Abstract
Urothelial bladder cancer (UBC) is a common genitourinary malignancy, accounting for more than 160.000 deaths per year worldwide. Overexpression and aberrant glycosylation of mucins are frequent traits of many human cancers derived from epithelial cells, and are found to have prognostic significance in various carcinomas. The aim of this study was to further elucidate the features and significance of mucin expression in UBC. We investigated the relationship between mucin expression and clinicopathological characteristics in 539 cases of UBC by immunohistochemical analysis of MUC1, MUC2, MUC4, MUC5AC and MUC6 expression profiles. MUC1 stained 61.8% of the tumors and correlated with high tumor grade (P = 0.013). The expression of MUC2 and MUC6 was associated with low tumor grade (P < 0.000 and P < 0.022, respectively), and low pathologic stage (P < 0.001 and P = 0.001, respectively). MUC2 negative tumors were more frequently associated with the finding of carcinoma in situ in tumor surroundings (P = 0.019). UBC with divergent differentiation correlated with MUC1, MUC4 and MUC5AC staining. MUC4 expression was directly linked to cancer specific death (P = 0.027), while MUC2 and MUC6 showed inverse correlation to cancer-specific death (P < 0.001 and P = 0.005, respectively). Kaplan-Meier analyses showed that expression of MUC2 and MUC6 in UBC was significantly associated with better overall survival of the patients (P < 0.001, respectively). In Cox regression model, the absence of MUC6 expression emerged as independent predictor of death outcome. In conclusion, this study identifies MUC2 and MUC6 expression as markers of UBC with less aggressive behavior and useful predictors of better survival.
Keywords: Urothelial blader cancer, mucins, outcome
Introduction
Urothelial bladder cancer (UBC) is a frequently occurring malignancy of the urinary tract. It is the 7th most common cancer in men and the 17th most common cancer in women, responsible for approximately 165.000 deaths per year worldwide [1,2]. Natural history and tumor biology of UBC have been studied extensively and are relatively well defined [1,3]. Approximately 75% of patients with UBCs present with superficially invasive UBC, which is prone to recurrence, but rarely progress to muscle invasive disease. Muscle invasive UBC and high grade carcinoma in situ is aggressive cancer with a poor response to therapy and tendency to early spread and metastasize to distant sites [1,3,4]. Neoplastic urothelium displays significant plasticity, therefore UBC may demonstrate variable architectural patterns and divergent differentiation, which may have prognostic and therapeutic implications [5-7]. Recently, three main pathways frequently dysregulated in UBC have been recognized: cell cycle regulation, kinase signaling pathways, and chromatin remodeling [1]. The use of molecular markers for characterization of UBC allows improved and more complete insight in nature of cancer than histologic evaluation alone [3], and they may serve as valuable tools for prediction of tumor recurrence and response to therapy and identification of therapeutic targets.
Mucins are high molecular weight glycoproteins with protective role and precisely ordered distribution among epithelia. To date, 20 different human mucins have been identified (MUC1-MUC20) [8]. Mucins are generally classified in two major groups: membrane bound (cell surface associated), including MUC1 and MUC4, and secreted or oligomeric mucus/gel forming mucins, like MUC2, MUC5AC and MUC6. Alterations of mucin genes expression, modifications of post-transcriptional and epigenetic regulation, are frequent traits of many human cancers derived from epithelial cells [9].
The knowledge about significance of mucin expression in UBC is scarce [10-14]. The aim of this research was to further clarify the relationship between mucin expression and biological behavior of UBC and clinical outcome of the disease. We examined the expression of MUC1, MUC2, MUC4, MUC5AC, and MUC6 in 539 UBCs using immunohistochemistry, and correlated the results with clinicopathological factors of the disease.
Materials and methods
Patients and tissue samples
Tissue samples of urothelial bladder cancer were obtained from 539 patients who underwent transurethral resection of bladder between January 2000 and December 2009 in Urology Clinic, Clinical Centre Nis, Serbia. The majority of samples were obtained from patients diagnosed with UBC during a four year period. All cases of UBC were diagnosed at the Institute of Pathology, Faculty of Medicine, Nis, Serbia. Tumors with any distinct adenocarcinoma component were excluded from the study.
The mean age of patients was 66.6±10.0 years and there were 78.5% male and 21.5% female patients. Patients’ clinical history, cancer characteristics and survival data including survival time, disease-free survival and recurrence were available for all patients included in the study. Patient follow-up was expressed as the number of months from the date of diagnostic transurethral resection to the date of last control visit (most recent cystoscopy) or death. The median follow-up was 59 months (29 to 237 months) for patients alive at the time of analysis. Cause of death for the patients who died during the follow-up period was determined by treating physician or by medical chart review and death certificate. Cancer-specific death was defined as that caused by bladder cancer. Patients who died of bladder cancer had progressive and widely disseminated disease.
Pathohistologic analysis and construction of tissue microarrays (TMA)
The histological sections of representative paraffin-embedded cancer tissue samples were processed by standard techniques, and stained with hematoxylin and eosin (H&E). H&E-stained slides were used to assess histological grade, pathologic stage, growth pattern of the tumor, presence of carcinoma in situ and variant urothelial histology. The 2002 TNM classification system [15] was used for pathologic staging, and the 2004 World Health Organization classification was used for histological grading of BC [16].
Tissue microarrays (TMA) containing representative cancer tissue samples were constructed using the manual tissue arrayer. Two core tissue biopsies with a diameter of 2 mm were punched from carefully selected region of cancer from each donor block and placed into recipient paraffin block. Presence of selected tumor tissue on TMA sections were verified on H&E-stained slides. In addition, every TMA block included two samples of normal bladder mucosa. Three ¼m thick sections were used for immunohistochemical evaluation. The loss of core sections on slides produced from TMAs was low (≤ 5%), and the number of interpretable specimens varied very little between the investigated markers.
Immunohistochemical analysis
TMA sections were deparaffinized in xylene and rehydrated in a graded ethanol series. A microwave antigen retrieval procedure was carried out for 20 minutes with citrate buffer (pH 6.0). Endogenous peroxydase activity was quenched by 10 minute immersion in 0.3% hydrogen peroxyde methanol solution. The sections were thoroughly washed with 0.01 phosphate buffered saline (PBS) and incubated with primary antibody for one hour at room temperature. A standard avidin-biotin immunoperoxidase complex detection system according to the manufacturer’s protocol (Dako LSAB2R system-HRP) was applied. Staining was developed using a liquid 3,3’-diaminobenzidine (DAB) substrate kit. Sections were counterstained with Mayer’s hematoxylin, dehydrated and mounted. Appropriate negative and positive controls were included in all immunostaining procedures.
The following primary antibodies were used: mouse monoclonal antibodies to glicoproteins MUC1, MUC2, MUC5AC, and MUC6, all obtained from Novocastra laboratories, Newcastle upon Tyne, United Kingdom, applied in dilutions of 1:100, 1:200, 1:100, 1:100, respectively, and mouse monoclonal anti-MUC4 antibody (Abcam, Cambridge, United Kingdom; dilution 1:100).
Immunostaining of MUC1, MUC2, MUC4 and MUC6 was considered positive if at least 10% of cancer cells were stained with intermediate or strong brown color intensity. Staining intensity was graded using a scale of 0 to 3 (0, no staining; 1, weak; 2, moderate; 3, intense). MUC5AC expression was considered positive if more than 1% of cancer cells were stained.
Statistical analysis
The expression of mucins and clinicopathologic features were tested for association by the χ 2 test. For each investigated marker, Kaplan-Meier survival curve was constructed to compare the patients with tumors of positive or negative mucin expression. The differences between survival curves were tested for statistical significance by log-rank test. Cox regression analysis with enter method was performed to explore the relationship between the survival of the patients and explanatory variables.
All data analyses were processed using the Statistical Package for Social Sciences, version 15.0 statistical software (SPSS, Chicago, IL). A P value of 0.05 or less was considered indicative of a statistically significant difference.
Results
Expression of mucins in urothelial bladder cancer and in non-neoplastic transitional cell epithelium of the bladder
In normal bladder urothelium MUC1 expression was noted exclusively on the apical membranes of the umbrella cells, while MUC2 staining was absent. MUC1 positivity was observed in 61.8% of the tumors (Table 1). In low grade papillary neoplasms, the staining was predominantly membranous, but only UBCs with stroma facing MUC1 activity were considered positive, according to the previous data [17]. In less differentiated neoplasms, MUC1 membranous and cytoplasmic staining was observed. MUC2 immunoexpression was noted in 40.1% of tumors. Focal or cluster-like cytoplasmic stain, comprising between 10% and 25% of tumor cells was the most frequent finding. MUC4 stained 26.5% of investigated tumor samples, predominantly in a form of distinct membranous pattern. MUC4 marked stromal vascular network, which provided internal control. MUC5AC displayed predominantly moderate expression in single tumor cells or clusters in a form of cytoplasmic granular pattern in 11.1% of investigated UBCs. MUC6 positivity was observed in 21.9% of cases, in a form of cytoplasmic or apical membranous with cytoplasmic perimembranous granular staining. Representative photomicrographs of mucins immunohistochemical expression are shown in Figures 1 and 2.
Table 1.
Association of the expression of MUC1, MUC2, MUC4, MUC5AC and MUC6 with clinicopathologic features of bladder cancer, occurrence of tumor relapse during the follow-up period and cancer specific death
| χ 2 Pearson | Patients | MUC1 | MUC2 | MUC4 | MUC5AC | MUC6 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
||||||||||||
| Characteristic | Positive | Positive | Positive | Positive | Positive | ||||||||||||
|
|
|
|
|
|
|||||||||||||
| No | % | No | % | P | No | % | P | No | % | P | No | % | P | No | % | P | |
| Total | 539 | 100 | 333 | 61.8 | 216 | 40.1 | 143 | 26.5 | 60 | 11.1 | 118 | 21.9 | |||||
| Gender | |||||||||||||||||
| Female | 116 | 21.5 | 81 | 24.3 | 0.044* | 55 | 25.5 | 0.069 | 34 | 23.8 | 0.444 | 16 | 26.7 | 0.304 | 30 | 25.4 | 0.243 |
| Male | 423 | 78.5 | 252 | 75.7 | 161 | 74.5 | 109 | 76.2 | 44 | 73.3 | 88 | 74.6 | |||||
| Pathological stage | |||||||||||||||||
| ≤pT1 | 393 | 72.9 | 240 | 72.1 | 0.577 | 188 | 87.0 | 0.000* | 96 | 67.1 | 0.070 | 48 | 80.0 | 0.190 | 100 | 84.7 | 0.001* |
| ≥T2 | 146 | 27.1 | 93 | 27.9 | 28 | 13.0 | 47 | 32.9 | 12 | 20.0 | 18 | 15.3 | |||||
| Pathological grade | |||||||||||||||||
| Low | 288 | 53.4 | 164 | 49.2 | 0.013* | 149 | 69.0 | 0.000* | 62 | 43.4 | 0.005* | 32 | 53.3 | 0.987 | 74 | 62.7 | 0.022* |
| High | 251 | 46.6 | 169 | 50.8 | 67 | 31.0 | 81 | 56.8 | 28 | 46.7 | 44 | 37.3 | |||||
| Carcinoma in situ | |||||||||||||||||
| Negative | 496 | 92.0 | 305 | 91.6 | 0.639 | 206 | 95.4 | 0.019* | 127 | 88.8 | 0.098 | 52 | 86.7 | 0.104 | 112 | 94.9 | 0.189 |
| Positive | 43 | 8.0 | 28 | 8.4 | 10 | 4.6 | 16 | 11.2 | 8 | 13.3 | 6 | 5.1 | |||||
| Divergent differentiation | |||||||||||||||||
| Negative | 470 | 87.2 | 277 | 83.2 | 0.000* | 194 | 89.8 | 0.137 | 114 | 79.7 | 0.002* | 47 | 78.3 | 0.029* | 106 | 89.8 | 0.333 |
| Positive | 69 | 12.8 | 56 | 16.8 | 22 | 10.2 | 29 | 20.3 | 13 | 21.7 | 12 | 10.2 | |||||
| Tumor relapse | |||||||||||||||||
| No | 347 | 64.4 | 218 | 65.5 | 0.503 | 125 | 57.9 | 0.010* | 90 | 62.9 | 0.675 | 34 | 56.7 | 0.186 | 69 | 59.5 | 0.130 |
| Yes | 192 | 35.6 | 115 | 34.5 | 91 | 42.1 | 53 | 37.1 | 26 | 43.3 | 49 | 41.5 | |||||
| Cancer specific death | |||||||||||||||||
| No | 357 | 66.2 | 215 | 64.6 | 0.297 | 164 | 75.9 | 0.000* | 84 | 58.7 | 0.027* | 45 | 75.0 | 0.128 | 91 | 77.1 | 0.005* |
| Yes | 182 | 33.8 | 118 | 35.4 | 52 | 24.1 | 59 | 41.3 | 15 | 25.0 | 27 | 22.9 | |||||
P≤0.05, the result is statistically significant.
Figure 1.
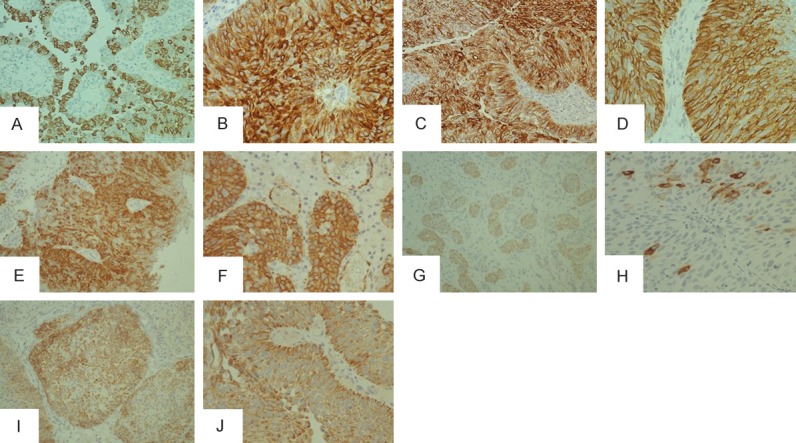
Immunohistochemical expression of mucins in urothelial bladder cancer (UBC): (A) Moderate expression of MUC1 in micropapillary-like foci of conventional UBC; (B) Strong diffuse staining of MUC1 in invasive UBC; (C) Intensive diffuse immunoreactivity of MUC2 in high grade, and (D) Low grade UBC; (E) Strong expression of MUC4 in invasive component of UBC, and (F) In UBC with squamous differentiation; (G) Weak to intermediate activity of MUC5AC in invasive nests of UBC, and (H) Focal, prominent granular staining of MUC5AC in single neoplastic cells within papilla; (I) Intermediate MUC6 expression in pT1, and (J) PTa UBC. Original magnification x 200 (A, C, E, G, I) and x 400 (B, D, F, H, J).
Figure 2.
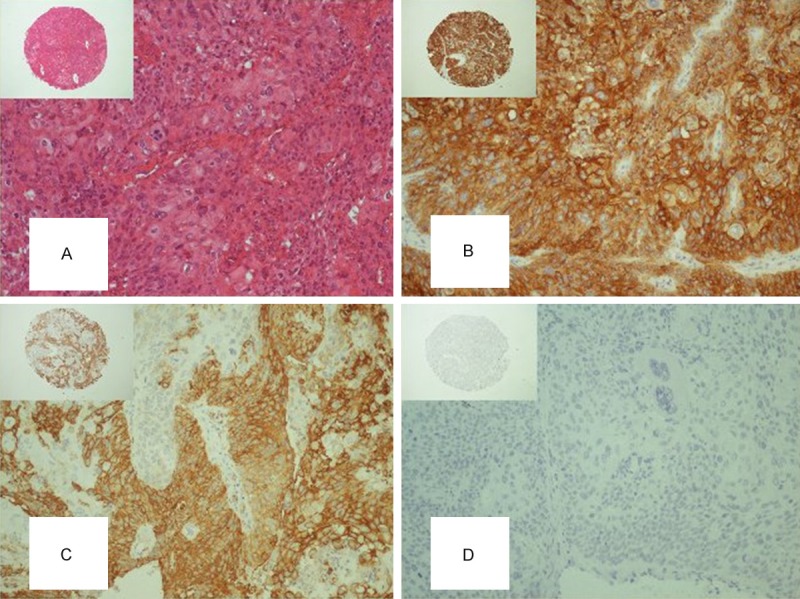
Differential protein expression patterns on same tissue microarray sections representating a case of high grade non-musle invasive urothelial bladder cancer: (A) H&E stain; (B) Strong, diffuse cytoplasmic and membranous immunoreactivity to MUC1; and (C) MUC4; (D) Absent immunoreactivity to MUC6. Original magnification x 400, for insets x 40.
In addition, we analyzed the simultaneous expression of mucins in UBC (Table 2). We found significant relationship between MUC1 and MUC2 positivity (P = 0.003), and between MUC1 and MUC5AC expression (P < 0.001), with 83% of UBCs positive for MUC5AC simultaneously expressing MUC1. Statistically strong inverse association was found between MUC5AC and MUC6 (P < 0.001). Positive correlation was observed between MUC2 and MUC6 expression (P < 0.001), with 75% of MUC6 positive tumors simultaneously coexpressing MUC2.
Table 2.
Coexpression of MUC1, MUC2, MUC4, MUC5AC and MUC6 in urothelial bladder cancer
| χ 2 Pearson | Patients | MUC1 | MUC2 | MUC4 | MUC5AC | MUC6 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
||||||||||||
| Characteristic | Positive | Positive | Positive | Positive | Positive | ||||||||||||
|
|
|
|
|
|
|||||||||||||
| No | % | No | % | P | No | % | P | No | % | P | No | % | P | No | % | P | |
| Total | 539 | 100 | 333 | 61.8 | 216 | 40.1 | 143 | 26.5 | 60 | 11.1 | 118 | 21.9 | |||||
| MUC1 | |||||||||||||||||
| Negative | 206 | 38.2 | - | - | 66 | 30.6 | 0.003* | 52 | 36.4 | 0.594 | 10 | 16.7 | 0.000* | 37 | 31.4 | 0.083 | |
| Positive | 333 | 61.8 | - | - | 150 | 69.4 | 91 | 63.6 | 50 | 83.3 | 81 | 68.6 | |||||
| MUC2 | |||||||||||||||||
| Negative | 323 | 59.9 | 183 | 55.0 | 0.003* | - | - | 82 | 57.3 | 0.462 | 26 | 43.3 | 0.005* | 37 | 31.4 | 0.000* | |
| Positive | 216 | 40.1 | 150 | 45.0 | - | - | 61 | 42.7 | 34 | 56.7 | 81 | 68.6 | |||||
| MUC4 | |||||||||||||||||
| Negative | 396 | 73.5 | 242 | 72.7 | 0.594 | 155 | 71.8 | 0.462 | - | - | 40 | 66.7 | 0.205 | 82 | 69.5 | 0.268 | |
| Positive | 143 | 26.5 | 91 | 27.3 | 61 | 28.2 | - | - | 20 | 33.3 | 36 | 30.5 | |||||
| MUC5AC | |||||||||||||||||
| Negative | 479 | 88.9 | 283 | 85.0 | 0.000* | 182 | 84.3 | 0.005* | 123 | 86.0 | 0.205 | - | - | 89 | 75.4 | 0.000* | |
| Positive | 60 | 11.1 | 50 | 15.0 | 34 | 15.7 | 20 | 14.0 | - | - | 29 | 24.6 | |||||
| MUC6 | |||||||||||||||||
| Negative | 421 | 78.1 | 252 | 75.7 | 0.083 | 135 | 62.5 | 0.000* | 107 | 74.8 | 0.268 | 31 | 51.7 | 0.000* | - | - | |
| Positive | 118 | 21.9 | 81 | 24.3 | 81 | 37.5 | 36 | 25.2 | 29 | 48.3 | - | - | |||||
P≤0.05, the result is statistically significant.
Correlation of mucins expression and clinicopathologic characteristics
Pathologic features of 539 patients with UBC and association of mucin expression with clinicopathologic features are shown in Table 1. Positivity to MUC1 and MUC4 correlated with high pathologic tumor grade (P = 0.013). In contrast, expression of MUC2 and MUC6 was more frequently observed in low grade tumors (P < 0.000 and P < 0.022, respectively).
MUC2 and MUC6 expression significantly correlated with low pathologic stage (P < 0.001 and P = 0.001, respectively). Almost half of the analyzed low stage tumors (188 out of 393) demonstrated MUC2 staining, in contrast to only 19.2% positive high grade UBC (28/146). In addition, MUC2 negative tumors were more frequently associated with the finding of carcinoma in situ in tumor surroundings (p = 0.019).
MUC1 expression was significantly associated with divergent differentiation of UBC (P < 0.001). MUC4 was positive in 42% of tumors with divergent differentiation and stained 45% (23/51) of carcinoma showing squamous differentiation and 50% (6/12) of invasive carcinoma with glandular differentiation. Moreover, 18.8% of tumors with divergent differentiation exhibited MUC5AC staining, with the highest and strongest expression in UBC with glandular differentiation (3/12, 25%). UBC with squamous differentiation demonstrated inverse association with MUC2: these tumors had lower tendency to stain for MUC2 (13/51 vs. 203/488, P = 0.026).
At the designated endpoint of the follow up period 282 patients (52.3%) were alive, and 257 (47.7%) had died. Cause of death was declared as cancer specific in 33.2% of patients. MUC4 expression was directly linked to cancer specific death (P = 0.027), while MUC2 and MUC6 showed inverse correlation to cancer-specific death (P < 0.001 and p = 0.005, respectively).
MUC2 expression correlated with tumor relapse: patients with MUC2 positive UBC had higher rate of disease recurrence (P = 0.01). Moreover, patients with MUC2 had higher probability to be treated with intravesical instillation of BCG (125 (No of MUC2 + UBC)/244 (patients treated with BCG) vs. 91 (MUC2 + UBC)/295 (patients treated with other therapeutic modalities), P < 0.001). In addition, these patients had decreased probability to undergo radical cystectomy (20/84 patients who underwent radical surgery) vs. 196/455 of patients who did not, p = 0.001), as well as patients with MUC6 positive tumors (11/84 vs. 107/455, P = 0.034). Patients with MUC4 positive UBC were more often submitted to chemotherapy, radiotherapy or combination of these two treatment modalities (41/108 vs. 102/431, P = 0.003).
Association of expression of mucins and overall survival
Survival after diagnosis, expressed as mean±SD, was 45.2±32.0 months, and recurrence free survival was 22.5±26.1 months.
Kaplan-Meier analyses (Figure 3) showed that positive expression of MUC2 and MUC6 in urothelial bladder cancer was significantly associated with better survival of the patients (P < 0.001, respectively). Immunohistochemical positivity to MUC1, MUC4, and MUC5AC did not correlate with survival.
Figure 3.
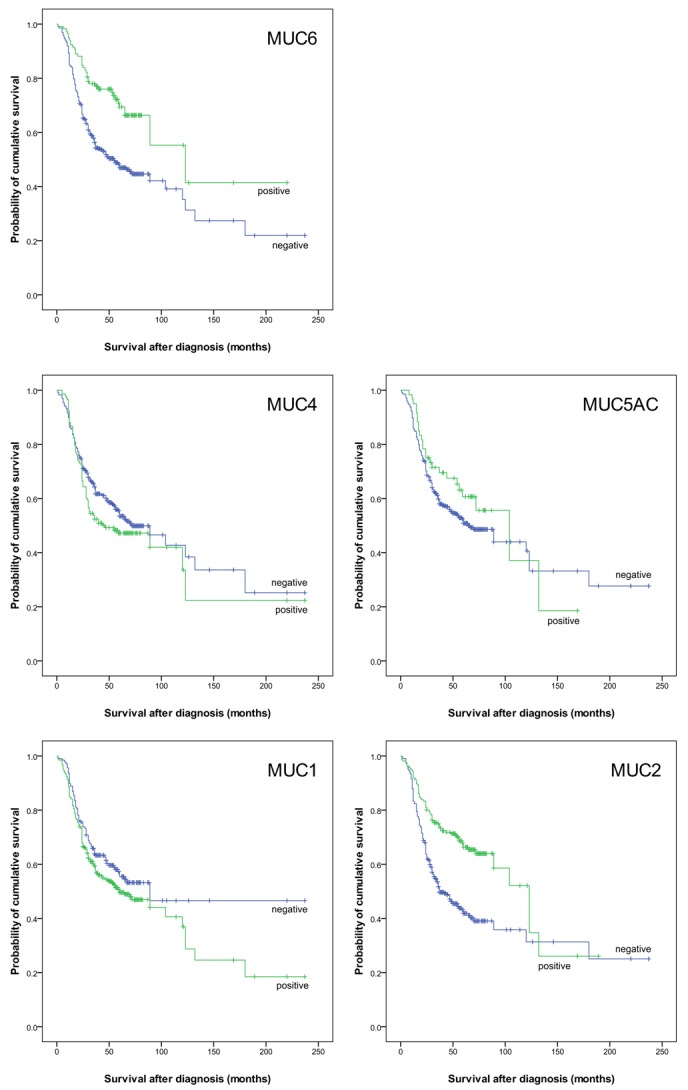
Kaplan-Meier survival curves showing overall survival in 539 patients with urothelial bladder cancer, with negative or positive MUC1, MUC2, MUC4, MUC5AC and MUC6 expression. For MUC1 P = 0.093; MUC2 P < 0.001; MUC4 P = 0.165; MUC5AC P = 0.209; MUC6 P < 0.001.
Cox regression model (Table 3) identified that the absence of MUC6 expression, patients’ age, high grade, advanced stage and radical cystectomy used as treatment modality were independent predictors of death outcome, after adjustment for the other explanatory variables in the model (Table 3). The hazard ratio of death for the patients with high grade UBC, compared with patients with low grade tumors, was 1.971 (95% CI: 1.408 to 2.759). Patients with invasive cancer (≥ pT2) had a 2.262 times (95% CI: 1.644 to 3.113) increased risk of death, compared with patients with superficial tumors. The hazard ratio of death for the patients who subsequently underwent radical cystectomy followed or not with radiation and/or chemotherapy, compared with other patients, was 1.696 (95% CI: 1.206 to 2.385). The estimated hazard or risk of death increases by 4.7% (95% CI: 3.1 to 6.4%) for each year of age. Hazard ratio of death decreased by 34.4% (95% CI: 4.4 to 54.9%) in patients with UBC that demonstrated positive MUC6 immunoexpression.
Table 3.
Multivariate Cox regression analysis showing significant variables with independent influence on the hazard ratio of death in 539 patients with urothelial bladder cancer
| Variable | Regression coefficient | Standard error | P-value | Hazard ratio | 95.0% CI for Hazard ratio | |
|---|---|---|---|---|---|---|
|
| ||||||
| Lower | Upper | |||||
| MUC1 | 0.087 | 0.142 | 0.540 | 1.091 | 0.826 | 1.441 |
| MUC2 | -0.197 | 0.158 | 0.214 | 0.821 | 0.602 | 1.120 |
| MUC4 | -0.037 | 0.142 | 0.794 | 0.964 | 0.730 | 1.273 |
| MUC5AC | -0.312 | 0.236 | 0.187 | 0.732 | 0.461 | 1.164 |
| MUC6 | -0.421 | 0.192 | 0.028 | 0.656 | 0.451 | 0.956 |
| Gender (male) | 0.154 | 0.179 | 0.389 | 1.166 | 0.822 | 1.656 |
| Age (years) | 0.046 | 0.008 | 0.000 | 1.047 | 1.031 | 1.064 |
| Pathological grade (high) | 0.679 | 0.172 | 0.000 | 1.971 | 1.408 | 2.759 |
| Pathological stage (invasive) | 0.816 | 0.163 | 0.000 | 2.262 | 1.644 | 3.113 |
| Glandular differentiation | 1.014 | 0.546 | 0.063 | 2.758 | 0.946 | 8.038 |
| Squamous differentiation | 0.584 | 0.607 | 0.336 | 1.793 | 0.546 | 5.888 |
| Divergent differentiation | -0.362 | 0.615 | 0.556 | 0.696 | 0.209 | 2.326 |
| Carcinoma in situ | -0.349 | 0.218 | 0.110 | 0.705 | 0.460 | 1.082 |
| Radical cystectomy | 0.528 | 0.174 | 0.002 | 1.696 | 1.206 | 2.385 |
Discussion
An estimated global incidence for bladder cancer is about 430.000 newly diagnosed cases annually, while more than 165.000 people die from the disease each year [2]. The frequency of bladder cancer is higher in more developed regions, and male sex is affected three times more often than female [2]. Although UBC is well studied cancer, therapeutic options available are quite limited, especially for high grade, muscle invasive disease. Median survival for patients with recurrent or metastatic bladder cancer remains about 15 months with cisplatin based chemotherapy, and there is no new widely recognized therapy [18]. UBC carcinogenesis involves alterations in multiple cellular pathways, which are included in signal transduction, cell cycle regulation, apoptosis and angiogenesis. Identification of biomarkers which would allow accurate and precise diagnosis, and stratification of patients in terms of optimal treatment response is of major importance.
Mucins are macromolecules with similar properties, consisting of large, polymorphic central domain with highly O-glicosylated serine and threonine residues and presence of tandem repeats [8,19]. They are closely involved in cell signaling, cell adhesions, differentiation of epithelial cells and immune response [19,20]. Alteration in glicosylation pattern of mucins in cancer creates tumor associated epitopes in carbohydrate side chains that can serve as biomarkers for diagnostic, prognostic and therapeutic purposes. These characteristic oligosaccharide sequences are extensively investigated as immunotargets of malignant cells. More than 200 clinical studies have been done over the last few decades to evaluate mucins as possible prognostic or therapeutic tools [20]. Overexpression and aberrant glycosylation of mucins are associated with aggressive biological behavior and poor clinical outcome in patients with adenocarcinomas of various sites [9,21-25]. Data about positive mucin staining and elecronomicroscopic evidence of intracellular mucin production and deposition within typical urothelial cancer cells [26] contributes to notion that neoplastic urothelial epithelium may aberrantly produce mucins.
In urothelial malignancy, MUC1 is the most studied tumor associated antigen [11-13], which elevation was linked to tumor invasion and metastasis in many carcinomas [21-25]. MUC1 serum levels were increased in patients with advanced stage bladder tumors [27]. Unlike in normal epithelium where MUC1 is confined to apical membranes of umbrella cells, immunohistochemical studies demonstrated aberrant MUC1 expression in basal and intermediate layers of neoplastic epithelium. It was found that staining pattern of MUC1 correlates well with invasiveness of the disease and that MUC1 represents a good predictor of cancer progression in superficial UBC [28]. A study that examined differential expression of three mucins in carcinomas of various sites concluded that immunophenotype MUC1+/MUC2–/MUC5AC– is the most likely for UBC, however the investigation included only 15 cancer samples [29]. In present study, high MUC1 expression rate was observed, with almost 62% of tumors positive to MUC1 immunostaining. Interestingly, expression of MUC1 did not correlate with tumor stage, but it was significantly associated with high grade UBC.
Aberrant overexpression of MUC1 is liked to inhibition of stress-induced apoptosis and promotion of epithelial to mesenchymal transition (EMT) [30,31]. MUC1 contributes to EMT and invasiveness through repression of E-cadherian and upregulation of Snail and Slug, EMT-associated transcription factors [31]. Recent study demonstrated that oncoprotein MUC1 contributes to progression of urothelial carcinomas. Silencing and suppression of MUC1 in KU7, human bladder cancer cell line, lead to cell cycle arrest and growth inhibition, and decreased the expression of genes associated with mesenchymal phenotype [4]. At the time of diagnosis around 25% of patients with UBC are at an advanced stage, where available therapeutic options (radical cystectomy, chemotherapy, radiotherapy, or their combinations) have only limited effects. In an attempt to surpass the limitations of conventional treatment, cancer vaccine therapies are rapidly evolving [32]. MUC1 emerged as highly immunogenic molecular candidate for several cancer types [21,33]. In parallel with the development of anti-MUC1 immunotherapeutic agents, further studies of MUC1 expression in UBC seem reasonable. In this study MUC1 did not correlate with outcome of patients with UBC. This may be at least partially explained by various glycoforms of MUC1 antigen, including underglycosylated, sialylated, and fully glycosylated forms [9].
Important immunomodulatory effects are attributed to MUC2, which is normally expressed predominantly in intestine. Previously, MUC2 expression was noted in about 40% of UBCs, as detected by the monoclonal antibody 4F1 [12], which is in accordance with our results. Overexpression of MUC2 accompanies pancreatobilliary neoplasms with indolent course and favorable prognosis [9,23], and is involved in suppression of colorectal carcinoma [34], although in carcinomas of other sites prognostic implications of MUC2 are somewhat controversial [21,35]. Galectin-3, which has been associated with tumor progression and adverse clinical outcome in patients with bladder cancer [36], upregulates the transcription of MUC2 [37]. However, many other molecules, including TNFa, Il4, Butyrate [8], also modulate transcription of MUC2. Moreover, it was found that epigenetic regulation (methylation and/or histone modifications) has major influence on MUC2 expression in epithelial cancer cells [38].
In our study, MUC2 generally correlated with favorable prognostic parameters (low tumor grade, low stage, UBC without divergent features). Lack of MUC2 expression was associated with carcinoma in situ found in the surrounding urothelium. Nevertheless, MUC2 significantly correlated with MUC1 expression and increased probability of tumor relapse. Better overall survival of patients with MUC2 positive disease may be associated with higher chance for development of UBC recurrence, and may be related to tumor suppressor activity of MUC2. Additional research is warranted to determine the functional significance of MUC2 accumulation in UBC cells.
MUC4, large transmembrane mucin normally found in tracheobronchial and colonic mucosa, is overexpressed in breast, pancreatic, lung adenocarcinoma, cholangiocarcinoma of the liver, extrahepatic bile duct carcinoma, gallbladder carcinoma, as well as in oral squamous cell carcinoma and is associated with invasive tumor and poor prognosis [39-44]. The results of a recent study suggested that MUC4 overexpression in pancreatic cancer cells contributes to invasion and metastasis by promotion of epithelial-mesenchimal transition [45]. In present investigation, MUC4 emerged as a predictor of cancer specific death. In addition, MUC4 correlated with tumor high grade and divergent differentiation, parameters associated with unfavorable prognosis.
Although the significance of gastric mucin MUC5AC in carcinogenesis remains undetermined, recent study has implied its tumorigenic inclination, since the presence of MUC5AC on the surface of pancreatic cancer cells contributed to evasion of immunosurveillance [46]. In addition, MUC5AC is recognized as a direct transcriptional target of GLI1 in Hedgehog signaling pathway, and is implicated in tumor proliferation and facilitation of invasion [47]. MUC5AC was found in intestinal type of glandular cystitis, metaplastic lesion of the bladder mucosa, which may develop into nonurachal adenocarcinoma of the bladder [48]. Tumor associated appearance of normally absent MUC5AC in malignant urothelial cells may indicate the reactivation of repressed pathways related to embryologic origin of urinary bladder from sinus urogenitalis. Kunze and coworkers analyzed the capability of UBC to secrete MUC5AC, using the monoclonal antibody 45M1. They found that 10.8% of 130 investigated UBCs expressed this apomucin, while UBCs with divergent differentiation stained positively in much higher percentage, 43.8% [14]. Compared to this, rate of immunohistochemical positivity to MUC5AC was a bit lower in our investigation for both UBC in general and for histologic variants. MUC4 and MUC5AC failed to show prognostic impact in patients with UBC included in this study.
Recent study implied that MUC6 inhibits tumor growth and hinders invasion by altering cell adhesion to matrix proteins in pancreatic, colorectal and breast cancer cell lines [49]. It was suggested that MUC6 expression in preneoplastic and early neoplastic lesions may slow the development of infiltrating carcinoma [49,50]. Downregulation of MUC6 was associated with poor prognosis of patients with gastric cancer [51]. Our results strongly suggested that MUC6 expression in UBC represents a significant predictor of improved survival, while the absence of MUC6 is one of the independent predictors of death outcome. Similar to MUC2, MUC6 staining was associated with less aggressive tumor phenotype: the expression of MUC6 significantly correlated with better differentiated and less invasive UBC. MUC6 positive UBCs were concordantly positive for MUC2 and far more frequently negative for MUC5AC.
Although conventional UBC accounts for most of transitional cell carcinoma, glandular, squamous, microcystic, micropapillary, plasmacytoid, lymphoepithelioma-like and other elements are often found in infiltrating UBC. Variant tumor histology, most often in a form of squamous or glandular differentiation in conventional UBC, has been associated with prognostic and potential therapeutic implications [5-7]. Tumors with mixed histology were found to have greater propensity to metastasize [52]. Recent study recognized squamous differentiation in UBCs as an adverse independent predictor of cancer specific survival and predictor of local recurrence after radical cystectomy [53]. However, no adjusted therapeutic paradigm for UBC with variant histology has been recommended so far. Study that comprised a series of 13 cases of invasive micropapillary carcinomas found MUC1 and MUC2 expression in every investigated tumor, while MUC5AC and MUC6 staining was negative [7]. In distinction between two similar entities, invasive micropapillary carcinoma and typical invasive UBC with retraction artifact, among the investigated markers (MUC1, CA125 and Her2Neu), MUC1 was the only significantly differentially expressed with 96% positivity rate in micropapillary vs. 63% in classical UBCs [17]. In this study, MUC1, MUC4 and MUC5AC correlated with variant tumor histology, while MUC2 was inversely liked to squamous differentiation in UBC.
This study attempted to further elucidate the features and significance of mucin expression in UBC, since there is only limited data available on this matter. However, increasing knowledge about numerous and often pleiotropic roles of mucins in carcinogenesis and their potential usefulness in developing anti-cancer immunotherapies should direct more attention to their expression in cancer.
Acknowledgements
This study was supported in part by a Grant No 175092 from the Ministry of Education Science and Technological Development of Serbia.
Disclosure of conflict of interest
None.
References
- 1.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F, editors. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed 14/03/2014. [Google Scholar]
- 3.Cheng L, Zhang S, MacLennan GT, Williamson SR, Lopez-Beltran A, Montironi R. Bladder cancer: translating molecular genetic insights into clinical practice. Hum Pathol. 2011;42:455–81. doi: 10.1016/j.humpath.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Fujii T, Shimada K, Anai S, Fujimoto K, Konishi N. ALKBH2, a novel AlkB homologue, contributes to human bladder cancer progression by regulating MUC1 expression. Cancer Sci. 2013;104:321–7. doi: 10.1111/cas.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanks JH, Iczkowski KA. Divergent differentiation in urothelial carcinoma and other bladder cancer subtypes with selected mimics. Histopathology. 2009;54:885–900. doi: 10.1111/j.1365-2559.2008.03167.x. [DOI] [PubMed] [Google Scholar]
- 6.Lim M, Adsay NV, Grignon D, Osunkoya AO. Urothelial carcinoma with villoglandular differentiation: a study of 14 cases. Mod Pathol. 2009;22:1280–6. doi: 10.1038/modpathol.2009.97. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Beltran A, Montironi R, Blanca A, Cheng L. Invasive micropapillary urothelial carcinoma of the bladder. Hum Pathol. 2010;41:1159–64. doi: 10.1016/j.humpath.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Theodoropoulos G, Carraway KL. Molecular signaling in the regulation of mucins. J Cell Biochem. 2007;102:1103–16. doi: 10.1002/jcb.21539. [DOI] [PubMed] [Google Scholar]
- 9.Yonezawa S, Higashi M, Yamada N, Yokoyama S, Kitamoto S, Kitajima S, Goto M. Mucins in human neoplasms: clinical pathology, gene expression and diagnostic application. Pathol Int. 2011;61:697–716. doi: 10.1111/j.1440-1827.2011.02734.x. [DOI] [PubMed] [Google Scholar]
- 10.Scholfield DP, Simms MS, Bishop MC. MUC1 mucin in urological malignancy. BJU Int. 2003;91:560–6. doi: 10.1046/j.1464-410x.2003.04132.x. [DOI] [PubMed] [Google Scholar]
- 11.Takashi M, Murase T, Kinjo T, Mitsuya H, Nagura H. Epithelial membrane antigen as an immunohistochemical marker for transitional cell carcinoma of the urinary bladder. Urol Int. 1987;42:170–5. doi: 10.1159/000281888. [DOI] [PubMed] [Google Scholar]
- 12.Walsh MD, Hohn BG, Thong W, Devine PL, Gardiner RA, Samaratunga ML, McGuckin MA. Mucin expression by transitional cell carcinomas of the bladder. Br J Urol. 1994;73:256–62. doi: 10.1111/j.1464-410x.1994.tb07514.x. [DOI] [PubMed] [Google Scholar]
- 13.Hughes OD, Bishop MC, Perkins AC, Wastie ML, Denton G, Price MR, Frier M, Denley H, Rutherford R, Schubiger PA. Targeting superficial bladder cancer by the intravesical administration of copper-67-labeled anti-MUC1 mucin monoclonal antibody C595. J. Clin. Oncol. 2000;18:363–70. doi: 10.1200/JCO.2000.18.2.363. [DOI] [PubMed] [Google Scholar]
- 14.Kunze E, Francksen B, Schulz H. Expression of MUC5AC apomucin in transitional cell carcinomas of the urinary bladder and its possible role in the development of mucus-secreting adenocarcinomas. Virchows Arch. 2001;439:609–15. doi: 10.1007/s004280100429. [DOI] [PubMed] [Google Scholar]
- 15.Sobin LH, Wittekind C. TNM Classification of Malignant Tumours. 6th edition. New York: International Union Against Cancer, Wiley-Liss; 2002. [Google Scholar]
- 16.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and genetics of tumors of the urinary system and male genital organ. Lyon: IARC Press; 2004. World Health Organization classification of tumors; pp. 89–120. [Google Scholar]
- 17.Sangoi AR, Higgins JP, Rouse RV, Schneider AG, McKenney JK. Immunohistochemical comparison of MUC1, CA125, and Her2Neu in invasive micropapillary carcinoma of the urinary tract and typical invasive urothelial carcinoma with retraction artifact. Mod Pathol. 2009;22:660–7. doi: 10.1038/modpathol.2009.16. [DOI] [PubMed] [Google Scholar]
- 18.Roberts JT, von der Maase H, Sengeløv L, Conte PF, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine/cisplatin and methotrexate/vinblastine/doxorubicin/cisplatin in patients with locally advanced and metastatic bladder cancer. Ann Oncol. 2006;17(Suppl 5):v118–22. doi: 10.1093/annonc/mdj965. [DOI] [PubMed] [Google Scholar]
- 19.Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Front Biosci. 2001;6:D1192–D1206. doi: 10.2741/moniaux. [DOI] [PubMed] [Google Scholar]
- 20.Rachagani S, Torres MP, Moniaux N, Batra SK. Current status of mucins in the diagnosis and therapy of cancer. Biofactors. 2009;35:509–27. doi: 10.1002/biof.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakha EA, Boyce RW, Abd El-Rehim D, Kurien T, Green AR, Paish EC, Robertson JF, Ellis IO. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005;18:1295–304. doi: 10.1038/modpathol.3800445. [DOI] [PubMed] [Google Scholar]
- 22.Kufe DW. Mucins in cancer: Function, prognosis and therapy. Nat Rev Cancer. 2009;9:874–85. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yonezawa S, Goto M, Yamada N, Higashi M, Nomoto M. Expression profiles of MUC1, MUC2, and MUC4 mucins in human neoplasms and their relationship with biological behavior. Proteomics. 2008;8:3329–41. doi: 10.1002/pmic.200800040. [DOI] [PubMed] [Google Scholar]
- 24.Yonezawa S, Higashi M, Yamada N, Yokoyama S, Goto M. Significance of mucin expression in pancreatobiliary neoplasms. J Hepatobiliary Pancreat Sci. 2010;17:108–24. doi: 10.1007/s00534-009-0174-7. [DOI] [PubMed] [Google Scholar]
- 25.Tsutsumida H, Goto M, Kitajima S, Kubota I, Hirotsu Y, Yonezawa S. Combined status of MUC1 mucin and surfactant apoprotein A expression can predict the outcome of patients with small-size lung adenocarcinoma. Histopathology. 2004;44:147–55. doi: 10.1111/j.1365-2559.2004.01797.x. [DOI] [PubMed] [Google Scholar]
- 26.Gilg MM, Wimmer B, Ott A, Langner C. Urothelial carcinoma with abundant myxoid stroma: evidence for mucus production by cancer cells. Virchows Arch. 2012;461:99–101. doi: 10.1007/s00428-012-1253-8. [DOI] [PubMed] [Google Scholar]
- 27.Simms MS, Hughes OD, Limb M, Price MR, Bishop MC. MUC1 mucin as a tumour marker in bladder cancer. BJU Int. 1999;84:350–2. doi: 10.1046/j.1464-410x.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 28.Conn IG, Crocker J, Emtage LA, Wallace DM. HMFG-2 as a prognostic indicator in superficial bladder cancer. J Clin Pathol. 1988;41:1191–5. doi: 10.1136/jcp.41.11.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau SK, Weiss LM, Chu PG. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol. 2004;122:61–9. doi: 10.1309/9R66-73QE-C06D-86Y4. [DOI] [PubMed] [Google Scholar]
- 30.Agata N, Ahmad R, Kawano T, Raina D, Kharbanda S, Kufe D. MUC1 oncoprotein blocks death receptor-mediated apoptosis by inhibiting recruitment of caspase-8. Cancer Res. 2008;68:6136–44. doi: 10.1158/0008-5472.CAN-08-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy LD, Sahraei M, Subramani DB, Besmer D, Nath S, Tinder TL, Bajaj E, Shanmugam K, Lee YY, Hwang SI, Gendler SJ, Mukherjee P. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene. 2011;30:1449–59. doi: 10.1038/onc.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obara W, Ohsawa R, Kanehira M, Takata R, Tsunoda T, Yoshida K, Takeda K, Katagiri T, Nakamura Y, Fujioka T. Cancer peptide vaccine therapy developed from oncoantigens identified through genome-wide expression profile analysis for bladder cancer. Jpn J Clin Oncol. 2012;42:591–600. doi: 10.1093/jjco/hys069. [DOI] [PubMed] [Google Scholar]
- 33.Kimura T, McKolanis JR, Dzubinski LA, Islam K, Potter DM, Salazar AM, Schoen RE, Finn OJ. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res (Phila) 2013;6:18–26. doi: 10.1158/1940-6207.CAPR-12-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–9. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 35.He YF, Zhang MY, Wu X, Sun XJ, Xu T, He QZ, Di W. High MUC2 expression in ovarian cancer is inversely associated with the M1/M2 ratio of tumor-associated macrophages and patient survival time. PLoS One. 2013;8:e79769. doi: 10.1371/journal.pone.0079769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canesin G, Gonzalez-Peramato P, Palou J, Urrutia M, Cordón-Cardo C, Sánchez-Carbayo M. Galectin-3 expression is associated with bladder cancer progression and clinical outcome. Tumor Biol. 2010;31:277–85. doi: 10.1007/s13277-010-0033-9. [DOI] [PubMed] [Google Scholar]
- 37.Song S, Byrd JC, Mazurek N, Liu K, Koo JS, Bresalier RS. Galectin-3 modulates MUC2 mucin expression in human colon cancer cells at the level of transcription via AP-1 activation. Gastroenterology. 2005;129:1581–91. doi: 10.1053/j.gastro.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Vincent A, Perrais M, Desseyn JL, Aubert JP, Pigny P, Van Seuningen I. Epigenetic regulation (DNA methylation, histone modifications) of the 11p15 mucin genes (MUC2, MUC5AC, MUC5B, MUC6) in epithelial cancer cells. Oncogene. 2007;26:6566–76. doi: 10.1038/sj.onc.1210479. [DOI] [PubMed] [Google Scholar]
- 39.Shibahara H, Tamada S, Higashi M, Goto M, Batra SK, Hollingsworth MA, Imai K, Yonezawa S. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology. 2004;39:220–29. doi: 10.1002/hep.20031. [DOI] [PubMed] [Google Scholar]
- 40.Saitou M, Goto M, Horinouchi M, Tamada S, Nagata K, Hamada T, Osako M, Takao S, Batra SK, Aikou T, Imai K, Yonezawa S. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58:845–52. doi: 10.1136/jcp.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamada S, Shibahara H, Higashi M, Goto M, Batra SK, Imai K, Yonezawa S. MUC4 is a novel prognostic factor of extrahepatic bile duct carcinoma. Clin Cancer Res. 2006;12:4257–64. doi: 10.1158/1078-0432.CCR-05-2814. [DOI] [PubMed] [Google Scholar]
- 42.Tsutsumida H, Goto M, Kitajima S, Kubota I, Hirotsu Y, Wakimoto J, Batra SK, Imai K, Yonezawa S. MUC4 expression correlates with poor prognosis in small-sized lung adenocarcinoma. Lung Cancer. 2007;55:195–203. doi: 10.1016/j.lungcan.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Hamada T, Wakamatsu T, Miyahara M, Nagata S, Nomura M, Kamikawa Y, Yamada N, Batra SK, Yonezawa S, Sugihara K. MUC4 is a novel prognostic factor of oral squamous cell carcinoma. Int J Cancer. 2011;130:1768–76. doi: 10.1002/ijc.26187. [DOI] [PubMed] [Google Scholar]
- 44.Lee HK, Cho MS, Kim TH. Prognostic significance of muc4 expression in gallbladder carcinoma. World J Surg Oncol. 2012;10:224. doi: 10.1186/1477-7819-10-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rachagani S, Macha MA, Ponnusamy MP, Haridas D, Kaur S, Jain M, Batra SK. MUC4 potentiates invasion and metastasis of pancreatic cancer cells through stabilization of fibroblast growth factor receptor 1. Carcinogenesis. 2012;33:1953–64. doi: 10.1093/carcin/bgs225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoshi H, Sawada T, Uchida M, Saito H, Iijima H, Toda-Agetsuma M, Wada T, Yamazoe S, Tanaka H, Kimura K, Kakehashi A, Wei M, Hirakawa K, Wanibuchi H. Tumor-associated MUC5AC stimulates in vivo tumorigenicity of human pancreatic cancer. Int J Oncol. 2011;38:619–27. doi: 10.3892/ijo.2011.911. [DOI] [PubMed] [Google Scholar]
- 47.Inaguma S, Kasai S, Ikeda H. GLI1 facilitates the migration and invasion of pancreatic cancer cells through MUC5AC mediated attenuation of E-cadherin. Oncogene. 2011;30:714–23. doi: 10.1038/onc.2010.459. [DOI] [PubMed] [Google Scholar]
- 48.Jankovic Velickovic L, Katic V, Hattori T, Kushima R, Marjanovic G, Stefanovic V. Differences in the expression of mucins in various forms of cystitis glandularis. Pathol Res Pract. 2007;203:653–8. doi: 10.1016/j.prp.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Leir SH, Harris A. MUC6 mucin expression inhibits tumor cell invasion. Exp Cell Res. 2011;317:2408–19. doi: 10.1016/j.yexcr.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 50.Owens SR, Chiosea SI, Kuan SF. Selective expression of gastric mucin MUC6 in colonic sessile serrated adenoma but not in hyperplastic polyp aids in morphological diagnosis of serrated polyps. Mod Pathol. 2008;21:660–9. doi: 10.1038/modpathol.2008.55. [DOI] [PubMed] [Google Scholar]
- 51.Zheng H, Takahashi H, Nakajima T, Murai Y, Cui Z, Nomoto K, Tsuneyama K, Takano Y. MUC6 down-regulation correlates with gastric carcinoma progression and a poor prognosis: an immunohistochemical study with tissue microarrays. J Cancer Res Clin Oncol. 2006;132:817–23. doi: 10.1007/s00432-006-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Domanowska E, Jozwicki W, Domaniewski J, Golda R, Skok Z, Wiśniewska H, Sujkowska R, Wolski Z, Jozwicka G. Muscle-invasive urothelial cell carcinoma of the human bladder: multidirectional differentiation and ability to metastasize. Hum Pathol. 2007;38:741–6. doi: 10.1016/j.humpath.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Antunes AA, Nesrallah LJ, Dall’Oglio MF, Maluf CE, Camara C, Leite KR, Srougi M. The role of squamous differentiation in patients with transitional cell carcinoma of the bladder treated with radical cystectomy. Int Braz J Urol. 2007;33:339–45. doi: 10.1590/s1677-55382007000300006. [DOI] [PubMed] [Google Scholar]


