Abstract
Pancreatic adenocarcinoma up-regulated factor (PAUF) expression is elevated in both ovarian tumors and pancreatic adenocarcinoma. However, PAUF expression in ovarian tumors according to histologic subtype and grade has not been investigated. In this study, we examined various clinicopathologic features of 24 patients with mucinous cystadenoma (MCA), 36 with mucinous borderline tumors (MBTs), and 46 with mucinous adenocarcinomas (MACs) according to PAUF expression status assessed using immunohistochemistry. We found that MACs more frequently stained positive for PAUF than did MCAs and MBTs (P < 0.0001). Although there was no significant differences with respect to other clinicopathologic characteristics of MACs according to PAUF expression status, patients with PAUF-weakly positive and PAUF-strongly positive MACs tended to have a shorter overall survival (OS) than those with PAUF-negative MAC, determined using a Kaplan–Meier analysis (P = 0.1885). After adjusting for various clinicopathologic parameters, PAUF positivity of MACs was a significant predictive factor for disease-free survival (DFS) (negative vs. weakly positive: P = 0.045, hazard ratio [HR] = 57.406, 95% confidence interval [CI]: 1.090-3022.596; and negative vs. strongly positive: P = 0.034, HR = 97.890, 95% CI: 1.412-6785.925). In conclusion, PAUF was more frequently expressed in MAC than in its benign and borderline counterparts, and was associated with a poor OS and DFS in MAC patients. Therefore, we suggest that PAUF may be a practical biomarker for histopathological categorization and a prognostic marker for patients with an ovarian mucinous tumor.
Keywords: Mucinous tumor, ovary, PAUF, prognostic factor
Introduction
Ovarian mucinous neoplasm is a common ovarian epithelial tumor. It is categorized as benign mucinous cystadenoma (MCA), mucinous borderline tumor (MBT) of intermediate grade, and malignant mucinous adenocarcinoma (MAC) based on its biological behavior [1,2]. Of these, MCA is the most common ovarian mucinous neoplasm, accounting for 14% of all ovarian tumors and approximately 80% of all primary ovarian mucinous tumors [3]. MBTs, which are associated with an excellent prognosis and have a survival rate of > 90%, are the most common type of borderline ovarian tumor in Asia and the second most common in North America and Europe [4-8]. In contrast, ovarian MAC accounts for 3-4% of all primary ovarian carcinomas and is known to have an aggressive behavior, often recurring early and showing resistance to chemotherapy and radiotherapy [9,10]. It is therefore important to distinguish between these tumor subtypes; however, there are no suitable biomarkers to that can help to differentiate between intermediate and malignant mucinous tumors.
Pancreatic adenocarcinoma up-regulated factor (PAUF) is a novel, tumor-specific protein [11]. It is known to play an important role in cancer progression and metastasis in pancreatic ductal adenocarcinoma [11,12]. In addition, Kim et al. demonstrated that PAUF was also highly expressed in colon and ovarian tumors using Northern blot analysis [11]. However, the expression profiles of PAUF in ovarian neoplasms according to histologic subtype or malignant potential are not known. In this study, we evaluated PAUF expression status according to the histologic grade of ovarian mucinous neoplasms and investigated its potential as a prognostic biomarker.
Materials and methods
Case selection and tumor samples
The study cohort consisted of 24 patients with MCA, 36 patients with MBT, and 46 patients with MAC who had undergone surgery and were diagnosed at Yonsei University Medical Center between 2001 and 2012. This retrospective study was approved by the institutional review board of Yonsei University Medical Center (IRB no. 4-2014-0034). The following clinical parameters were recorded: age at diagnosis, tumor stage, follow-up duration, and survival. We grouped cases of mucinous neoplasms using the International Federation of Gynecology and Obstetrics (FIGO) staging criteria at diagnosis. These groups were a localized stage including FIGO stages IA and IB, a regional stage including FIGO stages IC and II, and a distant stage including FIGO stages III and IV.
Tissue samples were fixed in 10% buffered formalin and embedded in paraffin. Archival tissues stained with hematoxylin and eosin (H&E) were reviewed by two obstetrics and gynecology pathologists (SK Kim and NH Cho). A representative area was selected on an H&E-stained slide and the corresponding area was marked on the formalin-fixed paraffin-embedded (FFPE) tissue block to make a tissue microarray (TMA), with 5 mm tissue cores. The pathologic parameters included the histologic subtype of MBT, invasion patterns of MAC, PAUF expression, estrogen receptor (ER) expression, and Ki-67 labeling index (LI).
Immunohistochemistry
We used an anti-recombinant human PAUF (antirhPAUF) polyclonal antibody (pAb) which was kindly provided by Dr. Sun A Kim [11]. Immunohistochemistry was performed using 5-μm-thick sections cut from a TMA block using a microtome. These were transferred onto adhesive slides and dried at 70°C for 30 min. Immunohistochemistry with anti-rhPAUF pAb (diluted 1:200), ER antibody (Thermo Scientific, diluted 1:100), and Ki-67 (Abcam, diluted 1:1000) was performed using a Dako Envision Kit following the manufacturers’ instructions.
Interpretation of immunohistochemical staining
We divided ovarian mucinous tumors into three groups based on the immunostaining intensity of PAUF: 0 (negative), 1 (weakly positive) and 2 (strongly positive). The proportion of PAUF-stained cells was scored as 0 (negative), 1 (< 10%) and 2 (> 10%). The final score for each tumor was calculated by multiplying the immunostaining intensity by the proportion of stained cells, and was then categorized as follows: 0-1, negative; 2, weakly positive; and 4, strongly positive.
A cut-off value of > 1% of nuclei that were strongly stained was used to define ER expression [13]. To establish the cutoff Ki-67 LI, we analyzed the Ki-67 LI of ovarian mucinous tumors using receiver operator characteristic (ROC) curves according to PAUF positivity and determined that 1.167% (sensitivity: 70.4%, 1 - specificity: 84.8%) was the optimal cutoff value.
Statistics
Statistical analyses were performed using GraphPad Prism 5 software, version 5.01 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA). For the analysis of age at diagnosis, a significant difference between means was determined by analysis of variances (ANOVA). Follow-up durations of patients with MBT and MAC were compared using a t-test. FIGO stage, histologic subtypes, invasion patterns, and the expressions of PAUF, ER, and Ki-67 LI were compared using the chi-square and Fisher’s exact test. Kaplan-Meier survival curves and log-rank statistics were employed to evaluate overall survival (OS) and disease-free survival (DFS). Univariate and multivariate regression analyses were performed using the Cox proportional hazards model. All reported P-values are two-sided, and P-values <0.05 were considered statistically significant.
Results
Clinicopathologic features of ovarian mucinous neoplasms
Ovarian mucinous neoplasms are categorized as benign, intermediate, or malignant based on their pathologic features, and are referred to as MCA, MBT, and MAC, respectively [2]. We selected 24 cases of MCA, 36 cases of MBT, and 46 cases of MAC and compared the clinicopathologic features of these ovarian mucinous neoplasms (Table 1).
Table 1.
Clinicopathologic features of ovarian mucinous neoplasms
| Mucinous cystadenoma | Mucinous borderline tumor | Mucinous adenocarcinoma | P-value | |
|---|---|---|---|---|
| Number of cases | 24 | 36 | 46 | |
| Age at diagnosis (years, mean ± SD) | 49.25 ± 3.632 | 44.36 ± 2.502 | 43.26 ± 2.122 | 0.2951 |
| Stage (%) | < 0.0001 | |||
| Localized | - | 34 (94.44) | 22 (47.83) | |
| Regional | - | 2 (5.56) | 14 (30.43) | |
| Distant | - | 0 | 10 (21.74) | |
| Histologic subtype (%) | ||||
| Intestinal | - | 30 (83.33) | - | |
| Endocervical | - | 6 (16.67) | - | |
| Invasion pattern (%) | ||||
| Expansile | - | - | 37 (80.43) | |
| Infiltrative | - | - | 9 (19.57) | |
| PAUF expression (%) | < 0.0001 | |||
| Negative | 16 (66.67) | 27 (75.00) | 10 (21.74) | |
| Weakly positive | 7 (29.17) | 5 (13.89) | 19 (41.30) | |
| Strongly positive | 1 (4.17) | 4 (11.11) | 17 (36.96) |
SD, standard deviation.
There was no significant difference in the median age at diagnosis of patients with MCA (49.25 ± 3.632 years), MBT (44.36 ± 2.502 years), and MAC (43.26 ± 2.122, P = 0.2951). We grouped cases of MBTs and MACs based on FIGO stage and categorized them as being localized, regional, or distant. Most MBT cases were at a localized stage, whereas most MCA cases were at a regional or distant stage (P < 0.0001).
For a more detailed analysis, we subdivided MBTs into two groups according to the histologic features of their epithelial component: intestinal type or endocervical type [2]. According to the most recent World Health Organization classification of female reproductive organs, endocervical-type MBTs are considered a subset of seromucinous tumors, referred to as seromucinous borderline tumors [14]. However, to date, seromucinous borderline tumors have been reported as endocervical-type MBTs and compared with other intestinal-type MBTs. Therefore, we included endocervical-type MBTs (seromucinous borderline tumors) in this study to clarify the clinicopathological features of intestinal-type MBTs. Thirty of the 36 MBTs (83.33%) had an epithelial component resembling intestinal epithelium, and the other six cases (16.67%) exhibited mucinous epithelial cells resembling endocervical epithelium. This finding is similar to those of previous reports, in which the intestinal type accounted for 85-90% of MBTs and the endocervical type for 10-15% [7,15-17].
MACs were subdivided into expansile and infiltrative types according to their invasion patterns, rather than a grading system, because it is well established that the current grading system for mucinous carcinoma can predict neither tumor behavior nor treatment response [15,16,18]. However, infiltrative stromal invasion has proved to be more biologically aggressive than expansile invasion [2]. We found infiltrative invasion growth in nine out of 46 MACs (19.57%) and an expansile invasion pattern in 37 out of 46 MACs (80.43%).
PAUF expression status according to subtypes of ovarian mucinous tumors
We performed immunohistochemical staining with antirhPAUF pAb on FFPE tissue of ovarian mucinous neoplasms, and interpreted PAUF reactivity as negative, weakly positive, or strongly positive as described above (Figure 1A). Interestingly, MACs most frequently demonstrated weak PAUF positivity (19 of 46 cases, 41.30%) and strong PAUF positivity (17 of 46 cases, 36.96%), in contrast to MCAs (weakly positive: 29.17%, strongly positive: 4.17%) and MBTs (weakly positive: 13.89%, strongly positive: 8.33%; Table 1 and Figure 1B, P < 0.0001). Thus, strongly positive PAUF staining was more common in ovarian mucinous tumors with more aggressive behavior.
Figure 1.
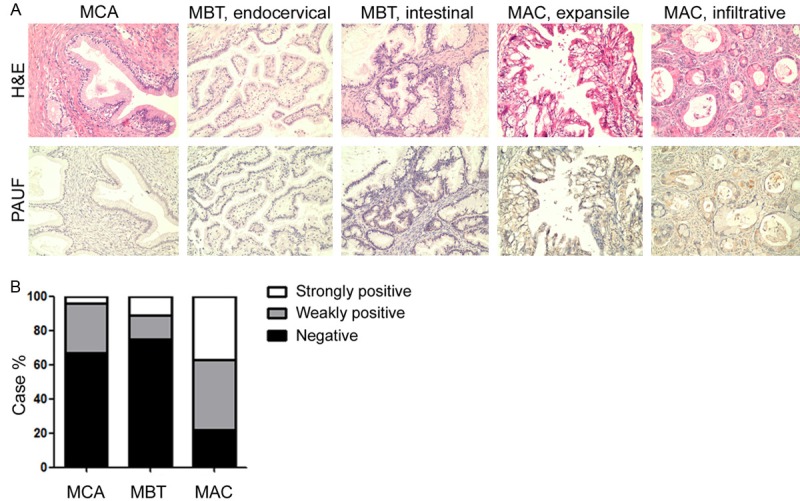
PAUF expression in ovarian mucinous neoplasms. A. Immunohistochemical staining for PAUF protein in mucinous neoplasms of the ovary. PAUF-negative tumor cells (bottom, two left panels), PAUF-weakly positive tumor cells (bottom, middle of the panels), and PAUF-strongly positive tumor cells (bottom, two right panels). Magnification, ×200. B. The proportion of tumors with strong, weak, or negative PAUF expression according to histologic grade. MCA, mucinous cystadenoma; MBT, mucinous borderline tumor; MAC, mucinous adenocarcinoma.
Clincopathologic features of MBTs according to PAUF expression status
We next compared the clinicopathologic features of MBTs according to their PAUF expression status (Table 2). Although only four of 36 MBTs were strongly positive for PAUF, these cases tended to occur 10 years earlier (31.00 ± 2.273 years) than their PAUF negative (45.96 ± 2.864 years) or weakly positive PAUF (46.40 ± 7.954 years) equivalents, although these differences were not statistically different (P = 0.1697). Differences in tumor stage according to PAUF expression status could not be statistically analyzed because most MBTs were at a localized stage, except for two cases at a regional stage.
Table 2.
Clinicopathologic features of 36 MBTs according to PAUF expression status
| PAUF expression | P-value | |||
|---|---|---|---|---|
|
|
||||
| Negative | Weakly positive | Strongly positive | ||
| Number of cases | 27 (75.00) | 5 (13.89) | 4 (11.11) | |
| Age at diagnosis (years, mean ± SD) | 45.96 ± 2.864 | 46.40 ± 7.954 | 31.00 ± 2.273 | 0.1697 |
| Stage | ||||
| Localized | 25 | 5 | 4 | |
| Regional | 2 | 0 | 0 | |
| Distant | 0 | 0 | 0 | |
| Histologic subtype | 0.1466 | |||
| Intestinal | 24 | 4 | 2 | |
| Endocervical | 3 | 1 | 2 | |
| ER status | 0.1466 | |||
| Negative | 24 | 4 | 2 | |
| Positive | 3 | 1 | 2 | |
| Ki-67 LI | 0.0863* | |||
| < 1.167% | 4 | 1 | 3 | |
| ≥ 1.167% | 23 | 4 | 1 | |
SD, standard deviation.
We calculated P-value using Fisher’s exact test after grouping PAUF weakly positive and strongly positive MBTs together as PAUF-positive MBTs and compared them with PAUF-negative MBTs, because the chi-square calculation is not valid when all expected values are < 1.
Chi-square calculations are usually only valid when all expected values are > 1, and this condition was not met when we assessed the pathologic features of MBTs according to PAUF expression status. Therefore, we combined MBTs that were either weakly or strongly positive for PAUF as a single PAUF-positive group (n = 9) and compared their pathologic parameters with those of the PAUF-negative group (n = 27) using Fisher’s exact test. This revealed no statistically significant differences with respect to histologic subtype or ER status. However, all of the endocervical MBTs were ER positive, as they are known [19]. There were also no significant differences between PAUF-positive and negative MBTs with respect to the Ki-67 LI, a marker of proliferative activity.
MAC PAUF expression is associated with shorter OS and DFS
As described above, MACs more frequently stain positive for PAUF than other ovarian mucinous tumors. We therefore compared the clinicopathologic features of MACs according to PAUF expression (Table 3). There was no significant difference in age at diagnosis, tumor stage, and invasion pattern in MACs with respect to PAUF expression.
Table 3.
Clinicopathologic features of 46 MACs according to PAUF expression status
| PAUF expression | P-value | |||
|---|---|---|---|---|
|
|
||||
| Negative | Weakly positive | Strongly positive | ||
| Number of cases | 10 | 19 | 17 | |
| Age at diagnosis (years, mean ± SD) | 40.40 ± 3.933 | 39.58 ± 2.838 | 49.06 ± 3.957 | 0.1095 |
| Follow-up (months, mean ± SD) | 69.10 ± 12.96 | 53.74 ± 8.538 | 37.53 ± 5.829 | 0.0696 |
| Overall survival (%) | 10/10 (100.000) | 14/19 (73.684) | 13/17 (76.471) | 0.1885 |
| Disease-free survival (%) | 9/10 (87.500) | 13/19 (60.014) | 12/17 (63.995) | 0.3732 |
| Stage | 0.2205 | |||
| Localized | 3 | 10 | 9 | |
| Regional | 3 | 4 | 7 | |
| Distant | 4 | 5 | 1 | |
| Invasion pattern (%) | 0.5464 | |||
| Expansile | 8 | 14 | 15 | |
| Infiltrative | 2 | 5 | 2 | |
SD, standard deviation.
When we compared the OS and DFS of MAC patients according to PAUF expression status using a Kaplan-Meier analysis, patients with MACs staining weakly or strongly for PAUF tended to have a shorter OS than those negative for PAUF (Figure 2, P = 0.1885). We conducted a statistical analysis to identify predictive factors for survival of ovarian MAC patients using a Cox regression assay (Table 4). In univariate analysis, age at diagnosis (P = 0.002, hazard ratio [HR] = 1.097, 95% confidence interval [CI]: 1.034-1.164), tumor stage (localized vs. distant, P = 0.016, HR = 7.099, 95% CI: 1.431-35.206), and invasion pattern (expansile vs. infiltrative, P = 0.091, HR = 3.117, 95% CI: 0.835-11.636) affected the OS of MAC patients. Age at diagnosis (P = 0.001, HR = 1.090, 95% CI: 1.038-1.146), tumor stage (localized vs. distant, P = 0.006, HR = 9.175, 95% CI: 1.899-44.334), and invasion pattern (expansile vs. infiltrative, P = 0.088, HR = 2.724, 95% CI: 0.863-8.599) also affected the DFS of MAC patients. There was a tendency towards a shorter DFS in patients with MACs that stained strongly for PAUF than that in those with PAUF-negative MACs (P = 0.197, HR = 4.134, 95% CI: 0.480-35.618). When we adjusted various parameters by multivariate analysis, older age at diagnosis and a distant tumor stage were associated with a shorter OS and DFS amongst MAC patients (Table 5). In particular, PAUF expression in MACs was an independent predictive factor for DFS (negative vs. weakly positive PAUF staining, P = 0.045, HR = 57.406, 95% CI: 1.090-3022.596 and negative vs. strongly positive PAUF staining, P = 0.034, HR = 97.890, 95% CI: 1.412-6785.95), although there was no statistical difference in DFS according to PAUF expression status using Kaplan-Meier survival analysis.
Figure 2.

Survival analysis of patients with ovarian mucinous adenocarcinoma according to PAUF expression. A. Overall survival of MAC patients according to PAUF expression status. B. Disease-free survival of MAC patients according to PAUF expression status.
Table 4.
Univariate analysis for overall survival and disease-free survival
| Parameter | Overall survival | Disease-free survival | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| P-value | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | |
| Age | 0.002 | 1.097 | 1.034-1.164 | 0.001 | 1.090 | 1.038-1.146 |
| Stage | 0.014 | 0.009 | ||||
| Localized vs. regional | 0.808 | 0.741 | 0.067-8.256 | 0.385 | 2.213 | 0.369-13.263 |
| Localized vs. distant | 0.016 | 7.099 | 1.431-35.206 | 0.006 | 9.175 | 1.899-44.334 |
| Invasion | ||||||
| Expansile vs. infiltrative | 0.091 | 3.117 | 0.835-11.636 | 0.088 | 2.724 | 0.863-8.599 |
| PAUF | 0.960 | 0.425 | ||||
| Negative vs. weakly positive | 0.948 | 0.230 | 3.655 | 0.440-30.369 | ||
| Negative vs. strongly positive | 0.947 | 0.197 | 4.134 | 0.480-35.618 | ||
Table 5.
Multivariate analysis for overall survival and disease-free survival
| Parameter | Overall survival | Disease-free survival | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| P-value | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | |
| Age | 0.042 | 1.116 | 1.004-1.240 | 0.044 | 1.083 | 1.002-1.170 |
| Stage | 0.024 | 0.080 | ||||
| Localized vs. regional | 0.378 | 0.313 | 0.024-4.131 | 0.882 | 1.159 | 0.164-8.184 |
| Localized vs. distant | 0.021 | 23.365 | 1.605-340.079 | 0.037 | 9.941 | 1.142-86.496 |
| Invasion | ||||||
| Expansile vs. infiltrative | 0.640 | 0.626 | 0.088-4.450 | 0.823 | 1.218 | 0.216-6.884 |
| PAUF | 0.809 | 0.099 | ||||
| Negative vs. weakly positive | 0.941 | 0.045 | 57.406 | 1.090-3022.596 | ||
| Negative vs. strongly positive | 0.937 | 0.034 | 97.890 | 1.412-6785.925 | ||
Discussion
PAUF, a novel tumor-specific protein, is known to be associated with cancer progression and metastasis in pancreatic ductal adenocarcinoma and ovarian tumors [11,12]. MBTs are the most common type of borderline ovarian tumor and generally have an excellent prognosis. In contrast, MACs, although far less common, show an aggressive behavior [4-10]. We therefore hypothesized that PAUF expression status might be different between MBTs and MACs.
As expected, we found that MACs were more frequently positive for PAUF expression than other ovarian mucinous tumors, with an increasing frequency of strong PAUF staining in MCA, MBT, and MAC tumor types. This suggests that PAUF could be a sensitive and specific biomarker for distinguishing between intermediate and malignant mucinous tumors of the ovary.
We also failed to find any significant differences in PAUF expression status of MBTs with respect to a range of clinicopathological features, which might reflect the generally low PAUF expression in these tumors. However, for MACs, PAUF expression tended to be higher in patients with a shorter OS. When we adjusted for possible confounders using multivariate Cox regression analysis, weak or strong PAUF expression in MAC was associated with a shorter DFS.
Our findings indicate that PAUF expression can help categorize ovarian mucinous tumors, and predicts shorter OS and DFS amongst MAC patients. Thus, PAUF could act as a prognostic and diagnostic biomarker for patients with an ovarian mucinous tumor.
Acknowledgements
This study was supported by grants from the National Research Foundation (NRF) of Korea Grant funded by the Korean Government (No. 2011-0010800 and 2014-002926; EJN) and the Basic Science Research Program through the NRF of Korea funded by the Mid-career Researcher Program by the MEST (No. 2012R1A2A4A01006435; CHO).
Disclosure of conflict of interest
None.
References
- 1.Zaloudek CF. Diagnostic Histopathology of Tumors. Philadelpiha, USA: Elsevier; 2007. [Google Scholar]
- 2.Lee KR, Tavassoli FA, Prat J, Dietel M, Gersell DJ, Karseladze AI, Hauptmann S, Rtugers JKL, Russell P, Buckley CH, Pisani P, Schwartz PE, Goldgar DE, Silva EG, Caduff R, Kubik-Huch R. Tumours of the Breast and Female Genital Organs. Lyon, France: International Agency for Research on Cancer (IARC); 2003. [Google Scholar]
- 3.Hart WR. Mucinous tumors of the ovary: a review. Int J Gynecol Pathol. 2005;24:4–25. [PubMed] [Google Scholar]
- 4.Khunamornpong S, Settakorn J, Sukpan K, Suprasert P, Siriaunkgul S. Mucinous tumor of low malignant potential (“borderline” or “atypical proliferative” tumor) of the ovary: a study of 171 cases with the assessment of intraepithelial carcinoma and microinvasion. Int J Gynecol Pathol. 2011;30:218–230. doi: 10.1097/PGP.0b013e3181fcf01a. [DOI] [PubMed] [Google Scholar]
- 5.Kaern J, Trope CG, Abeler VM. A retrospective study of 370 borderline tumors of the ovary treated at the Norwegian Radium Hospital from 1970 to 1982. A review of clinicopathologic features and treatment modalities. Cancer. 1993;71:1810–1820. doi: 10.1002/1097-0142(19930301)71:5<1810::aid-cncr2820710516>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Russell P, Merkur H. Proliferating ovarian “epithelial” tumours: a clinico-pathological analysis of 144 cases. Aust N Z J Obstet Gynaecol. 1979;19:45–51. doi: 10.1111/j.1479-828x.1979.tb01352.x. [DOI] [PubMed] [Google Scholar]
- 7.Siriaunkgul S, Robbins KM, McGowan L, Silverberg SG. Ovarian mucinous tumors of low malignant potential: a clinicopathologic study of 54 tumors of intestinal and mullerian type. Int J Gynecol Pathol. 1995;14:198–208. doi: 10.1097/00004347-199507000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kehoe S, Powell J. Long-term follow-up of women with borderline ovarian tumors. Int J Gynaecol Obstet. 1996;53:139–143. doi: 10.1016/0020-7292(95)02642-8. [DOI] [PubMed] [Google Scholar]
- 9.Schiavone MB, Herzog TJ, Lewin SN, Deutsch I, Sun X, Burke WM, Wright JD. Natural history and outcome of mucinous carcinoma of the ovary. Am J Obstet Gynecol. 2011;205:480, e1–8. doi: 10.1016/j.ajog.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 10.Zaino RJ, Brady MF, Lele SM, Michael H, Greer B, Bookman MA. Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal: a Gynecologic Oncology Group study. Cancer. 2011;117:554–562. doi: 10.1002/cncr.25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SA, Lee Y, Jung DE, Park KH, Park JY, Gang J, Jeon SB, Park EC, Kim YG, Lee B, Liu Q, Zeng W, Yeramilli S, Lee S, Koh SS, Song SY. Pancreatic adenocarcinoma up-regulated factor (PAUF), a novel up-regulated secretory protein in pancreatic ductal adenocarcinoma. Cancer Sci. 2009;100:828–836. doi: 10.1111/j.1349-7006.2009.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y, Kim SJ, Park HD, Park EH, Huang SM, Jeon SB, Kim JM, Lim DS, Koh SS. PAUF functions in the metastasis of human pancreatic cancer cells and upregulates CXCR4 expression. Oncogene. 2010;29:56–67. doi: 10.1038/onc.2009.298. [DOI] [PubMed] [Google Scholar]
- 13.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobel M, Bell DA, Carcangiu ML, Oliva E, Prat J, Shihl M, Soslow R, Vang R. WHO Classification of Tumours of Female Reproductive Organs. Lyon, France: International Agency for Research on Cancer (IARC); 2014. [Google Scholar]
- 15.Kikkawa F, Kawai M, Tamakoshi K, Suganuma N, Nakashima N, Furuhashi Y, Kuzuya K, Hattori S, Arii Y, Tomoda Y. Mucinous carcinoma of the ovary. Clinicopathologic analysis. Oncology. 1996;53:303–307. doi: 10.1159/000227577. [DOI] [PubMed] [Google Scholar]
- 16.Lee KR, Scully RE. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with ‘pseudomyxoma peritonei’. Am J Surg Pathol. 2000;24:1447–1464. doi: 10.1097/00000478-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Rutgers JL, Scully RE. Ovarian mullerian mucinous papillary cystadenomas of borderline malignancy. A clinicopathologic analysis. Cancer. 1988;61:340–348. doi: 10.1002/1097-0142(19880115)61:2<340::aid-cncr2820610225>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 18.Hoerl HD, Hart WR. Primary ovarian mucinous cystadenocarcinomas: a clinicopathologic study of 49 cases with long-term follow-up. Am J Surg Pathol. 1998;22:1449–1462. doi: 10.1097/00000478-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Vang R, Gown AM, Barry TS, Wheeler DT, Ronnett BM. Immunohistochemistry for estrogen and progesterone receptors in the distinction of primary and metastatic mucinous tumors in the ovary: an analysis of 124 cases. Mod Pathol. 2006;19:97–105. doi: 10.1038/modpathol.3800510. [DOI] [PubMed] [Google Scholar]


