Abstract
Objective: To investigate the expression profile of miR-140-3p in formalin-fixed paraffin-embedded (FFPE) tissues of spinal chordoma, and its correlation with the prognosis of spinal chordoma patients. Methods: Dysregulated miRNAs in FFPE tissues of spinal chordoma were identified by microarray analysis. MiR-140-3p expression in surgically removed spinal chordoma tissues of 42 spinal chordoma patients (27 males and 15 females, aged 29-76 years) and corresponding nucleus pulposus tissues of 14 patients with disc herniation as the healthy control group (8 males and 6 females, aged 24-73 years) was measured by real-time quantitative RT-PCR assay. The association of miR-140-3p expression with clinicopathologic characteristics of spinal chordoma patients was analyzed. Additionally, we investigated the prognostic significance of miR-140-3p with the use of Kaplan-Meier methods and a Cox proportional hazard model. Results: The expression of miR-140-3p was significantly higher in chordoma tissues than nucleus pulposus tissues (t = 3.530, P = 0.001). The expression of miR-140-3p positively correlated with surrounding muscle invasion. The Kapan-Meier survival analysis showed that the patients with high miR-140-3p expression had a significantly worse recurrence-free survival than those with a low expression (χ 2 = 31.270, P = 0.000, log-rank test). In addition, univariate and multivariate analyses for recurrence-free survival showed that miR-140-3p expression was an independent prognostic factor for patients with spinal chordoma (HR = 1.361, 95% CI: 1.135-1.633, P = 0.001). Conclusion: Over-expression of miR-140-3p is correlated with recurrence and tumor invasion, suggesting that miR-140-3p could be a new predictor for recurrence and prognosis in patients with spinal chordoma.
Keywords: MicroRNA, chordoma, spine, prognosis, recurrence, case-control study
Introduction
Chordoma is a relatively rare mesenchymal tissue tumor that accounts for 1%-4% of all bone malignancies with an age-adjusted incidence of 0.08/100000 [1,2]. Although comparatively low malignancy, chordoma is refractory to conventional chemotherapy or radiotherapy, which poses a therapeutic challenge. The patients are vulnerable to relapse after surgery, which significantly reduces their quality of life postoperatively, and seriously affects their prognosis. Currently, there is lack of reliable prognostic marker for this disease [3,4].
MicroRNAs (miRNAs) are a class of small non-coding single-stranded RNA molecules of about 19-22 nucleotides long, and can regulate gene expression and function by mainly interacting with 3’ UTRs of their target messenger RNA [5]. Previous studies have demonstrated that miRNAs acting as tumor promoters or tumor suppressors are involved in the regulation of a variety of malignancies [6-9]. However, reports focusing on miRNAs expression profiling in chordoma are limited [10-13]. Moreover, data regarding the prognostic value of miRNAs in spinal chordoma are almost nonexistent. Recently, a study by Duan et al. [14] showed that miR-1 expression for chordoma samples from non-survivors were significantly lower than that of survivors (P < 0.001), and their Kaplan-Meier survival analysis indicated that the patients group with a low miR-1 expression had a significantly worse clinical outcome than those with a high expression (P < 0.04).
Normally, fresh and frozen tissue samples are considered to be optimum for expression analysis of DNA, RNA and protein. But, because of the limitation and inefficiency in prospectively collecting enough fresh and frozen samples and the availability of only formalin fixed paraffin embedding (FFPE) tissues in most pathology departments, researchers are attempting to employ alternative tissues samples, such as FFPE tissues, for disease analysis [15]. Despite RNAs are found to degrade in FFPE tissue [16], there is evidence that miRNAs are stable in FFPE tissue due to shorter length or secondary structure [17,18], and degraded tissue samples are also suitable for miRNA studies [19-22].
Although emerging evidence has demonstrated the aberrant expression of miR-140-3p in fresh-frozen tissue samples of chordoma or other cancer patients [11,23-26], little is known about the miRNAs expression levels in FFPE tissue and their potential source as prognosis biomarkers. In the current study, we identified the elevated expression level of miR-140-3p in FFPE tissues from spinal chordoma patients by miRNA microarry analysis, and then investigated the association of differentially expressed miR-140-3p with clinico-pathologic characteristics of these patients. Meanwhile, we further explored the prognostic value of miR-140-3p for spinal chordoma patients.
Materials and methods
Patients and samples
From June 2002 to November 2013, FFPE tumor samples from 42 spinal chordoma patients (27 males and 15 females, aged 29-76 years, mean, 52.93 ± 11.50 years) and FFPE nucleus pulposus samples from 14 patients with disc herniation as the healthy control group (8 males and 6 females, aged 24-73 years, mean, 49.21 ± 13.35 years) were collected in this study. There are 32 primary spinal chordoma patients and 10 recurrent patients. All the patients were diagnosed with histopathologically and clinically confirmed spinal chordoma of conventional subtype. The tissue samples were obtained as per the guidelines of The second Xiangya Hospital’s protocol including patient consent and specimen collection. The clinical data were obtained in all patients (Table 1), including age, gender, tumor size, tumor location, surrounding muscle invasion and preoperative recurrence status. Preoperative recurrence means the patient had previously received surgical tumor excision for one time and had relapse on admission. Surrounding muscle invasion means the tumor invaded into surrounding muscle, which was evaluated by preoperative Magnetic Resonance Imaging [27]. Patients were followed-up clinically and radiographically at 3-month intervals in the first two years after surgery, and every six months three years postoperatively and annually thereafter. Recurrence-free survival time (RFS) was defined as the time interval from surgical resection to disease relapse, which was evaluated from clinical examinations and imaging [3]. No patients received chemotherapy or radiotherapy before the surgical excision.
Table 1.
Correlation of miR-140-3p relative expression level with clinico-pathological factors of spinal chordoma patients
| miR-140-3p expression | P-value | OR (95% CI) | ||
|---|---|---|---|---|
|
|
||||
| Clinicopathological factors | Low (n = 24) | High (n = 18) | ||
| Age (years) | ||||
| ≤ 50 | 12 | 7 | 0.474 | 1.211 (0.719-2.037) |
| > 50 | 12 | 11 | ||
| Sex | ||||
| Male | 15 | 12 | 0.780 | 1.080 (0.634-1.841) |
| Female | 9 | 6 | ||
| Tumor size | ||||
| ≤ 5 cm | 11 | 8 | 0.929 | 1.024 (0.606-1.731) |
| > 5 cm | 13 | 10 | ||
| Tumor location | ||||
| Sacral vertebra | 20 | 16 | 0.685 | 0.833 (0.441-1.575) |
| Cervical or thoracic vertebra | 4 | 2 | ||
| Surrounding muscle invasion | ||||
| Yes | 7 | 17 | 0.000* | 3.238 (1.719-6.101) |
| No | 17 | 1 | ||
| Preoperative recurrence | ||||
| Yes | 5 | 5 | 0.875 | 1.188 (0.600-2.351) |
| No | 19 | 13 | ||
P < 0.05.
Total RNA extraction
Total RNA was extracted from 42 FFPE tissue samples of spinal chordoma patients and 14 controls using mirVanaTM RNA Isolation Kit (Applied Biosystem, Cat.No. AM 1556) according to the manufacturer’s specifications. The yield of RNA was determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, USA), and the integrity was evaluated using agarose gel electrophoresis stained with ethidium bromide.
Microarray and hierarchical cluster analysis
To determine the global miRNA expression profiles in FFPE tissues of spinal chordoma and corresponding controls, we performed a microarray analysis using The Agilent Human miRNA Microarray Kit (Agilent Technologies, Santa Clara, CA, USA; Ver. 6.5, 8x60 K, 3 slides/kit, Cat. No. G4140-90040) comprising 2006 human mature miRNAs. The data of microarray analysis was imported into Genespring software (Version 12.5, Agilent) and normalized by quantile. Two-sided t tests and fold change were used to select differentially expressed miRNAs. After installing Java (Tm) platform SE Binary (Version 6.0, Sun Microsystems, Inc.), hierarchical cluster analysis was performed using MultiExperiment Viewer tool (Version 4.6, Dana-Farber Cancer Institute) for differentially expressed miRNAs. The selection criteria for differential miRNAs was defined as P-value < 0.05 (t test) and fold change > 5.
Real-time quantitative PCR of selected miRNA
There are seven significantly differentially expressed miRNAs (hsa-let-7b-3p, hsa-let-7f-1-3p, miR-4725-5p, miR-634, miR-1304-3p, miR-140-3p and miR-4649-3p) in FFPE tissues of spinal chordoma patients with more than 19-fold fold change. Among those, we focused on miR-140-3p because there is evidence suggesting that miR-140-3p was significantly up-regulated in skull base chorodma compared with normal tissues [11]. Moreover, miR-140-3p was also found to have altered expression in various human cancers [23-26]. Therefore, we further validated the expression level of miR-140-3p by subsequent real-time quantitative PCR assay, and investigated its correlation with clinico-pathological features of spinal chordoma patients as well as the potential role of miR-140-3p in chordoma progression.
Quantification was performed with a two-step reaction process: reverse transcription (RT) and PCR. Each RT reaction consisted of 1 μg RNA, 4 μl miScript HiSpec buffer, 2 μl Nucleics Mix and 2 μl miScript Reverse Transcriptase Mix (Qiagen, Germany), in a total volume of 20 μl. Reactions were performed in a GeneAmp® PCR System 9700 (Applied Biosystem, USA) for 60 minutes at 37°C, followed by heat inactivation of RT for 5 minutes at 95°C. The 20 μl RT reaction mix was then diluted 5 times in nuclease-free water and held at -20°C. Real-time PCR was performed using LightCycler® 480 II Real-time PCR Instrument (Roche, Swiss) with 10 μl PCR reaction mixture that included 1 μl cDNA, 5 μl LightCycler® 480 SYBR Green I Master (Roche, Swiss) that was diluted 2 times, 0.2 μl universal primer (Qiagen, Germany), 0.2 μl microRNA-specific primer and 3.6 μl nuclease-free water. Reactions were incubated in a 384-well optical plate (Roche, Swiss) at 95°C for 10 minutes, followed by 40 cycles of 95°C for 10 seconds, 60°C for 30 seconds. Each sample was run in triplicate for analysis. At the end of the PCR cycles, melting curve analysis was performed to validate the specific generation of the expected PCR product. The microRNA-specific primer sequences were designed in the laboratory and synthesized by Generay Biotech (Generay, PRC) based on the microRNA sequences obtained from the miRBase database (Release 20.0) as follows: TACCACAGGGTAGAACCACGG. The expression levels of microRNAs were normalized to U6 and were calculated using the 2-ΔΔCt method.
Statistical analysis
All statistical analyses were performed using SPSS17.0. The comparison of miRNA expression level between chordoma and controls was performed using Student’s t test. The χ 2 test was used to analyze the association of miR-140-3p expression level with clinicopathological characteristics. Kaplan-Meier method was used to estimate the survival rates and survival curves between low and high miR-140-3p expression group, whose difference was evaluated by Log-rank test. Cox proportional hazard model was used to analyze the correlation between multiple factors and survival time. All values showed are two-sided. A P-value < 0.05 was considered statistically significant.
Results
Differential expression of miRNAs in chordoma samples
When the selection criteria for differential miRNAs was P-value < 0.05 (t test) and fold change > 5, 72 of 2006 human mature miRNAs were differentially expressed in chordoma compared with nucleus pulposus tissues. Of the 72 miRNAs that were differentially expressed, 4 was upregulated and 68 was downregulated as compared to miRNA expression level in controls (Figure 1). Furthermore, there are seven significantly differentially expressed miRNAs (hsa-let-7b-3p, hsa-let-7f-1-3p, miR-4725-5p, miR-634, miR-1304-3p, miR-140-3p and miR-4649-3p) in FFPE tissues from spinal chordoma patients with more than 19-fold fold change.
Figure 1.
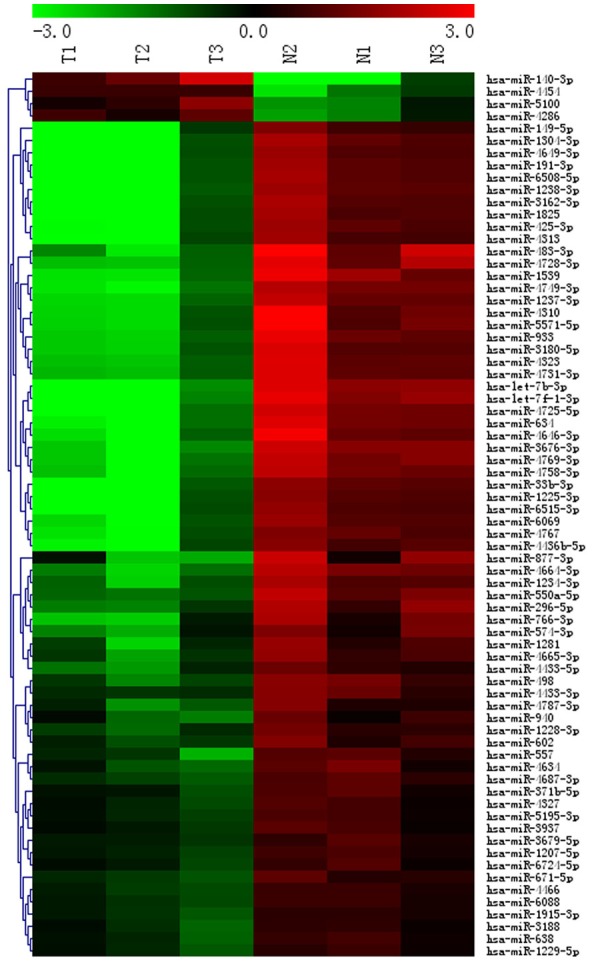
A heat-map representation of the differentially expressed miRNAs patterns in chordoma (T) versus nucleus pulposus tissues (N). Relative expression is presented as a colorgram (red: high expression).
Relative expression of miR-140-3p between patient and control group
The differences of sex composition and age distribution between case and control group were not statistically significant (χ 2 = 0.229, P = 0.633 and t = 1.005, P = 0.319, respectively). The expression level of miR-140-3p in chordoma tissues was significantly higher than that in nucleus pulposus samples (t = 3.530, P = 0.001). The average expression level of miR-140-3p in chordoma tissues was 3.56 ± 3.17. The comparision of miR-140-3p expression between patient and control group was shown in Figure 2.
Figure 2.
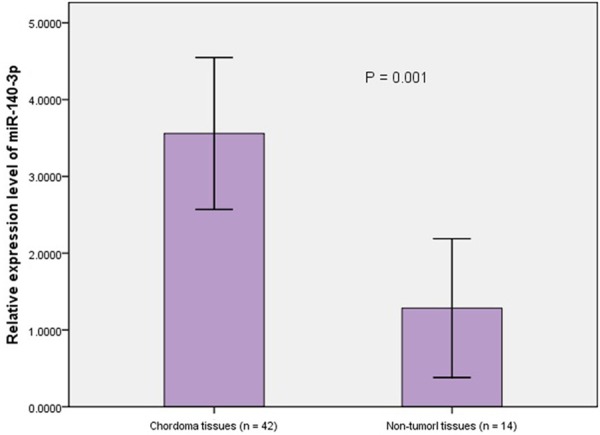
The detection of miR-140-3p expression in FFPE tissues from 42 spinal chordoma patients and 14 controls using qRT-PCR assay. After normalization to U6 RNA, the expression level of miR-140-3p was significantly higher in chordoma tissues (3.56 ± 3.17) compared with that in non-tumor tissues (1.28 ± 1.57, P = 0.001).
Association of miR-140-3p relative expresison with clinico-pathological characteristics of spinal chordoma patients
To evaluate whether the high expression of miR-140-3p was correlated with the clinical progression of spinal chordoma, the patients were classified into two groups according to the overall mean expression of miR-140-3p: low miR-140-3p expression group (≤ 3.56-fold, n = 24) and high miR-140-3p expression group (> 3.56-fold, n = 18). We analyzed the association of miR-140-3p relative expresison with clinico-pathological characteristics of these patients (Table 1). Our results revealed that the patients with a high miR-140-3p expression had a significantly higher risk of the occurrence of surrounding muscle invasion than those with a low expression (OR = 3.238, 95% CI: 1.719-6.101, P = 0.000). The expression of miR-140-3p showed no significantly difference in age, gender, tumor size, tumor location and preoperative recurrence in these patients (Table 2).
Table 2.
Univariate analysis of prognostic factors for Recurrence-free survival rates of spinal chordoma patients
| Factors | Categories | χ 2 | P-value |
|---|---|---|---|
| Sex | Male/Female | 0.033 | 0.857 |
| Age | ≤ 50/> 50 | 1.536 | 0.215 |
| Tumor size | ≤ 5 cm/> 5 cm | 0.044 | 0.834 |
| Tumor location | Sacral vertebra/Cervical or thoracic vertebra | 0.611 | 0.434 |
| Preoperative recurrence | Yes/No | 0.000 | 0.984 |
| Surrounding muscle invasion | Yes/No | 26.917 | 0.000* |
| miR-140-3p expression | High/Low | 31.270 | 0.000* |
P < 0.05.
Comparison of survival rate between different miR-140-3p expression and surrounding muscle invasion group using Kaplan-Meier method
The median RFS time was 28.0 months (range, 5-72 months). During follow-up, 32 patients (76.19%) had relapse. Patients with a high miR-140-3p expression in chordoma had a significantly shorter RFS as compared to those with a low expression (χ 2 = 31.270, P = 0.000, log-rank test). The survival curve of high and low miR-140-3p expression group was shown in Figure 3. Similarly, the patients with preoperative surrounding muscle invasion had a significantly shorter RFS than those without surrounding muscle invasion (χ 2 = 26.917, P = 0.000, log-rank test). The survival curve of different surrounding muscle invasion status in these patients was shown in Figure 4.
Figure 3.
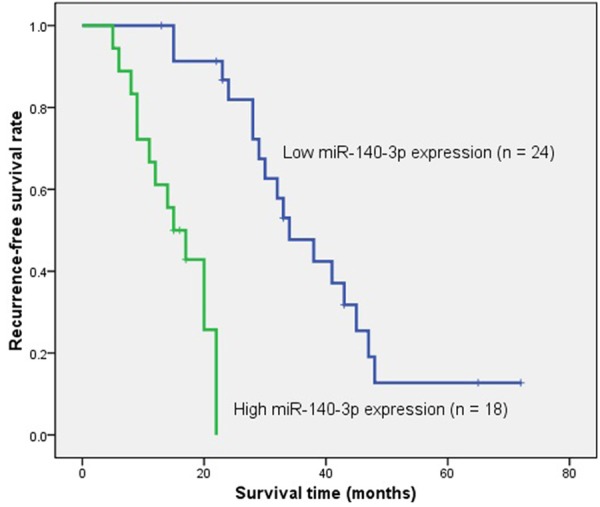
Kaplan-Meier survival curve of spinal chordoma patients with high versus low miR-140-3p expression. The recurrence-free survival rate of patients with high miR-140-3p expression was significantly lower that those with a low expression (χ 2 = 31.270, P = 0.000, log-rank test).
Figure 4.
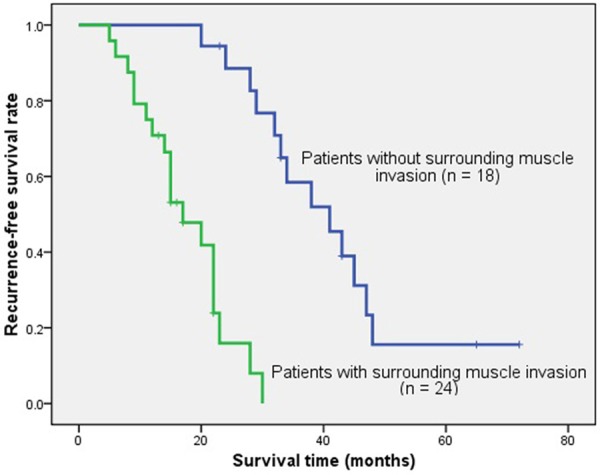
Kaplan-Meier survival curve of spinal chordoma patients with versus without surrounding muscle invasion. The recurrence-free survival rate of patients with surrounding muscle invasion was significantly lower that those without surrounding muscle invasion (χ 2 = 26.917, P = 0.000, log-rank test).
Analyze the impact of multiple factors on the survival time using Cox proportional hazard model
Cox’s multivariate analysis showed that miR-140-3p expression (HR = 1.361, 95% CI: 1.135-1.633, P = 0.001) and surrounding muscle invasion (HR = 8.483, 95% CI: 2.176-33.062, P = 0.002) were significantly associated with RFS of spinal chordoma patients as independent prognostic factors. The results of the Cox’s multivariate analysis were summarized in Table 3.
Table 3.
The results of risk factors selection and parameter estimation by Cox proportional hazard modela
| Parameters | B | SE | Wald | Sig. | HR | 95% CI for HR | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Lower | Upper | ||||||
| Sex | -0.357 | 0.418 | 0.727 | 0.394 | 0.700 | 0.308 | 1.589 |
| Age | -0.036 | 0.024 | 2.188 | 0.139 | 0.965 | 0.920 | 1.012 |
| Tumor size | -0.012 | 0.096 | 0.017 | 0.897 | 0.988 | 0.818 | 1.192 |
| Tumor location | 0.657 | 0.627 | 1.096 | 0.295 | 1.928 | 0.564 | 6.591 |
| Preoperative recurrence | -0.376 | 0.509 | 0.545 | 0.460 | 0.687 | 0.253 | 1.862 |
| Surrounding muscle invasion | 2.138 | 0.694 | 9.489 | 0.002* | 8.483 | 2.176 | 33.062 |
| miR-140-3p expression level | 0.309 | 0.093 | 11.056 | 0.001* | 1.361 | 1.135 | 1.633 |
Model Summary (χ 2 = 47.914, P = 0.000, Score test);
P < 0.05.
Discussion
Originating from embryonic remnants of the notochord, chordoma is a relatively rare and locally invasive mesenchymal tissue tumor with an annual incidence rate of 1/8000000 [2]. Due to its resistance to conventional chemotherapy or radiotherapy, the main treatment strategy of choice for this disease is thorough surgical excision. However, 40% of patients with chordoma can still have local recurrence after surgery [28,29], which significantly compromises their prognosis in this population. Given above, studies focus on identifying new biomarkers for recurrence or prognosis in chordoma are more than warranted. Currently, accumulating evidences have showed that deregulated protein expression or genetic aberration in chordoma is related to its recurrence or progression. But the prognostic role of miRNA using FFPE tissues in this entity is nonexistent. FFPE tissues are common specimen source of archival material and easily available in most histological departements because of their feasibility of long-term storage and ability to permanently preserve the structure of the tissue. miRNAs have been proven to be stable in FFPE tissue due to shorter length or secondary structure [17,18], and they are found to exhibit stable expression level in FFPE samples as compared to frozen tissues in several studies [17,30,31].
The expression profile of miR-140-3p in different human cancers is controversial as it can be up-regulated or down-regulated in tumor tissues compared with normal samples. Previous studies have found that miR-140-3p was down-regulated in cutaneous squamous cell carcinoma or basal cell carcinoma [23,24], ovarian cancer [25] and lung squamous cell carcinoma [26]. But miRNA expression profiles have revealed that miR-140-3p was significantly up-regulated in chordoma tissue [11], which was similarly noted in our study. This finding suggests that the over-expression of miR-140-3p may have a role in progression or recurrence of chordoma. To examine this assumption, we investigated the possible relationship between miR-140-3p expression and clinico-pathological information of these patients. We found that patients with over-expressed miR-140-3p had a increased risk of occurrence of surrounding muscle invasion. Further, our study identified the altered miR-140-3p expression as a independent predictor for short recurrence-free survival of spinal chordoma patients. The correlation of miR-140-3p up-expression with chordoma invasion and recurrence suggests the aberrant miR-140-3p expression may promote the malignant differentiation of this tumor, thus resulting in poor prognosis in this population.
Currently, the definite role that miRNAs play in the tumorigenesis or progression of chordoma remains unclear. Recent studies have shown that the differentially expressed miRNAs in chordoma could cause apoptosis of tumor cells, regulate tumor malignancy and inhibit cell growth and proliferation by regulating various targets, such as c-MET [11,13,14], epidermal growth factor receptor (EGFR) and Bcl-xL [13]. Meanwhile, in the study of Long et al., [12] researchers detected that five altered expression of miRNAs in chordoma likely had an important effect on the regulation of mitogen-activated protein kinase (MAPK) signal pathways. It is well-known that c-MET and EGFR are family members of receptor tyrosine kinase (RTKs), which has been demonstrated to exhibit abnormal expression in chordoma [32,33]. Moreover, RTKs are involved in pathways including RAS/ERK, PI3K/AKT, STAT and MAPK signaling pathways that have been shown to play key role in chordoma [32-35]. Considering aforementioned findings, we postulate that the altered expression of miR-140-3p might have a effect on recurrence and invasion of chordoma by partially regulating targets in signal pathways with the involvement of RTKs.
In conclusion, the over-expression of miR-140-3p in FFPE tissues of spinal chordoma patients was correlated with recurrence and tumor invasion, suggesting that miR-140-3p obtained from FFPE tissues could be a new predictor for recurrence and prognosis in these patients. Further studies with large sample size in order to target the precise molecular mechanisms by which miR-140-3p contributes to the recurrence and invasion of spinal chordoma are needed.
Disclosure of conflict of interest
None.
References
- 1.Bydon M, Papadimitriou K, Witham T, Wolinsky JP, Bydon A, Sciubba D, Gokaslan Z. Novel therapeutic targets in chordoma. Expert Opin Ther Targets. 2012;16:1139–43. doi: 10.1517/14728222.2012.714772. [DOI] [PubMed] [Google Scholar]
- 2.McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12:1–11. doi: 10.1023/a:1008947301735. [DOI] [PubMed] [Google Scholar]
- 3.Hu H, Yang HL, Lu J, Chen KW, Qiu YH, Liu W, Luo ZP. Association of telomerase expression with recurrence of sacral chordoma. Ann Oncol. 2012;23:2772. doi: 10.1093/annonc/mds462. [DOI] [PubMed] [Google Scholar]
- 4.Horbinski C, Oakley GJ, Cieply K, Mantha GS, Nikiforova MN, Dacic S, Seethala RR. The prognostic value of Ki-67, p53, epidermal growth factor receptor, 1p36, 9p21, 10q23, and 17p13 in skull base chordomas. Arch Pathol Lab Med. 2010;134:1170–6. doi: 10.1043/2009-0380-OA.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medina PP, Slack FJ. MicroRNAs and cancer: an overview. Cell Cycle. 2008;7:2485–2492. doi: 10.4161/cc.7.16.6453. [DOI] [PubMed] [Google Scholar]
- 6.Yu SL, Chen HY, Chang GC, Chen CY, Chen HW, Singh S, Cheng CL, Yu CJ, Lee YC, Chen HS, Su TJ, Chiang CC, Li HN, Hong QS, Su HY, Chen CC, Chen WJ, Liu CC, Chan WK, Chen WJ, Li KC, Chen JJ, Yang PC. MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell. 2008;13:48–57. doi: 10.1016/j.ccr.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Ozen M, Creighton CJ, Ozdemir M, Ittmann M. Wide-spread deregulation of microRNA expression in human prostate cancer. Oncogene. 2008;27:1788–1793. doi: 10.1038/sj.onc.1210809. [DOI] [PubMed] [Google Scholar]
- 8.Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, Poon TC, Ng SS, Sung JJ. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375–1381. doi: 10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- 9.Frenquelli M, Muzio M, Scielzo C, Fazi C, Scarfò L, Rossi C, Ferrari G, Ghia P, Caligaris-Cappio F. MicroRNA and proliferation control in chronic lymphocytic leukemia: functional relationship between miR-221/222 cluster and p27. Blood. 2010;115:3949–3959. doi: 10.1182/blood-2009-11-254656. [DOI] [PubMed] [Google Scholar]
- 10.Duan Z, Choy E, Nielsen GP, Rosenberg A, Iafrate J, Yang C, Schwab J, Mankin H, Xavier R, Hornicek FJ. Differential Expression of microRNA (miRNA) in Chordoma Reveals a Role for miRNA-1 in Met Expression. J Orthop Res. 2010;28:746–52. doi: 10.1002/jor.21055. [DOI] [PubMed] [Google Scholar]
- 11.Bayrak OF, Gulluoglu S, Aydemir E, Ture U, Acar H, Atalay B, Demir Z, Sevli S, Creighton CJ, Ittmann M, Sahin F, Ozen M. MicroRNA expression profiling reveals the potential function of microRNA-31 in chordomas. J Neurooncol. 2013;115:143–51. doi: 10.1007/s11060-013-1211-6. [DOI] [PubMed] [Google Scholar]
- 12.Long C, Jiang L, Wei F, Ma C, Zhou H, Yang S, Liu X, Liu Z. Integrated miRNA-mRNA analysis revealing the potential roles of miRNAs in chordomas. PLoS One. 2013;8:e66676. doi: 10.1371/journal.pone.0066676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Schiff D, Park D, Abounader R. MicroRNA-608 and MicroRNA-34a Regulate Chordoma Malignancy by Targeting EGFR, Bcl-xL and MET. PLoS One. 2014;9:e91546. doi: 10.1371/journal.pone.0091546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan Z, Shen J, Yang X, Yang P, Osaka E, Choy E, Cote G, Harmon D, Zhang Y, Nielsen GP, Spentzos D, Mankin H, Hornicek F. Prognostic significance of miRNA-1 (miR-1) expression in patients with chordoma. J Orthop Res. 2014;32:695–701. doi: 10.1002/jor.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goswami RS, Waldron L, Machado J, Cervigne NK, Xu W, Reis PP, Bailey DJ, Jurisica I, Crump MR, Kamel-Reid S. Optimization and analysis of a quantitative real-time PCR-based technique to determine microRNA expression in formalin-fixed paraffin-embedded samples. BMC Biotechnol. 2010;10:47–59. doi: 10.1186/1472-6750-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macabeo-Ong M, Ginzinger DG, Dekker N, Mc-Millan A, Regezi JA, Wong DT, Jordan RC. Effect of duration of fixation on quantitative reverse transcription polymerase chain reaction analy-ses. Mod Pathol. 2002;15:979–987. doi: 10.1097/01.MP.0000026054.62220.FC. [DOI] [PubMed] [Google Scholar]
- 17.Xi Y, Nakajima G, Gavin E, Morris CG, Kudo K, Hayashi K, Ju J. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixedparaffin-embedded samples. RNA. 2007;13:1668–74. doi: 10.1261/rna.642907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng ZQ, Yin JY, Tang Q, Liu FQ, Qian J, Lin J, Shao R, Zhang M, He L. Over-expression of miR-98 in FFPE tissues might serve as a valuable source for biomarkerdiscovery in breast cancer patients. Int J Clin Exp Pathol. 2014;7:1166–71. [PMC free article] [PubMed] [Google Scholar]
- 19.Peiró-Chova L, Peña-Chilet M, López-Guerrero JA, García-Giménez JL, Alonso-Yuste E, Burgues O, Lluch A, Ferrer-Lozano J, Ribas G. High stability of microRNAs in tissue samples of compromised quality. Virchows Arch. 2013;463:765–74. doi: 10.1007/s00428-013-1485-2. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Lu Y, Chen Y, Lu W, Xie X. MicroRNA profile of paclitaxel-resistant serous ovarian carcino-ma based on formalin-fixed paraffin-embed-ded samples. BMC Cancer. 2013;13:216–223. doi: 10.1186/1471-2407-13-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu SW, Chen L, Man YG. miRNA Biomarkers in Breast Cancer Detection and Management. J Cancer. 2011;2:116–22. doi: 10.7150/jca.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Chen J, Radcliffe T, Lebrun DP, Tron VA, Feilotter H. An array-based analysis of microRNA expression comparing matched frozen and formalin-fixed paraffin-embedded human tissue samples. J Mol Diagn. 2008;10:513–9. doi: 10.2353/jmoldx.2008.080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sand M, Skrygan M, Georgas D, Sand D, Hahn SA, Gambichler T, Altmeyer P, Bechara FG. Microarray analysis of microRNA expression in cutaneous squamous cell carcinoma. J Dermatol Sci. 2012;68:119–26. doi: 10.1016/j.jdermsci.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Sand M, Skrygan M, Sand D, Georgas D, Hahn SA, Gambichler T, Altmeyer P, Bechara FG. Expression of microRNAs in basal cell carcinoma. Br J Dermatol. 2012;167:847–55. doi: 10.1111/j.1365-2133.2012.11022.x. [DOI] [PubMed] [Google Scholar]
- 25.Miles GD, Seiler M, Rodriguez L, Rajagopal G, Bhanot G. Identifying microRNA/mRNA dysregulations in ovarian cancer. BMC Res Notes. 2012;5:164. doi: 10.1186/1756-0500-5-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan X, Qin W, Zhang L, Hang J, Li B, Zhang C, Wan J, Zhou F, Shao K, Sun Y, Wu J, Zhang X, Qiu B, Li N, Shi S, Feng X, Zhao S, Wang Z, Zhao X, Chen Z, Mitchelson K, Cheng J, Guo Y, He J. A 5-microRNA signature for lung squamous cell carcinoma diagnosis and hsa-miR-31 for prognosis. Clin Cancer Res. 2011;17:6802–11. doi: 10.1158/1078-0432.CCR-11-0419. [DOI] [PubMed] [Google Scholar]
- 27.Chen K, Mo J, Zhou M, Wang G, Wu G, Chen H, Zhang K, Yang H. Expression of PTEN and mTOR in sacral chordoma and association with poor prognosis. Med Oncol. 2014;31:886. doi: 10.1007/s12032-014-0886-7. [DOI] [PubMed] [Google Scholar]
- 28.Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH. Chordoma: the nonsarcoma primary bone tumor. Oncologist. 2007;12:1344–1350. doi: 10.1634/theoncologist.12-11-1344. [DOI] [PubMed] [Google Scholar]
- 29.Muro K, Das S, Raizer JJ. Chordomas of the craniospinal axis: multimodality surgical, radiation and medical management strategies. Expert Rev Neurother. 2007;7:1295–1312. doi: 10.1586/14737175.7.10.1295. [DOI] [PubMed] [Google Scholar]
- 30.Hoefig KP, Thorns C, Roehle A, Kaehler C, We-sche KO, Repsilber D, Branke B, Thiere M, Feller AC, Merz H. Unlocking pathology ar-chives for microRNA-profiling. Anticancer Res. 2008;28:119–123. [PubMed] [Google Scholar]
- 31.Laios A, O’Toole S, Flavin R, Martin C, Kelly L, Ring M, Finn SP, Barrett C, Loda M, Gleeson N, D’Arcy T, McGuinness E, Sheils O, Sheppard B, O’Leary J. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer. 2008;7:35–49. doi: 10.1186/1476-4598-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barry JJ, Jian BJ, Sughrue ME, Kane AJ, Mills SA, Tihan T, Parsa AT. The next step: innovative molecular targeted therapies for treatment of intracranial chordoma patients. Neurosurgery. 2011;68:231–40. doi: 10.1227/NEU.0b013e3181fd2ac5. discussion 240-1. [DOI] [PubMed] [Google Scholar]
- 33.Diaz RJ, Cusimano MD. The biological basis for modern treatment of chordoma. J Neurooncol. 2011;104:411–22. doi: 10.1007/s11060-011-0559-8. [DOI] [PubMed] [Google Scholar]
- 34.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 35.Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RASRAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]


