Abstract
A new category, “EBV positive T-cell lymphoproliferative disorders (LPD) of childhood”, was proposed in the 2008 World Health Organization’s (WHO) classifications of lymphoma. This series of lymphoproliferative disorders is rare. There are two major types of this series of disorders: systemic EBV positive T-cell LPD of childhood and hydroa vacciniforme-like lymphoma (HVLL). In this study, we describe the distinct features of four cases of EBV positive T-cell LPD of childhood in China. Two were systemic EBV positive T-cell LPD of childhood, one was HVLL and one was chronic active EBV (CAEBV). The main manifestations were lymphadenopathy, fever, hepatosplenomegaly and skin rashes. The structure of the lymph nodes in the patients ranged from preserved to partially or totally destroyed. Small- to medium-sized, atypical T cells had infiltrated the lymph nodes. In HVLL, the neoplastic cells had infiltrated the dermis and subcutaneous region surrounding sweat glands and nerves. All of the cases tested positive for CD8, other T cells, cytotoxic markers and EBV-encoded RNA (EBER) without CD56 expression. Molecular analysis was performed in three cases. All of the three analyses showed a TCRγ rearrangement and one case also had an IGH rearrangement. One of the patients with systemic EBV positive T-cell LPD of childhood experienced rapid evolved and died within five months of onset. CAEBV, systemic EBV-positive T-cell LPD of childhood and HVLL are distinct but overlapping diseases within the category of EBV-positive T-cell LPD of childhood. They constitute a continuous spectrum of EBV-infected associated disorders.
Keywords: Epstein-Barr virus, T cells, lymphoproliferative disease, pediatric
Introduction
It has been proven that the Epstein-Barr virus (EBV) can infect B cells, T cells and natural killer (NK) cells, and can result in various types of lymphoproliferative disorders containing B-cell, T-cell, NK-cell and Hodgkin lymphomas. EBV positive T-cell LPD of childhood is fairly rare and predominantly occurs in Asians and Native Americans. According to the 2008 WHO classification of tumors of the hematopoietic and lymphoid tissues, EBV-positive T-cell LPD of childhood can be divided into two major types: systemic EBV-positive T-cell LPD of childhood and HVLL [1]. The first type usually occurs shortly after primary acute EBV infection or in the setting of CAEBV. It is characterized by a clonal proliferation of EBV-infected T cells with an activated cytotoxic phenotype. It generally occurs in the liver, spleen, bone marrow, lymph nodes, skin and lungs. Patients with this disease most commonly show an acute or subacute clinical course and usually die of multiple organ failure days to months after the onset of the disease. The second type, HVLL, is a cutaneous T-cell lymphoma with an indolent clinical process. It is characterized by a papulovesicular eruption in a sun-exposed area. System symptoms may be present, particularly late in the course of the disease. Reviewing the literature, it transpires that the majority cases of reported HVLL have been from Asia, particularly Japan [2], with a few from China. In this study, we report the clinicopathological features of four Chinese pediatric patients with EBV-positive T-cell LPD to accumulate more information and diagnostic experience about this series of diseases.
Materials and methods
Patients
Four cases of EBV-positive T-cell LPD of childhood were studied at the Cancer Institute and Hospital of the Chinese Academy of Medical Sciences (CICAMS) in Beijing between February 2011 and 2013. The morphology and immunophenotype of each case were analyzed. All four cases were discussed and finally diagnosed consistently by several pathological professors in the department.
Immunohistochemistry
Immunostaining was performed on 4 µm-thick FFPE tissue sections using an autostainer, a Ventana Benchmark XT (Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer’s instructions. The primary antibodies used included CD2, CD3, CD5, CD7, CD43, CD4, CD8, CD20, PAX-5, CD56, TIA-1, granzyme B (GrB), CD30 and Ki-67.
In situ hybridization for EBER
The presence of EBV RNA was analyzed by non-isotopic in-situ hybridization with EBV-encoded RNA (EBER) 1 and 2 oligonucleotide probes (Dako Cytomation, Carpinteria, CA, USA) in paraffin-embedded tissue sections, as previously described.
B-cell and T-cell clonality analysis
A BIOMED-2 polymerase chain reaction (PCR) was performed to analyze the clonal expansion of T and B cells. DNA was extracted from the paraffin sections and the quality of the DNA was assessed. T-cell clonal expansion was detected by analysis of TCRβ and TCRγ gene rearrangement and B-cell clonal expansion by IGH and IGK gene rearrangement. The PCR products were analyzed using capillary electrophoresis. Appropriate positive and negative controls were included in all of the experiments.
Results
Clinical features and follow up data
The patients were three males and one female, aged from seven to 14 years. All of the patients were Chinese. None of them had any previous history of immunodeficiency. Three of the patients presented fever. All had multiple lymphadenopathy. Two patients presented with hepatosplenomegaly and cytopenia. One exhibited hypersensitivity to mosquito bites. One had a recurrent skin rash on the right toe and on two sites on the trunk (summarized in Table 1).
Table 1.
Clinical characteristics and follow-up data of the four cases of EBV-positive T-cell LPD of childhood
| Patient number | Age (years) | Gender | Clinical presentation | Therapy | Outcome |
|---|---|---|---|---|---|
| 1 | 14 | Male | Fever, hepatosplenomegaly, lymphadenopathy, anemia, sensitivity to insect bites | Chemotherapy, interferon | Alive. Currently completely healthy, 38 months from onset |
| 2 | 7 | Male | Fever, lymphadenopathy | Oral drugs (non-chemotherapy, not clear) | Alive. Currently completely healthy, 32 months from onset |
| 3 | 9 | Male | Fever, hepatosplenomegaly, lymphadenopathy, pancytopenia, then progressed to multiorgan failure | Chemotherapy | Dead at 5 months from onset |
| 4 | 12 | Female | Recurrent skin rash, lymphadenopathy | Chemotherapy | Alive. Under treatment, more than 12 months from onset |
All patients proceeded with treatment; three of them with chemotherapy. One patient’s condition progressed rapidly and he died within five months of onset. The other three patients are alive. Two of these patients are currently completely healthy.
Histological analysis
The architecture of the lymph nodes differed between the patients. Case two showed a preserved architecture, with the interfollicular area and lymphatic sinusoid remarkably expanded. In cases one and three some to all of the lymph node structure was destroyed (Figure 1A). Small- to medium-sized neoplastic lymphoid cells had infiltrated the expanded T zone, with mild atypia (Figure 1B). Marked proliferation of high endothelial venules was seen in all lymph nodes. Case four was analyzed with a skin biopsy, which revealed that a large number of neoplastic cells had infiltrated the dermis and subcutaneous areas around the sweat glands and nerves (Figure 1C and 1D).
Figure 1.
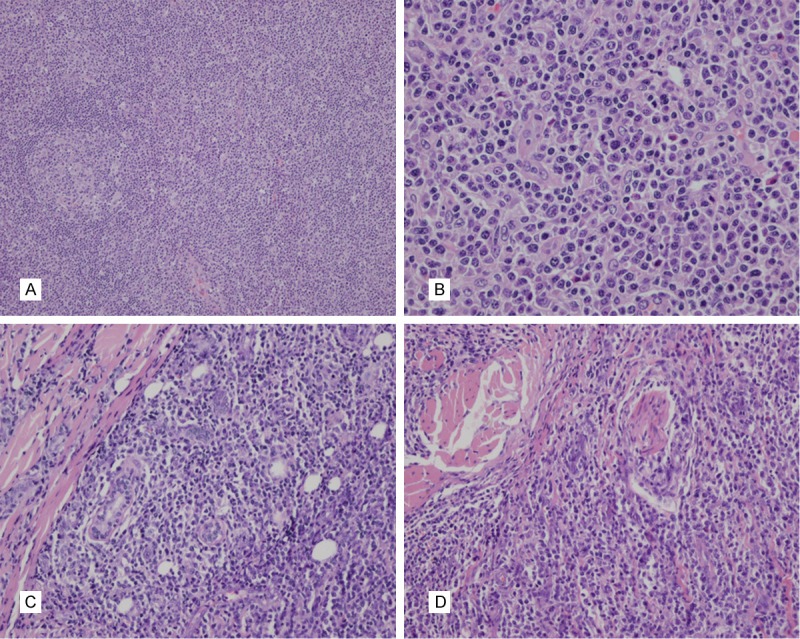
Histologic features of EBV positive T-cell LPD of childhood. A: Lymph node structure partially destroyed, neoplastic cells proliferated actively in an expanded T-zone. B: Small- to medium-sized neoplastic lymphoid cells had infiltrated the expanded T zone, with mild atypia. C and D: A large number of neoplastic cells infiltrated around the sweat glands and nerves.
Based on the clinical and morphologic features, immunophenotype and ISH data, and molecular analysis, the four cases were classified as follows: cases one and three were systemic EBV-positive T-cell LPD of childhood, case four was HVLL and case two was CAEBV.
Immunohistochemistry and ISH analysis
In situ hybridization for EBV using an EBER probe demonstrated a marked positivity in the majority of the small-sized lymphocytes. It also expressed cytotoxic molecules GrB and TIA-1. All of the cases expressed CD8. Only one expressed CD4 (Figure 2). CD56 was negative in all of the cases. Two cases were positive for CD30. The Ki-67 labeling index was high (40% to 80%) in all of the cases (Table 2).
Figure 2.
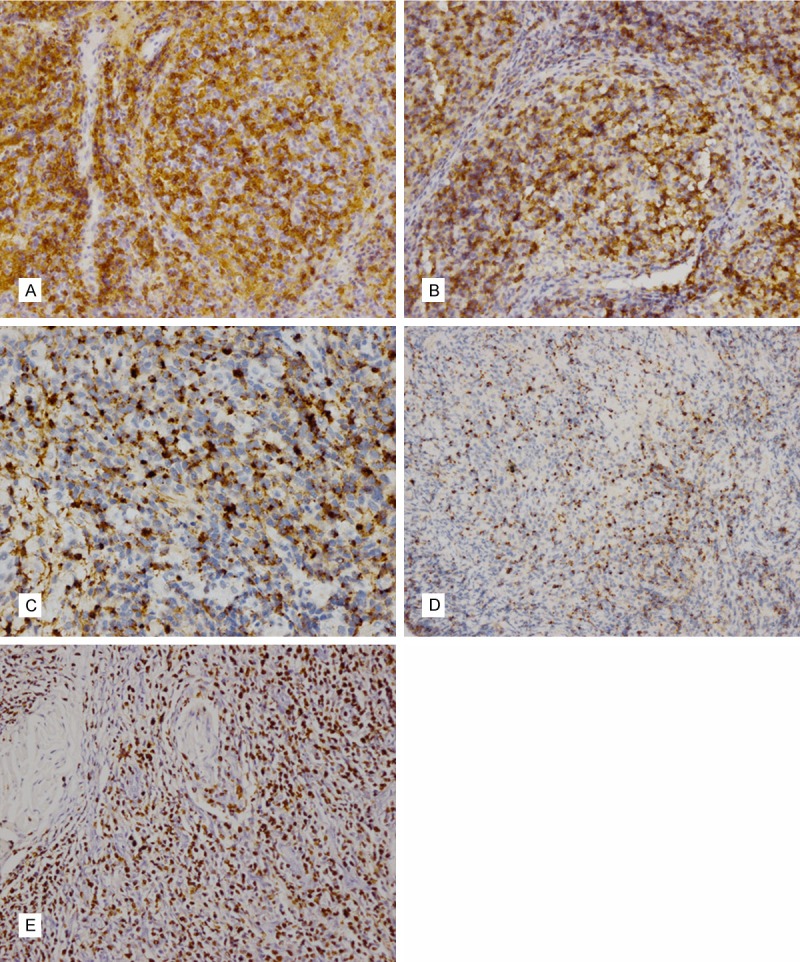
Immunophenotypic features of EBV-positive T-cell LPD of childhood. Case 3 showed both CD4 (A) and CD8 (B) positive. Neoplastic cells were positive for GrB (C), TIA-1 (D) and EBER (E).
Table 2.
Morphologic, immunophenotypic and molecular features of the patients with EBV-positive T-cell LPD of childhood
| Patient number | Diagnosis | Biopsied organ | Morphologic features | Immunophenotype and EBER | PCR |
|---|---|---|---|---|---|
| 1 | Systemic EBV-positive T-cell LPD of childhood | Lymph node | Lymph node structure was partially destroyed, neoplastic cells proliferated actively in an expanded T-zone | CD2+, CD3+, CD5+, CD7+, CD4-, CD8+, CD30+, CD56-, TIA-1+, GrB+, CD20-, Ki-67 > 60%, EBER+ | TCRγ |
| 2 | CAEBV | Lymph node | Lymph node structure was preserved, T-zone and lymphatic sinusoid was expanded and infiltrated with neoplastic cells | CD2+, CD3+, CD43+, CD4-, CD8+, CD30+, CD56-, TIA-1+, GrB+, CD20-, PAX-5-, Ki-67 > 50%, EBER+ | NV |
| 3 | Systemic EBV-positive T-cell LPD of childhood | Lymph node | Lymph node structure was totally destroyed, FDC was diminished or disappeared, neoplastic cells diffusedly infiltrated | CD2+, CD5+, CD7+, CD4+, CD8+, CD56-, TIA-1+, GrB+, CD20-, Ki-67 40%, EBER+ | TCRγ |
| 4 | HVLL | Skin | Marked degree of neoplastic cells infiltrated the dermis | CD2+, CD3+, CD5+, CD7+, CD4-, CD8+, CD30+, CD56-, TIA-1+, GrB-, CD20-, PAX-5-, Ki-67-Li 80%, EBER+ | TCRγ and IGH |
NV, not evaluable.
Molecular studies
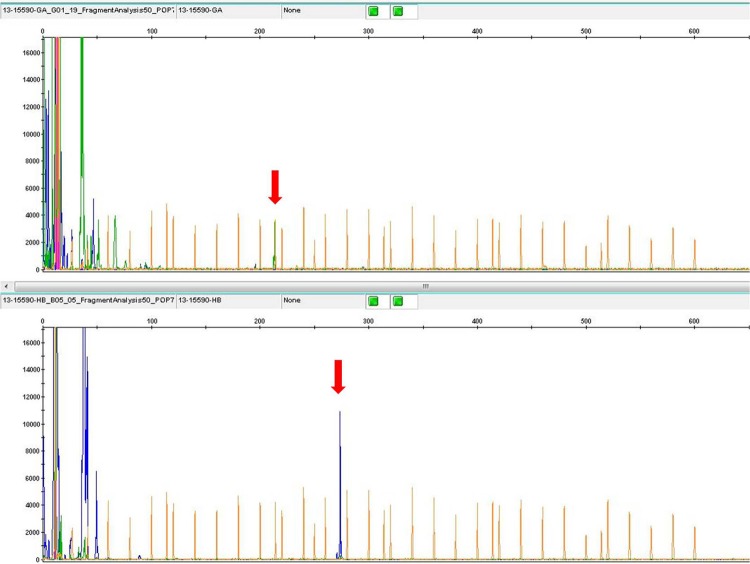
Three cases displayed B-cell receptor (BCR) and TCR rearrangements. All of the cases examined exhibited TCR rearrangement. Interestingly, case four also displayed a monoclonal IGH gene rearrangement (Figure 3).
Figure 3.
Molecular features of Case 4. It showed both TCRγ and IGH rearrangement.
Discussion
Recently, extensive attention has been paid to EBV because infection may lead to a proliferation of different subsets of lymphocytes and cause various types of lymphoproliferative disorders involving B cells, T cells and NK cells. In 2008, the WHO classification of tumors of the hematopoietic and lymphoid tissues introduced a new category of T-cell lymphoproliferative disorders, called “EBV-positive T-cell LPD of childhood” [1]. This disorder may be associated with acute EBV infection or CAEBV [2-4]. It usually affects children and young adults [5-7]. This disorder is fairly rare and appears to have a racial predilection. It is more prevalent in Asia and South America [8-10] and is seldom found in Western countries [4,7]. The majority of the cases reported in Asia were Japanese and only a few Chinese cases have been described in the literature. In our study, all four patients were Chinese. The patients ranged in age from seven to 14 years (with a median age of 10.5 years). This age range is consistent with Jin’s study [9].
Systemic EBV-positive T-cell LPD of childhood and HVLL are the two main types of EBV-positive T-cell LPD of childhood. Systemic EBV-positive T-cell LPD of childhood encompasses a variety of entities, including fulminant EBV+ T-cell LPD of childhood, fatal infectious mononucleosis (FIM) and severe CAEBV with monoclonal EBV+ T-cell proliferation.
CAEBV has been mostly seen in Western populations. However, it rarely progresses to systemic lymphoproliferative disorder in such patients and it has usually been associated with EBV-infected B cell proliferation [11]. The main clinical symptom of CAEBV in Western populations is an infectious mononucleosis (IM)-like syndrome with high titers of antibodies to EBV-capsid antigen (VCA-IgG) and early antigen (EA-IgG) [1]. In contrast, the CAEBV that has been found in Japanese and other East Asian patients often has a poor prognosis and a high mortality rate, and is characterized by a clonal expansion of T cells or NK cells [2,12,13]. Thus, it has been called “severe CAEBV”. Patients with this disorder usually have a fever, lymphadenopathy, hepatosplenomegaly and pancytopenia, which often evolve into fatal complications such as multi-organ failure, sepsis, disseminated intravascular coagulopathy (DIC) and hemophagocytic syndrome. To avoid confusion between the two different CAEBVs affecting different populations, the form of severe CAEBV with monoclonal EBV-positive T-cell proliferation was included by the WHO in the systemic EBV-positive T-cell LPD of childhood classification of lymphoma in 2008.
Cutaneous complications, including hypersensitivity to mosquito bites and hydroa vacciniforme (HV), may occur independently or in association with CAEBV. A few cases of HV overlapping with CAEBV subsequently developed into EBV associated T-cell or NK-cell lymphoma, which was categorized in the 2008 WHO classification of lymphoma as HVLL [14]. The clinical characteristics of HVLL are facial edema and recurrent vesiculopapular rash, followed by ulceration and crusting. In some patients, this disorder may develop into a systemic disease with systemic symptoms including fever, lymphadenopathy and hepatosplenomegaly [15-17]. However, systemic EBV-positive T-cell LPD of childhood may also be accompanied by hypersensitivity to insect bites and HV. Thus, HVLL with systemic symptoms and systemic EBV-positive T-cell LPD of childhood with accompanying skin lesion are overlapping conditions.
Given this fact, we consider CAEBV, systemic EBV-positive T-cell LPD of childhood and HVLL to be distinct but overlapping entities within the category of EBV-positive T-cell LPD of childhood. These three conditions constitute a continuous spectrum of EBV-infected associated disorders.
In our study, all of the patients presented with lymphadenopathy regardless of the different entities. One case of systemic EBV-positive T-cell LPD of childhood presented with a mosquito bite allergy. The patient with HVLL in our study showed multiple skin rashes and accompanying lymphadenopathy in several regions. Thus, the clinical manifestations of our series support our previous inference.
To some extent, the histological changes in each case revealed the severity of the disease. The distinction between different entities also manifested a continuous spectrum of EBV-infected associated disorders. Compared with the case of CAEBV, which showed a largely preserved lymph node structure, the two cases of systemic EBV-positive T-cell LPD of childhood in our series exhibited a partial to complete destruction of the lymph node architecture. The number of atypical T cells infiltrating the lymph node in the CAEBV case was less than that of the two cases of systemic EBV-positive T-cell LPD of childhood. Furthermore, the two cases of systemic EBV-positive T-cell LPD of childhood exhibited a higher degree of atypia of neoplastic cells than the case of CAEBV. Moreover, the outcome of the CAEBV patient was very positive. The patient was completely healthy after a non-chemotherapy treatment. In contrast, the case of systemic EBV-positive T-cell LPD of childhood in which the normal lymph node structure was destroyed and had the highest proportion of the neoplastic cells rapidly progressed. This patient died of the disease within five months of its onset. Thus, we hypothesize that CAEBV, which has a lack of systemic clinical presentations and does not show T-cell or NK-cell monoclonal proliferation, may be a preneoplastic disorder of systemic lymphoma. The morphological changes may reveal the seriousness of the disorder and help predict its prognosis.
EBV-positive T-cell LPD of childhood is characterized by a clonal proliferation of EBV-infected T-cells and NK-cells. An investigation of Japanese CAEBV patients suggested that the T cell type had a poorer prognosis than the NK cell type [18]. In our study, all four cases had T-cell type expressed cytotoxic granules such as TIA-1 and/or GrB. All four cases were negative for CD56, a marker of NK-cell immunophenotype. The neoplastic cells in all four cases expressed CD8, which is consistent with the results of other reported studies [19,20]. Only one case was positive for CD4. The WHO classification of lymphoma suggested that cases that occur in the setting of CAEBV are prone to be CD4 positive [1].
Most interestingly, the case of HVLL had not only TCRγ but also IGH rearrangement, which has been described in one other study [20]. It is considered that this result could be due to CD20 positive B-blasts intermingling with neoplastic cells.
EBV-positive T-cell LPD of childhood is a rare series of disorders involving many entities. Definitive diagnosis requires correlations to be made among clinical, pathological and ancillary investigatory findings. EBV detection by ISH and molecular cloning technique analysis could contribute to the diagnosis. Due to the low incidence of this disease, research should continue to accumulate more cases in an attempt to better understand its morphological and clinical characteristics.
Disclosure of conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. Lyon: IARC; 2008. [Google Scholar]
- 2.Fujiwara S, Kimura H, Imadome K, Arai A, Kodama E, Morio T, Shimizu N, Wakiguchi H. Current research on chronic active Epstein-Barr virus infection in Japan. Pediatr Int. 2014;56:159–166. doi: 10.1111/ped.12314. [DOI] [PubMed] [Google Scholar]
- 3.Park S, Ko YH. Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disorders. J Dermatol. 2014;41:29–39. doi: 10.1111/1346-8138.12322. [DOI] [PubMed] [Google Scholar]
- 4.Quintanilla-Martinez L, Kumar S, Fend F, Reyes E, Teruya-Feldstein J, Kingma DW, Sorbara L, Raffeld M, Straus SE, Jaffe ES. Fulminant EBV(+) T-cell lymphoproliferative disorder following acute/chronic EBV infection: a distinct clinicopathologic syndrome. Blood. 2000;96:443–451. [PubMed] [Google Scholar]
- 5.Suzuki K, Ohshima K, Karube K, Suzumiya J, Ohga S, Ishihara S, Tamura K, Kikuchi M. Clinicopathological states of Epstein-Barr virus-associated T/NK-cell lymphoproliferative disorders (severe chronic active EBV infection) of children and young adults. Int J Oncol. 2004;24:1165–1174. [PubMed] [Google Scholar]
- 6.Ohshima K, Kimura H, Yoshino T, Kim CW, Ko YH, Lee SS, Peh SC, Chan JK CAEBV Study Group. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Int. 2008;58:209–217. doi: 10.1111/j.1440-1827.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 7.Tabanelli V, Agostinelli C, Sabattini E, Gazzola A, Bacci F, Capria S, Mannu C, Righi S, Sista MT, Meloni G, Pileri SA, Piccaluga PP. Systemic Epstein-Barr-virus-positive T cell lymphoproliferative childhood disease in a 22-year-old Caucasian man: A case report and review of the literature. J Med Case Rep. 2011;5:218. doi: 10.1186/1752-1947-5-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshii M, Ishida M, Hodohara K, Okuno H, Nakanishi R, Yoshida T, Okabe H. Systemic Epstein-Barr virus-positive T-cell lymphoproliferative disease of childhood: Report of a case with review of the literature. Oncol Lett. 2012;4:381–384. doi: 10.3892/ol.2012.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Y, Zhou XG, He LJ, Xie JL, Zheng YY, Zhang YN, Zhang SH. Clinicopathologic features of systemic EBV-positive T-cell lymphoproliferative disease of childhood. Zhonghua Bing Li Xue Za Zhi. 2009;38:600–608. [PubMed] [Google Scholar]
- 10.Wang W, Ti HJ, Nong L, Zhang S, Zhang Y, Li T. Clinical and pathological analysis of three cases of childhood systemic EB virus positive T-cell lymphoproliferative disease. Beijing Da Xue Xue Bao. 2012;44:594–598. [PubMed] [Google Scholar]
- 11.Ohga S, Ishimura M, Yoshimoto G, Miyamoto T, Takada H, Tanaka T, Ohshima K, Ogawa Y, Imadome K, Abe Y, Akashi K, Hara T. Clonal origin of Epstein-Barr virus (EBV)-infected T/NK-cell subpopulations in EBV-positive T/NK-cell lymphoproliferative disorders of childhood. J Clin Virol. 2011;51:31–37. doi: 10.1016/j.jcv.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Su IJ, Chen RL, Lin DT, Lin KS, Chen CC. Epstein-Barr virus (EBV) infects T lymphocytes in childhood EBV-associated hemophagocytic syndrome in Taiwan. Am J Pathol. 1994;144:1219–1225. [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen JI, Jaffe ES, Dale JK, Pittaluga S, Heslop HE, Rooney CM, Gottschalk S, Bollard CM, Rao VK, Marques A, Burbelo PD, Turk SP, Fulton R, Wayne AS, Little RF, Cairo MS, El-Mallawany NK, Fowler D, Sportes C, Bishop MR, Wilson W, Straus SE. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood. 2011;17:5835–5849. doi: 10.1182/blood-2010-11-316745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quintanilla-Martinez L, Ridaura C, Nagl F, Saez-de-Ocariz M, Durán-McKinster C, Ruiz-Maldonado R, Alderete G, Grube P, Lome-Maldonado C, Bonzheim I, Fend F. Hydroa vacciniforme-like lymphoma: a chronic EBV+ lymphoproliferative disorder with risk to develop a systemic lymphoma. Blood. 2013;122:3101–3110. doi: 10.1182/blood-2013-05-502203. [DOI] [PubMed] [Google Scholar]
- 15.Xu Z, Lian S. Epstein-Barr virus-associated hydroa vacciniforme-like cutaneous lymphoma in seven Chinese children. Pediatr Dermatol. 2010;27:463–469. doi: 10.1111/j.1525-1470.2010.01094.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang M, Wang S, Yang QP, Liu YM, Gao LM, Sun H, Liu WP. Hydroa vacciniforme-like lymphoma of an adult: a case report with review of the literature. Diagn Pathol. 2013;8:72. doi: 10.1186/1746-1596-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HY, Baek JO, Lee JR, Park SH, Jeon IS, Roh JY. Atypical hydroa vacciniforme-like epstein-barr virus associated T/NK-cell lymphoproliferative disorder. Am J Dermatopathol. 2012;34:e119–124. doi: 10.1097/DAD.0b013e3181c036de. [DOI] [PubMed] [Google Scholar]
- 18.Kimura H, Morishima T, Kanegane H, Ohga S, Hoshino Y, Maeda A, Imai S, Okano M, Morio T, Yokota S, Tsuchiya S, Yachie A, Imashuku S, Kawa K, Wakiguchi H Japanese Association for Research on Epstein-Barr Virus and Related Diseases. Prognostic factors for chronic active Epstein-Barr virus infection. J Infect Dis. 2003;187:527–533. doi: 10.1086/367988. [DOI] [PubMed] [Google Scholar]
- 19.Yoshii M, Ishida M, Hodohara K, Okuno H, Nakanishi R, Yoshida T, Okabe H. Systemic Epstein-Barr virus-positive T-cell lymphoproliferative disease of childhood: Report of a case with review of the literature. Oncol Lett. 2012;4:381–384. doi: 10.3892/ol.2012.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Pinilla SM, Barrionuevo C, Garcia J, de los Ángeles M, Pajares R, Casavilca S, Montes J, Martínez A, Montes-Moreno S, Sánchez L, Piris MÁ. Epstein-Barr virus-positive systemic NK/T-cell lymphomas in children: report of six cases. Histopathology. 2011;59:1183–1193. doi: 10.1111/j.1365-2559.2011.04047.x. [DOI] [PubMed] [Google Scholar]