Abstract
Astrocyte Elevated Gene-1 (AEG-1) has been proposed as a biomarker for a variety of cancers. This study aimed to investigate the expression of AEG-1 in human astrocytomas and the correlation between AEG-1 expression and clinicopathologic variables of astrocytomas. AEG-1 expression in four pairs of matched astrocytomas tissues and 5 cell lines was detected by immunohistochemical and Western blot analysis. In addition, AEG-1 protein expression was examined by immunohistochemical staining in 204 cases, including 32 normal brain tissues, 80 Low-malignant astrocytomas (LMAs) and 92 High-Malignant astrocytomas (HMAs). AEG-1 expression in 31 LMAs and 29 HMAs samples was detected by RT-PCR and Western blot analysis. We detected AEG-1 expression in normal neurons and glioma cell lines U87, U251 and M059K, but not in normal glial cells. Immunohistochemical analysis showed that 128 of 172 (74.4%) paraffin-embedded archival astrocytomas samples exhibited positive AEG-1 expression. Statistical analysis suggested that higher AEG-1 level was significantly correlated with histological grade of astrocytomas. In addition, AEG-1 mRNA and protein expression was higher in HMAs than in LMAs. AEG-1 expression had no correlation with the gender or age of astrocytoma patients. In summary, our data suggest that AEG-1 may represent a novel prognostic marker for astrocytomas.
Keywords: Astrocytoma, AEG-1, prognosis
Introduction
Astrocytomas are the most common primary tumor in central nervous system (CNS), accounting for roughly 75% of neuroepithelial tumors. Astrocytomas are graded as pilocytic astrocytoma (grade I), diffuse astrocytoma (grade II), anaplastic astrocytoma (grade III) and glioblastoma (grade IV) by World Health Organization (WHO) [1]. Grade I and II astrocytomas are classified into Low-Malignant Astrocytomas (LMAs), while grade III and IV astrocytomas are classified into High-Malignant Astrocytomas (HMAs). Some LMAs patients respond to chemotherapy, but most HMAs patients are chemoresistant [2,3]. Despite contemporary surgery and radiotherapy, survival rate of astrocytomas patients mostly depends on chemotherapy. Several molecules have been proposed as prognostic factors for astrocytomas [4,5].
Astrocyte elevated gene-1 (AEG-1), also known as metadherin (MTDH) and lysine-rich CEACAM1 co-isolated (LYRIC), is implicated in cancer chemoresistance [6]. AEG-1 has been proposed as a biomarker for a variety of cancers including cervical cancer, hepatocellular carcinoma and osteosarcoma [7]. The up-regulation of AEG-1 promotes the growth, invasion and metastasis of cancers via several signaling pathways such as PI3K/AKT, NF-Kb and MAPK pathways [8]. AEG-1 contributes to broad spectrum resistance to various chemotherapeutics such as paclitaxel, doxorubicin and cisplatin by increasing the expression of multidrug resistance genes [9,10]. AEG-1 upregulates matrix metalloproteinase-9 and enhanced the migration and invasion of human glioma cells [11,12]. In addition, knockdown of AEG-1 enhanced chemosensitivity of neuroblastoma cells [13].
In current study, we aimed to investigate the expression of AEG-1 in human astrocytomas and the correlation between AEG-1 expression and clinicopathologic variables of astrocytomas. Our results showed that AEG-1 expression was significantly correlated with histological grade of astrocytomas.
Materials and methods
Patients and tissue specimens
Total 204 paraffin-embedded glioma specimens were collected, which had been histopathologically and clinically diagnosed at the First Hospital Affiliated with Harbin Medical University from 2008 to 2012. 32 normal brain tissues were used as controls. Among 204 glioma specimens, 92 were LMAs tissues, and 80 were HMAs tissues. The 204 tissues were from 128 males and 76 females with a median age of 42 years (range 2-80 years old). In addition, 31 LMAs and 29 HMAs samples from 204 cases were collected and immediately frozen in liquid nitrogen, and subsequently stored at -80°C. The clinicopathologic variables, such as age, gender and histopathologic classification were recorded. Prior informed consent was obtained from the patients for collection of glioma specimens in accordance with the guidelines of the Hospital, and the study protocols have been approved by Ethics Committee of Harbin Medical University.
Real-time quantitative RT-PCR
Total RNA was extracted from the tissues or cells by using Trizol (Invitrogen). Reverse transcription was performed with 2 μg total RNA and M-MLV reverse transcriptase (Takara), and 2 μg cDNA was used for PCR reaction. Primer sequences of AEG-1 and GAPDH were designed by Primer-5.0 primer and synthesized by Sangon Biological Engineering (Shanghai, China): AEG-1 forward, AAATAgCCAgCCTATCAAgACTC’, reverse, TTCAgACTTggTCTgTGAAggAg; GAPDH forward, ATCACTgCCCACCCAgAAgAC, reverse, TTTCTAgACggCAggTCAgg. PCR parameters were as follows: 95°C for 30 s (one cycle), 20 s at 95°C, 20 s at 60°C and 20 s at 72°C (45 cycles), and 1 min at 72°C (one cycle). Relative AEG-1 mRNA level was defined as the ratio of A value of AEG-1 to that of GAPDH.
Western blot analysis
The protein was extracted from tissues by using RIPA (Beyotime) and protein concentration was determined by Bradford assay using BSA. Equal quantities of protein were separated electrophoretically on 10% SDS-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Millipore Corporation). Next, the membranes were probed with AEG-1 rabbit antibody (Epitomics, 1:1000 dilution), or GAPDH mouse monoclonal antibody (Zhongshanjin-qiao, 1:1000 dilution), followed by incubation with horseradish peroxidase conjugated anti-rabbit IgG (1:4000 dilution). The membranes were developed by using enhanced chemiluminescence kit (Thermo) according to the manufacturer’s protocols, and exposed to X-ray film.
Immunohistochemical staining
Formalin-fixed, paraffin embedded tissue sections were deparaffinized and rehydrated through a xylene and ethanol series and then treated with 3% hydrogen peroxide for 5 min to block endogenous peroxidase activity. Subs-equently, slides were washed in distilled water, and then pretreated with boiling citric acid buffer (pH 6.0) for 5 min. The slides were incubated with 10% normal goat serum in phosphate-buffered saline for 10 min. The sections were probed with primary monoclonal antibodies to AEG-1 (1:150), followed by secondary goat anti-rabbit IgG conjugated to horseradish peroxidase (1:1000). Then the sections were stained with 0.02% diaminobenzidine and 0.02% H2O2 in 0.05 M Tris-HCl buffer for 10 min. Finally, the sections were counterstained with 10% Mayer hematoxylin. For negative controls, rabbit serum was used instead of the primary antibody. The positive controls were prostate cancer with positive expression of AEG-1.
Evaluation of immunohistochemical staining
The sections were scored by combining the proportion and intensity of positively stained tumor cells in a series of 10 randomly selected high-power fields. The proportion of positively stained tumor cells was scored as follows: 0 (no positive tumor cells), 1 (<10% positive tumor cells), 2 (10-50% positive tumor cells) and 3 (>50% positive tumor cells). Staining intensity was classified according to the following criteria: 0 (no staining), 1 (weak staining, light yellow), 2 (moderate staining, yellow brown) and 3 (strong staining, brown). AEG-1 expression was evaluated by determining the staining index (SI), calculated as the staining intensity score x the proportion score.
Statistical analysis
All statistical analyses were carried out using SPSS16.0 statistical software package. Quan-titative values were presented as mean ± SD or median (range). Independent Student’s t test was used to compare AEG-1 mRNA and protein expression in LGA and HMAs samples. The Chi-Square test was performed to analyze the relationship between AEG-1 expression and histopathologic classification. Bivariate correlations between study variables were calculated by Spearman’s rank correlation coefficients. All tests were two tailed and P-value of <0.05 was considered statistically significant.
Results
AEG-1 expression in matched astrocytomas tissues and glioma cell lines
The immunohistochemical staining of AEG-1 was performed with 4 matched astrocytomas and their adjacent noncancerous tissues (ANT) from 4 patients, including two-paired LMAs tissues and two-paired HMAs tissues. AEG-1 expression was detected in all four astrocytomas tissues and AEG-1 was localized mainly in the cytoplasm as in many other cancer tissues. In contrast, weak or no AEG-1 was observed in glial cells of the 4 adjacent noncancerous brain tissues (Figure 1A). Immunohistochemistry showed that AEG-1 protein level was at least 2-fold higher in all 4 tumor tissues compared with tissues adjacent to the tumors (Figure 1B). In adjacent noncancerous brain tissues, neurons showed high level of AEG-1 protein expression (Figure 1A-a, 1A-c).
Figure 1.
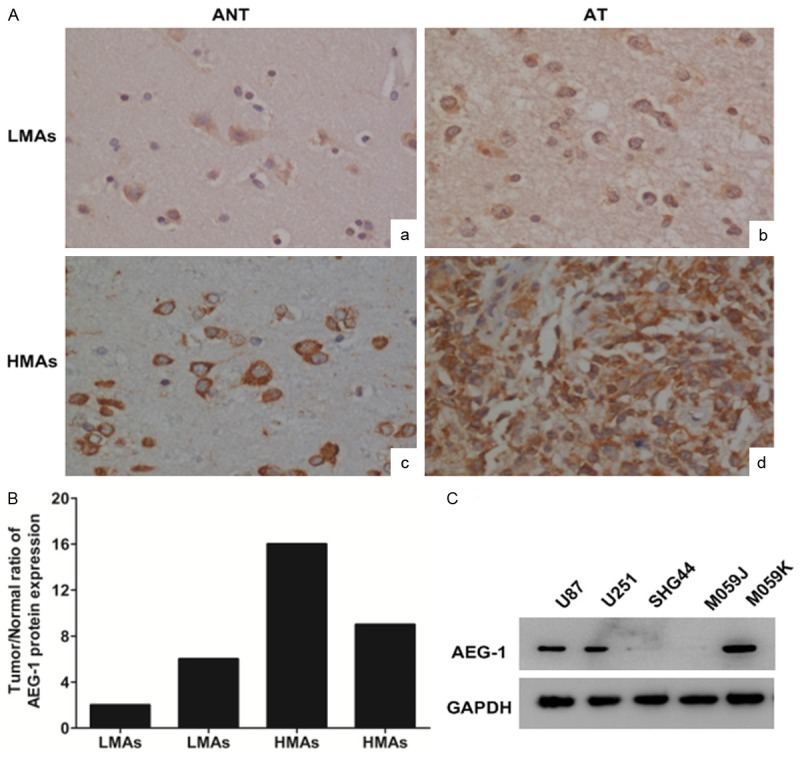
Immunohistochemical analysis of AEG-1 protein expression in astrocytomas tissues (AT) and their adjacent noncancerous tissues (ANT) paired from the same patient. (A-a) The adjacent noncancerous tissue from the Low-Malignant Astrocytomas (LMAs) patient; (A-b) The astrocytomas tissue from the same patient as (A-a); (A-c) The adjacent noncancerous tissue from High-Malignant Astrocytomas (HMAs) patient; (A-d) The astrocytomas tissue from the same patient as (A-c). Original magnification ×400; (B) Quantitative analysis of AEG-1 staining in astrocytomas tissues; (C) Western blot analysis of AEG-1 expression in human glioma U87, U251, SHG44, M059J and M059K cell lines.
Western blot analysis showed that AEG-1 expression was higher in HMAs cell lines U87, U251 and M059K than in LMAs cell line SHG44. AEG-1 expression in HMAs cell line M059J, deficient in DNA dependent protein kinase (DNA-PK), was low (Figure 1C).
AEG-1 expression is higher in HMAs tissues than in LMAs tissues
Next we analyzed the association between AEG-1 expression level and histological grade of astrocytomas. We performed immunochemical staining on 32 normal brain tissues, 92 LMAs tissues and 80 HMAs tissues. The results showed that the frequency and intensity of AEG-1 expression were gradually elevated from normal brain tissues to astrocytoma tissues and were the highest in GBM tissues. For the transition from LMAs tissues to HMAs tissues, AEG-1 staining was more intense in HMAs tissues than in LMAs tissues (Figure 2). AEG-1 expression was significantly different between normal brain tissues and LMAs tissues (P<0.001), between normal brain tissues and HMAs tissues (P<0.001), and between LMAs tissues and HMAs (P<0.001) (Table 1). Western blot and RT-PCR analysis showed that AEG-1 protein and mRNA levels of HMAs tissue were significantly increased compared to those of LMAs tissues (Figure 3).
Figure 2.
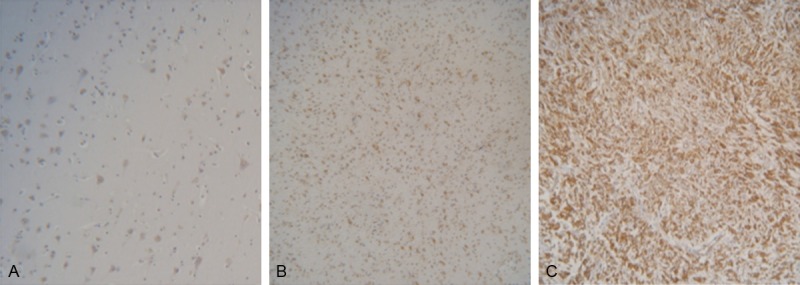
Immunohistochemical analysis of AEG-1 protein expression in formalin-fixed paraffin-embedded normal brain tissues and astrocytomas tissues. A. Normal brain tissue; B. Low-Malignant Astrocytoma tissue; C. High-Malignant Astrocytoma tissue. Original magnification × 100.
Table 1.
AEG-1 expression in astrocytomas normal and tumor tissues
| Patients | AEG-1 expression | |||
|---|---|---|---|---|
|
|
||||
| Group | n | Negative | Positive | P |
| N | 32 | 30 | 2 | 0.000* |
| LMAs | 92 | 34 | 58 | |
| HMAs | 80 | 10 | 70 | |
Statistical analysis was performed using the chi squared test; a P-value <0.05 was considered significant. N, Normal brain tissues; LMAs, Low-Malignant Astrocytomas tissues; HMAs, High-Malignant Astrocytomas tissues.
P<0.05.
Figure 3.
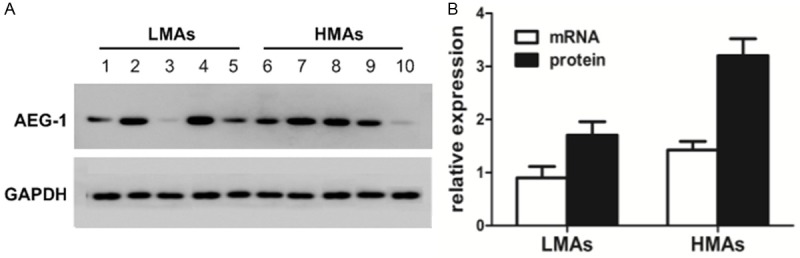
AEG-1 expression is higher in HMAs tissues than in LMAs tissues. A. Western blot analysis of AEG-1 expression in HMAs tissues and LMAs tissues. Ten representative LMAs and HMAs tissues were shown. Glyceraldehyde 3-phosphate dehydrogenase was used as loading control. B. Quantitative analysis of AEG-1 mRNA and protein levels in 31 LMAs and 29 HMAs tissues. LMAs, Low-Malignant Astrocytomas; HMAs, High-Malignant Astrocytomas.
Correlations between AEG-1 expression and clinicopathologic variables of astrocytomas
We analyzed 172 astrocytomas tissues, which were from 100 males and 72 females. As shown in Table 2, for males, 40 were negative of AEG-1 expression and 60 were positive; for females, 38 were negative of AEG-1 expression and 34 were positive. For subjects younger than 45 years old, 36 were negative of AEG-1 expression and 50 were positive; for subjects older than 45 years old, 38 were negative of AEG-1 expression and 48 were positive. Spearman’s correlation analysis showed that th-ere was no correlation between AEG-1 expressi-on and the gender (P = 0.098) or the age (P = 0.363).
Table 2.
Correlations between AEG-1 expression and clinicopathologic variables of astrocytomas
| Patients | AEG-1 expression | P | Spearman Rank Correlations | ||
|---|---|---|---|---|---|
|
|
|
||||
| Variables | n | Negative | Positive | r (P) | |
| Gender | 0.097 | 0.127 (0.098) | |||
| Male | 100 | 40 | 60 | ||
| Female | 72 | 38 | 34 | ||
| Age | 0.758 | 0.070 (0.363) | |||
| <45 | 86 | 36 | 50 | ||
| ≥45 | 86 | 38 | 48 | ||
AEG-1 expression was determined in 172 astrocytomas samples by immunohistochemical staining. Bivariate correlations between study variables were calculated by Spearman’s rank correlation coefficients.
Discussion
In this study, we found that AEG-1 was expressed in normal brain tissues and the expression of AEG-1 in normal neurons was much higher than that in glial cells. As AEG-1 could prevents serum starvation-induced cell death in primary human fetal astrocytes (PHFA) through PI3K-Akt signaling, the expression of AEG-1 may be important for the survival of normal neurons in bad situation [14]. Further experiments are needed to elucidate the exact function of AEG-1 in normal neurons. However, up-regulation of AEG-1 expression in glioma can cause neuronal cell death by negatively regulating EAAT2 expression and glutamate uptake [15,16]. Interestingly, AEG-1 gene is located at 8q22.1 on which there is a common susceptibility variant for migraine, indicating that AEG-1 may be implicated in migraine [17].
LMAs are well-differentiated and slow growing tumors. In most astrocytomas patients, the progression into HMAs occurs during the course of this ultimately fatal disease [18]. HMAs are infiltrative and diffuses in nature. In this study we found that AEG-1 expression was higher in HMAs tissues and glioma cells than in LMAs, normal brain tissues and glial cells, in agreement with the results of other researchers [19]. AEG-1 has been shown to be overexpressed in a variety of cancers and proposed as a negative prognostic and predictive factor for these cancers owing to its multiple contributions to chemoresistance, vasculogenesis, invasiveness and metastasis [20]. Our data suggest that AEG-1 may indicate poor prognosis in astrocytomas patients as well.
In conclusion, AEG-1 may serve as a new biomarker for the diagnosis, therapy and prognosis of astrocytomas patients, and appears as an ideal adjuvant reagent to decrease the chemoresistance in astrocytomas therapy.
Acknowledgements
This study was supported by The Natural Science Foundation of Harbin City (2011RFLXS025).
Disclosure of conflict of interest
None.
References
- 1.Lind-Landstrom T, Habberstad AH, Sundstrom S, Torp SH. Prognostic value of histological features in diffuse astrocytomas WHO grade II. Int J Clin Exp Pathol. 2012;5:152–158. [PMC free article] [PubMed] [Google Scholar]
- 2.Henriksson R, Asklund T, Poulsen HS. Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: a review. J Neurooncol. 2011;104:639–646. doi: 10.1007/s11060-011-0565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker DG, Kaye AH. Diagnosis and management of astrocytomas, oligodendrogliomas and mixed gliomas: a review. Australas Radiol. 2001;45:472–482. doi: 10.1046/j.1440-1673.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni A, Thota B, Srividya MR, Thennarasu K, Arivazhagan A, Santosh V, Chandramouli BA. Expression pattern and prognostic significance of IGFBP isoforms in anaplastic astrocytoma. Pathol Oncol Res. 2012 Oct;18:961–7. doi: 10.1007/s12253-012-9526-8. [DOI] [PubMed] [Google Scholar]
- 5.Zhang LY, Jiang LN, Li FF, Li H, Liu F, Gu Y, Song Y, Zhang F, Ye J, Li Q. Reduced beta-catenin expression is associated with good prognosis in Astrocytoma. Pathol Oncol Res. 2010 Jun;16:253–7. doi: 10.1007/s12253-009-9219-0. [DOI] [PubMed] [Google Scholar]
- 6.Hu G, Chong RA, Yang Q, Wei Y, Blanco MA, Li F, Reiss M, Au JL, Haffty BG, Kang Y. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell. 2009;15:9–20. doi: 10.1016/j.ccr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Moran MS, Yang Q, Liu Q, Yuan C, Hong S, Kong B. Metadherin regulates radioresistance in cervical cancer cells. Oncol Rep. 2012;27:1520–1526. doi: 10.3892/or.2012.1692. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar D, Emdad L, Lee SG, Yoo BK, Su ZZ, Fisher PB. Astrocyte elevated gene-1: far more than just a gene regulated in astrocytes. Cancer Res. 2009;69:8529–8535. doi: 10.1158/0008-5472.CAN-09-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu G, Wei Y, Kang Y. The multifaceted role of MTDH/AEG-1 in cancer progression. Clin Cancer Res. 2009;15:5615–5620. doi: 10.1158/1078-0432.CCR-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo BK, Chen D, Su ZZ, Gredler R, Yoo J, Shah K, Fisher PB, Sarkar D. Molecular mechanism of chemoresistance by astrocyte elevated gene-1. Cancer Res. 2010;70:3249–3258. doi: 10.1158/0008-5472.CAN-09-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Wu J, Ying Z, Chen B, Han A, Liang Y, Song L, Yuan J, Li J, Li M. Astrocyte Elevated Gene-1 Upregulates Matrix Metalloproteinase-9 and Induces Human Glioma Invasion. Cancer Res. 2010;70:3750–3759. doi: 10.1158/0008-5472.CAN-09-3838. [DOI] [PubMed] [Google Scholar]
- 12.Emdad L, Sarkar D, Lee SG, Su ZZ, Yoo BK, Dash R, Yacoub A, Fuller CE, Shah K, Dent P, Bruce JN, Fisher PB. Astrocyte elevated gene-1: a novel target for human glioma therapy. Mol Cancer Ther. 2010;9:79–88. doi: 10.1158/1535-7163.MCT-09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Song X, Liu C, Xie L, Wei L, Sun R. Knockdown of astrocyte elevated gene-1 inhibits proliferation and enhancing chemo-sensitivity to cisplatin or doxorubicin in neuroblastoma cells. J Exp Clin Cancer Res. 2009;28:19. doi: 10.1186/1756-9966-28-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–1121. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 15.Lee SG, Kim K, Kegelman TP, Dash R, Das SK, Choi JK, Emdad L, Howlett EL, Jeon HY, Su ZZ, Yoo BK, Sarkar D, Kim SH, Kang DC, Fisher PB. Oncogene AEG-1 Promotes Glioma-Induced Neurodegeneration by Increasing Glutamate Excitotoxicity. Cancer Res. 2011;71:6514–6523. doi: 10.1158/0008-5472.CAN-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int. 2007;51:333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anttila V, Stefansson H, Kallela M, Todt U, Terwindt GM, Calafato MS, Nyholt DR, Dimas AS, Freilinger T, Muller-Myhsok B, Artto V, Inouye M, Alakurtti K, Kaunisto MA, Hamalainen E, de Vries B, Stam AH, Weller CM, Heinze A, Heinze-Kuhn K, Goebel I, Borck G, Gobel H, Steinberg S, Wolf C, Bjornsson A, Gudmundsson G, Kirchmann M, Hauge A, Werge T, Schoenen J, Eriksson JG, Hagen K, Stovner L, Wichmann HE, Meitinger T, Alexander M, Moebus S, Schreiber S, Aulchenko YS, Breteler MM, Uitterlinden AG, Hofman A, van Duijn CM, Tikka-Kleemola P, Vepsalainen S, Lucae S, Tozzi F, Muglia P, Barrett J, Kaprio J, Farkkila M, Peltonen L, Stefansson K, Zwart JA, Ferrari MD, Olesen J, Daly M, Wessman M, van den Maagdenberg AM, Dichgans M, Kubisch C, Dermitzakis ET, Frants RR, Palotie A. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nat Genet. 2010;42:869–873. doi: 10.1038/ng.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durmaz R, Vural M, Isildi E, Cosan E, Ozkara E, Bal C, Ciftci E, Arslantas A, Atasoy MA. Efficacy of prognostic factors on survival in patients with low grade glioma. Turk Neurosurg. 2008;18:336–344. [PubMed] [Google Scholar]
- 19.Emdad L, Dent P, Sarkar D, Fisher PB. Future approaches for the therapy of malignant glioma: targeting genes mediating invasion. Future Oncol. 2012;8:343–346. doi: 10.2217/fon.12.16. [DOI] [PubMed] [Google Scholar]
- 20.Emdad L, Sarkar D, Su ZZ, Lee SG, Kang DC, Bruce JN, Volsky DJ, Fisher PB. Astrocyte elevated gene-1: recent insights into a novel gene involved in tumor progression, metastasis and neurodegeneration. Pharmacol Ther. 2007;114:155–170. doi: 10.1016/j.pharmthera.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


