Abstract
Emerging evidence has indicated that apoptotic cells have a compensatory effect on the proliferation of neighboring cells. Recent studies have shown that apoptotic tumor cells stimulate the repopulation of tumors from a small number of surviving cells by cleaved caspase-3 regulation and elevated tumor cleaved (and thus activated) caspase-3 expression levels predict worse treatment outcomes in cancer patients. The prognostic significance of cleaved caspase-3 should be demonstrated in more human cancer types and larger subjects. Here, we examined the cleaved caspase-3 expression in 367 human tumor samples (gastric cancer: 97 cases, ovarian cancer: 65 cases, cervical cancer: 104 cases; colorectal cancer: 101 cases) with immunohistochemistry (IHC) and the relationship between the expression of cleaved caspase-3 and various clinicopathological factors were also detected. We found that, cleaved caspase-3 was significantly associated with pathological risk factors (P < 0.005) for the studied cancers, such as tumor stage, lymph-node metastasis, differentiation and so on. In univariate and multivariate analysis, patients with high expression of cleaved caspase-3 had a significant shorter overall survival time compared with those with low cleaved caspase-3 expression in gastric cancer (P < 0.001), ovarian cancer (P < 0.001), cervical cancer (P = 0.002), colorectal cancer (P < 0.001) individually and in the patients combined (P < 0.001). Cox regression results suggested cleaved caspase-3 as an independent prognosis predictor for the studied four cancer types. Our study showed cleaved caspase-3 was well correlated to progression, aggressive behaviors in the studied cancer, and implicated it as a potential predictive factor for the prognosis of the four cancer types. It also indicated cleaved caspase-3 as a potential therapeutic target for cancer patients.
Keywords: Cleaved caspase-3 expression, overall survival of cancer patients, multivariate Cox proportional hazards model analysis, prognosis
Introduction
In cancer treatment, apoptosis is a well-recognized cell death mechanism through which cytotoxic agents kill tumor cells. Studies conducted more than 40 years ago [1-4] indicated that tumors initiate a process called accelerated repopulation after radiotherapy. This phenomenon has become the major obstacle in modern radiotherapy and chemotherapy [5]. Hence the understanding of tumor repopulation is crucial for improving our current therapies. Investigators have made tremendous efforts to understand the molecular mechanism of tumor repopulation after cytotoxic therapy. Recent evidences showed that dying tumor cells use the apoptotic process to generate potent growth-stimulating signals to stimulate the repopulation of tumors undergoing radiotherapy [5,6]. Mao et al revealed a paracrine signal released from dying ECs which promotes the proliferation of surrounding glioma cells [7]. Indeed, the phenomenon of apoptosisstimulated tissue regeneration has been observed by other investigators in lower organisms such as Drosophila and hydra systems [8-10]. In these cases, it has been proposed that apoptotic cells stimulate socalled compensatory proliferation for tissue regeneration. Cleaved caspase-3, a key executor in apoptosis, was involved in the growth stimulation [5-7,11]. Deficiency of caspase-3 either in tumor cells or in tumor stroma resulted in substantial tumor sensitivity to radiotherapy in xenograft or mouse tumors [5]. Caspase-3 inhibition attenuated the growth-stimulating effect of dying melanoma cells on living melanoma cells in vitro [6]. In human subjects with head and neck cancer and advanced stage breast cancer, higher amounts of cleaved caspase-3 in tumor tissues were correlated with markedly increased rate of recurrence ,death and shorter survival time [5]. In 249 subjects with breast cancer from Sweden and Singapore, elevated caspase-3 mRNA levels correlated with significantly elevated risk of relapse [5]. They concluded that elevated tumor cleaved caspase-3 levels predict worse outcomes in people with cancer [5]. But the result has not been confirmed in other human cancer types and more subjects with cancer.
In this study, we examined cleaved caspase-3 level in four cancer types pretreatment biopsies, namely human gastric cancer, ovarian cancer, cervical cancer, and colorectal cancer, which are the main causes of tumor-associated death in series of cancer for women. The result that its over-expression correlated with reduced patient overall survival time (OS) is consistent with the previous study [5]. To our knowledge, it is the first time to detect the prognostic value of cleaved caspase-3 in the four cancer types. Supported by data from immunohistochemical (IHC) analyses of human tumor samples, our study potentiated the use of cleaved caspase-3 as a biomarker to predict tumor prognosis. And it also implicated cleaved caspase-3 as a potential therapeutic target of cancer patient.
Materials and methods
Patients and tissue specimens
For this retrospective study, archival formalin-fixed paraffin-embedded (FFPE) specimens from 367 cancer patients admitted to West China Hospital and West China Second University Hospital, Sichuan University from 2001 to 2007 pretreated were attained. The four types of malignant tumors included in the study are shown in Table 1. Cases (141 males, 226 females) with available follow-up and complete clinical data were included for immunohistochemical studies. Routine follow-up was continued up to at least 5 years. The primary endpoint in this study was defined as the time to death related to studied cancer. Patients receiving chemotherapy or radiation therapy before surgery were excluded. Information on sex, age, stage of disease, and histopathologic parameters were retrieved from the medical records. The tumors were confirmed as malignant by pathologists from West China Hospital and West China Second University Hospital. The study was approved by the Ethics Committees of Sichuan University. Informed consent was obtained from all participating patients.
Table 1.
Characteristics of patients
| Characteristics | n |
|---|---|
| Total no. with assessable stains | 367 |
| Mean age (range) | 52.94 (18-87) |
| Sex | |
| Male | 141 |
| Female | 226 |
| Tumor type | |
| Gastric cancer | 97 |
| Ovarian cancer | 65 |
| Cervical cancer | 104 |
| Colorectal cancer | 101 |
| Caspase-3 | |
| High | 116 |
| Low | 251 |
Immunohistochemistry (IHC)
For IHC analysis, 4 µm-thick sections cut from the FFPE tissue blocks were deparaffinized and rehydrated using xylene and a graded series of ethanol (absolute, 95%, 80%, 50%), followed by two 5 min washes in phosphate buffered saline with tween-20 (PBST). Antigen retrieval was performed in 10 mmol sodium citrate buffer (pH 6.0), which was microwaved at 90-100°C for 20 min and washed in PBST for 2×5 min. The sections were then incubated for 30 min in 3% (v/v) hydrogen peroxide in methanol to block endogenous peroxidase activity, washed in PBST for 3×5 min, blocked at room temperature for 30 min by using 2% normal goat serum, 2% bovine serum albumin (BSA), and 0.1% triton-X in phosphate buffered saline (PBS), and incubated in a humidified chamber overnight at 4°C with the primary antibodies anti-cleaved caspase-3 (1:150 dilution; Cell Signaling Technology, USA). The sections were then washed in PBST (3×5 min) and incubated at room temperature for 1 h with the secondary antibodies (goat-anti-rabbit, SP-9002, Zhongshan Golden Bridge Inc, China). After a wash with PBST (3×5 min), the sections were incubated with ready-to-use streptavidin peroxidase at room temperature for 30 min and well rinsed with distilled water. Colors were developed with a DAB kit. The sections were then counterstained with hematoxylin, dehydrated, and mounted. Negative controls were prepared by substituting PBS for the primary antibodies.
Immunoreactivity scoring
A staining score of cleaved caspase-3 was calculated as the percentage of immunostained cancer cells to all cancer cells in three view fields. Expression level was categorized as high (> 10% cells stained) or low (staining cells ≤ 10%) as previously described [5]. Brown cytoplasmic and/or nuclear staining was counted as positive. The results from the immunohistochemically stained slides were scored by two of the histopathologists independently, who were blinded to patient information.
Statistical analysis
As the study endpoint, overall survival (OS) was calculated from the date of primary surgery to the date of death or the last follow-up. All patients were followed until death or the end of the follow-up period (March, 2012). Patients were censored at the date of last visit or at the time of death not related to the studied cancer. To assess correlations of demographic and clinical variables with cleaved caspase-3 expression, chi-square test and Fisher’s exact test were used for categorical variables and two-sample t-test for continuous variables. Hazard ratios (HR) and their 95% confidence intervals (CI) were estimated using multivariate Cox’s proportional hazards model adjusted for age, sex, clinical stage, hitologic grade, and if necessary, therapeutic modality and chemotherapy regimen. Overall patient survival was estimated with Kaplan-Meier analysis with a log-rank score for determining statistical significance. For all the test, a two-tailed P ≤ 0.05 was considered statistically significant. All statistical analyses were performed using SPSS16.0 for Windows (SPSS Inc., Chicago, IL).
Results
IHC and correlations of cleaved caspase-3 expression with clinicopathological parameters
Expression levels of cleaved caspase-3 in different cancers were determined by IHC (Figure 1). Cleaved caspase-3 protein was mainly presented in the cytoplasm of the tumor cell, and partially localized in the nucleus. In order to know the clinical role of cleaved caspase-3 in cancer, we further assessed the correlations between cleaved caspase-3 expression and clinicopathological parameters for different cancer separately. High cleaved caspase-3 expression was defined as positive staining in greater than 10% of primary tumor cells. In all, 116 of 367 specimens (31.6%) were designated as cases with high cleaved caspase-3 expression (Table 1). As was shown in Table 2, among the 97 gastric cancer cases, 55 cases were with high cleaved caspase-3 expression (56.7%). Interestingly, gastric cancer cases with lymph node metastasis was more likely to had high cleaved caspase-3 expression compared with those without it (68.8% vs. 33.3%, P = 0.001). High cleaved caspase-3 expression was found less in Stage I+II than Stage III+IV gastric cancer patients (39.4% vs.70.7%, P = 0.017). Among the poorly differentiated gastric cancer cases, 67.9% were found with high cleaved caspase-3 expression, compared to 41.5% of the well differentiated cases (P = 0.010). Gastric cancer patients without serosal invasion were more likely to have a low level of cleaved caspase-3 expression than those with it (46.6% vs. 73.0%, P = 0.011). Cleaved caspase-3 expression was not significantly correlated with other clinical parameters as age, sex, tumor site, and therapeutic modality in the gastric cancer patients. Of the 65 ovarian cancer cases, 18 had high cleaved caspase-3 expression (27.7%). Cleaved caspase-3 expression was significantly higher in patients with advanced stages (42.1% vs. 7.4%, P = 0.002), in patients with lymph node metastasis (47.8% vs. 1.6.7%, P = 0.007), and in patients with residual tumor left after primary surgery (50% vs. 8.6%, P < 0.001), as well as in patients with serous adenocarcinoma (42.3% vs.17.9%, P = 0.032) (Table 3). No other significant associations were found between cleaved caspase-3 expression and clinicopathological variables. In the 104 cervical cancer cases, only 11 cases had high cleaved caspase-3 expression (10.6%). We detected a clear trend that more cases with stromal invasion (13.3% vs. 6.8%) or with vaginal wall extension (15% vs. 9.5%) or with intravascular space involvement (30.4% vs. 20.7%) or with lymph node metastasis (17.6% vs. 9.2%) or tumor size larger than 4 cm (13.6% vs. 8.1%) or with advanced stages (16.7% vs. 8.7%) had high cleaved caspase-3 expression, though no statistical significance was noted (P > 0.05, Table 4). Maybe the relatively low number of patients with high cleaved caspase-3 expression biased the results. As shown in Table 5, in the 101 colorectal cancer cases, 32 cases had high cleaved caspase-3 expression (31.7%). A well-association can be detected between cleaved caspase-3 expression with lymph node metastasis (P = 0.025), depth of invasion (P = 0.040) and Dukes classification (P = 0.018), colorectal cancer samples with high expression of cleaved caspase-3 had higher frequencies of cases with lymph node metastasis (46.9% vs. 24.6%) or cases with serosal invasion (44.4% vs. 24.6%). And high expression of cleaved caspase-3 was more prevalent in Dukes C and D cases than Dukes A and B cases (47.1% vs. 23.9).
Figure 1.
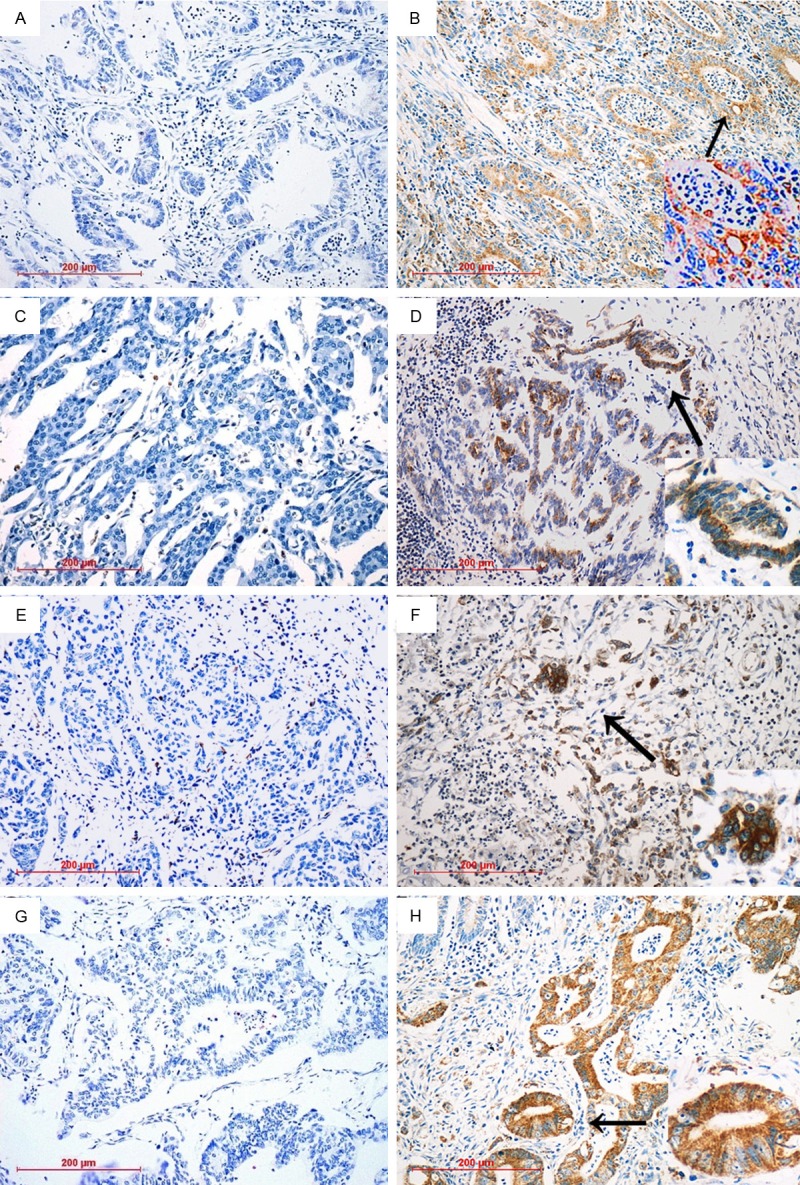
IHC analysis of cleaved caspase-3 in human cancer smples. Paraffin-embedded tissue sections were stained using an immunoperoxidase method, as described in Materials and methods. Representative images (200×) are shown. A. Representative IHC staining patterns for samples with low cleaved caspase-3 in gastric cancer; B. Representative IHC staining patterns for samples with high levels of cleaved caspase-3 in gastric cancer; C. Representative IHC staining patterns for samples with low cleaved caspase-3 in ovarian cancer; D. Representative IHC staining patterns for samples with high levels of cleaved caspase-3 in ovarian cancer; E. Representative IHC staining patterns for samples with low cleaved caspase-3 in cervical cancer; F. Representative IHC staining patterns for samples with high levels of cleaved caspase-3 in cervical cancer; G. Representative IHC staining patterns for samples with low cleaved caspase-3 in colorectal cancer; H. Representative IHC staining patterns for samples with high levels of cleaved caspase-3 in colorectal cancer. As shown above, intense immunostaining for cleaved caspase-3 protein was mainly presented in the cytoplasm of the tumor cell, and partially localized in the nucleus.
Table 2.
Correlation between caspase-3 expression and various clinicopathological features of patients with gastric cancer
| Caspase-3 expression | P value | ||
|---|---|---|---|
|
|
|||
| low | high | ||
| n | 42 | 55 | |
| Age | |||
| ≤ 50 | 7 | 17 | |
| > 50 | 35 | 38 | 0.107 |
| Sex | |||
| Male | 33 | 41 | |
| Female | 9 | 14 | 0.644 |
| Tumor site | |||
| Corpus gastricum | 18 | 24 | |
| Fundus gastricus | 18 | 24 | |
| Sinus ventriculi | 6 | 7 | 0.975 |
| Lymph node metastasis | |||
| Negative | 22 | 11 | |
| Positive | 20 | 44 | 0.001 |
| Stage | |||
| I+II | 30 | 26 | |
| III+IV | 12 | 29 | 0.017 |
| Therapy | |||
| Surgery | 22 | 25 | |
| Surgery+others | 20 | 30 | 0.499 |
| Serosal invation | |||
| Negative | 32 | 28 | |
| Positive | 10 | 27 | 0.011 |
| Differentiation | |||
| Poor | 18 | 38 | |
| Otherwise* | 24 | 17 | 0.010 |
Otherwise means well-differentiated carcinoma and moderately-differentiated carcinoma.
Table 3.
Correlation between caspase-3 expression and various clinicopathological features ofpatients with ovarian cancer
| Caspase-3 expression | P value | ||
|---|---|---|---|
|
|
|||
| Low | High | ||
| n | 47 | 18 | |
| Age | |||
| ≤ 55 | 29 | 8 | |
| > 55 | 18 | 10 | 0.209 |
| Residual tumor | |||
| Negative | 32 | 3 | |
| Positive | 15 | 15 | < 0.001 |
| Lymphnode metastasis | |||
| Negative | 35 | 7 | |
| Positive | 12 | 11 | 0.007 |
| Stage | |||
| I+II | 25 | 2 | |
| III+IV | 22 | 16 | 0.002 |
| Therapy | |||
| Surgery | 2 | 2 | |
| Surgery+others | 45 | 16 | 0.651 |
| Differentiation | |||
| Poorly | 38 | 16 | |
| Moderately | 8 | 2 | |
| Well | 1 | 0 | 0.792 |
| Histology type | |||
| Serous adenocarcinoma | 15 | 11 | |
| Non-Serous adenocarcinoma | 32 | 7 | 0.032 |
Table 4.
Correlation between Caspase-3 expression and various clinicopathological features of patients with cervical cancer
| Caspase-3 expression | P value | ||
|---|---|---|---|
|
|
|||
| Low | High | ||
| n | 93 | 11 | |
| Age | |||
| < 55 | 78 | 10 | |
| ≥ 55 | 15 | 1 | 0.865 |
| Therapy | |||
| Operation | 17 | 2 | |
| Operation+others | 76 | 9 | 1.000 |
| Stromal invasion | |||
| Negative | 41 | 3 | |
| Positive | 52 | 8 | 0.456 |
| Vaginal wall extension | |||
| Negative | 76 | 8 | |
| Positive | 17 | 3 | 1.000 |
| Parametrial extension | |||
| Negative | 52 | 6 | |
| Positive | 41 | 5 | 1.000 |
| Intravascular space involvement | |||
| Absent | 46 | 12 | |
| Present | 32 | 14 | 0.254 |
| Lymph node metastasis | |||
| Negative | 79 | 8 | |
| Positive | 14 | 3 | 0.545 |
| Stage | |||
| Early (in situ, I) | 73 | 7 | |
| Advanced (II, III, IV) | 20 | 4 | 0.467 |
| Differentiation | |||
| Poorly | 66 | 9 | |
| Moderately | 23 | 1 | |
| Well | 4 | 1 | 0.320 |
| Tumor size | |||
| < 4cm | 34 | 3 | |
| ≥ 4cm | 19 | 3 | |
| Unknown | 40 | 5 | 0.790 |
| Histology type | |||
| SCC | 82 | 11 | |
| ADC | 6 | 0 | |
| ASC | 5 | 0 | 1.000 |
Abbreviations: SCC: squamous cell carcinoma; ADC: adenocarcinoma; ASC: adenosquamous carcinoma.
Table 5.
Correlation between caspase-3 expression and various clinicopathological features ofpatients with colorectal cancer
| Caspase-3 expression | P value | ||
|---|---|---|---|
|
|
|||
| low | high | ||
| n | 69 | 32 | |
| Age | |||
| ≤ 50 | 23 | 8 | |
| > 50 | 46 | 24 | 0.398 |
| Sex | |||
| Male | 46 | 21 | |
| Female | 23 | 11 | 0.918 |
| Lymph node status | |||
| Negative | 52 | 17 | |
| Positive | 17 | 15 | 0.025 |
| Therapy | |||
| Surgery | 25 | 9 | |
| Surgery+others | 44 | 23 | 0.422 |
| Depth of tumor invation | |||
| Subserosa | 49 | 16 | |
| Serosa | 20 | 16 | 0.040 |
| Differentiation | |||
| Well | 7 | 6 | |
| Moderately | 52 | 22 | 0.502 |
| Poorly | 10 | 4 | |
| Dukes classification | |||
| A+B | 51 | 16 | |
| C+D | 18 | 16 | 0.018 |
| Tumor diameter, cm | |||
| < 5 | 19 | 12 | |
| ≥ 5 | 50 | 20 | 0.312 |
High cleaved caspase-3 expression correlating with reduced patient overall survival time
Kaplan-Meier survival analysis demonstrated that patient overall survival time (OS) was negatively correlated with the cleaved caspase-3 expression level, where higher expression of cleaved caspase-3 resulted in shorter OS (Figure 2A). Of the 367 tumor cases, 116 were with high cleaved caspase-3 expression with a median survival time (MST) of 24 months and a 5-year survival rate (5-YSR) of 27.6%, whereas the remaining 251 cases with low cleaved caspase-3 expression saw an MST over 136 months and a 5-YSR of 80.1% (P < 0.001, Figure 2A).
Figure 2.
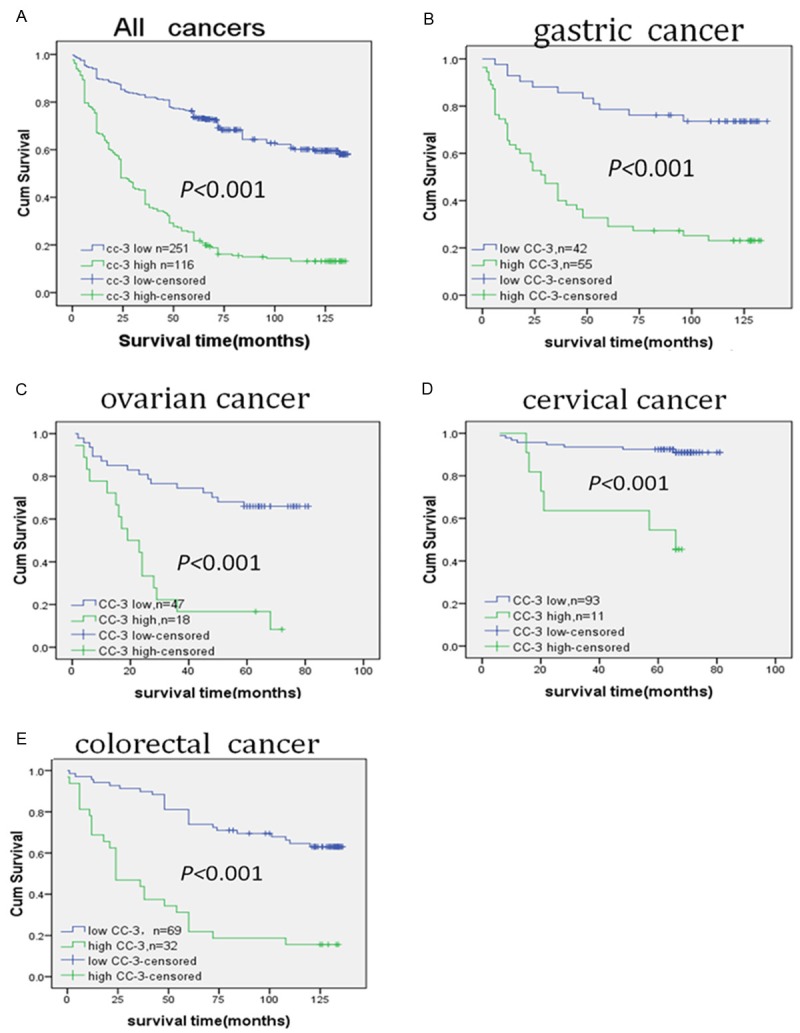
Kaplan-Meier survival analysis of survival of cancer patients according to cleaved caspase-3 expression. A. Kaplan-Meier survival analysis of overall survival of total Cancer patients in our study according to expression level of cleaved caspase-3 protein, n = 367, the 5-year survival rate of patients with high cleaved caspase-3 expression was27.6%, compared to 80.1% for patients with low cleaved caspase-3 expression (P < 0.001). B. Kaplan-Meier survival analysis of overall survival of gastric Cancer patients, n = 97, the 5-year survival rate of patients with high cleaved caspase-3 expression was29.1%, compared to 70.2% for patients with low cleaved caspase-3 expression (P < 0.001); C. Kaplan-Meier survival analysis of overall survival of ovarian Cancer patients, n = 65, the 5-year survival rate of patients with high cleaved caspase-3 expression was16.7%, compared to 66% for patients with low cleaved caspase-3 expression (P < 0.001). D. Kaplan-Meier survival analysis of overall survival of cervical Cancer patients. n = 104, the 5-year survival rate of patients with high cleaved caspase-3 expression was 54%, compared to 92.5% for patients with low cleaved caspase-3 expression (P = 0.002). E. Kaplan-Meier survival analysis of overall survival of colorectal Cancer patients, n = 101, the 5-year survival rate of patients with high cleaved caspase-3 expression was21.9%, compared to 73.9% for patients with low cleaved caspase-3 expression (P < 0.001). High cleaved caspase-3 staining in the four types of cancers correlates with reduced patient survival.
At univariate analysis, lymph node metastasis, stage, serosal invasion, cleaved caspase-3 expression as well as grade could predict the prognosis of gastric cancer (P = 0.001, P < 0.001, P = 0.003, P < 0.001, P = 0.001, respectively; Table 6). Elevation of cleaved caspase-3 correlated with poorer OS in patient with gastric cancer. The 5-YSR was 29.1% in the patients with high cleaved caspase-3 expression (MST, 30 months), whereas in patients with low cleaved caspase-3 expression it was 70.2% (MST > 136 months, P < 0.001, Figure 2B). The multivariate analysis revealed that stage and cleaved caspase-3 expression were suggested as independent predictive factors for OS in gastric cancer [Hazard ratio (HR) = 1.768 and P = 0.046, HR = 3.987 and P < 0.001, respectively; Table 6]. For ovarian cancer, the stage, lymph node metastasis, presence of residual tumor and cleaved caspase-3 expression, were found to be significant predictors of patient OS (P < 0.001, P < 0.001, P < 0.001, P < 0.001; respectively; Table 7) according to the univariate analysis. The cases with high cleaved caspase-3 expression saw an MST over 19 months and a 5-YSR of 16.7%, whereas the other remaining patients with low cleaved caspas-e3 expression had an MST over 81 months and a 5-YSR of 66% (P < 0.001, Figure 2C). When all other variables were controlled in the multivariate analysis, stage and cleaved caspase-3 expression retained its prognostic significance (HR = 13.961 and P < 0.001, HR = 2.264 and P = 0.026, respectively; Table 7) in ovarian cancer. Similar results were obtained in cervical cancer. Using Kaplan-Meier analysis method, we found that the protein expression of cleaved caspase-3, stromal invasion, lymph node metastasis, and stage, in cervical cancer, was significantly correlated with patient OS (P = 0.023, P = 0.001, P = 0.014, P < 0.001, respectively; Table 8). The 5-YSR was 54% in the patients with high cleaved caspase-3 expression (MST, 66months), whereas in patients with low cleaved caspase-3 expression it was 92.5% (MST > 81 months, P = 0.002, Figure 2D). To determine whether the expression of cleaved caspase-3 was an independent prognostic factor of outcomes, multivariate survival analysis including stromal invasion, vaginal wall extension, Lymph node metastasis, stage and cleaved caspase-3 were done. Results showed that the expression of cleaved caspase-3 and lymph node metastasis were two potential independent prognostic factor of outcomes of cervical cancer patients in our study (HR = 4.208 and P = 0.012, HR = 5.134 and P = 0.012, respectively; Table 8). In a univariate Cox regression analysis in colorectal cancer patients, the poor overall survival correlated with depth of invasion (P = 0.032), regional lymph node metastasis (P = 0.004), Dukes classification (P < 0.001) and cleaved caspase-3 expression (P < 0.001). A significant reduced MST and 5-YSR for the patients with high cleaved caspase-3 expression was shown comparing to the remaining patients with low cleaved caspase-3 expression (MST: 24 month vs. more than 136 months; 5-YSR: 21.9% vs. 73.9%; P < 0.001, Figure 2E). Moving ahead with the multivariate analysis, we noted that lymph node metastasis (HR = 0.240, P = 0.042) Dukes classification (HR = 6.950, P = 0.005) and cleaved caspase-3 expression (HR = 1.696, P < 0.001) remained significantly associated with OS (Table 9).
Table 6.
Results of univariate and multivariate Cox’s models for overall survival of gastric tumor patients
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
|
|
|||||
| N | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | |||||
| ≤ 50 | 24 | 0.749 (0.416-1.346) | 0.333 | ||
| > 50 | 73 | ||||
| Sex | |||||
| Male | 74 | 0.777 (0.400-1.511) | 0.457 | ||
| Female | 23 | ||||
| Tumor site | |||||
| Corpus gastricum | 13 | 0.949 (0.648-1.388) | 0.786 | ||
| Fundus gastricus | 42 | ||||
| Sinus ventriculi | 42 | ||||
| Lymph node metastasis | |||||
| Negative | 33 | 3.572 (1.739-7.338) | 0.001 | 1.711 (0.711-4.119) | 0.231 |
| Positive | 64 | ||||
| Stage | |||||
| I+II | 56 | 2.759 (1.590-4.787) | < 0.001 | 1.768 (1.009-3.096) | 0.046 |
| III+IV | 41 | ||||
| Therapy | |||||
| Surgery | 47 | 1.594 (0.918-2.769) | 0.098 | ||
| Surgery+others | 50 | ||||
| Serosal invasion | |||||
| Negative | 60 | 2.266 (1.318-3.896) | 0.003 | 1.468 (0.716-3.011) | 0.295 |
| Positive | 37 | ||||
| Caspase-3 | |||||
| Low | 42 | 4.816 (2.465-9.410) | < 0.001 | 3.987 (2.029-7.835) | < 0.001 |
| High | 55 | ||||
| Histology Grade | |||||
| Poor | 56 | 2.853 (1.545-5.268) | 0.001 | 1.225 (0.626-2.396) | 0.554 |
| Otherwise | 41 | ||||
Abbreviations: HR: hazard ratio; 95% CI: 95% confidence interval.
Table 7.
Results of univariate and multivariate Cox’s models for overall survival of ovarian cancer patients
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
|
|
|||||
| N | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | |||||
| < 55 | 37 | 1.906 (0.949-3.828) | 0.070 | ||
| ≥ 55 | 28 | ||||
| Therapy | |||||
| Operation | 4 | 0.738 (0.176-3.090) | 0.677 | ||
| Operation+others | 61 | ||||
| Histological type | |||||
| Serous adenocarcinoma | 26 | 0.548 (0.274-1.099) | 0.090 | ||
| Non-Serous adenocarcinoma | 39 | ||||
| Stage | |||||
| I+II | 27 | 18.180 (4.315-76.591) | < 0.001 | 13.961 (3.220-60.528) | < 0.001 |
| III+IV | 38 | ||||
| Tumor differentiation | |||||
| Poorly | 54 | 0.371 (0.134-1.026) | 0.056 | 1.788 (0.791-4.041) | 0.163 |
| Otherwise* | 11 | ||||
| Lymph node metastasis | |||||
| Negative | 42 | 5.010 (2.421-10.370) | < 0.001 | 0.817 (0.267-2.494) | 0.722 |
| Positive | 23 | ||||
| Residual tumor | |||||
| Negative | 35 | 13.906 (5.241-36.900) | < 0.001 | ||
| Positive | 30 | ||||
| Caspase-3 | |||||
| Low | 47 | 4.538 (2.225-9.259) | 0.001 | 2.264 (1.100-4.661) | 0.026 |
| High | 18 | ||||
Otherwise means well-differentiated carcinoma and moderately-differentiated carcinoma.
Table 8.
Results of univariate and multivariate Cox’s models for overall survival of cervical cancer patients
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
|
|
|||||
| N | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | |||||
| < 55 | 88 | 0.869 (0.195-3.886) | 0.855 | ||
| ≥ 55 | 16 | ||||
| Therapy | |||||
| Operation | 19 | 28.268 (0.098-8.183) | 0.248 | ||
| Operation+others | 85 | ||||
| Stromal invasion | |||||
| Negative | 44 | 10.552 (1.380-80.693) | 0.023 | 3.756 (0.416-33.934) | 0.239 |
| Positive | 60 | ||||
| Vaginal wall extension | |||||
| Negative | 85 | 1.765 (0.553-5.631) | 0.337 | 0.873 (0.243-3.142) | 0.835 |
| Positive | 19 | ||||
| Parametrial extension | |||||
| Negative | 99 | 1.495 (0.195-11.439) | 0.698 | ||
| Positive | 5 | ||||
| Intravascular space involvement | |||||
| Absent | 58 | 1.253 (0.439-3.573) | 0.674 | ||
| Present | 46 | ||||
| Lymph node metastasis | |||||
| Negative | 76 | 7.363 (2.307-23.499) | 0.001 | 5.134 (1.514-17.415) | 0.009 |
| Positive | 28 | ||||
| Histological type | |||||
| SCC | 93 | 1.352 (0.302-6.040) | 0.693 | ||
| ADC+ASC | 11 | ||||
| Stage | |||||
| Early (in situ, I) | 80 | 4.267 (1.337-13.619) | 0.014 | 2.248 (0.621-8.137) | 0.217 |
| Advanced(II, III, IV) | 24 | ||||
| Caspase-3 | |||||
| Low | 93 | 7.478 (2.585-21.632) | <0.001 | 4.208 (1.373-12.894) | 0.012 |
| High | 11 | ||||
Abbreviation: ADC+ASC: adenocarcinoma+adenosquamous carcinoma.
Table 9.
Results of univariate and multivariate Cox’s models for overall survival of colorectal cancer patients
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
|
|
|||||
| N | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | |||||
| ≤ 50 | 31 | 0.242 (0.673-2.292) | 0.488 | ||
| > 50 | 70 | ||||
| Sex | |||||
| Male | 67 | 1.216 (0.696-2.127) | 00.492 | ||
| Female | 34 | ||||
| Tumor diameter, cm | |||||
| < 5 | 31 | 1.148 (0.630-2.093) | 0.652 | ||
| ≥ 5 | 70 | ||||
| Lymph node metastasis | |||||
| Negative | 69 | 2.257 (1.300-3.917) | 0.004 | 0.240 (0.061-0.949) | 0.042 |
| Positive | 32 | ||||
| Dukes classification | |||||
| A+B | 67 | 2.781 (1.606-4.815) | < 0.001 | 6.950 (1.783-27.093) | 0.005 |
| C+D | 34 | ||||
| Therapy | |||||
| Surgery | 34 | 0.864 (0.488-1.531) | 0.617 | ||
| Surgery+others | 67 | ||||
| Depth of tumor invasion | |||||
| Subserosal | 65 | 1.821 (1.052-3.154) | 0.032 | 1.245 (0.664-2.334) | 0.494 |
| Serosal | 36 | ||||
| Caspase-3 | |||||
| Low | 69 | 4.486 (2.573-7.819) | < 0.001 | 1.696 (1.352-2.127) | < 0.001 |
| High | 32 | ||||
| Tumor differentiation | |||||
| Poor | 14 | 1.258 (0.723-2.188) | 0.416 | 0.862 (0.478-1.555) | 0.622 |
| Moderate | 73 | ||||
| Well | 13 | ||||
All these above results showed that high amount of cleaved caspase-3 is significantly associated with shorter survival time in the four kinds of cancer patients and indicated it as an independent prognostic factor.
Discussion
Programmed cell death or apoptosis is a genetically controlled process with important roles during development of multi-cellular organisms. One common feature in the cell death program is this activation of caspases, a highly specialized class of cell death proteases [9]. In addition to an essential role of caspases for apoptosis, recent findings have demonstrated that caspases also have important functions in non-apoptotic processes [12,13]. One such important non-apoptotic process is the induction of compensatory proliferation in apoptotic tissue to maintain tissue homeostasis in multi-cellular organisms. For example, in differentiating eye tissue of Drosophila, apoptosis induces compensatory proliferation through a novel mechanism requiring the effector caspases DrICE and Dcp-1, which induce Hh signaling in a non-apoptotic function [9]. These study have important implications for tumorigenesis in human. A similar argument can be made for chemotherapy and radiotherapy in which the majority of cells are killed by the cytotoxic treatment, whereas only a few cells survive and go on to repopulate the tumor in the case of relapse, thus predict a poor prognosis for the patient. In this process, the few surviving cells that escaped death after exposure to radiotherapy or chemotherapy can rapidly repopulate the badly damaged tumor by proliferating at a markedly accelerated pace [1,2]. This is very obvious in the apoptotic wing or anterior eye discs in Drosophila when apoptosis is blocked by P35. Under these conditions, overgrown wing and eye tissues are observed [8,14,15]. Furthermore, recent evidence showed that caspase-3, the “executor” protease that is the key machinery of cellular death and functions at the terminal stages of apoptosis, hold the key for mobilizing tissue stem and progenitor cells and promoting tumor regeneration [5-7,16]. Thus, dissecting the molecular mechanisms of apoptosis-induced compensatory proliferation could contribute to the understanding of several common diseases like cancer and might provide potential therapeutic targets for treatment. Evidence reveals the key role of the caspase 3-iPLA2-PGE2 signaling pathway in the growth stimulation process, as demonstrated by in vitro gene knockdown and overexpression [5-7]. In this pathway, dying tumor cells activate caspase-3 that results in the ultimate effect of increased surviving tumor cell growth through a mechanism involving secretion of growth-stimulating factors such as PGE2. Studies finding cleaved caspase-3 regulated tumor repopulation in vitro and in vivo have profound implications for the understanding of cancer biology and treatment.
Using IHC analysis, we found that cleaved caspase-3 protein expression predicted stage, serosal invasion, lymph nodes metastasis, and tumor differentiation of gastric cancer patients. As we know, the stage and lymph node status are two prognostic indexes widely used in clinic for gastric cancer [17,18] and poorly differentiated cancer cells of gastric cancer often show stronger aggressive and metastatic ability [19].The relevance between cleaved caspase-3 expression and the above clinicopathological characteristics indicates that cleaved caspase-3 could be used as a potential factor to predict tumor progression and poor prognosis in gastric cancer. Similarly in ovarian cancer, we also observed the level of cleaved caspase-3 expression was well-associated with tumor stage and lymph node metastasis, residual tumor and histology type. Advanced stage, lymph node metastasis and residual tumor left after operation have been reported to be well established conventional poorly prognostic indicators that correlate with outcome of patients with ovarian carcinoma [20]. On the basis of our results, cleaved caspase-3 over-expression may predict ovarian cancer more aggressive and metastatic with poor prognostic potential. In cervical cancer, no statistic significance was found between cleaved caspase-3 expression and other pathological risk factors, such as tumor stage, lymph-node metastasis, stromal invasion, as well as tumor size and vaginal wall invasion, though it displayed a clear trend. Interestingly, we could notice that in all the 104 cervical cancer cases, only 11 cases had high cleaved caspase-3 expression. This may explain the phenomenon that most cervical cancer cases were sensitive to cytotoxic like radiotherapy, especially in the patients with risky prognostic factors [21]. This discovery was compatible with previous finding that caspase-3-mediated repopulation during cytotoxic cancer therapeutics like radiotherapy played a important place causing cancer relapse and insensitivity during therapy [5,7]. The results suggested that enough samples are needed to know the exact role of cleaved caspase-3 in cervical cancer. Meanwhile our results in colorectal cancer also revealed that cleaved caspase-3 expression status was well-associated with lymph node metastasis, depth of invasion and Dukes classification, thus marking cleaved caspase-3 a potential independent prognostic factor of outcomes of colorectal cancer patients.
We further evaluated the prognostic value of cleaved caspase-3 in the four cancer types. The results of the current work indicated a strong correlation between the expression of cleaved caspase-3 and the OS of the 367 cancer patients. Kaplan-Meier analysis showed that patient OS is negatively correlated with the cleaved caspase-3 expression level in gastric cancer, ovarian cancer, and cervical cancer and colorectal cancer, where higher cleaved caspase-3 expression pointed to shorter OS in the four types of cancers individually and in the patients combined. Multivariate analysis results suggested cleaved caspase-3 as an independent prognosis predictor for the four cancer types in the current study.
Cleaved caspase-3 as a prognosis predictor has drawn much attention in recent years. Recent studies, using mouse model and in vitro cell systems has supplied functional evidence that cleaved caspase-3, a cysteine protease involved in the ‘execution’ phase of cellular apoptosis, is a key regulator of tumor repopulation promoting generated from the dying cells [5-7]. To determine the relevance of caspasemediated tumor repopulation in human cancer treatment, Huang et al evaluated cleaved caspase-3 status in two cohorts of human subjects with cancer, one was head and neck cancer subjects, and the other was subjects with advanced stage breast cancer. On the basis of their results, they concluded that elevated tumor cleaved caspase-3 levels predict worse treatment outcomes in people with the two cancer types, and these conclusion has also be demonstrated in the current study. Although we do not fully understand the mechanism by which caspase-3 is activated in pretreatment biopsies, we speculate that cell death is common during tumor growth, and growthpromoting signals from the dying cells may have a role in regulating overall tumor growth even in the absence of cytotoxic treatments. Although further study need to be done to determine a direct role for caspase-3 in stimulating tumor repopulation, The current literature contains several examples of caspases acting in pathways other than apoptosis in mammalian biology, such as caspasemediated stimulation of differentiation [22-24], dedifferentiation of fibroblasts into pluripotent stem cells [16] and T cell activation [25]. In melanoma, basal caspase 3 expression correlates with invasion of melanoma cells [26]. Overall, the clinical survey of cleaved caspase-3 in tumor prognosis are still lacking.
In conclusion, our findings demonstrate that cleaved caspase-3 is well correlated to progression, aggressive behaviors in the studied cancer. Elevated cleaved caspase-3 is associated with shortened OS, pointing it as a potential predictive factor for the prognosis of four types of cancers, namely gastric cancer, ovarian cancer, cervical cancer, colorectal cancer, though our study has some limitations such as failing to describe its prognostic value in terms of tumor recurrence. Currently, pan-caspase inhibitor drugs are in clinical trials for hepatitis C, nonalcoholic steatohepatitis, and liver reperfusion injury with promising results [27-29]. In essence, we believe our finding indicates cleaved caspase-3 as a potential therapeutic target of cancer patients, such as combining caspase-3 inhibitors with the current cytotoxic therapy. Other forms of human cancer and larger studies will be necessary to confirm this data and other prognosis end as tumor relapse should be taken into account to further our understanding of the predictive value of cleaved caspase-3 protein.
Acknowledgements
This work was Supported by the Science Grant from Science and Technology Department of Sichuan Province, China (2012SZ0136) to Shanling Liu, also Supported by the Science Grant from National Natural Science Foundation of China (81270660) to He Wang, and the Young Scientific Innovation Team in Neurological Disorders grant 2011JTD0005 from the Department of Science and Technology of Sichuan Province, China.
Disclosure of conflict of interest
None.
References
- 1.Hermens AF, Barendsen GW. Changes of cell proliferation characteristics in a rat rhabdomyosarcoma before and after x-irradiation. EUR J Cancer. 1969;5:173–189. doi: 10.1016/0014-2964(69)90065-6. [DOI] [PubMed] [Google Scholar]
- 2.Stephens TC, Currie GA, Peacock JH. Repopulation of gamma-irradiated Lewis lung carcinoma by malignant cells and host macrophage progenitors. BR J Cancer. 1978;38:573–582. doi: 10.1038/bjc.1978.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tubiana M. Repopulation in human tumors: A biological background for fractionation in radiotherapy. Acta Oncol. 1988;27:83–8. doi: 10.3109/02841868809090328. [DOI] [PubMed] [Google Scholar]
- 4.Trott KR. Cell repopulation and overall treatment time. Int J Radiat Oncol Biol Phys. 1990;19:1071–5. doi: 10.1016/0360-3016(90)90036-j. [DOI] [PubMed] [Google Scholar]
- 5.Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, O’Sullivan B, He Z, Peng Y, Tan AC, Zhou L, Shen J, Han G, Wang XJ, Thorburn J, Thorburn A, Jimeno A, Raben D, Bedford JS, Li CY. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donato AL, Huang Q, Liu X, Li F, Zimmerman MA, Li CY. Caspase 3 Promotes Surviving Melanoma Tumor Cell Growth after Cytotoxic Therapy. J Invest Dermatol. 2014;134:1686–92. doi: 10.1038/jid.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao P, Smith L, Xie W, Wang M. Dying endothelial cells stimulate proliferation of malignant glioma cells via a caspase 3-mediated pathway. Oncol Lett. 2013;5:1615–1620. doi: 10.3892/ol.2013.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell. 2008;14:399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chera S, Ghila L, Dobretz K, Wenger Y, Bauer C, Buzgariu W, Martinou JC, Galliot B. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev Cell. 2009;17:279–89. doi: 10.1016/j.devcel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, Li CY. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3:ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuranaga E, Miura M. Nonapoptotic functions of caspases: caspases as regulatory molecules for immunity and cell-fate determination. Trends Cell Biol. 2007;17:135–144. doi: 10.1016/j.tcb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007;14:44–55. doi: 10.1038/sj.cdd.4402047. [DOI] [PubMed] [Google Scholar]
- 14.Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol. 2004;14:1262–1266. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Garijo A, Martin FA, Morata G. Caspase inhibition during apoptosis causes abnormal signaling and developmental aberrations in Drosophila. Development. 2004;131:5591–5598. doi: 10.1242/dev.01432. [DOI] [PubMed] [Google Scholar]
- 16.Li F, He Z, Shen J, Huang Q, Li W, Liu X, He Y, Wolf F, Li CY. Apoptotic caspases regulate induction of iPSCs from human fibroblasts. Cell Stem Cell. 2010;7:508–520. doi: 10.1016/j.stem.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Xu H, Wang S, Chen J. Relationship between new TNM classifi cation and the prognosis and biological behavior of gastric cancer. Zhonghua Wai Ke Za Zhi. 2000;38:493–505. [PubMed] [Google Scholar]
- 18.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 19.Strosberg JR, Nasir A, Hodul P, Kvols L. Biology and treatment of metastatic gastrointestinal neuroendocrine tumors. Gastrointest Cancer Res. 2008;2:113–25. [PMC free article] [PubMed] [Google Scholar]
- 20.Trope C. Prognostic factors in ovarian cancer. Cancer Treat Res. 1998;95:287–352. doi: 10.1007/978-1-4615-5447-9_11. [DOI] [PubMed] [Google Scholar]
- 21.Rotman M, Sedlis A Piedmonte MR, Bundy B, Lentz SS, Muderspach LI, Zaino RJ. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2006;65:169–176. doi: 10.1016/j.ijrobp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, Waisman A, Brenner O, Haffner R, Gustafsson E, Ramakrishnan P, Lapidot T, Wallach D. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–2984. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- 23.Szymczyk KH, Freeman TA, Adams CS, Srinivas V, Steinbeck MJ. Active caspase-3 is required for osteoclast differentiation. J Cell Physiol. 2006;209:836–844. doi: 10.1002/jcp.20770. [DOI] [PubMed] [Google Scholar]
- 24.Fujita J, Crane AM, Souza MK, Dejosez M, Kyba M, Flavell RA, Thomson JA, Zwaka TP. Caspase activity mediates the differentiation of embryonic stem cells. Cell Stem Cell. 2008;2:595–601. doi: 10.1016/j.stem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy NJ, Kataoka T, Tschopp J, Budd RC. Caspase activation is required for T cell proliferation. J Exp Med. 1999;190:1891–1896. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu YR, Sun B, Zhao XL, Gu Q, Liu ZY, Dong XY, Che N, Mo J. Basal caspase-3 activity promotes migration, invasion, and vasculogenic mimicry formation of melanoma cells. Melanoma Res. 2013;23:243–53. doi: 10.1097/CMR.0b013e3283625498. [DOI] [PubMed] [Google Scholar]
- 27.Baskin-Bey ES, Washburn K, Feng S, Oltersdorf T, Shapiro D, Huyghe M, Burgart L, Garrity-Park M, van Vilsteren FG, Oliver LK, Rosen CB, Gores GJ. Clinical trial of the pancaspase inhibitor, IDN-6556, in human liver preservation injury. Am J Transplant. 2007;7:218–25. doi: 10.1111/j.1600-6143.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- 28.Shiffman ML, Pockros P, McHutchison JG, Schiff ER, Morris M, Burgess G. Clinical trial: the effi cacy and safety of oral PF-03491390, a pancaspase inhibitor - a randomized placebo-controlled study in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2010;31:969–78. doi: 10.1111/j.1365-2036.2010.04264.x. [DOI] [PubMed] [Google Scholar]
- 29.Ratziu V, Sheikh MY, Sanyal AJ, Lim JK, Conjeevaram H, Chalasani N, Abdelmalek M, Bakken A, Renou C, Palmer M, Levine RA, Bhandari BR, Cornpropst M, Liang W, King B, Mondou E, Rousseau FS, McHutchison J, Chojkier M. A phase 2, randomized, double-blind, placebo-controlled study of GS-9450 in subjects with nonalcoholic steatohepatitis. Hepatology. 2012;55:419–28. doi: 10.1002/hep.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]


