may be MYOC. Future studies will need to determine how the increased expression of MYOC in TM cells contributes to the global response to TGF-β2 exposure.
Our observations are in conflict with the data reported by Howell et al. [37], who found no significant correlation between myocilin secretion and TGF-β2 levels in patients with POAG. However, this study was performed in a small number of patients and deserves further investigation. Our findings also differ from those of Resch et al [51]. These authors treated cultures of primary human TM cells with 10, 100, or 1000 ng/ml of TGF-β2 but did not observe increased secretion of MYOC in conditioned media. We believe that this discrepancy arises from differences in experimental procedures. First, Resch and colleagues used Western blots to analyze the expression of MYOC in the conditioned media of TM cells treated with TGF-β2, while we used ELISA. Western blotting and ELISA analyses differ in sensitivity and in the nature of quantitative data that is obtained. Although not large in magnitude, we observed significant differences in the quantity of MYOC protein in the media of TM cells after exposure to TGF-β2. Importantly, we complemented our examination of the expression of secreted MYOC protein with analyses of the expression of intracellular MYOC protein and MYOC mRNA, and our results support the finding that treatment with TGF-β2 increases the secretion of MYOC in the TM. Second, Resch et al. report that the TM cells used in their experiments were passaged four to eight times, while the cells used in our experiments were at the third passage. The possibility exists that extended passage of TM cells leads to a different response profile after exposure to TGF-β2. After consideration of these factors, we conclude that regulation of MYOC by TGF-β2 in vitro is sensitive to experimental conditions and that additional studies of the relationship between these two proteins must account for this sensitivity.
Based on the results of this study, we conclude that TGF-β2 promotes the expression and secretion of MYOC in human primary cultured TM cells. However, further research is needed to determine the signaling events that include these two factors in the pathogenesis of POAG. An improved understanding of the etiology of POAG is essential for diagnosis and the development of therapeutic strategies.
Acknowledgements
This work was supported by the Natural Science Foundation of Fujian Province (Grant No. 2010J01148) and the Nursery Backbone of Scientific Research Foundation of Second Affiliated Hospital of Fujian Medical University. The authors would like to thank the laboratory of Molecular Biology, Quanzhou Medical College, Fujian for the technical assistance. The authors are also grateful for the acquisition of donor eyes from Eye and Tissue Bank, Fuzhou General Hospital of Nanjing Military Command.
Disclosure of conflict of interest
None.
References
- 1.Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82:887–888. [PMC free article] [PubMed] [Google Scholar]
- 2.Foets B, van den Oord J, Engelmann K, Missotten L. A comparative immunohistochemical study of human corneotrabecular tissue. Graefes Arch Clin Exp Ophthalmol. 1992;230:269–274. doi: 10.1007/BF00176303. [DOI] [PubMed] [Google Scholar]
- 3.Lin S, Lee OT, Minasi P, Wong J. Isolation, culture, and characterization of human fetal trabecular meshwork cells. Curr Eye Res. 2007;32:43–50. doi: 10.1080/02713680601107058. [DOI] [PubMed] [Google Scholar]
- 4.Stone RA, Kuwayama Y, Laties AM, Marangos PJ. Neuron-specific enolase-containing cells in the rhesus monkey trabecular meshwork. Invest Ophthalmol Vis Sci. 1984;25:1332–1334. [PubMed] [Google Scholar]
- 5.Tamm ER, Russell P, Piatigorsky J. Development of characterization of a immortal and differentiated murine trabecular meshwork cell line. Invest Ophthalmol Vis Sci. 1999;40:1392–1403. [PubMed] [Google Scholar]
- 6.Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol. 2001;85:1277–1282. doi: 10.1136/bjo.85.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng JW, Cheng SW, Ma XY, Cai JP, Li Y, Lu GC, Wei RL. Myocilin polymorphisms and primary open-angle glaucoma: a systematic review and meta-analysis. PLoS One. 2012;7:e46632. doi: 10.1371/journal.pone.0046632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renard JP, Rouland JF, Bron A, Sellem E, Nordmann JP, Baudouin C, Denis P, Villain M, Chaine G, Colin J, de Pouvourville G, Pinchinat S, Moore N, Estephan M, Delcourt C. Nutritional, lifestyle and environmental factors in ocular hypertension and primary open-angle glaucoma: an exploratory case-control study. Acta Ophthalmol. 2013;91:505–513. doi: 10.1111/j.1755-3768.2011.02356.x. [DOI] [PubMed] [Google Scholar]
- 10.The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 11.Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 12.The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 13.Junglas B, Kuespert S, Seleem AA, Struller T, Ullmann S, Bosl M, Bosserhoff A, Kostler J, Wagner R, Tamm ER, Fuchshofer R. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am J Pathol. 2012;180:2386–2403. doi: 10.1016/j.ajpath.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Stamer WD, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Curr Opin Ophthalmol. 2012;23:135–143. doi: 10.1097/ICU.0b013e32834ff23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tektas OY, Lutjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Takai Y, Tanito M, Ohira A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Invest Ophthalmol Vis Sci. 2012;53:241–247. doi: 10.1167/iovs.11-8434. [DOI] [PubMed] [Google Scholar]
- 17.Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta2. Exp Eye Res. 1994;59:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- 18.Fuchshofer R, Tamm ER. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012;347:279–290. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]
- 19.Prendes MA, Harris A, Wirostko BM, Gerber AL, Siesky B. The role of transforming growth factor beta in glaucoma and the therapeutic implications. Br J Ophthalmol. 2013;97:680–686. doi: 10.1136/bjophthalmol-2011-301132. [DOI] [PubMed] [Google Scholar]
- 20.Johnson DH. Myocilin and glaucoma: A TIGR by the tail? Arch Ophthalmol. 2000;118:974–978. [PubMed] [Google Scholar]
- 21.Hewitt AW, Mackey DA, Craig JE. Myocilin allele-specific glaucoma phenotype database. Hum Mutat. 2008;29:207–211. doi: 10.1002/humu.20634. [DOI] [PubMed] [Google Scholar]
- 22.Ueda J, Wentz-Hunter KK, Cheng EL, Fukuchi T, Abe H, Yue BY. Ultrastructural localization of myocilin in human trabecular meshwork cells and tissues. J Histochem Cytochem. 2000;48:1321–1330. doi: 10.1177/002215540004801003. [DOI] [PubMed] [Google Scholar]
- 23.Kubota R, Noda S, Wang Y, Minoshima S, Asakawa S, Kudoh J, Mashima Y, Oguchi Y, Shimizu N. A novel myosin-like protein (myocilin) expressed in the connecting cilium of the photoreceptor: molecular cloning, tissue expression, and chromosomal mapping. Genomics. 1997;41:360–369. doi: 10.1006/geno.1997.4682. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- 25.Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002;21:395–428. doi: 10.1016/s1350-9462(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 26.Anderssohn AM, Cox K, O’Malley K, Dees S, Hosseini M, Boren L, Wagner A, Bradley JM, Kelley MJ, Acott TS. Molecular chaperone function for myocilin. Invest Ophthalmol Vis Sci. 2011;52:7548–7555. doi: 10.1167/iovs.11-7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donegan RK, Hill SE, Turnage KC, Orwig SD, Lieberman RL. The glaucoma-associated olfactomedin domain of myocilin is a novel calcium binding protein. J Biol Chem. 2012;287:43370–43377. doi: 10.1074/jbc.M112.408906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon HS, Tomarev SI. Myocilin, a glaucoma-associated protein, promotes cell migration through activation of integrin-focal adhesion kinase-serine/threonine kinase signaling pathway. J Cell Physiol. 2011;226:3392–3402. doi: 10.1002/jcp.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKay BS, Congrove NR, Johnson AA, Dismuke WM, Bowen TJ, Stamer WD. A role for myocilin in receptor-mediated endocytosis. PLoS One. 2013;8:e82301. doi: 10.1371/journal.pone.0082301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aroca-Aguilar JD, Sanchez-Sanchez F, Ghosh S, Fernandez-Navarro A, Coca-Prados M, Escribano J. Interaction of recombinant myocilin with the matricellular protein SPARC: functional implications. Invest Ophthalmol Vis Sci. 2011;52:179–189. doi: 10.1167/iovs.09-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fautsch MP, Vrabel AM, Johnson DH. The identification of myocilin-associated proteins in the human trabecular meshwork. Exp Eye Res. 2006;82:1046–1052. doi: 10.1016/j.exer.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Joe MK, Kee C, Tomarev SI. Myocilin interacts with syntrophins and is member of dystrophin-associated protein complex. J Biol Chem. 2012;287:13216–13227. doi: 10.1074/jbc.M111.224063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda J, Wentz-Hunter K, Yue BY. Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Invest Ophthalmol Vis Sci. 2002;43:1068–1076. [PubMed] [Google Scholar]
- 34.Wentz-Hunter K, Ueda J, Yue BY. Protein interactions with myocilin. Invest Ophthalmol Vis Sci. 2002;43:176–182. [PubMed] [Google Scholar]
- 35.Burns JN, Turnage KC, Walker CA, Lieberman RL. The stability of myocilin olfactomedin domain variants provides new insight into glaucoma as a protein misfolding disorder. Biochemistry. 2011;50:5824–5833. doi: 10.1021/bi200231x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gobeil S, Letartre L, Raymond V. Functional analysis of the glaucoma-causing TIGR/myocilin protein: integrity of amino-terminal coiled-coil regions and olfactomedin homology domain is essential for extracellular adhesion and secretion. Exp Eye Res. 2006;82:1017–1029. doi: 10.1016/j.exer.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Howell KG, Vrabel AM, Chowdhury UR, Stamer WD, Fautsch MP. Myocilin levels in primary open-angle glaucoma and pseudoexfoliation glaucoma human aqueous humor. J Glaucoma. 2010;19:569–575. doi: 10.1097/IJG.0b013e3181d13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fautsch MP, Bahler CK, Jewison DJ, Johnson DH. Recombinant TIGR/MYOC increases outflow resistance in the human anterior segment. Invest Ophthalmol Vis Sci. 2000;41:4163–4168. [PubMed] [Google Scholar]
- 39.Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001;239:109–113. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- 40.Menaa F, Braghini CA, Vasconcellos JP, Menaa B, Costa VP, Figueiredo ES, Melo MB. Keeping an eye on myocilin: a complex molecule associated with primary open-angle glaucoma susceptibility. Molecules. 2011;16:5402–5421. doi: 10.3390/molecules16075402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo MS, Wu YY, Liang ZB. Hyaluronic acid increases MMP-2 and MMP-9 expressions in cultured trabecular meshwork cells from patients with primary open-angle glaucoma. Mol Vis. 2012;18:1175–1181. [PMC free article] [PubMed] [Google Scholar]
- 42.Tamm ER, Russell P, Epstein DL, Johnson DH, Piatigorsky J. Modulation of myocilin/TIGR expression in human trabecular meshwork. Invest Ophthalmol Vis Sci. 1999;40:2577–2582. [PubMed] [Google Scholar]
- 43.Picht G, Welge-Luessen U, Grehn F, Lutjen-Drecoll E. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthalmol. 2001;239:199–207. doi: 10.1007/s004170000252. [DOI] [PubMed] [Google Scholar]
- 44.Fuchshofer R, Welge-Lussen U, Lutjen-Drecoll E. The effect of TGF-beta2 on human trabecular meshwork extracellular proteolytic system. Exp Eye Res. 2003;77:757–765. doi: 10.1016/s0014-4835(03)00220-3. [DOI] [PubMed] [Google Scholar]
- 45.Paper W, Kroeber M, Heersink S, Stephan DA, Fuchshofer R, Russell P, Tamm ER. Elevated amounts of myocilin in the aqueous humor of transgenic mice cause significant changes in ocular gene expression. Exp Eye Res. 2008;87:257–267. doi: 10.1016/j.exer.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filla MS, Schwinn MK, Nosie AK, Clark RW, Peters DM. Dexamethasone-associated cross-linked actin network formation in human trabecular meshwork cells involves beta3 integrin signaling. Invest Ophthalmol Vis Sci. 2011;52:2952–2959. doi: 10.1167/iovs.10-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Reilly S, Pollock N, Currie L, Paraoan L, Clark AF, Grierson I. Inducers of cross-linked actin networks in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:7316–7324. doi: 10.1167/iovs.10-6692. [DOI] [PubMed] [Google Scholar]
- 48.Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010;298:C749–763. doi: 10.1152/ajpcell.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark AF, Wilson K, McCartney MD, Miggans ST, Kunkle M, Howe W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1994;35:281–294. [PubMed] [Google Scholar]
- 50.Wentz-Hunter K, Shen X, Okazaki K, Tanihara H, Yue BY. Overexpression of myocilin in cultured human trabecular meshwork cells. Exp Cell Res. 2004;297:39–48. doi: 10.1016/j.yexcr.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 51.Resch ZT, Hann CR, Cook KA, Fautsch MP. Aqueous humor rapidly stimulates myocilin secretion from human trabecular meshwork cells. Exp Eye Res. 2010;91:901–908. doi: 10.1016/j.exer.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


