Abstract
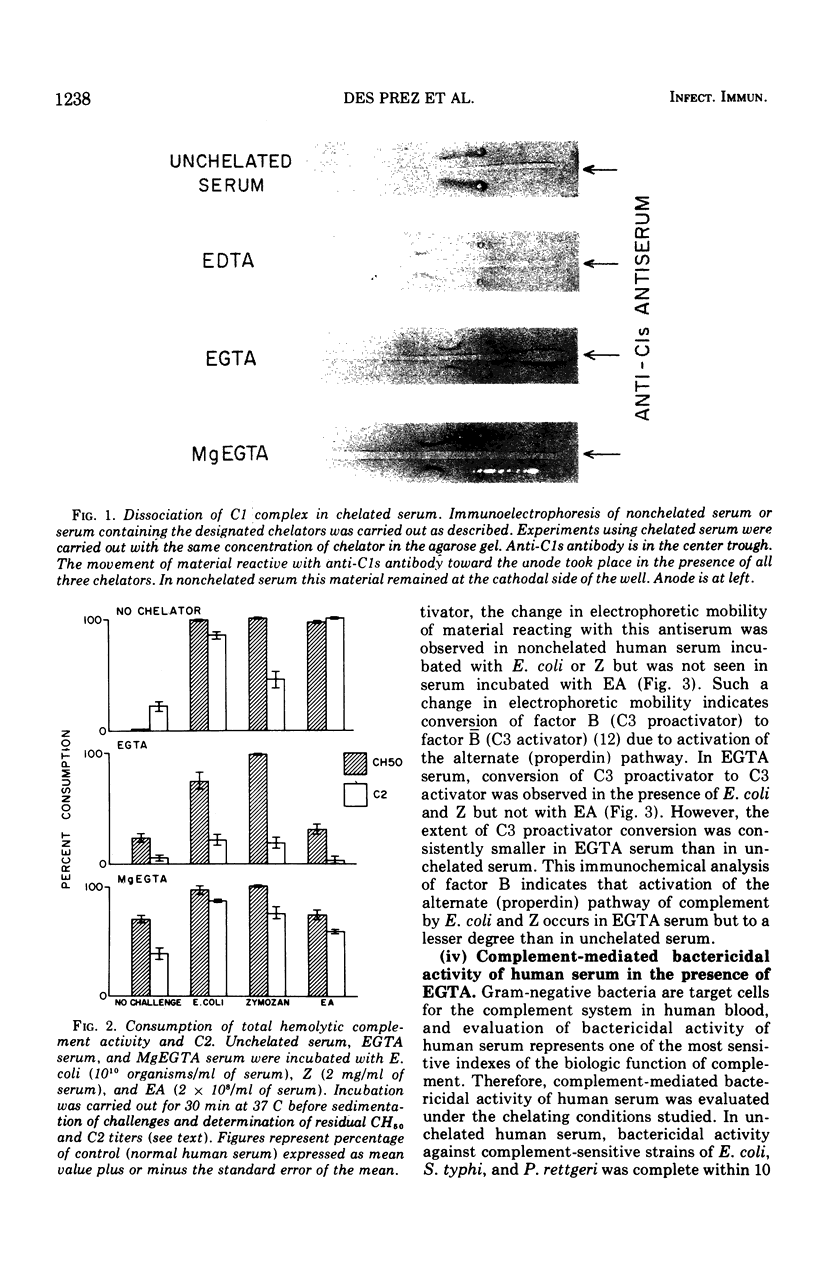
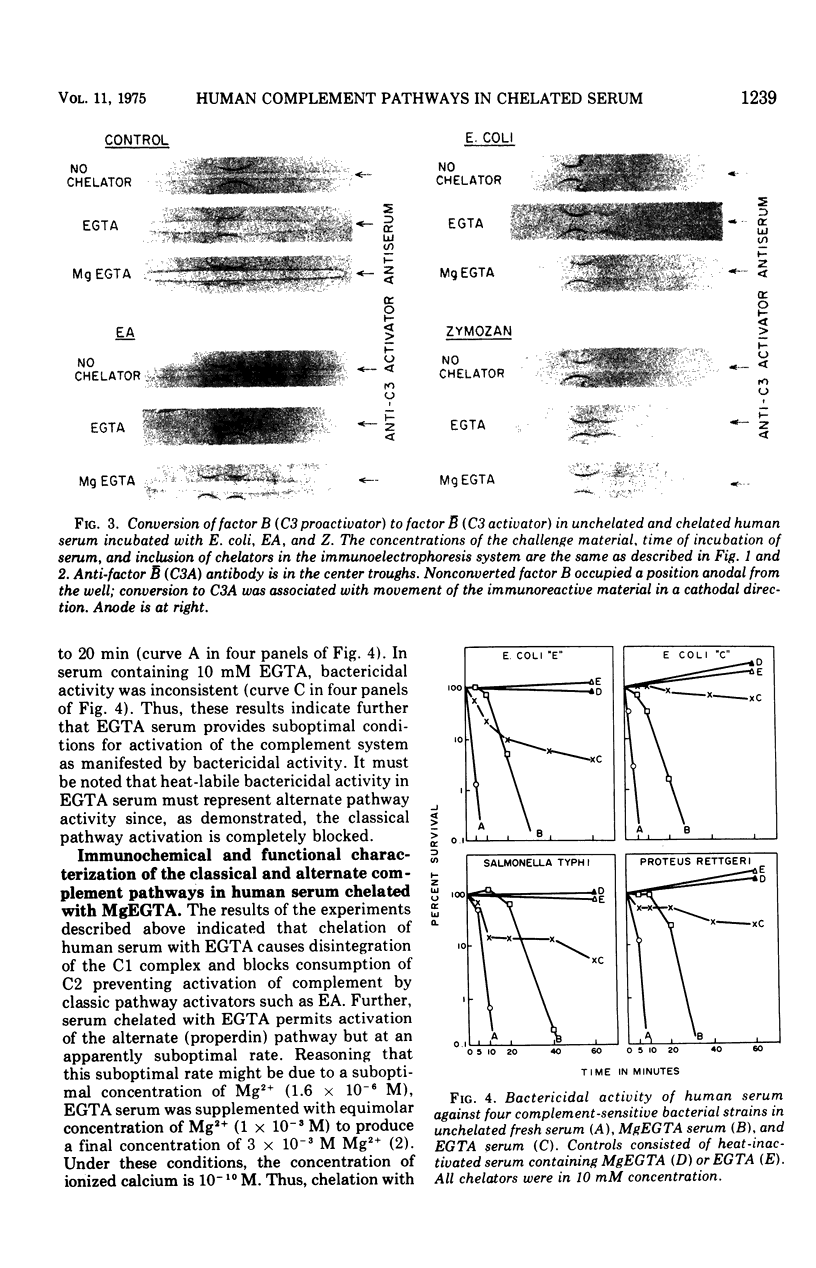
An immunochemical and functional analysis of the classical and alternate complement pathways in human serum was performed in the presence of 10 mM ethylene glycol tetraacetic acid (EGTA) and MgCl2-EGTA (MgEGTA), chelating agents which have been recently utilized as a means of distinguishing between these two complement pathways. Total hemolytic activity, integrity of the C1 complex, hemolytic activity of C2, conversion of factor B (C3 proactivator), and complement-dependent bactericidal activity were studied. The effect of these chelators on activation of complement pathways by Escherichia coli, by sensitized erythrocytes as a prototype of activators of the classical pathway, and by zymosan as a prototype of alternate (properdin) pathway activators was studied. Human serum containing 10 mM EGTA, which provides almost no ionized calcium and considerably less ionized magnesium than unchelated serum, allowed consumption of complement via the alternate (properdin) pathway, but blocked the classical pathway as judged by disintegration of the C1 complex and lack of utilization of C2. However, activity of the alternate complement pathway in EGTA serum, as judged by conversion of factor B and bactericidal activity against gram-negative bacteria, was distinctly suboptimal. Addition of magnesium ion in a concentration equimolar to EGTA (MgEGTA serum), while still providing conditions in which the C1 complex dissociated, significantly enhanced alternate complement pathway-mediated bactericidal activity. However, in MgEGTA serum considerable fluid-phase activation of the alternate pathway, as indicated by decrease in 50% hemolytic complement (CH5 0) titers and conversion of factor B to its active form in the absence of any activating challenge, was observed. Moreover, some fluid-phase consumption of C2 was observed in MgEGTA serum, even though, as mentioned, the C1 complex was shown to be dissociated under these conditions. MgEGTA-related activation of C2 and of the alternate (properdin) pathway of complement was significantly enhanced by the presence of zymosan and E. coli. These results indicate that use of the chelating agents EGTA and MgEGTA to differentiate between classical and alternate pathway activation of human complement is more complex than has hitherto been suggested. In EGTA serum, spontaneous activation of either pathway does not occur but bactericidal activity, as a measure of biologic function of complement, is suboptimal. In MgEGTA serum, bactericidal activity is fully expressed, but there is considerable instability, in terms of fluid-phase activation, in Mg2+-dependent components of both pathways. Thus, caution is indicated in the use and interpretation of the effects of these chelating agents on biologic functions mediated by either pathway of human complement.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan C. S. Sensitization of E. coli to the serum bactericidal system and to lysozyme by ethyleneglycoltetraacetic acid. Proc Soc Exp Biol Med. 1974 Apr;145(4):1431–1433. doi: 10.3181/00379727-145-38028. [DOI] [PubMed] [Google Scholar]
- Bryant R. E., Jenkins D. E., Jr Calcium requirements for complement dependent hemolytic reactions. J Immunol. 1968 Oct;101(4):664–668. [PubMed] [Google Scholar]
- DeMeo A. N., Andersen B. R. Defective chemotaxis associated with a serum inhibitor in cirrhotic patients. N Engl J Med. 1972 Apr 6;286(14):735–740. doi: 10.1056/NEJM197204062861401. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., May J. E., Kane M. A., Frank M. M., Bennett J. E. The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol. 1974 Jun;112(6):2260–2270. [PubMed] [Google Scholar]
- Fierer J., Finley F., Braude A. I. A plaque assay on agar for detection of gram-negative bacilli sensitive to complement. J Immunol. 1972 Nov;109(5):1156–1158. [PubMed] [Google Scholar]
- Fine D. P. Activation of the classic and alternate complement pathways by endotoxin. J Immunol. 1974 Feb;112(2):763–769. [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Gewurz H., Pickering R. J., Mergenhagen S. E., Good R. A. The complement profile in acute glomerulonephritis systemic lupus erythematosus and hypocomplementemic chronic glomerulonephritis. Contrasts and experimental correlations. Int Arch Allergy Appl Immunol. 1968;34(6):556–570. doi: 10.1159/000230149. [DOI] [PubMed] [Google Scholar]
- Gigli I., Austen K. F. Fluid phase destruction of C2hu by C1hu. II. Unmasking by C4ihu of C1hu specificity for C2hu. J Exp Med. 1969 Oct 1;130(4):833–846. doi: 10.1084/jem.130.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- HOVIG T. THE EFFECT OF CALCIUM AND MAGNESIUM ON RABBIT BLOOD PLATELET AGGREGATION IN VITRO. Thromb Diath Haemorrh. 1964 Oct 15;12:179–200. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Newman S. L., Struth A. G. An abnormality of the alternate pathway of complement activation in sickle-cell disease. N Engl J Med. 1973 Apr 19;288(16):803–808. doi: 10.1056/NEJM197304192881601. [DOI] [PubMed] [Google Scholar]
- LEPOW I. H., NAFF G. B., TODD E. W., PENSKY J., HINZ C. F. Chromatographic resolution of the first component of human complement into three activities. J Exp Med. 1963 Jun 1;117:983–1008. doi: 10.1084/jem.117.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE L., MAYER M. M., RAPP H. J. Kinetic studies on immune hemolysis. VI. Resolution of the C'y reaction step into two successive processes involving C'2 and C'3. J Immunol. 1954 Dec;73(6):435–442. [PubMed] [Google Scholar]
- LEVINE L., OSLER A. G., MAYER M. M. Studies on the role of Ca++ and Mg++ in complement fixation and immune hemolysis. III. The respective roles of Ca++ and Mg++ in immune hemolysis. J Immunol. 1953 Nov;71(5):374–379. [PubMed] [Google Scholar]
- Marcus R. L., Shin H. S., Mayer M. M. An alternate complement pathway: C-3 cleaving activity, not due to C4,2a, on endotoxic lipopolysaccharide after treatment with guinea pig serum; relation to properdin. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1351–1354. doi: 10.1073/pnas.68.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J. E., Frank M. M. Hemolysis of sheep erythrocytes in guinea pig serum deficient in the fourth component of complement. II. Evidence for involvement of C1 and components of the alternate complement pathway. J Immunol. 1973 Dec;111(6):1668–1676. [PubMed] [Google Scholar]
- May J. E., Rosse W., Frank M. M. Paroxysmal nocturnal hemoglobinuria. Alternate-complement-pathway-mediated lysis induced by magnesium. N Engl J Med. 1973 Oct 4;289(14):705–709. doi: 10.1056/NEJM197310042891401. [DOI] [PubMed] [Google Scholar]
- Miller G. W., Saluk P. H., Nussenzweig V. Complement-dependent release of immune complexes from the lymphocyte membrane. J Exp Med. 1973 Sep 1;138(3):495–507. doi: 10.1084/jem.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Götze O. C3 proactivator convertase and its mode of action. J Exp Med. 1972 Apr 1;135(4):1003–1008. doi: 10.1084/jem.135.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaki K., Stroud R. M. Specific antisera to C1s: detection of different electrophoretic species of C1s. J Immunol. 1969 Jul;103(1):141–145. [PubMed] [Google Scholar]
- PILLEMER L., BLUM L., LEPOW I. H., ROSS O. A., TODD E. W., WARDLAW A. C. The properdin system and immunity. I. Demonstration and isolation of a new serum protein, properdin, and its role in immune phenomena. Science. 1954 Aug 20;120(3112):279–285. doi: 10.1126/science.120.3112.279. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Lambert P. H., Nydegger U. E., Miescher P. A. Quantitation of C3PA (properdin factor B) and other complement components in diseases associated with a low C3 level. Clin Immunol Immunopathol. 1973 Nov;2(1):16–27. doi: 10.1016/0090-1229(73)90032-9. [DOI] [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. The second component of human complement: its isolation, fragmentation by C'1 esterase, and incorporation into C'3 convertase. J Exp Med. 1968 Sep 1;128(3):533–551. doi: 10.1084/jem.128.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Gigli I., Austen K. F. The complement system of man. I. N Engl J Med. 1972 Sep 7;287(10):489–495. doi: 10.1056/NEJM197209072871005. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Sandberg A. L., Osler A. G. Dual pathways of complement interaction with guinea pig immunoglobulins. J Immunol. 1971 Nov;107(5):1268–1273. [PubMed] [Google Scholar]