Abstract
Glioblastoma tumor cells release microvesicles, which contain mRNA, miRNA and angiogenic proteins. These tumor-derived microvesicles transfer genetic information and proteins to normal cells. Previous reports demonstrated that the increased microvesicles in cerebrospinal fluid (CSF) of patients with glioblastoma up-regulate procoagulant activity. The concentration of microvesicles was closely related to thromboembolism incidence and clinical therapeutic effects of glioblastoma patients. However, it is still not clear how CSF microvesicles and what factors affect glioblastoma development. In this study, we collected the plasma and CSF from glioblastoma patients and healthy volunteers. Microvesicles acquired from serum or CSF were added to cultured endothelial cells. And the effects of these microvesicles on endothelial cells were examined. Our results showed that microvesicles from CSF of patients, but not from circulating blood, promoted endothelial cells migration and proliferation in vitro. In addition, the degree of endothelial cell proliferation triggered by microvesicles from CSF was reduced when treated with siRNA targeting Akt/beta-catenin, suggesting that the Akt/beta-catenin pathway is involved in the microvesicle-initiated endothelial cell proliferation. In conclusion, glioblastoma mainly affects microvesicles within CSF without showing significant impact on microvesicles in circulating blood. Microvesicles from the CSF of glioblastoma patients may initiate endothelial cell growth and thus promote cell invasion. This effect may be directly exerted by activated Akt/beta-catenin pathway.
Keywords: Microvesicles, glioblastoma, Akt/beta-catenin, endothelial cells
Introduction
Glioblastomas (GBMs) are the most common malignant primary brain tumors in humans. They are highly aggressive and heterogeneous, and remain to be a major therapeutic challenge [1]. The median survival of GBM patients after diagnosis ranges from only 3 months (without therapy) to no more than 15 months even with advanced medical care.
Microvesicles (MVs) are small membrane-enclosed vesicles secreted from different types of cells, and are important mediators of intercellular communication by transferring effector bio-molecules [2]. MVs are categorized into three major groups according to their origins, including endothelial microvesicles (EMVs), platelet-derived microvesicles (PMVs) and leukocytes-derived microvesicles (LMVs). They have recently been reported to serve as indicators in the diagnosis, prognosis and surveillance of different types of cancers, such as ovarian cancer [3,4], bladder cancer [5], mesothelioma [6], colorectal cancer [7], and liver tumor [8]. Increase of MV content is associated with endothelial dysfunction, abnormal hemostasis/thrombosis, pro-inflammatory states [9] and poor clinical prognosis for cancer patients. For example, cancer features a prothrombotic state marked with increased prevalence of venous thromboembolism (VTE) [10]. PMV has been reported to contribute to the VTE state in cancer patients [11], as PMVs-associated Factor Va, Factor VIII as well as tissue factor (TF) increase prothrombinase activity and exaggerate VTE condition.
MVs are easier to be isolated and of larger content than circulating tumor cells (CTCs). Moreover, MVs shed from cancer cells may contain specific microRNAs and proteins that strongly correlate with specific cancer progression stages [12]. Thus, MVs released into microenvironment from various tumer cells represent a group of novel tumor diagnostic biomarkers.
Glioblastoma releases tumor-promoting microvesicles [13]. EMVs and LMVs shed from glioblastomas have been shown to exhibit procoagulant properties due to their richness of phosphatidylserine [14,15]. On the other hand, endothelial cells have been shown to function in the formation of perivascular niche to promote the self-renewal of CSCs in GBM [16]. Recently it has been shown that MVs released by glioblastoma tumor (though comprised of various cell types) can be taken up by normal cells, and followed by the translation of proteins or microRNA known for gliomagenesis in normal cells [17]. However, the effect of MVs generated by GBM on endothelial cells remains less studied.
The conserved beta-catenin pathway regulates stem cell pluripotency and cell fate decisions during development and adult life, while aberrant beta-catenin signaling is associated with a wide range of diseases, including cancers [18]. Beta-catenin has also been demonstrated as a highly predictive biomarker of the short survival for GBM patients. It plays a critical role in glioblastoma exaggeration through the phosphoinositide 3-kinase (PI3K)/Akt/glycogen synthase kinase-3 beta (GSK-3β) and wnt/beta-catenin pathways, which initiates subsequent transcriptional activation of oncogenes [19]. Endothelial wnt/β-catenin signaling is necessary for angiogenesis of the central nervous system and blood-brain barrier (BBB) differentiation. Furthermore, GSK-3β inhibitor can suppress glioma cell growth through abovementioned two pathways [20]. Recently, Ghosh et al. reported that MVs mediate Akt activation and further modulate beta-catenin pathway in leukemic disease progression through increased expression of cyclin D1 and c-myc in bone marrow stromal cells (BMSCs) [21], and further confirmed that MVs played more roles than just biomarkers during tumor progression.
Here, by quantification of MV content in plasma and CSF samples from GBM patients and control samples from healthy volunteers, the MVs content change in GBM patients was demonstrated for the first time. In addition, by incubating endothelial cells with CSF MVs from GBM patients, we observed activated and significantly enhanced beta-catenin pathway activity. We also reported here that the stimulated endothelial cells by concentrated MVs could promote in vitro angiogenesis and cell invasion, and this stimulation is caused by the activated beta-catenin pathway.
Materials and methods
Patients recruitment
A total of 105 GBM patients were recruited for collecting plasma or CSF samples from the Department of Neurosurgery, The Second Affiliated Hospital of Suzhou University and Department of Surgical Oncology, Zhejiang University School of Medicine from 2005 to 2011. Informed consent from patients was obtained, and the study was approved by local ethics committee.
Sample collection
Samples from a total of 105 patients were analyzed. CSF specimens was obtained either during brain tumor surgery by lumbar puncture as part of the routine procedure aimed at controlling brain pressure during surgery (n = 65) or during chemotherapy of glioma patients (n = 40). Vacuum blood collection tube with sodium citrate as anticoagulation (BD vacutainer, citrate) was used for sample collection. Blood samples were centrifuged at 3000 rpm for 15 minutes to separate plasma, then at 13000 g for 2 minutes to collect platelet-poor plasma (PPP). CSF (n = 40) and paired plasma (n = 40) for control were obtained from healthy volunteers. Patients continued to take necessary medications to control blood pressure or glucose.
MV detection by flow cytometry
50 μL plasma sample was transferred to flow cytometry testing tubes (Becton, Dickinson and Company, BD falcon) and was mixed with four fluorescence stains (4 μL of each, KeyGen Biotech Company) and 4 μL heparin. Plasma samples were incubated at room temperature (25°C) for 15 minutes in the dark and then mixed with 200 μL loading buffer (KeyGen Biotech). Beckman Coulter Gallios flow cytometry was used to detect MVs, which has been proved of high accuracy in MVs detecting and enumeration for MVs of different origins. EMV, PMV and LMV were identified by CD144-Phycoerythrin (PE), CD41b-fluorescein isothiocyanate (FITC) and CD45-PerCP-Cy5.5 (KeyGen Biotech Company), respectively. Enumeration of subsets of MVs was confirmed by dual-positive Annexin V and fluorescence label staining. The detecting protocol was generally complied with a previous report by Robert et al. [22]. During MVs quantization, the background to isotypic irrelevant IgG was subtracted. Megamix contains 0.5 μm and 0.9 μm fluorescent beads were applied in our method to ensure the accuracy of MVs measurement. Procoagulant PMVs are identified as CD41 and AnnexinV positive. EMVs and LMVs are characterized as CD 144 and AnnexinV positive, or CD45 and AnnexinV positive, respectively.
Cell culture
Human umbilical vein endothelial cells (HUVECs, ATCC) were maintained in endothelial cell growth medium (EGM) containing 10% fetal bovine serum (FBS) (GIBCO) in cell culture dishes. The human endothelial cell line EA.hy926 (ATCC) which were generated by fusion of HUVECs with the permanent human lung carcinoma cell line A549, was cultured in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO) supplemented with 10% fetal calf serum (GIBCO). Cell cultures were maintained at 37°C in a humidified atmosphere containing (5% CO2, 95% air).
Endothelial cells treated with concentrated MVs
PPP or CSF samples were ultracentrifugated (Hitachi CP-WX, Japan) at 100000 g (4°C, 1 hour) to get MV precipitation, which was resuspended in DMEM. Different concentrations (102, 103, 104, 105/ml) of MVs were tested and the activation effect to beta-catenin were noticed to be enhanced with the increase of MV concentration, thus the highest concentration 105/ml was used in endothelial cells treatment. Cells were incubated with MVs for 24 hrs before examination.
Knockdown beta-catenin expression in endothelial cells by siRNA transfection
EA.hy926 cultured in six well plates in DMEM with 10% FBS. 24 hours after plating, DMEM medium was removed and replaced with serum-free medium. EA.hy926 was transfected with 20 ng/ml siRNA via lipofectamine (Invitrogen). 6 hours later, serum-free medium was removed and replaced with complete medium and cells were kept culturing at 37°C in 5% CO2. Targeted siRNA sequences against beta-catenin is 5’-CAGGGGGUUGUGGUUAAGCUCUU-3’. A scramble sequence was used as negative control [23].
Western blotting analysis
72 hours after beta-catenin siRNA transfection, total proteins of transfected HUVEC or EA.hy926 cells lysis buffer were extracted (30 min, on ice) and the total protein concentration was determined by the bicinchoninic acid assay (BCA assay). Equal amount of proteins (50 ug) from lysates was separated by electrophoresis in 8% SDS-polyacrylamide gel. Separated proteins were transferred to a NC membrane. After blocking with 5% non-fat milk, membranes were incubated with primary antibodies against PI3K p85α (1:500 dilution, Santa Cruz), Akt, p-Akt, beta-catenin, p-beta-catenin (1:500 dilution, Cell Signaling) at room temperature for 4 hours, washed and incubated with horseradish peroxidase-conjugated secondary antibodies for 2 hours at room temperature. After extensive washing, membranes were developed with enhance chemiluminescence reagents (Thermo). The band density of specific proteins was quantified after normalization with the density of β-actin.
MTT test
The viability of siRNA-transfected EA. Hy926 and control cells were measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, 5*103 cells were plated into each well of a 96-well plate. At 24, 48 and 72 h after plating and incubation with complete DMEM, respectively, 20 μl of MTT (5 mg/ml) was added to each well and incubated for 4 h at room temperature. 200 μl DMSO was added into each well and incubated for 5 min. Optical density were measured at 570 nm and cell viability were expressed as percentage of negative control.
In vitro tube-formation assay
Tube formation assays were performed as described [24]. Briefly, HUVEC and siRNA transfected cells were seeded in 96-well and incubated in reduced serum (0.2% FBS) to test angiogenesis activators and full serum media (2% FBS) to test angiogenesis inhibitors. Cells were incubated at 37°C for 6 h, fixed in formalin, stained with Oregon Green 488 Phalloidin (Molecular Probes), and imaged using fluorescence microscopy. Number of tube branches (in pixels) was quantified using the ImageJ software.
TCF reporter luciferase assay
The TCF reporter luciferase assay was performed following manufacture’s protocol (Qiagen). Briefly, cells were transfected with TCF/catenin reporter plasmid and expression plasmid. As an internal control to monitor Renilla plasmid (pRL) were added. Luciferase activities were determined 24 hr after transfection using the Dual-Luciferase Assay System.
Statistical analysis
Data were expressed as mean ± SEM. Data analysis and concentration-response curves were obtained with Prism (GraphPad Software). The comparison between groups was performed by one-way ANOVA followed by Bonferroni’s test as appropriate. Statistical significance was determined at P < 0.05 level.
Results
MVs content change in peripheral blood and cerebrospinal fluid (CSF) in GBM patients compared with healthy control
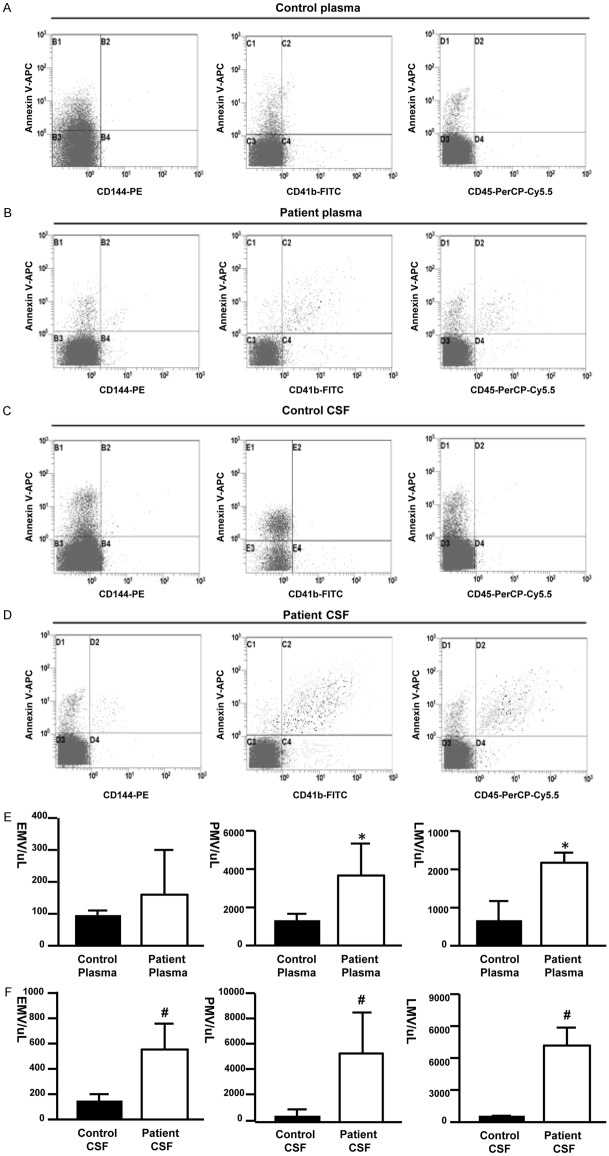
To investigate whether glioblastoma is associated with changes of circulating microvesicles, plasma and CSF samples from both GBM patients and healthy volunteers were examined by flow cytometry. Three subgroups of MVs, EMV, PMV and LMV, were measured and identified as with CD144-PE positive, CD41b-FITC positive and CD45-PerCP-Cy5.5 positive signals, respectively (Figure 1A-D). MVs content increased significantly in CSF samples from patients compared with CSF samples from controls, represented by the up-regulation of all three subpopulations of MVs (Figure 1F). EMV, PMV and LMV were also mildly increased in plasma samples from GBM patients comparing to those from controls (Figure 1E).
Figure 1.
MVs levels were up-regulated in GBM patients. (A-D) Representative scatter plot of microvesicles obtained by flow cytometry. Representative quadrants derived from the microvesicles collected from healthy control plasma (A), GBM patient plasma (B), healthy control CSF (C) and GBM patient CSF (D). The horizontal and vertical axes represent labeling with cell marker antibodies and Annexin V-APC, respectively (E, F). *P < 0.05 denotes significant difference.
MVs released by glioblastoma affect cell viablilty of endothelia cells
To determine the effect of GBM-derived MVs on normal cells in the tumor environment, endothelial cells (EA. hy.926 and HUVEC) were treated with MVs drawn from patients’ plasma or patients’ CSF, respectively, and the cytoactivity was measured by MTT method (Materials and methods). For EA. hy.926 cells, cytoactivity of control cells was measured as 0.028 ± 0.013 (n = 20) (Figure 2A). Cell viability of cells treated with MVs drawn from patients’ plasma (0.034 ± 0.015, n = 20, Figure 2A) showed no significant change compared with controls. While cells treated with CSF MVs from GBM patients exhibited significant increase in cell viability (0.153 ± 0.073, n = 20, *P < 0.05) compared to controls. These data suggest that MVs released in CSF of GBM patients effectively increased cell viability of endothelial cells.
Figure 2.
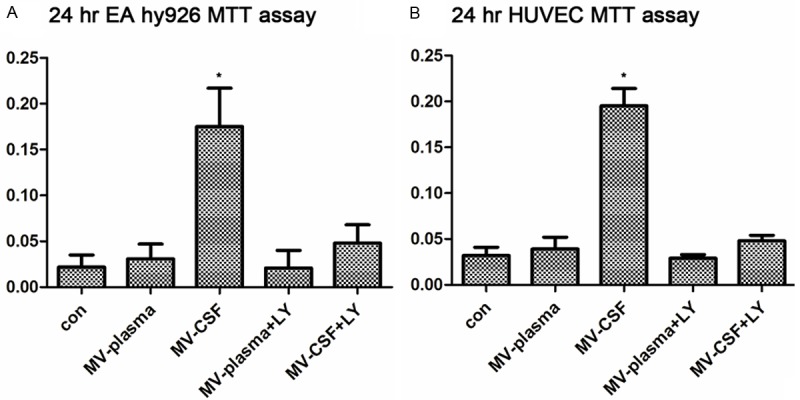
MVs derived from CSF of GBM patients enhanced viability of in vitro endothelial cells. Effects of MVs were tested on the viability of endothelial cell line EA. hy926 (A) and primary cell culture HUVEC (B). (A) Cell viability of EA.hy926 treated with different MVs is shown after 24 h incubation. (B) Cell viability of HUVEC treated with different MVs is shown after 24 h incubation. The viability was measured by MTT assay. The different MVs are from: con: non MVs; MV-plasma: MVs from plasma; MV-CSF: MVs from CSF; MV-plasma+LY: MVs from plasma, with inhibitor LY294002; MV-plasma+LY: MVs from CSF, with inhibitor LY294002. Data are represented as the mean ± SD from three independent experiments. An asterisk indicates P < 0.05.
Moreover, to test if the increased cell viability is due to Akt/beta-catenin pathway, Akt pathway inhibitor (LY294002) was added to these cells together with MVs from patients’ plasma or patients’ CSF, respectively. When LY294002 was added, the cell viability was tested to be 0.021 ± 0.069 (n = 20) for cells treated with plasma MVs and 0.051 ± 0.063 (n = 20) for cells incubated with CSF MVs. Thus, LY294002, the inhibitor of phosphoinositide 3-kinases (PI3K), can significantly abrogate the stimulating effect of MVs from patients’ CSF, and this effect might be related to the (PI3K) pathway, in which beta-catenin might be the key effector.
While for HUVEC, similar trends were observed (Figure 2B). MTT test for untreated control HUVEC was 0.0471 ± 0.012 (n = 20), which increased to 0.193 ± 0.032 (n = 20) when treated with CSF MVs from GBM patients. While treatment with MVs from plasma did not lead to significant change in MTT test (0.047 ± 0.015, n = 20), cell viability of MV-treated HUVEC decreased to control level when treated with LY294002 (Figure 2B).
GBM derived MVs stimulates endothelial cell angiogenesis in vitro
Capillary tube formation ability is a key feature of mature endothelial cells and has been used as an in vitro method to screen for factors that promote or inhibit angiogenesis [25]. Using this method, we examined whether MVs from patients’ plasma or CSF can drive capillary tube formation of HUVEC cells in the collagen culture (Materials and methods). Tube lengths of capillaries were recorded with a light microscope and the length of each capillary was quantified. As shown in Figure 3, HUVEC formed capillary tube networks with variable lengths in collagen gel under different treatments. Compared with non-treated control (tube length as 100%), cells treated with CSF MV only (105/ml) (Figure 3D) exhibited significantly enhanced tube formation ability (295.7%, n = 5, *P < 0.05, Figure 3G). While HUVEC cells treated with plasma MVs did not show significantly change in tube formation length. Furthermore, both siRNA (against beta-catenin) and LY294002 significantly abolished the enhanced tube formation ability of HUVEC cells triggered by CSF MVs (Figure 3F, 3G), suggesting that the tube formation ability of HUVEC cells incubated with CSF MVs was enhanced through beta-catenin-associated signaling pathways.
Figure 3.
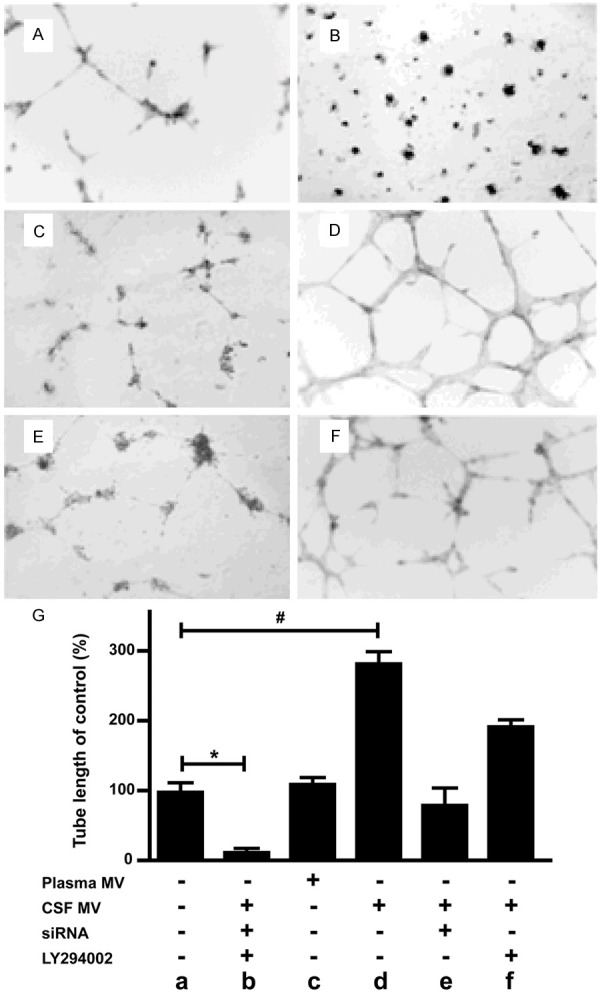
MVs derived from CSF of GBM patients promote angiogenesis in an in vitro endothelial tube formation assay, and tube formation is sensitive to Beta-catenin signaling. (A-F) are representative images. (G) Histogram of quantified branches for MVs effect; mean ± s.e.m., n = 5, *, #, P < 0.05 compared to control.
MVs activates beta-catenin pathway in endothelial cells in an Akt-dependent manner
The MTT assay and tube formation results indicate that Akt/beta-catenin pathway was involved in effect of CSF MVs on endothelial cells. Therefore, we next compared the protein levels of Akt and beta-catenin in HUVEC cells treated with CSF MVs, and HUVEC cells treated with plasma MVs from either patients or controls to determine whether MVs derived from GBM increased the expression of Akt or Beta-catenin in recipient cells. As shown in Figure 4A, the beta-catenin and Akt protein levels in HUVEC cells significantly increased after incubated with MVs for 12 hours, indicating involvement of MVs in activation of beta-catnin pathway in these endothelial cells.
Figure 4.
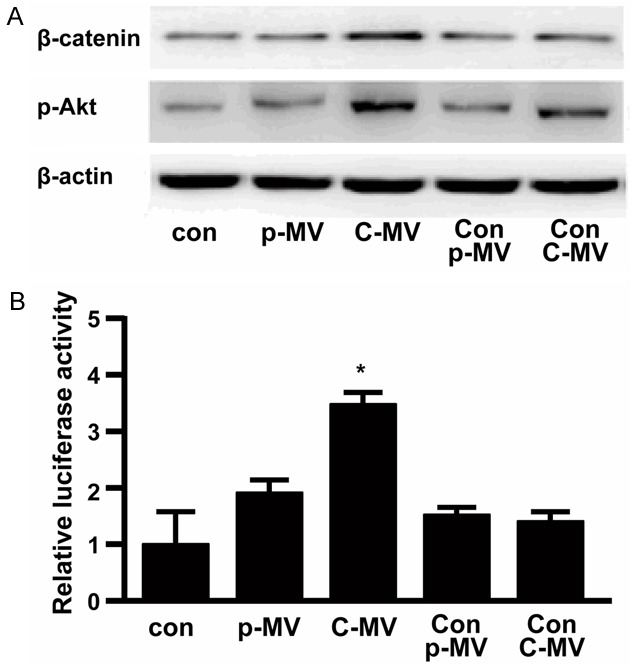
A. Western blotting demonstrating changed β-catenin and phosphorylated Akt expression in endothelial cells treated with different MVs conditions. con: non-treated cells; p-MV: cells treated with plasma MV from GBM patient; C-MV: cells treated with CSF MV from GBM patient; Con-p-MV: cells treated with plasma MV from control volunteer; Con-C-MV: cells treated with CSF MV from control volunteer. The experiment was performed three times with similar results. β-actin was measured as a loading and lysis control. B. Relative luciferase activity driven by TCF-responsive promoter in differently treated cells are shown.
Beta-catenin recruits TCF/LEF transcription factors in the nucleus and binds to enhancers of target genes, leading to transcription and expression of Wnt-responsive genes. To test the effects of activation of beta-catenin pathway initiated by tumor-derived MVs, we also tested whether CSF MVs can lead to the activation of downstream genes of the beta-catenin pathway. The ratio between luciferase activity of a TCF-responsive reporter construct and that of a control luciferase reporter gene construct represents TCF/beta-catenin-mediated gene activation, which was increased by approximately three times after CSF MVs treatment (Figure 4B). Thus, only CSF MVs from GBM patients can significantly increase TCF activity compared with the non-treated control, indicating the phosphorylation of Akt and beta-catenin, which result in the elevated expression of downstream target genes.
Discussion
In this study, we used several experimental approaches to demonstrate that micorvesicle (MV) levels are significantly increased in cerebrospinal fluid (CSF) of GBM patients and these MVs can induce angiogenic activity by stimulating endothelial cell tube formation. Our data also provide evidence that MVs released from glioblastoma is associated with the activation of beta-catenin pathways in endothelial cells treated with these MVs. And the activation of beta-catenin pathway in endothelial cells is critical for the enhanced cell viability and angiogenesis. The results have several important implications, including the finding of the novel extracellular function of MVs, and potentially linking MVs with angiogenic signaling in other cancers. However, the interaction between GBM MVs and endothelial cells, the effectors carried by MVs triggering the beta-catenin activity and responsiveness of other cell types to GBM MVs remain to be identified.
Glioblastoma is the most common type of primary malignant brain tumor with poor prognosis despite of advances in surgical and chemo-radiation therapies. Angiogenesis is considered to be an important factor in the development of malignant brain tumors, especially for glioblastoma. Abnormal vascular construction with a glomeruloid appearance is characteristic of GBM. Beta-catenin is known as one of the adhesive molecules associated not only with cell adhesion and cell polarity, but also with carcinogenesis [26]. Moreover, beta-catenin signaling was associated with proliferative responses in GBM. We first put forward the hypothesis that up-regulated microvesicles in glioblastoma patients (circulating blood or CSF) may play an important role in surrounding normal cells, as to serve as a transporter of cancer effector molecules. In this study, we demonstrated that MVs concentrated from CSF of GBM patients stimulated endothelial cell proliferation and enhanced cyto-viablity. By comparison the in vitro effect of differently-originated MVs, we demonstrated that only CSF MVs from GBM patients significantly induced angiogenesis capability of endothelial cells (Figure 3).
However, there are several questions remain to be explored. First, we could not exclude the effect of exosomes during the microvesicle-triggered endothelial cell transformation, since exosoms have emerged as novel subcellular transduction channels for signal molecules and microRNAs [27,28]. Microvesicles and exosomes share a lot of biological characteristics. Both are indicated as therapeutic carriers and transducers for certain reactive signals [29]. Currently the collection of exosomes and microvesicles are mainly relied on ultracentrifugation (exosomes: 130000 g, 30 min; MVs: 100000 g, 60 min), yet no studies has reported any method to separate these two groups of particles from each other. Both particles can stimulate angiogenesis and mediate Reactive oxygen species (ROS) level when they were treated with cells [30]. Besides, exosomes characterized with even smaller size (30 to 100 nm in diameter) compared to MVs (100 to 1000 nm in diameter), presenting higher demand for accuracy and detecting resolution for testing exosomes by cytometry.
Also we focused on MVs in plasma and CSF, which reflect the overall condition of patients, instead of MVs specifically extracted from tumor tissue. Methods to isolate MVs originated directly from tumor issues remain to be studied. After all, MVs directly originated from glioblastoma in GBM patients in vivo can tell us more about roles played by MVs under certain conditions.
Various mechanisms have connected MVs with other bio-chemical signals or effectors such as C-reactive protein [31], Annexin I/phosphatidylserine receptor [32], P-selectin [33], beta-1 integrin [34], T-cadherin [35], p38 [36], and etc. Beta-catenin has been proven to play a significant role in tumorigenicity via two separate signal pathways: the wnt/beta-catenin pathway and GSK-3beta/beta-catenin pathway, of which beta-catenin serves as the intersection. In GBM patients, the expression level of wnt/beta-catenin pathway has been proven to correlate with GBM patient survival time. Wnt signaling is required for central nervous system (CNS) development and pattern formation of the embryonic nervous system. Over-expression of Wnt3/beta-catenin increases hippocampal progenitor and stem cell neurogenesis in vivo [37]. And siRNA knockdown of Wnt2 and β-catenin inhibits cell proliferation and invasion and induces apoptosis in human U251 glioma cells [37].
In summary, we explored the mechanism underlying the interaction between MVs and endothelial cells, where MVs promote cell viability and proliferation of endothelial cells by activating beta-catenin pathway. In the future, treatment methods targeting on MVs by inhibiting beta-catenin signaling may be a new approach to improve prognosis in glioblastoma patients.
Disclosure of conflict of interest
None.
References
- 1.Wang Y, Jiang T. Understanding high grade glioma: Molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331:139–146. doi: 10.1016/j.canlet.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Principe S, Hui AB, Bruce J, Sinha A, Liu FF, Kislinger T. Tumor-derived exosomes and microvesicles in head and neck cancer: Implications for tumor biology and biomarker discovery. Proteomics. 2013;13:1608–23. doi: 10.1002/pmic.201200533. [DOI] [PubMed] [Google Scholar]
- 3.Yang Q, Kang YQ, Wang HJ, Yin GF, Fang K, Yang K. [Controlled release of paclitaxel from microparticles containing PLLA and its anti-tumor activity on human ovarian carcinoma cell line] . Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:212–216. [PubMed] [Google Scholar]
- 4.Chablani L, Tawde SA, Akalkotkar A, D’Souza C, Selvaraj P, D’Souza MJ. Formulation and evaluation of a particulate oral breast cancer vaccine. J Pharm Sci. 2012;101:3661–3671. doi: 10.1002/jps.23275. [DOI] [PubMed] [Google Scholar]
- 5.Perry JR. Thromboembolic disease in patients with high-grade glioma. Neuro Oncol. 2012;14(Suppl 4):iv73–iv80. doi: 10.1093/neuonc/nos197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macura SL, Hillegass JM, Steinbacher JL, MacPherson MB, Shukla A, Beuschel SL, Perkins TN, Butnor KJ, Lathrop MJ, Sayan M, Hekmatyar K, Taatjes DJ, Kauppinen RA, Landry CC, Mossman BT. A multifunctional mesothelin antibody-tagged microparticle targets human mesotheliomas. J Histochem Cytochem. 2012;60:658–674. doi: 10.1369/0022155412452567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urbanska AM, Karagiannis ED, Guajardo G, Langer RS, Anderson DG. Therapeutic effect of orally administered microencapsulated oxaliplatin for colorectal cancer. Biomaterials. 2012;33:4752–4761. doi: 10.1016/j.biomaterials.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy A, Coldwell D, Sangro B, Wasan H, Salem R. Radioembolization for the treatment of liver tumors general principles. Am J Clin Oncol. 2012;35:91–99. doi: 10.1097/coc.0b013e3181f47583. [DOI] [PubMed] [Google Scholar]
- 9.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, László V, Pállinger E, Pap E, Kittel A, Nagy G, Falus A, Buzás EI. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakkar AK. Cancer-associated thrombosis. Br J Cancer. 2010;102(Suppl 1):S1. doi: 10.1038/sj.bjc.6605598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owens AP 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Principe S, Hui AB, Bruce J, Sinha A, Liu FF, Kislinger T. Tumor-derived exosomes and microvesicles in head and neck cancer: Implications for tumor biology and biomarker discovery. Proteomics. 2013;13:1608–23. doi: 10.1002/pmic.201200533. [DOI] [PubMed] [Google Scholar]
- 13.Glioblastoma produces tumor-promoting microvesicles. Nat Clin Pract Neurol. 2009;5:120–121. [PubMed] [Google Scholar]
- 14.Hrachovinova I, Cambien B, Hafezi-Moghadam A, Kappelmayer J, Camphausen RT, Widom A, Xia L, Kazazian HH Jr, Schaub RG, McEver RP, Wagner DD. Interaction of P-selectin and PSGL-1 generates microparticles that correct hemostasis in a mouse model of hemophilia A. Nat Med. 2003;9:1020–1025. doi: 10.1038/nm899. [DOI] [PubMed] [Google Scholar]
- 15.Tans G, Rosing J, Thomassen MC, Heeb MJ, Zwaal RF, Griffin JH. Comparison of anticoagulant and procoagulant activities of stimulated platelets and platelet-derived microparticles. Blood. 1991;77:2641–2648. [PubMed] [Google Scholar]
- 16.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 17.D’Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012;26:1287–1299. doi: 10.1101/gad.192351.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Atkins RJ, Dimou J, Paradiso L, Morokoff AP, Kaye AH, Drummond KJ, Hovens CM. Regulation of glycogen synthase kinase-3 beta (GSK-3β) by the Akt pathway in gliomas. J Clin Neurosci. 2012;19:1558–1563. doi: 10.1016/j.jocn.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 20.D’Asti E, Garnier D, Lee TH, Montermini L, Meehan B, Rak J. Oncogenic extracellular vesicles in brain tumor progression. Front Physiol. 2012;3:294. doi: 10.3389/fphys.2012.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh AK, Secreto CR, Knox TR, Ding W, Mukhopadhyay D, Kay NE. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: Implications for disease progression. Blood. 2010;115:1755–1764. doi: 10.1182/blood-2009-09-242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert S, Lacroix R, Poncelet P, Harhouri K, Bouriche T, Judicone C, Wischhusen J, Arnaud L, Dignat-George F. High-sensitivity flow cytometry provides access to standardized measurement of small-size microparticles--brief report. Arterioscler Thromb Vasc Biol. 2012;32:1054–1058. doi: 10.1161/ATVBAHA.111.244616. [DOI] [PubMed] [Google Scholar]
- 23.Rossi M, Magnoni L, Miracco C, Mori E, Tosi P, Pirtoli L, Tini P, Oliveri G, Cosci E, Bakker A. β-catenin and Gli1 are prognostic markers in glioblastoma. Cancer Biol Ther. 2011;11:753–761. doi: 10.4161/cbt.11.8.14894. [DOI] [PubMed] [Google Scholar]
- 24.Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc. 2010;5:628–35. doi: 10.1038/nprot.2010.6. [DOI] [PubMed] [Google Scholar]
- 25.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yano H, Hara A, Takenaka K, Nakatani K, Shinoda J, Shimokawa K, Yoshimi N, Mori H, Sakai N. Differential expression of beta-catenin in human glioblastoma multiforme and normal brain tissue. Neurol Res. 2000;22:650–656. doi: 10.1080/01616412.2000.11740735. [DOI] [PubMed] [Google Scholar]
- 27.El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C, Alvarez-Erviti L, Sargent IL, Wood MJ. Exosome-mediated delivery of sirna in vitro and in vivo. Nat Protoc. 2012;7:2112–2126. doi: 10.1038/nprot.2012.131. [DOI] [PubMed] [Google Scholar]
- 28.Sharma P, Cosme J, Gramolini AO. Recent advances in cardiovascular proteomics. J Proteomics. 2013;81:3–14. doi: 10.1016/j.jprot.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thebaud B, Stewart DJ. Exosomes: cell garbage can, therapeutic carrier, or trojan horse? Circulation. 2012;126:2553–5. doi: 10.1161/CIRCULATIONAHA.112.146738. [DOI] [PubMed] [Google Scholar]
- 30.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–11. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habersberger J, Strang F, Scheichl A, Htun N, Bassler N, Merivirta RM, Diehl P, Krippner G, Meikle P, Eisenhardt SU, Meredith I, Peter K. Circulating microparticles generate and transport monomeric C-reactive protein in patients with myocardial infarction. Cardiovasc Res. 2012;96:64–72. doi: 10.1093/cvr/cvs237. [DOI] [PubMed] [Google Scholar]
- 32.Jansen F, Yang X, Hoyer FF, Paul K, Heiermann N, Becher MU, Abu Hussein N, Kebschull M, Bedorf J, Franklin BS, Latz E, Nickenig G, Werner N. Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1925–1935. doi: 10.1161/ATVBAHA.112.253229. [DOI] [PubMed] [Google Scholar]
- 33.Mayne E, Funderburg NT, Sieg SF, Asaad R, Kalinowska M, Rodriguez B, Schmaier AH, Stevens W, Lederman MM. Increased platelet and microparticle activation in HIV infection: upregulation of P-selectin and tissue factor expression. J Acquir Immune Defic Syndr. 2012;59:340–346. doi: 10.1097/QAI.0b013e3182439355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collier ME, Ettelaie C. Induction of endothelial cell proliferation by recombinant and microparticle-tissue factor involves beta1-integrin and extracellular signal regulated kinase activation. Arterioscler Thromb Vasc Biol. 2010;30:1810–1817. doi: 10.1161/ATVBAHA.110.211854. [DOI] [PubMed] [Google Scholar]
- 35.Philippova M, Suter Y, Toggweiler S, Schoenenberger AW, Joshi MB, Kyriakakis E, Erne P, Resink TJ. T-cadherin is present on endothelial microparticles and is elevated in plasma in early atherosclerosis. Eur Heart J. 2011;32:760–771. doi: 10.1093/eurheartj/ehq206. [DOI] [PubMed] [Google Scholar]
- 36.Curtis AM, Wilkinson PF, Gui M, Gales TL, Hu E, Edelberg JM. P38 mitogen-activated protein kinase targets the production of proinflammatory endothelial microparticles. J Thromb Haemost. 2009;7:701–709. doi: 10.1111/j.1538-7836.2009.03304.x. [DOI] [PubMed] [Google Scholar]
- 37.Gong A, Huang S. FoxM1 and Wnt/β-catenin signaling in glioma stem cells. Cancer Res. 2012;72:5658–5662. doi: 10.1158/0008-5472.CAN-12-0953. [DOI] [PMC free article] [PubMed] [Google Scholar]